Summary
Background and objectives
Kidney re-transplantation (KRT) candidates are considered at high risk for graft failure. Most of these patients are kept on a chronic steroid maintenance (CSM) regimen. The safety of early steroid withdrawal (ESW) remains unanswered in KRT.
Design, setting, participants, & measurements
This study was aimed at comparing the outcomes of ESW and CSM in KRT. Retrospective analysis of 113 KRT patients (ESW, n = 59; CSM, n = 54) was performed. All patients received rabbit anti-thymocyte globulin/steroid induction and were maintained on mycophenolate/tacrolimus (±steroids).
Results
One- and 5-year patient survival for the ESW and the CSM group were not significantly different (98 versus 96% and 91 versus 88%, respectively; P = 0.991). No significant difference was seen in the graft survival for both groups at 1 and 5 years (98 versus 93% and 80 versus 74%, respectively; P = 0.779). Mean 1- and 5-year estimated GFR was not statistically different between the groups (P = 0.773 and 0.790, respectively). The incidence of acute rejection at 1 year was 17 and 22% in ESW and CSM patients, respectively (P = 0.635). Compared with the ESW group, patients in the CSM group were more likely to be hyperlipidemic (P = 0.044), osteoporotic (P = 0.010), post-transplant diabetics (P = 0.051) and required more medications to control BP (P = 0.004).
Conclusions
ESW seems to be a reasonable approach in KRT recipients because the short and intermediate patient survival, graft survival, and graft function is comparable to CSM immunosuppression.
Introduction
The survival of kidney transplants has improved during the last few decades, but long-term graft loss remains an important problem (1). Immunologic (rejection) and nonimmunologic factors (glomerular disease, fibrosis, medical, unknown) contribute to graft loss (2). After losing a graft, some patients are listed for kidney re-transplantation (KRT). This group of re-listed patients has experienced steady growth during the last decade (3). In 2008 in the United States, 18% of wait listed and 13% of those receiving a kidney transplant had been transplanted previously (4). The survival of KRT is only slightly lower than that of the first transplant (5–8).
A patient's survival is improved by KRT compared with dialysis after primary graft failure (9,10). KRT recipients have traditionally been considered as a higher graft failure risk than those transplanted for the first time (9). Most of these patients therefore traditionally have been kept on chronic steroid maintenance (CSM). This increased risk for graft loss is frequently attributed to increased levels of preformed allo-antibodies (11).
The potential benefits of steroid-free maintenance after renal transplantation are well recognized (12–15). The long-term complications associated with CSM therapy include osteopenia, weight gain, hypertension, hyperlipidemia, hyperglycemia, increased cardiovascular morbidity, and increased susceptibility to infections, making aggressive steroid withdrawal an attractive goal (13,15,16). Most kidney transplant recipients, when asked which immunosuppressive agent they would most like to discontinue, choose steroids (17).
Modern immunosuppressive regimens have made rapid steroid withdrawal more feasible. The safety of discontinuing steroid therapy within the first week after transplant has been confirmed in patients receiving a calcineurin inhibitor, mycophenolate mofetil, and induction with an IL-2 receptor antagonist, alemtuzumab, or a polyclonal antibody (rabbit anti-thymocyte globulin) (12,14–16,18–23). Almost all of these studies address the primary kidney transplant. The safety of this approach in the KRT population remains unanswered.
This single center retrospective analysis compares the short and intermediate outcomes for KRT recipients that received early steroid withdrawal (ESW) with those who remained on the CSM regimen. To our knowledge, this is the first analysis of ESW in KRT patients compared with CSM.
Materials and Methods
Study Population
Approval for the study was obtained from the Institutional Review Board at the Indiana University School of Medicine. The data were collected from the kidney transplant program database at the Indiana University School of Medicine, Indianapolis, IN. Reviewed data consisted of 113 KRT patients who met the following inclusion criteria: (1) kidney retransplantations performed between January 1, 2003 and April 30, 2009 in patients with only a kidney transplant in the past and (2) recipients above the age of 18 years at the time of transplantation. The patients who received pretransplant desensitization or were undergoing re-transplant with another visceral organ were excluded. The studied KRT recipients were divided into two groups based on maintenance immunosuppression regimen, ESW and CSM, as defined below.
Immunosuppression
Rabbit anti-thymocyte globulin (r-ATG) was used for induction at a dose of 1.5 mg/kg per day for 4 days, along with methylprednisolone 500 mg (first doses for both given intraoperatively), followed by prednisone 120 mg on postoperative day (POD) 1; 80 mg on POD 2; 40 mg on POD 3; and 20 mg on POD 4. Maintenance immunosuppression regimen included tacrolimus to target blood trough levels of 6 to 8 ng/ml for the first year and 5 to 6 ng/ml thereafter, along with mycophenolate mofetil at 1000 mg oral twice daily. CSM was defined as a postdischarge daily maintenance prednisone dose of 5 mg orally. ESW was defined as no steroids after POD 4. Steroids were a part of our routine maintenance immunosuppression regimen for all KRT patients. Since early 2005, we switched to ESW for all KRT regardless of the cause of prior kidney allograft loss and the duration of previous allograft survival, so the likelihood of a selection bias is extremely low. This also explains a longer follow-up of the CSM group patients.
