Summary
Background and objectives
This study characterizes the pathologic and clinical relationships of thrombotic microangiopathy (TMA) to antibody-mediated rejection (AMR) in renal allograft biopsies.
Design, setting, participants, & measurements
Consecutive renal allograft biopsies, routinely stained for C4d over a period of 51 months (n = 1101), were reviewed. For comparative analysis of histology and clinical features, additional patients with TMA and peritubular capillary (PTC) C4d (n = 5) were combined with those identified in the 51-month period of review (n = 6).
Results
One hundred eighty-two of 1073 adequate biopsies from 563 allografts had PTC C4d in the study period. Six of 37 biopsies with TMA had PTC C4d (five at ≤90 days and one at 213 days). Early (≤90 days) C4d+ biopsies (n = 5) had more frequent TMA (11.9% C4d+ versus 3.4% C4d−; odds ratio, 3.84; P = 0.03). Graft loss was significantly greater in an early C4d+TMA+ group (n = 5 study + 2 archival patients) than in C4d+ controls without TMA (n = 21) (57% versus 9.5%; P = 0.02). Early TMA+C4d+ biopsies had more severe glomerulopathy and less severe arteriolopathy than TMA+C4d− and had more frequent neutrophilic capillaritis than TMA−C4d+ biopsies.
Conclusions
TMA was infrequent in this series of unselected, consecutive, renal allograft biopsies (3.4%). PTC C4d may be a significant risk factor for early TMA, and TMA is associated with glomerular thrombi and neutrophilic capillaritis. TMA in allografts with suspected AMR may portend a higher risk of graft loss.
Introduction
Thrombotic microangiopathy (TMA) in the transplant kidney is a form of renal vascular injury that may be associated with many disorders including calcineurin inhibitor (CI) toxicity, antibody-mediated rejection (AMR), infections, malignant hypertension, and recurrent diseases like hemolytic uremic syndrome or scleroderma (1). Linear peritubular capillary (PTC) C4d staining is a sensitive and specific marker of AMR (2–4); however, diagnosis requires demonstration of donor-specific antibodies and graft injury (5). TMA has been noted in 4 to 46% of patients with AMR, with the highest frequency in the early post-transplantation period (3,6–8). Glomerular thrombi are described in AMR, and arteriolar thrombi have been described in some (9,10). Thrombi in PTC are described rarely (10). PTC mural platelet deposits may be observed at sites of severe capillary injury, with endothelial loss and interstitial hemorrhage in AMR (11).
Diagnosis of AMR requires identification of PTC C4d, graft injury, and donor-specific antibodies (5). Many patients in this series lacked serologic data, and so these allografts had immunopathologic lesions suspicious for AMR. Here we hypothesized that allografts with immunopathologic features of AMR, identified by PTC C4d and graft injury, are at greater risk for the development of TMA. To test the hypothesis, we examined the concurrence of TMA and PTC C4d in unselected consecutive renal allograft biopsies, routinely stained for C4d, over a period of 51 months. We combined the patients from the observation period with the few other examples from our files (n = 6) to facilitate morphologic and clinical analyses. The pathologic features in TMA+C4d+ allograft biopsies were compared with TMA+C4d− and TMA−C4d+ biopsies to elucidate differences in morphology. The effect of TMA on graft survival in suspected AMR was determined by comparing the outcome of C4d+TMA+ and a matched control group of C4d+TMA− allografts in the year after biopsy diagnosis.
Materials and Methods
Consecutive unselected renal allograft biopsies accessioned between December 1, 2004 and February 1, 2009 were included in the study. Approval for this study was obtained from the University of Chicago Institutional Review Board (protocol number 16619B). Biopsies had been obtained for allograft dysfunction and not by protocol. Renal allograft biopsies (n = 1101) were routinely stained for C4d over this period of 51 months and had been reviewed by one of three renal pathologists. Twenty-eight biopsies were excluded for insufficiently representative tissue (n = 21) and inadequate clinical data (n = 7). TMA was defined by the following histologic features: occlusive fibrin-platelet thrombi in at least one glomerulus or one arteriole, with one or more of the following: (1) glomerular endothelial swelling and detachment, capillary wall thickening and double contour formation, mesangial lysis with microhemorrhage, and erythrocytolysis and/or (2) obliterative arteriolopathy defined as luminal occlusion with mural myxoid or fibrinoid change, thickening of the vessel wall, with or without erythrocytolysis, luminal thrombosis, and concentric spindle cell proliferation or hypercellularity.
Light microscopic diagnoses and Banff 1997 histologic indices (5) were tabulated from hematoxylin and periodic acid Schiff stained sections (n = 12 to 18 per biopsy). Polymorphonuclear neutrophilic (PMN) inflammation in capillaries was graded as follows: (1) glomeruli: 0, none; 0.5+, 2 or less PMN; 1+, 3 to 5 PMN; 2+, 6 to 10 PMN; 3+, more than 10 PMN in the most affected glomeruli and (2) peritubular capillaries: 0, none; 0.5+, 2 or less PMN in 10% or less of the PTC; 1+, >2 PMN in 11 to 25% of PTC; 2+, >2 PMN in 26 to 50% of PTC; 3+, >2 PMN in >50% PTC.
