Summary
Background and objectives
Mesenchymal stromal cells (MSCs) abrogate alloimmune response in vitro, suggesting a novel cell-based approach in transplantation. Moving this concept toward clinical application in organ transplantation should be critically assessed.
Design, setting, participants & measurements
A safety and clinical feasibility study (ClinicalTrials.gov, NCT00752479) of autologous MSC infusion was conducted in two recipients of kidneys from living-related donors. Patients were given T cell–depleting induction therapy and maintenance immunosuppression with cyclosporine and mycophenolate mofetil. On day 7 posttransplant, MSCs were administered intravenously. Clinical and immunomonitoring of MSC-treated patients was performed up to day 360 postsurgery.
Results
Serum creatinine levels increased 7 to 14 days after cell infusion in both MSC-treated patients. A graft biopsy in patient 2 excluded acute graft rejection, but showed a focal inflammatory infiltrate, mostly granulocytes. In patient 1 protocol biopsy at 1-year posttransplant showed a normal graft. Both MSC-treated patients are in good health with stable graft function. A progressive increase of the percentage of CD4+CD25highFoxP3+CD127− Treg and a marked inhibition of memory CD45RO+RA−CD8+ T cell expansion were observed posttransplant. Patient T cells showed a profound reduction of CD8+ T cell activity.
Conclusions
Findings from this study in the two patients show that MSC infusion in kidney transplant recipients is feasible, allows enlargement of Treg in the peripheral blood, and controls memory CD8+ T cell function. Future clinical trials with MSCs to look with the greatest care for unwanted side effects is advised.
Introduction
Transplant patients rely on life-long immunosuppressive drugs to prevent T cell activation and graft loss, but are exposed to drug-related complications (1). Induction of immune tolerance would overcome these shortcomings, possibly allowing indefinite graft survival (2). Attempts to translate successful strategies to induce tolerance in animal models to humans have been disappointing (3–5) and only anecdotal examples are available (6). Regulation of specific effector T cell function might be a suitable strategy to control alloimmune response (7). In this regard, induction protocols including T cell–depleting agents have been used with the aim of resetting the immune system to promote a tolerance-permissive environment (4,5). The rational for this approach rests on the evidence that transient lymphopenia following T cell–depleting therapy would result in homeostatic expansion of a unique population of regulatory T cells (Treg) with potent in vitro and in vivo immunoregulatory effects (8). Nevertheless, in human kidney transplantation T cell–depleting induction therapy with monoclonal anti-CD52 antibody alemtuzumab or polyclonal rabbit antithymocyte globulin (RATG) did not appreciably protect renal transplant recipients from chronic allograft injury and dysfunction (9), despite enhancing circulating Treg count and preserving their suppressive activity (10,11). Recent advances in experimental transplantation have also demonstrated that memory T cells ultimately compromise the development of transplant tolerance, with the presence of donor-specific memory T cells often being associated with poor allograft outcome (12).
Bone marrow–derived mesenchymal stromal cells (MSCs)—unique for their immunologic characteristics, such as low immunogenicity and immunoregulatory properties (13)—in vitro increase the percentage of Treg at coculture with T lymphocytes (14) and inhibit the proliferative response of antigen-specific memory T cells (15,16), suggesting a novel cell-based approach for immunotherapy which also targets Treg and memory T cells. By infusing either autologous or donor-derived MSCs in unconditioned mice, we were able to induce tolerance to a semiallogeneic heart transplantation (17).
For clinical studies with MSCs in organ transplantation, however, unwanted side effects of cell infusion must be assessed with the greatest care before planning large efficacy trials for tolerance induction. This particularly for the concern of possible MSC maldifferentiation in vivo and their potential for facilitating the growth of pre-existing but occult tumors (18,19). Although these side effects have been so far observed only in very few experimental animal models (20–22), the question of risk and benefit must be well assessed in pilot clinical studies, especially when MSCs meet additional immunosuppressive drugs. Also the question of whether patients should be treated with autologous, donor-derived, or third-party MSCs remains to be addressed. Applying recipient-unrelated MSCs in organ transplantation at this point in time may raise objections because they may cause recipient sensitization. To prevent activation of immune cells and sensitization of transplant recipients, the introduction of foreign antigen should be avoided and first pilot studies should thus begin with autologous MSCs, making safety the first objective. Moreover, experimental evidence indicates that autologous MSCs are equally capable of inhibiting the antidonor immune response as donor-derived MSCs (17).