Diagnosis and Treatment of Rejection
Transplant biopsies were performed when clinically indicated for elevated serum creatinine (after excluding reversible causes) and/or significant proteinuria (>1 g). All acute rejection episodes were documented by histopathologic examination and reported according to Banff classification. Treatment for acute rejection consisted of Solu-Medrol pulse (three doses) and then an oral prednisone taper over 10 days. Steroid-resistant acute rejection was treated with r-ATG (1.25 mg/kg daily for 7 to 10 days). There was selective use of plasmapheresis/intravenous immune globulin/rituximab for biopsy-proven acute antibody-mediated rejection.
Donor and Recipient Characteristics
Several variables were investigated in both groups, including (1) demographic characteristics of the recipients (age, gender, race, original kidney disease, time on dialysis); (2) donor characteristics (living, deceased, age, race, cause of death, kidney pulsatile perfusion, cold ischemia time); and (3) factors related to current transplant (number of prior transplants, panel reactive antibody levels, number of HLA mismatches, type of induction, and maintenance immunosuppression and acute rejection). Data on metabolic profile of the recipients 1 year after KRT in both groups (systolic and diastolic BP, number of BP medications, fasting blood sugars, number of patient with post-transplant diabetes mellitus, LDL levels, serum cholesterol, use of anti-lipid medications, and use of bisphosphonate therapy) was also evaluated.
Graft loss was defined as initiation of chronic renal replacement therapy with any form of dialytic therapy or death with a functioning graft.
Outcome measures included (1) patient and graft survival over 5 years, (2) graft function as assessed by serum creatinine (mg/dl) and reported as estimated GFR (eGFR) using the Modification of Diet in Renal Disease equation, and (3) time to biopsy-proven rejection.
Statistical Analyses
Although this is a retrospective review, all donor and recipient data for this analysis were collected prospectively in an electronic database used for clinical management and research. Continuous variables are presented as mean ± SD or median with range; categorical values are presented as percentages. Comparison between categorical variables was made by Fisher exact test. Comparison between continuous variables was made with the two-tailed unpaired t test. Survival analysis was performed by the methods of Kaplan-Meier with patient and graft survival censored on day of last follow-up; survival curve differences were compared using the log-rank test. Cox proportional model was used for multivariate analysis of the effect of two or more variables on survival. For the purpose of this study, P ≤ 0.05 was considered statistically significant. ESW versus CSM patients was analyzed on intent-to-treat basis.
Results
Characteristics of the study population are presented in Table 1. The majority of the patients were maintained on steroids as a part of their previous immunosuppression protocol. Nine patients (15%) undergoing pre-emptive transplantation in the ESW group were still on steroids at the time of KRT.
Table 1.
Donor and recipient characteristics
| ESW (n = 59) | CSM (n = 54) | P | |
|---|---|---|---|
| Recipient | |||
| age (years) | 42 + 13 | 43 + 12 | 0.975 |
| male | 66% (n = 39) | 59% (32) | 0.452 |
| ethnicity | |||
| white | 78% (46) | 81% (44) | 0.642 |
| African American | 19% (11) | 11% (6) | 0.263 |
| others | 3% (2) | 8% (4) | 0.341 |
| ESRD diagnosis | |||