Indirect immunofluorescence staining using standard protocols was routinely performed on 4-μ frozen sections of core biopsies frozen at −20°C. The primary antibody was a monoclonal mouse anti-human C4d (clone 10-11; Biogenesis, Sandown, NH). Paraffin sections were stained in <5% of patients using previously published methods (12). C4d status, defined as the extent of involvement of PTC by linear deposition of C4d, was also recorded and correlated with histology. Focal C4d deposition was defined as 5 to 50%, and diffuse was defined as >50% mural staining of the peritubular capillary network of cortex and medulla. Kidney transplant nephrectomies were excluded.
Clinical data were obtained by retrospective chart review including age, sex, primary kidney disease, donor status, HLA mismatch, induction therapy, maintenance immunosuppressive agents, CI blood levels, clinical diagnosis at time of biopsy, treatment immediately postbiopsy, and 1-year outcomes. Serum creatinine at baseline was defined as the single lowest serum creatinine in up to 3 months before biopsy. Serum creatinine at the time of biopsy and at 1-year follow-up were also recorded. Patients with delayed graft function, defined as a requirement for hemodialysis in the first week post-transplantation, were considered to have a serum creatinine of 5 mg/dl. The CI levels included were from the time of biopsy and averaged over a 3-week period before biopsy, when available, and the results are expressed in ng/ml. All of the grafts were ABO blood group-compatible.
Induction therapy consisted of methylprednisolone plus either anti-IL-2 receptor antibody or anti-thymocyte globulin. The most common maintenance immunosuppressive regime consisted of tacrolimus, mycophenolic acid (mycophenolate mofetil or mycophenolate sodium), and prednisone. Patients who had a renal allograft nephrectomy and a creatinine of 5.0 mg/dl or more and those who required renal replacement therapy for greater than 8 weeks were defined as graft failure. At 1-year of follow-up, patients with graft failure were arbitrarily assigned a creatinine of 5 mg/dl for statistical analysis. To provide a reference end point for TMA episodes occurring at various time points in the post-transplantation period, graft failure in the first year after biopsy diagnosis of TMA was chosen as the most suitable index of graft loss.
Anti-donor antibodies were detected using flow cytometric cross-match and solid-phase assays. Three color flow cytometric cross-match analysis was performed by modification of a previously described dual-color technique (13). Donor-specific antibodies were detected by solid-phase assay. Antibody specificities were identified using the Luminex platform and the HLA phenotype and the single antigen panels as targets (14–16). The phenotype panels used were Lifematch classes I and II (Tepnel LifeCodes, Stamford, CT) and Labscreen classes I and II (One Lambda, Inc., Canoga Park, CA), and the single antigen panels were single antigen beads (One Lambda, Inc.). Donor-specific antibodies were defined as antibodies present in recipient sera with mean fluorescence intensity ≥1000 and directed against HLA antigens present on the donor cells.
Clinical records were evaluated to exclude other possible causes of allograft TMA including malignant hypertension, hepatitis C infection, parvovirus B-19 infection, cytomegalovirus infection, graft irradiation, lupus anticoagulants, and primary or recurrent hemolytic uremic syndrome or thrombotic thrombocytopenic purpura, to the extent possible.
For comparative analysis of the effect of TMA on graft loss, five additional C4d+TMA+ archival patients were combined with the biopsies identified in the initial observational analysis (n = 6 of 1073 biopsies). Histologic features of early TMA+C4d+ biopsies (n = 5 + 2 = 7) were compared with TMA+C4d− biopsies from the early post-transplantation period (90 days or less) identified in the initial analysis (n = 9). Causes of end-stage disease in the TMA+C4d+ group included insulin-dependent diabetes mellitus (n = 3), hypertension (n = 2), scleroderma (n = 1), and an unknown cause (n = 1). Causes of end-stage kidney disease in the TMA+C4d− (n = 9) group included insulin-dependent diabetes (n = 2), hypertension (n = 3), membranous glomerulopathy (n = 2), medullary cystic disease (n = 1), and an unknown cause (n = 1).
Biopsies in the C4d+ control group were frequency-matched for time post-transplantation, recipient age, type of graft, induction, and baseline immunosuppression (n = 35). The effect of TMA on AMR was evaluated by comparison of clinical features of TMA+C4d+ patients and controls with PTC C4d but without TMA (TMA−C4d+, n = 21 early biopsies and n = 14 late biopsies).
Group proportions were analyzed using the χ2 and Fisher exact tests. Two group comparisons were made using the Mann-Whitney test for nonparametric data.