Here we have extended our experimental work to define the safety and clinical feasibility of the autologous MSC approach in two human recipients of kidneys from living-related donors (ClinicalTrials. gov Identifier: NCT00752479).
Materials and Methods
Patients
A 22-year-old man (patient 1) on hemodialysis due to ESRD of unknown etiology received a renal transplant from his mother, mismatched for two HLA haplotypes (one mismatch on HLA-A and one on HLA-B whereas HLA-DR alleles were coincidental) (Figure 1A).
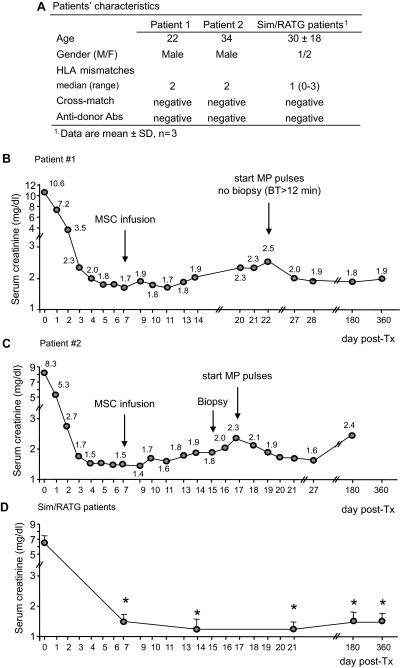
Figure 1.
Characteristics and posttransplant course of serum creatinine in patients given MSCs and in control patients: Patients' characteristics (A) and profile of serum creatinine levels before and after MSC infusion in patient 1 (B) and patient 2 (C) and profile of serum creatinine in Sim/RATG patients (D) during the first year after kidney transplantation are shown. Sim/RATG patients are control living donor-kidney transplant recipients given the same induction therapy but not MSCs. Data are means ± SEM; *P < 0.05 versus time 0.
A second 34-year-old man (patient 2) on ESRD secondary to IgA nephropathy received a pre-emptive renal transplant from his father, mismatched for two HLA haplotypes (one mismatch on HLA-A and one on HLA-B while HLA-DR alleles were coincidental) (Figure 1A). Four months before transplantation both of them underwent sterna bone marrow aspiration under local anesthesia. MSCs were isolated and ex vivo expanded according to Good-Manufacturing-Practice procedures (Cell-Therapy Laboratory “G. Lanzani”, Ospedali Riuniti di Bergamo, authorization no. aM-189/2008 Agenzia Italiana del Farmaco, AIFA) (23,24). On day 7 after kidney transplant, autologous MSCs were administered intravenously (1.7 × 106 cells and 2.0 × 106 cells per kg body weight, respectively) after premedication with chlorphenamine and acetaminophen. Three patients receiving a living-related kidney who were transplanted previously to patients 1 and 2 were taken as the control group. They were given the same induction therapy, but not MSCs (Figure 1A). In all transplant recipients immunophenotyping of peripheral blood T cell populations and also monitoring of T lymphocyte function were performed before and up to day 360 postsurgery.
Written informed consent was obtained from all recipients and living donors. All treatment protocols were approved by the Istituto Superiore di Sanità (ISS, Rome, Italy, authorization no. 45253(06)-PRE.21-882) and by the Institutional Review Board of the Ospedali Riuniti Bergamo (authorization no. 352, March 18, 2008).
All patients received induction regimen with basiliximab (20 mg intravenous pretransplant and on day 4 posttransplant) and low-dose rabbit antithymocyte globulin (RATG) infusion (thymoglobulin, 0.5 mg/kg, daily from day 0 to day 6 posttransplant) as per center practice (25). Maintenance immunosuppression was with cyclosporine A (CsA, target trough blood levels of 300 to 400 ng/ml up to day 7 postsurgery, and 100 to 150 ng/ml at month 5 posttransplantation), mycophenolate mofetil (plasma trough mycophenolic acid [MPA] levels of 0.5 to 1.5 μg/ml) (26), and steroids. Five hundred milligrams of methylprednisolone were administered before the first RATG infusion to minimize the possible cytokine release reaction related to the antibodies, and continued for 2 more days posttransplant (250 and 125 mg, respectively). Subsequently, oral prednisone (75 mg) was administered, which was progressively tapered and discontinued after day 7 postsurgery.