| GN | 34% (20) | 48% (26) | 0.123 |
| congenital | 24% (14) | 21% (11) | 0.667 |
| hypertension | 17% (10) | 11% (6) | 0.373 |
| diabetes mellitus | 13% (8) | 7% (4) | 0.289 |
| others | 12% (7) | 13% (7) | 0.859 |
| PRA | 34 + 25 | 37 + 31 | 0.562 |
| HLA mismatch(A,B,DR) | 3.4 + 1.7 | 3.3 + 1.8 | 0.821 |
| follow-up (days) | 1105 (118 to 2105) | 1786 (31 to 2608) | <0.0001 |
| time on HD (years) | 2.6 (1.0 to 6.0) | 1.6 (0.4 to 5.7) | 0.422 |
| graft survival first Tx (days) | 3249 (3 to 10,105) | 2892 (8 to 12,784) | 0.601 |
| pre-emptive KRT | 15% (9) | 17% (9) | 0.837 |
| number of Tx | |||
| 2 | 87% (51) | 83% (45) | 0.644 |
| 3 | 8% (5) | 13% (7) | 0.439 |
| 4 | 5% (3) | 4% (2) | 0.721 |
| Donor | |||
| living donor | 37% (22) | 31% (17) | 0.516 |
| deceased donor | 63% (37) | 69% (37) | 0.516 |
| age (years) | 35 + 15 | 37 + 13 | 0.455 |
| male | 49% (29) | 59% (32) | 0.281 |
| BMI | 27 + 5 | 26 + 5 | 0.626 |
| ethnicity | |||
| white | 88% (52) | 85% (46) | 0.644 |
| African American | 9% (5) | 11% (6) | 0.636 |
| others | 3% (2) | 4% (2) | 0.928 |
| cause of death | |||
| trauma | 35% (13) | 38% (14) | 0.809 |
| anoxia | 41% (15) | 32% (12) | 0.468 |
| CVA | 19% (7) | 27% (10) | 0.789 |
| others | 5% (2) | 3% (1) | 0.555 |
| Kidney | |||
| delayed graft function | 7% (4) | 9% (5) | 0.722 |
| pulsatile perfusion | |||
| yes | 78% (29) | 68% (25) | 0.295 |
| no | 14% (5) | 19% (7) | 0.528 |
| unknown | 8% (3) | 13% (5) | 0.454 |
| total warm ischemia time (minutes) | 33 + 5 | 31 + 5 | 0.103 |
| cold ischemia time (hours) | 16 + 7 | 14 + 5 | 0.165 |
The groups did not significantly differ in recipient and donor characteristics, etiology of primary kidney disease, cause of previous graft loss, and duration of first graft survival. There was no significant difference in time on dialysis before the most recent transplant in both groups (P = 0.422). The majority of patients in both groups being re-transplanted were receiving their second transplant (P = 0.644). There was no significant difference in the number of HLA (A, B, and DR) mismatches or the panel reactive antibodies level between the ESW and CSM groups (P = 0.821 and 0.562, respectively). There was no significant difference in the warm and cold ischemia times and the cause of donor death in either group as shown in Table 1.
Rejections
One-year biopsy-proven acute rejection rates was 17% (n = 10) in the ESW group and 16% (n = 9) in the CSM group (P = 0.89). During the entire follow-up, 20% of patients in the ESW group and 26% in the CSM group had biopsy-proven rejections, as shown in Table 2 (P = 0.641). There was no significant difference between the groups in median time to rejection (P = 0.819). The incidence of subsequent or a second biopsy-proven rejection in the ESW group was 17% (n = 2) compared with 7% (n = 1) in the CSM group (P = 0.579). Seven patients in the ESW group were switched to steroid maintenance (four because of rejection, two for de novo/recurrence of glomerular disease, and one for steroid withdrawal symptoms). One of nine patients with pre-emptive KRT i.e., 9% had to be re-initiated on steroids for steroid withdrawal symptoms. The rate of rejection in pre-emptive KRT with ESW was 9%. At 1 and 5 years, 92 and 88% of the ESW patients, respectively, were steroid free.
Table 2.
Rejection, graft loss, steroid exposure and metabolic profile comparison
| ESW | CSM | P | |
|---|---|---|---|
| Biopsy proven rejection | 20% (n = 12) | 26% (14) | 0.509 |
| Time to rejection (days) | 63 (14 to 1258) | 205 (6 to 1285) | 0.819 |