Results
Association of TMA and AMR
One thousand seventy-three biopsies from 563 allografts had adequate tissue and clinical data for inclusion in the study. One hundred eighty-two had PTC C4d expression (16.8%). C4d expression was focal in 52 and diffuse in 130. Thirty-seven of 1073 biopsies had TMA (3.4% of the total). Six biopsies (0.6% of total) had TMA and PTC C4d expression (focal in one and diffuse in five). Thirty-one biopsies had TMA without C4d expression in the PTC (3.5% of all C4d negative biopsies). Tables 1 and 2 summarize the association of TMA and C4d expression. The probability of observing TMA in C4d+ biopsies for the group as a whole (n = 182), given by the odds ratio (OR), is 0.95 (95% confidence interval, 0.4 to 2.3; P = 0.92). This observation is, however, confounded by the time after transplantation at which the biopsy was obtained. Coincidence of TMA+ and C4d+ was observed more frequently in early post-transplantation biopsies, obtained at 90 days or less after transplantation, compared with later biopsies (1.6% versus 0.13%; P = 0.003). The probability of observing TMA with PTC C4d in early biopsies (11.9%) was significantly greater than in C4d− biopsies (3.4%; OR, 3.8; 95% confidence interval, 1.2 to 12.1; P = 0.03). In contrast, TMA+C4d+ biopsies were less frequent (0.72%) than TMA+C4d− biopsies (3.5%; OR, 0.2; 95% confidence interval, 0.03 to 1.5; P = 0.09) for renal allograft biopsies obtained 90 days or more after transplantation. PTC C4d positivity is therefore a significant risk factor for TMA only in the early post-transplantation period.
Table 1.
Summary of results of TMA and PTC C4d status
| All |
Early (≤90 Days) |
Late |
||||
|---|---|---|---|---|---|---|
| n | % | n | % | n | % | |
| C4d+ | 182 | 17 | 43 | 16.3 | 139 | 22.2 |
| TMA+ | 37 | 3.4 | 14 | 4.6 | 23 | 3 |
| TMA+C4d+ | 6 | 0.6 | 5 | 1.6 | 1 | 0.13 |
| TMA+C4d− | 31 | 2.9 | 9 | 3 | 22 | 2.87 |
| TMA+C4d+/TMA+ | 6/37 | 16.2 | 5/14 | 35.7 | 1/23 | 4.5 |
| Total | 1073 | 307 | 766 | |||
Table 2.
Association of TMA and PTC C4d
| TMA+ |
TMA− |
OR | P | |||
|---|---|---|---|---|---|---|
| n | % | n | % | |||
| For all biopsies | ||||||
| C4d+ | 6 | 3.3 | 176 | 96.7 | 0.95 | 0.92 |
| C4d− | 31 | 3.5 | 860 | 96.5 | 95% CI (0.4 to 2.3) | |
| By time of biopsy | ||||||
| early (≤90 days) | ||||||
| C4d+ | 5 | 11.9 | 38 | 88.1 | 3.84 | 0.03 |
| C4d− | 9 | 3.4 | 255 | 96.6 | 95% CI (1.2 to 12.1) | |
| late | ||||||
| C4d+ | 1 | 0.72 | 138 | 99.28 | 0.2 | 0.09 |
| C4d− | 22 | 3.5 | 605 | 96.5 | 95% CI (0.03 to 1.5) | |
Comparative Analysis of TMA with and without PTC C4d
Histologic findings in early TMA+C4d+ and TMA+C4d− biopsies are given in Table 3. Nine TMA+C4d− biopsies were obtained ≤90 days, and 22 were obtained >90 days after transplantation. Glomerular thrombi (Figure 1A) were more frequent (100% versus 33.3%) and more extensive (28.3% versus 1.9% of glomeruli; P = 0.0015) in TMA+C4d+ biopsies from the early period. In contrast, acute arteriolopathic lesions (thrombi and/or obliterative arteriolopathy) were seen with higher frequency in early C4d− biopsies (78% versus 43%; P = 0.3), and these lesions tended to be more extensive in C4d− biopsies (lesions in >25% of sampled arterioles in 55.6% versus 0%; P = 0.03). The frequency and severity of arteriolar hyalinization was greater in the C4d− biopsies (55.6% versus 42.9%; P = 0.9; and Banff arteriolar hyalinosis score >1 in 44.4% versus 0%; P = 0.08). TMA in the absence of PTC C4d was attributable to CI toxicity (n = 7), malignant hypertension (n = 1), and transplanted TMA due to disseminated intravascular coagulation (n = 1). Diagnosis of CI toxicity was on the basis of histologic findings of hyaline or obliterative arteriolopathy (n = 7), with or without elevated blood levels of CI, return of serum creatinine to within 10% of baseline on reduction of the administered dose of CI, and the absence of other plausible explanations for arteriolopathy. Neutrophilic glomerulitis and peritubular capillaritis were more frequent in C4d+ biopsies (P = 0.0009 and 0.001, respectively). Intimal arteritis was also more frequent in the C4d+ biopsies (57% versus 0%; P = 0.019). The other histologic parameters assessed were not significantly different between these groups (Table 3). The TMA+C4d+ biopsy group had higher levels of serum creatinine at the time of biopsy compared with TMA+C4d− biopsies. Tacrolimus blood levels were not significantly different (14.8 versus 10.5 ng/ml; P = 0.57). The clinical details are summarized in Table 4.
Table 3.
Comparative histologic findings in early TMA
| C4d+ | C4d− | P | |
|---|---|---|---|
| n | 7 | 9 | |
| Glomeruli | |||
| total glomeruli (mean) | 24 | 18 | |
| thrombi - segmental | 100 | 33.3 | 0.01 |
| thrombi - global | 28.6 | 0 | 0.2 |
| mesangiolysis | 28.6 | 0 | 0.2 |
| double contours | 42.9 | 22.2 | 0.6 |
| neutrophilic glomerulitis | 100 | 0 | 0.0009 |
| transplant glomerulitis | 42.9 | 0 | 0.06 |
| global glomerulosclerosis | 71.4 | 88.9 | 0.55 |
| segmental glomerulosclerosis | 0 | 22.2 | 0.48 |
| ischemic glomerulopathy | 85.7 | 66 | 0.6 |
| Peritubular capillaries | |||
| peritubular capillaritis - neutrophils | 100 | 11.1 | 0.001 |
| peritubular capillaritis - mononuclear | 57.1 | 11.1 | 0.1 |
| Arterioles | |||
| obliterative arteriolopathy | 42.9 | 77.8 | 0.3 |
| arteriolar thrombi | 42.9 | 44.4 | 1 |
| arteriolar hyalinosis | 42.9 | 77.8 | 0.3 |
| Arteries | |||
| endothelialitis | 57.1 | 0 | 0.01 |
| arterial intimal fibrosis | 42.9 | 44.4 | 1 |
| Tubules and interstitium | |||
| ATN | 85.7 | 66 | 0.58 |
| interstitial mononuclear cells | 85.7 | 100 | 0.4 |
| tubulitis | 42.9 | 44.4 | 1 |
| interstitial hemorrhage | 42.9 | 0 | 0.06 |
| interstitial fibrosis | 42.9 | 55.6 | 0.99 |
| tubular atrophy | 28.6 | 55.6 | 0.36 |
The values are the percentages of biopsies with lesions in each group. ATN, acute tubular necrosis.
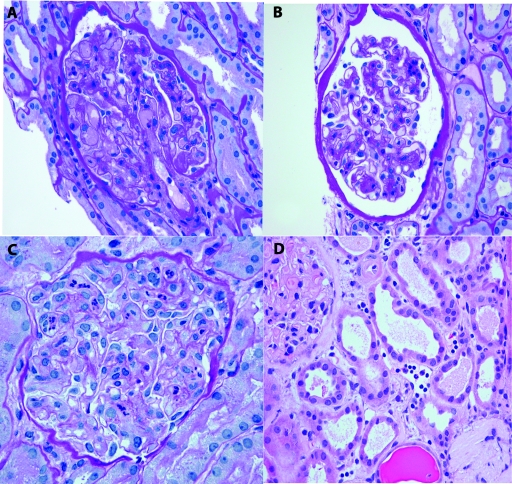
Figure 1.
(A) Glomerular capillary thrombi, segmental ischemia, and neutrophil infiltrates in a kidney allograft biopsy from post-transplant day 10 (periodic acid-Schiff (PAS)). (B) Neutrophilic glomerulitis (score 2+) in a nonthrombosed glomerulus from the same biopsy as A (PAS). (C) Neutrophilic glomerulitis (score 3+) without capillary thrombi (PAS). (D) Peritubular capillaritis with neutrophils and mononuclear cells. Tubules have acute tubular injury (hematoxylin and eosin).
Table 4.