| Type of rejection | |||
| acute cellular rejection | 92% (11) | 72% (10) | 0.330 |
| acute antibody mediated rejection | 8% (1) | 28% (4) | 0.330 |
| Graft loss | 7% (4) | 15% (8) | 0.231 |
| Cause for current graft loss | |||
| rejection | 25% (1) | 50% (4) | 0.575 |
| BK nephropathy | 25% (1) | 0 | |
| death with functioning graft | 50% (2) | 50% (4) | 1.00 |
| Cumulative steroid exposure prior to recent Tx (days) | 3834 (3 to 10,105) | 3600 (8 to 12,784) | 0.552 |
| Cause of prior graft loss | |||
| chronic transplant glomerulopathy | 68% (40) | 79% (43) | 0.363 |
| rejection | 17% (10) | 13% (7) | 0.839 |
| glomerulonephritis | 5% (3) | 4% (2) | 0.934 |
| others | 10% (6) | 4% (2) | 0.408 |
| Metabolic Profile | |||
| systolic blood pressure (mmHg) | 128 + 12 | 129 + 17 | 0.792 |
| diastolic blood pressure (mmHg) | 72 + 12 | 74 + 11 | 0.314 |
| serum albumin (gm/dl) | 4 + 0.5 | 3.7 + 0.5 | 0.005 |
| fasting blood glucose (mg/dl) | 104 + 27 | 101 + 30 | 0.741 |
| serum cholesterol (mg/dl) | 154 + 33 | 153 + 34 | 0.866 |
| serum LDL (mg/dl) | 84 + 29 | 80 + 22 | 0.457 |
| post transplant diabetes mellitus | 1.6% (1) | 11% (6) | 0.051 |
| anti lipid therapy | 44% (26) | 70% (38) | 0.007 |
| no. of blood pressure medications | 1.8 + 1 | 2.6 + 1.3 | 0.004 |
| bisphosphonate therapy | 10% (6) | 35% (19) | 0.010 |
Patient and Graft Survival
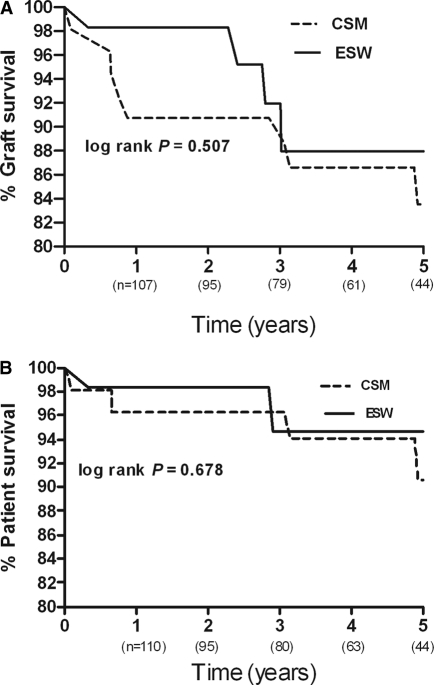
Five-year patient and graft survival time in the ESW and CSM groups was similar, as shown in Figure 1, A and B. Patient survival at 1, 3, and 5 years in the ESW group was 98, 95, and 95%, respectively, whereas in the CSM group, 1-, 3-, and 5-year patient survival was 96, 96, and 91%, respectively. The graft survival at 1, 3, and 5 years was noted to be 98, 88, and 88%, respectively, for the ESW group and 91, 90, and 84%, respectively, for the CSM group. The differences in patient and graft survival curves among these two groups were not statistically different (log rank, P = 0.678 and 0.507, respectively). No significant differences in 5-year graft survival of the ESW and CSM groups was found when they were divided into living and deceased donor sources. Kaplan-Meier survival analysis did not show a significant difference in the rejection-free graft survival between the ESW group and the CSM group at all time points (log rank, P = 0.245).
Figure 1.
Kaplan-Meier (A) graft survival and (B) patient survival of KRT recipients with the ESW and CSM immunosuppression regimen.
A Cox proportional model was used to assess the association between steroid use and combined survival outcome of graft loss and death. We adjusted the model for potential variables such as recipient age (P = 0.330; 95% confidence interval [CI] = 0.628 to 0.013), panel reactive antibodies (P = 0.101, 95% CI = 0.996 to 1.039), donor source (P = 0.211, 95% CI = 0.469 to 1.883), HLA mismatch (P = 0.664, 95% CI = 0.649 to 1.316), and time on hemodialysis (P = 0.685, 95% CI = 0.897 to 1.179). We did not find a significant association between steroid use and combined survival outcome (P = 0.507, hazard ratio = 0.674, 95% CI = 0.214 to 2.117).
Graft Outcomes
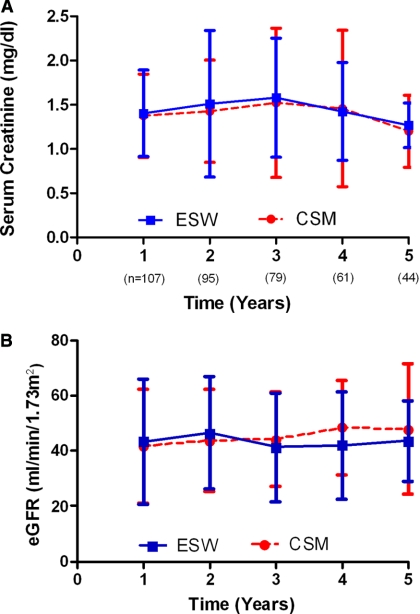
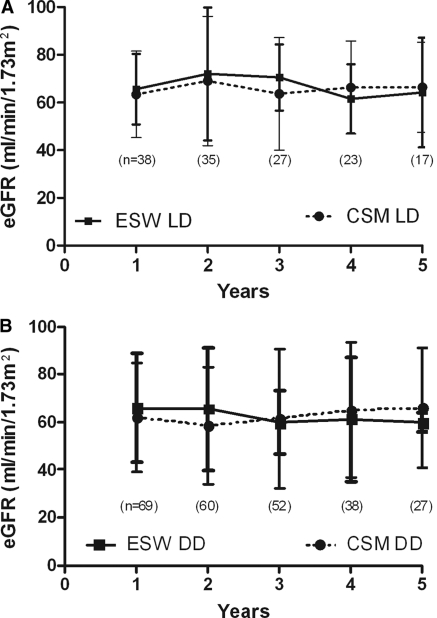
Graft function at 1, 3, and 5 years was comparable between the ESW and CSM groups (P = 0.773, 0.813, and 0.790 at 1, 3, and 5 years, respectively) as shown in Figure 2, A and B. When the groups were subanalyzed by splitting into living and deceased donor sources, no significant difference was noted in the mean eGFR at any time point (Figure 3, A and B).