Clinical data in early allograft biopsy groups
| TMA+C4d+ (n = 7) | TMA+C4d− (n = 9) | Pa | TMA−C4d+ (n = 21) | Pb | |
|---|---|---|---|---|---|
| Mean age (years) | 46 | 45.3 | 36.4 | ||
| Gender (women:men) | 3:4 | 2:7 | 11:10 | ||
| Cause of ESRD | |||||
| diabetic nephropathy | 3 | 2 | 0.6 | 3 | 0.29 |
| hypertensive nephrosclerosis | 2 | 3 | 1 | 6 | 1 |
| primary TMA | 0 | 0 | – | 1 | – |
| primary glomerular disease | 0 | 2 | 0.48 | 6 | 0.28 |
| other | 2 | 2 | 1 | 5 | 1 |
| Type of transplant | |||||
| deceased donor | 6 | 7 | 1 | 14 | 0.63 |
| living donor | 1 | 2 | 1 | 7 | 0.63 |
| kidney only | 6 | 7 | 1 | 16 | 1 |
| first transplant | 6 | 9 | 0.44 | 11 | 0.19 |
| HLA mismatches (mean) | 5 | 5.25 | 0.7 | 4.5 | 0.63 |
| Pretransplant cross-match | |||||
| positive | 1 | 0 | 2 | ||
| negative | 5 | 9 | 0.4 | 14 | 1 |
| unknown | 1 | 0 | 5 | ||
| Immunosuppression induction | |||||
| T cell depleting antibody | 5 | 5 | 0.63 | 14 | 1 |
| anti-IL-2 receptor antibody | 0 | 5 | 0.03 | 5 | 0.37 |
| steroids | 5 | 7 | 1 | 14 | 1 |
| unknown | 0 | 0 | 3 | ||
| Baseline | |||||
| tacrolimus | 7 | 7 | 0.5 | 14 | 0.29 |
| cyclosporin | 0 | 0 | 1 | ||
| sirolimus | 0 | 0 | 0 | ||
| mycophenolate mofetil | 7 | 9 | 1 | 16 | 1 |
| prednisone | 7 | 8 | 1 | 17 | 1 |
| unknown | 0 | 0 | 3 | ||
| Mean time of biopsy (days) | 25.9 | 26.3 | 0.9 | 21 | 0.33 |
| Serum creatinine | |||||
| baseline (median) | 1.8 | 1.5 | 0.9 | 4.5 | 0.75 |
| at biopsy (median) | 4.4 | 2.2 | 0.06 | 2.5 | 0.24 |
| at 1-year follow up (median) | 5 | 1.9 | 0.1 | 1.5 | 0.05 |
| Calcineurin inhibitor levels | |||||
| tacrolimus, at biopsy | 14.8 (6) | 10.5 | 0.57 | 9.5 (12) | 0.14 |
| tacrolimus, 3-week average | 9.9 (5) | 10.6 | 0.92 | 10.7 (12) | 0.75 |
| Treatment after biopsy | |||||
| steroid pulse | 4 | 0 | – | 6 | 0.65 |
| plasmapheresis | 2 | 0 | – | 3 | 0.9 |
| thymoglobulin | 3 | 0 | – | 7 | 1 |
| IvIg | 4 | 0 | – | 13 | 0.32 |
| rituximab | 2 | 0 | – | 6 | 1 |
| discontinuation of CI | 0 | 1 | – | 0 | – |
| reduction of CI dose | 0 | 4 | – | 0 | – |
| OKT3 | 1 | 0 | – | 0 | – |
| unknown | 1 | 1 | 5 | ||
| Graft loss | 4 | 1 | 2 | ||
| Graft survival | 3 | 8 | 0.1 | 19 | 0.02 |
TMA+C4d+ versus TMA+C4d−.
TMA+C4d+ versus TMA−C4d+.
In late biopsies the frequency and severity of glomerular capillary thromboses were similar in C4d+ (n = 1 from the observation period + 3 archival patients) and C4d− biopsies (n = 22). All of the late TMA+C4d+ biopsies had diffuse glomerular capillary double contours, and all had diffuse C4d deposits in glomerular capillary walls and PTC. Glomerular capillary double contours were evident less frequently in late TMA−C4d+ biopsies (100% TMA+ versus 32% TMA−; P = 0.4). The frequency of acute transplant glomerulitis (75% versus 13.6%; P = 0.027), neutrophilic glomerulitis and peritubular capillaritis (50% versus 4.5%; P = 0.052), and mononuclear peritubular capillaritis (100% versus 18.2%; P = 0.005) were significantly greater in the C4d+ group. Other histologic parameters assessed were not significantly different between these groups (data not shown).
Effects of TMA on C4d-positive Rejection Episodes
Baseline demographic and clinical data for the early TMA+C4d+ and TMA−C4d+ groups are shown in Table 4. Pretransplant standard and flow cytometric cross-matches were positive in one of seven patients in the TMA+C4d+ group and negative in all of the patients in the TMA−C4d+ group. Serologic evidence of post-transplantation anti-donor alloreactivity was available in two patients from the TMA+C4d+ group; one had a positive flow cytometric cross-match, and one was donor-specific antibody (DSA)-positive. Eleven of 21 controls had serologic evaluation, nine were DSA-positive, and two had undetectable DSAs. Allograft function was not significantly different between the groups at baseline and at the time of biopsy. At 1 year of follow-up after the biopsy, the median serum creatinine was higher in C4d+ patients with TMA compared with those without TMA (5 versus 1.5 mg/dl; P = 0.05). Treatment for rejection was similar in each group. Graft failure occurred in 57.1% of TMA+C4d+ patients compared with 9.5% of the TMA−C4d+ group at follow-up 1 year after the biopsy (P = 0.02). In allograft biopsies obtained >3 months after transplantation, graft loss was 100% in three of three TMA+C4d+ allografts with available follow-up data, compared with 28.6% of TMA−C4d+ biopsies (n = 14; P = 0.05).