Figure 2.
Graft function comparison expressed as (A) serum creatinine (mg/dl) and (B) eGFR (ml/min per 1.73 m2) of KRT recipients with the ESW and CSM immunosuppression regimen. The P value was not statistically significant for all time points.
Figure 3.
Graft function comparison using eGFR (ml/min per 1.73 m2) of KRT recipients with the ESW and CSM immunosuppression regimen by dividing them according to organ source: (A) living donor (LD) and (B) deceased donor (DD). The P value was not statistically significant for all time points.
Postrejection mean eGFR in the ESW group was comparable to the CSM groups (eGFR = 54 versus 53 ml/min per 1.73 m2, P = 0.83, at 1 year and 44 versus 47 ml/min per 1.73 m2, P = 0.82, at 4 years).
The mean eGFR of those ESW patients maintained steroid free after an episode of rejection (59 and 44 ml/min per 1.73 m2 at 1 and 4 years, respectively) was not significantly different from the CSM patients with rejection (52 and 47 ml/min per 1.73 m2 at 1 and 4 years; P = 0.51 and 0.82, respectively).
Metabolic Profile
In both groups, there was no significant difference in systolic and diastolic BP, serum cholesterol levels, LDL levels, and fasting blood sugar levels at 1 year (Table 2). However, a significant difference was noted in the incidence of posttransplant diabetes mellitus (P = 0.051), number of anti-hypertensive medications (P = 0.004), anti-lipid treatment (P = 0.044), and serum albumin levels (P = 0.005). We also observed that a significantly higher number of patients in the CSM group were on bisphosphonate therapy (P = 0.010).
Discussion
With an increasing number of patients undergoing repeat kidney transplantation, an evaluation of ESW to steroid maintenance approach is imperative in this set of patients. To our knowledge, this is the first and largest retrospective study describing and comparing the outcomes for ESW and CSM in KRT. Traditionally, KRT recipients have been labeled as “high-risk” and are maintained on long-term steroids. Although steroid-free regimens have been used by some transplant centers in KRT, most reports in the literature have not directly compared the outcomes or have only reported short-term data in a smaller number of patients (24,25). The purpose of this analysis was to determine whether ESW in an era of modern induction and maintenance immunosuppression could be successfully performed in KRT recipients, thus offering these recipients the benefits of steroid-free immunosuppression (15,19,22,25–30).
There are important outcomes of our study to be noted. The short and intermediate patient and graft survival rates of KRT were similar regardless of steroid use in maintenance immunosuppression. We did not find significant difference in the incidence of rejection and time to rejection with early steroid withdrawal. There was no significant difference in the graft function until 5 years of follow-up, and the rejection-free graft survival rates were comparable in both groups.
A number of studies have reported graft survival in KRT patients maintained on long-term steroids. Universally reported KRT graft survival has been lower than primary transplant, but re-transplantation is still advantageous over maintenance hemodialysis or peritoneal dialysis in terms of improved patient survival, better quality of life, and cost effectiveness (5–7,9,10,31–35). This study showed that the short and intermediate graft survival rates of repeat kidney transplantation were not significantly different between the two groups. Our results of 92% patient survival and 80% graft survival at 5 years in KRT with ESW are comparable to the reported survival data in primary kidney transplant recipients with similar immunosuppression (13,15,25,30). The graft survival showed the same trend when the same groups were subdivided into deceased and living donor categories. The graft function between the ESW and CSM groups remained comparable and consistent with reported data on primary transplants (15,23,34).
Risk factors for rejection with ESW regimens under modern immunosuppression include repeat transplantation, African-American race, high panel reactive antibodies >25%, delayed graft function, and deceased donor source (34). In a recent large multivariate analysis, re-transplant candidates remained at high risk for new initiation of steroids despite initial steroid-free discharge medication regimens (36). In our study population, over 5 years, the rejection rate in all re-transplant recipients was 23% (26% in the CSM group and 20% in the ESW group). The rejection rate in our retransplant ESW patients is consistent with the previously reported rejection rate of 10 to 30% in primary kidney transplants with ESW regimens (15,19,22,23,27). The data on the incidence of rejection and time to rejection with ESW are conflicting (15,23,37). Our study did not show a higher incidence of rejection and/or early time to rejection in patients with ESW. We attribute this improved outcome at least to some degree to modern induction therapy. Our overall incidence of second or subsequent rejection was 12.5%, which has been reported to be as high as 32% for primary kidney transplant (28).
Our group has previously reported successful steroid withdrawal for pancreas after kidney transplantation in the recipients who were on CSM immunosuppression (38). The same trend was seen in patients with a pre-emptive KRT who were subsequently switched to an ESW regimen.