Banff 97 histologic lesions of acute transplant glomerulitis had comparable frequency in C4d+ biopsies with and without TMA (Table 5). Careful evaluation revealed at least one PMN in one glomerulus in 82% and one PMN in one PTC in 68% of the C4d+ biopsies in these two groups. Neutrophilic glomerulitis (Figure 1, B and C), with more than two neutrophils per glomerulus (score 1+ or more) was more frequent in TMA+ biopsies (100% versus 14.3%; P = 0.002). The proportion of glomeruli with any neutrophils was greater in TMA+ biopsies than in biopsies without TMA (53% (range, 29 to 87%) versus 20.3% (range, 3 to 45%); P = 0.0021). Neutrophilic peritubular capillaritis (Figure 1D) with more than two PMN in more than 10% of the PTC (score 1+ or more) was more frequent in TMA+ biopsies (100% versus 23.8%; P = 0.007). Ischemic glomerulopathy, defined as segmental or global wrinkling of the glomerular basement membrane, reduced tuft area, and periglomerular fibrosis, was more frequent in the TMA+ biopsies (85.7% versus 23.8%; P = 0.007). The frequency of type 1 (14.3% versus 9.6%) and 2 (57% versus 23.8%) T cell-mediated rejection was not significantly different between these groups. TMA+C4d+ grafts lost in the year of follow-up tended to have a higher frequency of transplant glomerulopathy with mesangial lysis and capillary basement membrane duplication (100% versus 33.3%; P = 0.1) than grafts surviving more than 1 year.
Table 5.
Histologic comparison of early C4d+ biopsies with and without TMA
| TMA+ | TMA− | P | |
|---|---|---|---|
| n | 7 | 21 | |
| Glomeruli | |||
| total glomeruli (mean) | 24 | 21 | |
| thrombi | 100 | 0 | <0.0001 |
| mesangiolysis | 42.8 | 0 | 0.01 |
| double contours | 42.8 | 0 | 0.01 |
| neutrophilic glomerulitisa | 100 | 14.3 | 0.0001 |
| acute transplant glomerulitis | 57.1 | 47.6 | 0.99 |
| global glomerulosclerosis | 71.4 | 23.8 | 0.06 |
| segmental glomerulosclerosis | 0 | 4.8 | – |
| ischemic glomerulopathy | 85.7 | 23.8 | 0.007 |
| Peritubular capillaries | |||
| peritubular capillaritis - neutrophilsb | 100 | 23.8 | 0.007 |
| peritubular capillaritis - mononuclear | 57.1 | 52.4 | 1 |
| Arterioles | |||
| obliterative arteriolopathy | 57.1 | 0 | 0.002 |
| arteriolar thrombi | 42.8 | 0 | 0.01 |
| arteriolar hyalinosis | 42.8 | 47.6 | 1 |
| Arteries | |||
| endothelialitis | 57.1 | 23.8 | 0.16 |
| arterial intimal fibrosis | 42.8 | 47.6 | 1 |
| Tubules and interstitium | |||
| ATN | 100 | 76.2 | 0.29 |
| interstitial mononuclear cells | 85.7 | 71.4 | 0.64 |
| tubulitis | 42.8 | 28.6 | 0.65 |
| interstitial hemorrhage | 42.8 | 28.6 | 0.65 |
| interstitial fibrosis | 57.1 | 14.3 | 0.04 |
| tubular atrophy | 11.8 | 14.3 | 0.57 |
The values are the percentages of biopsies with lesions in each group. ATN, acute tubular necrosis.
Proportion of biopsies with more than two neutrophils in the worst affected glomeruli.
Proportion of biopsies with more than two neutrophils in more than 10% of the PTC.
Discussion
The study finds a low frequency of concurrence of TMA and PTC C4d staining in renal allograft biopsy specimens obtained for graft dysfunction. Although the number of index TMA+C4d+ patients is small, the study population was derived from unselected consecutive biopsy specimens obtained over more than 4 years. Only six of 182 biopsies with PTC C4d had TMA, and 83% of these were from the early post-transplantation period (<3 months). Most patients in this series lacked donor-specific antibody data, and so these allografts had immunopathologic lesions suspicious for AMR. However, if we accept that PTC C4d staining and tissue injury are reliable diagnostic markers of AMR, then the hypothesis that allografts with immunopathologic features of AMR are at increased risk for TMA is supported by the observations of this study only in the early period after transplantation. These findings are in general agreement with the observations of others (6,9,17). The presence of TMA with AMR appears to predict a worse prognosis for graft survival in the year after diagnosis.
The reported frequency of TMA in suspected AMR varies from 4.1% (16) to as high as 49.5% in a study of 21 high-risk patients with PTC C4d (7). Such variation in frequency is difficult to explain but may reflect selectivity of subjects, variation in criteria used for definition of TMA, variation in immunosuppressive regimes, clinical course after transplantation, and primary diseases. Analysis of studies that provide numerical data on the association of PTC C4d deposition with TMA (summarized in Table 6) indicates that significant association of these lesions is observed only in biopsies from the first few months after transplantation consistent with the observations of this study. One study (9) identified a high frequency of TMA in AMR (43%), defined as graft dysfunction with circulating DSAs; however, the study predated recognition of the significance of C4d.
Table 6.
Odds ratios by individual studies
| Study | C4d | TMA |
OR | P | Time of Biopsy | |
|---|---|---|---|---|---|---|
| + | − | |||||
| Regele (17) | + | 3 | 39 | 4.5 (0.45 to 45.2) | 0.3 | Median 14 days (1 to 532 days) |
| − | 1 | 59 | ||||
| Nickeleit (3) | + | 12 | 108 | 1.6 (0.7 to 3.4) | 0.3 | Median 38 days (7 to 5646 days) |
| − | 18 | 260 | ||||
| Mauiyyedi (6) | + | 4 | 16 | incalculable | 0.006 | <3 months |
| − | 0 | 47 | ||||
| Trpkov (9)a | + | 11 | 13 | 5.6 (1.3 to 24.2) | 0.02 | <45 days |
| DSA data | − | 3 | 20 | |||
| Regele (4) | + | 3 | 70 | 3 (0.5 to 18) | 0.34 | >1 year |
| − | 2 | 138 | ||||
| This study | + | 5 | 38 | 3.8 (1.2 to 12.1) | 0.03 | ≤90 days |
| − | 9 | 255 | ||||
| + | 1 | 138 | 0.2 (0.03 to 1.5) | 0.09 | >90 days | |
| − | 22 | 605 | ||||
Data are for DSA status as C4d data were not available.
Lesions of TMA in TMA+C4d+ biopsies differed from the TMA+C4d− biopsies. Glomerular thrombi, mesangial lysis, and capillary double contours were more frequent and severe in C4d+ biopsies. Arteriolar lesions were more severe in the C4d− biopsies. One study of early biopsies with rejection and DSAs (9) described severe arteriolopathy resembling hemolytic uremic syndrome; however, the patients in that study had been exposed to cyclosporine immunosuppression, and hence the possible contribution of CI toxicity is hard to exclude. Observation of predominantly arteriolar lesions in TMA+C4d− biopsies in this study is consistent with patterns of injury described in CI toxicity (18,19) and malignant hypertension.
Neutrophilic glomerulitis and peritubular capillaritis were more prominent in the TMA+C4d+ biopsies compared with TMA−C4d+ biopsies. It is possible that neutrophilic inflammation predisposes to microvascular thrombosis; however, the possibility that these infiltrates may be a consequence rather than a cause of thrombosis needs to be excluded. The absence of significant intracapillary neutrophils in TMA+C4d− biopsies in our cohort suggests that microthrombi do not necessarily incite neutrophil infiltrates. One study (20) identified a strong association of TMA and PTC neutrophil margination in HLA-incompatible grafts with PTC C4d expression and a positive cross-match. Thus it is possible that an association of intravascular neutrophils and the development of thrombi may be specific to AMR (8,20).
In conclusion, the study findings suggest that AMR is a risk factor for TMA in renal allografts in the early post-transplantation period. Biopsies with suspected AMR had TMA in a minority of patients (11.9%). Prominent glomerular capillary thrombi and neutrophilic capillaritis involving the glomeruli and peritubular capillaries are characteristic histologic features. The concurrence of immunopathologic features of AMR and TMA may portend a greater likelihood of graft loss attributable to the influence of TMA.
Disclosures
None.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1. Chiurchiu C, Ruggenenti P, Remuzzi G: Thrombotic microangiopathy in renal transplantation. Ann Transplant 7: 28–33, 2002 [PubMed] [Google Scholar]
- 2. Collins AB, Schneeberger EE, Pascual MA, Saidman SL, Williams WW, Tolkoff-Rubin N, Cosimi AB, Colvin RB: Complement activation in acute humoral renal allograft rejection: Diagnostic significance of C4d deposits in peritubular capillaries. J Am Soc Nephrol 10: 2208–2214, 1999 [DOI] [PubMed] [Google Scholar]
- 3. Nickeleit V, Zeiler M, Gudat F, Thiel G, Mihatsch MJ: Detection of the complement degradation product C4d in renal allografts: Diagnostic and therapeutic implications. J Am Soc Nephrol 13: 242–251, 2002 [DOI] [PubMed] [Google Scholar]
- 4. Bohmig GA, Exner M, Habicht A, Schillinger M, Lang U, Kletzmayr J, Säemann MD, Hörl WH, Watschinger B, Regele H: Capillary C4d deposition in kidney allografts: A specific marker of alloantibody-dependent graft injury. J Am Soc Nephrol 13: 1091–1099, 2002 [DOI] [PubMed] [Google Scholar]