The most compelling reason for ESW is the potential to decrease the cardiovascular risk associated with the chronic use of steroids (29,39,40). In agreement with previously reported data, it seems that, with ESW, there is both a trend toward fewer medications required to control metabolic and hypertensive issues and a trend toward a lower incidence of post-transplant diabetes mellitus (15). It is likely that the long-term effects of steroid use might be more deleterious in this particular group of patients because many of them have been exposed to long-term steroids throughout the functional life of their prior transplant.
Our results are subject to the limitations inherent in a retrospective analysis of a nonrandomized single center study. The majority of our patients were white. The limitation of Kaplan-Meier analysis needs to be recognized for both patient and graft survival because only 44 patients were available at 5 years. Another limitation is the lack of protocol biopsies. Although the number of subjects in the study is relatively small, it is larger than any comparable study done to date with similar induction therapy. Further long-term randomized trials are needed to evaluate steroid-free maintenance immunosuppression in KRT.
We conclude that, in our experience, short- and intermediate-term graft survival, patient survival, and graft quality in KRT recipients undergoing ESW seems to be comparable to patients managed with CSM. We suggest that ESW immunosuppression should be tried in patients undergoing KRT with modern induction therapies.
Disclosures
None.
Acknowledgment
Part of these data was presented at the American Transplant Congress 2010 meeting; May 2 through 5, 2010; San Diego, CA.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
Access to UpToDate on-line is available for additional clinical information at www.cjasn.org.
References
- 1. Nankivell BJ, Borrows RJ, Fung CL, O'Connell PJ, Allen RD, Chapman JR, Nankivell BJ, Borrows RJ, Fung CLS, O'Connell PJ, Allen RDM, Chapman JR: The natural history of chronic allograft nephropathy. N Engl J Med 349: 2326–2333, 2003 [DOI] [PubMed] [Google Scholar]
- 2. El-Zoghby ZM, Stegall MD, Lager DJ, Kremers WK, Am H, Gloor JM, Cosio FG: Identifying specific causes of kidney allograft loss. Am J Transplant 9: 527–535, 2009 [DOI] [PubMed] [Google Scholar]
- 3. Cecka JM, Cecka JM: The UNOS Renal Transplant Registry. Clin Transplants 16: 1–20, 2002 [PubMed] [Google Scholar]
- 4. 2010 Annual Report of the U.S. Organ Procurement and Transplantation Network and the Scientific Registry of Transplant Recipients: Transplant Data 2004–2009, Rockville, MD, U.S. Department of Health and Human Services, Health Resources and Services Administration, Healthcare Systems Bureau, Division of Transplantation [Google Scholar]
- 5. Ahmed K, Ahmad N, Khan MS, Koffman G, Calder F, Taylor J, Mamode N: Influence of number of retransplants on renal graft outcome. Transplant Proc 40: 1349–1352, 2008 [DOI] [PubMed] [Google Scholar]
- 6. Barocci S, Valente U, Fontana I, Tagliamacco A, Santori G, Mossa M, Ferrari E, Trovatello G, Centore C, Lorenzi S, Rolla D, Nocera A: Long-term outcome on kidney retransplantation: A review of 100 cases from a single center. Transplant Proc 41: 1156–1158, 2009 [DOI] [PubMed] [Google Scholar]
- 7. El-Agroudy AE, Wafa EW, Bakr MA, Donia AF, Ismail AM, Shokeir AA, El-Dein ABS, Ghoneim M: Living-donor kidney retransplantation: Risk factors and outcome. BJU Int 94: 369–373, 2004 [DOI] [PubMed] [Google Scholar]
- 8. Hirata M, Terasaki PI: Renal retransplantation. Clin Transplants 8: 419–433, 1994 [PubMed] [Google Scholar]
- 9. Ojo A, Wolfe RA, Agodoa LY, Held PJ, Port FK, Leavey SF, Callard SE, Dickinson DM, Schmouder RL, Leichtman AB: Prognosis after primary renal transplant failure and the beneficial effects of repeat transplantation: multivariate analyses from the United States Renal Data System. Transplantation 66: 1651–1659, 1998 [DOI] [PubMed] [Google Scholar]
- 10. Rao PS, Schaubel DE, Wei G, Fenton SSA: Evaluating the survival benefit of kidney retransplantation. Transplantation 82: 669–674, 2006 [DOI] [PubMed] [Google Scholar]