- 5. Solez K, Colvin RB, Racusen LC, Haas M, Sis B, Mengel M, Halloran PF, Baldwin W, Banfi G, Collins AB, Cosio F, David DS, Drachenberg C, Einecke G, Fogo AB, Gibson IW, Glotz D, Iskandar SS, Kraus E, Lerut E, Mannon RB, Mihatsch M, Nankivell BJ, Nickeleit V, Papadimitriou JC, Randhawa P, Regele H, Renaudin K, Roberts I, Seron D, Smith RN, Valente M: Banff 07 classification of renal allograft pathology: Updates and future directions. Am J Transplantation 8: 753–760, 2008 [DOI] [PubMed] [Google Scholar]
- 6. Mauiyyedi S, Crespo M, Collins AB, Schneeberger EE, Pascual MA, Saidman SL, Tolkoff-Rubin NE, Williams WW, Delmonico FL, Cosimi AB, Colvin RB: Acute humoral rejection in kidney transplantation: II. Morphology, immunopathology, and pathologic classification. J Am Soc Nephrol 13: 779–787, 2002 [DOI] [PubMed] [Google Scholar]
- 7. Lefaucher C, Nochy D, Hill GS, Suberbielle-Boissel C, Antoine C, Charron D, Glotz D: Determinants of poor graft outcome in patients with antibody-mediated acute rejection. Am J Transplant 7: 832–841, 2007 [DOI] [PubMed] [Google Scholar]
- 8. Truong LD, Barrios R, Adrogue HE, Gaber LW. Acute antibody-mediated rejection of renal transplant: pathogenetic and diagnostic considerations. Arch Pathol Lab Med 131: 1200–1208, 2007 [DOI] [PubMed] [Google Scholar]
- 9. Trpkov K, Campbell P, Pazderka F, Cockfield S, Solez K, Halloran PF: Pathologic features of acute renal allograft rejection associated with donor specific antibody. Transplantation 61: 1586–1592, 1996 [DOI] [PubMed] [Google Scholar]
- 10. Halloran PF, Wadgymar A, Ritchie S, Falk J, Solez K, Srinivasa NS: The significance of the anti-class I response: I. Clinical and pathologic features of anti-class I mediated rejection. Transplantation 49: 85–91, 1990 [DOI] [PubMed] [Google Scholar]
- 11. Meehan SM, Limsrichamrern S, Manaligod JR, Junsanto T, Josephson MA, Thistlethwaite JR, Haas M: Platelets and capillary injury in acute humoral rejection of renal allografts. Hum Pathol 34: 533–540, 2003 [DOI] [PubMed] [Google Scholar]
- 12. Poduval RD, Kadambi PV, Josephson MA, Cohn RA, Harland RC, Javaid B, Huo D, Manaligod JR, Thistlethwaite JR, Meehan SM: Implications of immunohistochemical detection of C4d along peritubular capillaries in late acute renal allograft rejection. Transplantation 79: 228–235, 2005 [DOI] [PubMed] [Google Scholar]
- 13. Bray RA, Lebeck LK, Gebel HM: The flow cytometric crossmatch: Dual-color analysis of T cell and B cell reactivities. Transplantation 48: 834–840, 1989 [PubMed] [Google Scholar]
- 14. Kao KJ, Scornik JC, Small SJ: Enzyme-linked immunoassay for anti-HLA antibodies: An alternative to panel studies by lymphocytotoxicity. Transplantation 55: 192–196, 1993 [DOI] [PubMed] [Google Scholar]
- 15. Pei R, Wang G, Tarsitani C, Rojo S, Chen T, Takemura S, Liu A, Lee J: Simultaneous HLA class I and class II antibodies screening with flow cytometry. Hum Immunol 5: 313–322, 1998 [DOI] [PubMed] [Google Scholar]
- 16. Pei R, Lee J-H, Chen T, Rojo S, Terasaki PI: Flow cytometric detection of HLA antibodies using a spectrum of microbeads. Human Immunol 60: 1293–1302, 1999 [DOI] [PubMed] [Google Scholar]
- 17. Regele H, Exner M, Watschinger B, Wenter C, Wahrmann M, Österreicher C, Saemann M, Merisch N, Horl WH, Zlabinger GJ, Bohmig GA: Endothelial C4d deposition is associated with inferior kidney allograft outcome independently of cellular rejection. Nephrol Dial Transplant 16: 2058–2066, 2001 [DOI] [PubMed] [Google Scholar]
- 18. Mihatsch MJ, Thiel G, Ryffel B. Histopathology of cyclosporine nephrotoxicity. Transplant Proc 20: 759–771, 1988 [PubMed] [Google Scholar]
- 19. Mihatsch MJ, Kyo M, Morozumi K, Yamaguchi Y, Nickeleit V, Ryffel B: The side effects of ciclosporine-A and tacrolimus. Clin Nephrol 49: 356–363, 1998 [PubMed] [Google Scholar]
- 20. Haas M, Rahman MH, Racusen LC, Kraus ES, Bagnasco SM, Segev DL, Simpkins CE, Warren DS, King KE, Zachary AA, Montgomery RA: C4d and C3d staining in biopsies of ABO- and HLA-incompatible renal allografts: Correlation with histologic findings. Am J Transplantation 6: 1829–1840, 2006 [DOI] [PubMed] [Google Scholar]