- 11. Scornik JC: Detection of alloantibodies by flow cytometry: Relevance to clinical transplantation. Cytometry 22: 259–263, 1995 [DOI] [PubMed] [Google Scholar]
- 12. Hricik DE: Steroid-free immunosuppression in kidney transplantation: An editorial review. Am J Transplant 2: 19–24, 2002 [DOI] [PubMed] [Google Scholar]
- 13. Matas AJ, Kandaswamy R, Gillingham KJ, McHugh L, Ibrahim H, Kasiske B, Humar A: Prednisone-free maintenance immunosuppression: A 5-year experience. Am J Transplant 5: 2473–2478, 2005 [DOI] [PubMed] [Google Scholar]
- 14. Veenstra DL, Best JH, Hornberger J, Sullivan SD, Hricik DE: Incidence and long-term cost of steroid-related side effects after renal transplantation. Am J Kidney Dis 33: 829–839,1999 [DOI] [PubMed] [Google Scholar]
- 15. Vincenti F, Schena FP, Paraskevas S, Hauser IA, Walker RG, Grinyo J, Group FS: A randomized, multicenter study of steroid avoidance, early steroid withdrawal or standard steroid therapy in kidney transplant recipients. Am J Transplantat 8: 307–316, 2008 [DOI] [PubMed] [Google Scholar]
- 16. Miller LW: Cardiovascular toxicities of immunosuppressive agents. Am J Transplant 2: 807–818, 2002 [DOI] [PubMed] [Google Scholar]
- 17. Prasad GVR, Nash MM, McFarlane PA, Zaltzman JS: Renal transplant recipient attitudes toward steroid use and steroid withdrawal. Clin Transplant 17: 135–139, 2003 [DOI] [PubMed] [Google Scholar]
- 18. Hricik D, O'Toole M, Schulak J, Herson J: Steroid-free immunosuppression in cyclosporine-treated renal transplant recipients: A meta-analysis. J Am Soc Nephrol 4: 1300–1305, 1993 [DOI] [PubMed] [Google Scholar]
- 19. Kaufman DB, Leventhal JR, Axelrod D, Gallon LG, Parker MA, Stuart FP, Kaufman DB, Leventhal JR, Axelrod D, Gallon LG, Parker MA, Stuart FP: Alemtuzumab induction and prednisone-free maintenance immunotherapy in kidney transplantation: Comparison with basiliximab induction—long-term results. Am J Transplant 5: 2539–2548, 2005 [DOI] [PubMed] [Google Scholar]
- 20. Tan HP, Donaldson J, Basu A, Unruh M, Randhawa P, Sharma V, Morgan C, McCauley J, Wu C, Shah N, Zeevi A, Shapiro R: Two hundred living donor kidney transplantations under alemtuzumab induction and tacrolimus monotherapy: 3-year follow-up. Am J Transplant 9: 355–366, 2009 [DOI] [PubMed] [Google Scholar]
- 21. Tan HP, Kaczorowski D, Basu A, McCauley J, Marcos A, Donaldson J, Unruh M, Randhawa P, Zeevi A, Shapiro R: Steroid-free tacrolimus monotherapy after pretransplantation thymoglobulin or campath and laparoscopy in living donor renal transplantation. Transplant Proc 37: 4235–4240, 2005 [DOI] [PubMed] [Google Scholar]
- 22. Kandaswamy R, Melancon JK, Dunn T, Tan M, Casingal V, Humar A, Payne WD, Gruessner RWG, Dunn DL, Najarian JS, Sutherland DER, Gillingham KJ, Matas AJ: A prospective randomized trial of steroid-free maintenance regimens in kidney transplant recipients. An interim analysis. Am J Transplant 5: 1529–1536, 2005 [DOI] [PubMed] [Google Scholar]
- 23. Rostaing L, Cantarovich D, Mourad G, Budde K, Rigotti P, Mariat C, Margreiter R, Capdevilla L, Lang P, Vialtel P, Ortuno-Mirete J, Charpentier B, Legendre C, Sanchez-Plumed J, Oppenheimer F, Kessler M, Group CS, Rostaing L, Cantarovich D, Mourad G, Budde K, Rigotti P, Mariat C, Margreiter R, Capdevilla L, Lang P, Vialtel P, Ortuno-Mirete J, Charpentier B, Legendre C, Sanchez-Plumed J, Oppenheimer F, Kessler M: Corticosteroid-free immunosuppression with tacrolimus, mycophenolate mofetil, and daclizumab induction in renal transplantation. Transplantation 79: 807–814, 2005 [DOI] [PubMed] [Google Scholar]
- 24. Alloway RR, Hanaway MJ, Trofe J, Boardman R, Rogers CC, Buell JF, Munda R, Alexander JW, Thomas MJ, Roy-Chaudhury P, Cardi M, Woodle ES: A prospective, pilot study of early corticosteroid cessation in high-immunologic-risk patients: The Cincinnati experience. Transplant Proc 37: 802–803, 2005 [DOI] [PubMed] [Google Scholar]
- 25. Khwaja K, Asolati M, Harmon JV, Melancon JK, Dunn TB, Gillingham KJ, Kandaswamy R, Humar A, Gruessner RWG, Payne WD, Najarian JS, Dunn DL, Sutherland DER, Matas AJ: Rapid discontinuation of prednisone in higher-risk kidney transplant recipients. Transplantation 78: 1397–1399, 2004 [DOI] [PubMed] [Google Scholar]
- 26. Birkeland SA: Steroid-free immunosuppression after kidney transplantation with antithymocyte globulin and cyclosporine and mycophenolate mofetil maintenance therapy. Transplantation 66: 1207–1210, 1998 [DOI] [PubMed] [Google Scholar]
- 27. Borrows R, Chan K, Loucaidou M, Lawrence C, Van Tromp J, Cairns T, Griffith M, Hakim N, McLean A, Palmer A, Papalois V, Taube D: Five years of steroid sparing in renal transplantation with tacrolimus and mycophenolate mofetil. Transplantation 81: 125–128, 2006 [DOI] [PubMed] [Google Scholar]
- 28. Humar A, Gillingham K, Kandaswamy R, Payne W, Matas A: Steroid avoidance regimens: A comparison of outcomes with maintenance steroids versus continued steroid avoidance in recipients having an acute rejection episode. Am J Transplant 7: 1948–1953, 2007 [DOI] [PubMed] [Google Scholar]
- 29. Knight SR, Morris PJ: Steroid avoidance or withdrawal after renal transplantation increases the risk of acute rejection but decreases cardiovascular risk. A meta-analysis. Transplantation 89: 1–14, 2010 [DOI] [PubMed] [Google Scholar]
- 30. Luan FL, Steffick DE, Gadegbeku C, Norman SP, Wolfe R, Ojo AO: Graft and patient survival in kidney transplant recipients selected for steroid-free maintenance immunosuppression. Am J Transplant 9: 160–168, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Etienne T, Goumaz C, Ruedin P, Jeannet M: Renal retransplantation in Switzerland: Poor HLA matching of first and subsequent allografts does not appear to affect overall graft survival. Transplant Int 5[Suppl 1]: S65–S66, 1992 [DOI] [PubMed] [Google Scholar]
- 32. Hornberger JC, Best JH, Garrison LP, Jr: Cost-effectiveness of repeat medical procedures: Kidney transplantation as an example. Med Decision Making 17: 363–372, 1997 [DOI] [PubMed] [Google Scholar]
- 33. Mouquet C, Benalia H, Chartier-Kastler E, Sylla C, Coriat P, Bitker MO, Richard F: [Renal retransplantation in adults. Comparative prognostic study]. Prog Urol 9: 239–243, 1999 [PubMed] [Google Scholar]
- 34. Woodle ES, Alloway RR, Buell JF, Alexander JW, Munda R, Roy-Chaudhury P, First MR, Cardi M, Trofe J: Multivariate analysis of risk factors for acute rejection in early corticosteroid cessation regimens under modern immunosuppression. Am J Transplant 5: 2740–2744, 2005 [DOI] [PubMed] [Google Scholar]
- 35. Yagmurdur MC, Emiroglu R, Ayvaz I, Sozen H, Karakayali H, Haberal M: The effect of graft nephrectomy on long-term graft function and survival in kidney retransplantation. Transplant Proc 37: 2957–2961, 2005 [DOI] [PubMed] [Google Scholar]
- 36. Schold JD, Santos A, Rehman S, Magliocca J, Meier-Kriesche H-U: The success of continued steroid avoidance after kidney transplantation in the US. Am J Transplant 9: 2768–2776, 2009 [DOI] [PubMed] [Google Scholar]
- 37. ter Meulen CG, van Riemsdijk I, Hene RJ, Christiaans MH, Borm GF, van Gelder T, Hilbrands LB, Weimar W, Hoitsma AJ: Steroid-withdrawal at 3 days after renal transplantation with anti-IL-2 receptor alpha therapy: A prospective, randomized, multicenter study. Am J Transplant 4: 803–810, 2004 [DOI] [PubMed] [Google Scholar]
- 38. Fridell JA, Agarwal A, Powelson JA, Goggins WC, Milgrom M, Pescovitz MD, Tector AJ: Steroid withdrawal for pancreas after kidney transplantation in recipients on maintenance prednisone immunosuppression. Transplantation 82: 389–392, 2006 [DOI] [PubMed] [Google Scholar]
- 39. Arnol M, de Mattos AM, Chung JS, Prather JC, Mittalhenkle A, Norman DJ: Late steroid withdrawal and cardiovascular events in kidney transplant recipients, Transplantation 86: 1844–1848, 2008 [DOI] [PubMed] [Google Scholar]
- 40. Rike AH, Mogilishetty G, Alloway RR, Succop P, Roy-Chaudhury P, Cardi M, Kaiser TE, Thomas M, Woodle ES: Cardiovascular risk, cardiovascular events, and metabolic syndrome in renal transplantation: Comparison of early steroid withdrawal and chronic steroids. Clin Transplant 22: 229–235, 2008 [DOI] [PubMed] [Google Scholar]