MSC Isolation and Expansion
MSCs were processed and cultured as previously reported (23,24). In brief, bone marrow aspirates were collected and nucleated cells were plated at 500,000 cells per cm2 in minimum essential medium-α in the presence of 5% human platelet lysate. The use of human platelet lysate avoids the need of fetal calf serum, which is a source of xenogenic antigens that may induce an adverse immune response. Nonadherent cells were removed after 2 to 3 days and a 50% medium change was performed twice weekly until 80% confluence. Then cells were recovered and subsequently replated at 200 cells per cm2 and cultured for an additional 12 days. The cells were classified as MSCs based on their ability to differentiate into bone, fat, and cartilage and by flow cytometric analysis (positive for CD44, CD29, CD73, HLA-ABC, CD90, and CD105, but negative for CD14, CD34, CD45, and HLA-DR) responding to defined criteria for MSCs recently stated by the International Society of Cell Therapy (ISCT) (27). The final product was characterized with respect to viability, purity, and therapeutic potential. Because a major concern with MSCs is the potential for malignant transformation, the MSC preparation underwent cytogenetic analysis, which showed a normal karyotype. Safety has been judged on the basis of negativity for all of the tested contaminants and lack of genetic lesions at cytogenetic analysis. Criteria for MSC batch release were the following: expression of CD105, CD73, and CD90 >70%, expression of CD34, CD45, and CD14 <10%; negative for mycoplasma, Gram-positive, and Gram-negative bacteria and fungi; endotoxin below 5 EU/kg; viability >80%. MSCs were frozen in liquid nitrogen until the day of the infusion into the kidney transplant recipient (see Supplemental Text).
Phenotypic and Functional Immunological Assays
To establish the ideal timing for MSC infusion, we first assessed in vitro whether RATG binds to MSC and affects their inhibitory properties on peripheral blood mononuclear cell (PBMC) proliferation. Immunophenotyping of peripheral blood T cell subpopulations was monitored by FACS analysis (10). Alloimmune response against donor and third-party antigens were assessed by ELISPOT for IFN-γ (10) and for Granzyme-B and by cell-mediated lympholysis. Anti-HLA class I and II antibodies were sequentially monitored by means of flow-panel reactive antibody assay.
Histology and Immunohistochemistry
Paraffin-embedded sections of kidney tissue stained with Massons trichrome, hematoxylin and eosin, and periodic acid–Schiff were evaluated by an independent pathologist. Graft infiltrating cells, MSC localization, and complement deposition were assessed by immunofluorescence or immunoperoxidase technique (see Supplemental Text).
Statistical Analyses
Variations in serum creatinine concentration, peripheral blood CD4+ and CD8+ T cell counts, percentages of T cell subpopulation, immunologic assay data from control living donor-kidney transplant recipients given the same induction therapy but not MSCs (Sim/RATG patients) were assessed by ANOVA for repeated measures. The statistical significance level was defined as P < 0.05.
Results
In Vitro Pretransplant Studies
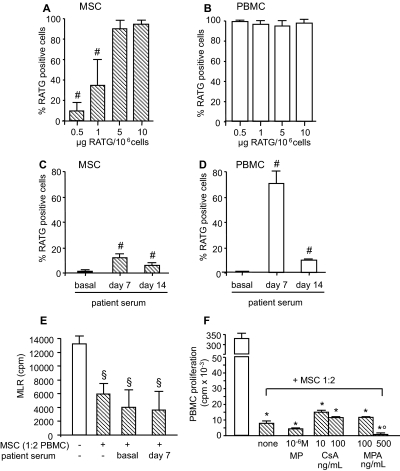
In culture medium RATG bound in a dose-dependent manner to human MSCs, with 85% ± 13% and 10% ± 8% binding at the antibody concentrations of 5 and 0.5 μg per 106 cells, respectively (Figure 2A). These concentrations are expected to be achieved in vivo in kidney recipients given 0.5 mg/kg RATG at day 0 (after the first RATG dose) and day 7 (24 hours after the last RATG dose) posttransplant, respectively. Conversely, >95% of PBMCs bound RATG independently of the antibody concentration in the medium (Figure 2B). To confirm these findings, we exposed MSCs to serum from RATG-treated kidney transplant patients taken 7 and 14 days postsurgery. We found a very low percentage of RATG bound to MSCs compared with >70% of RATG bound to PBMCs (Figure 2, C and D). After exposure to patients' serum, MSC viability ranged from 88% to 100% (by trypan blue dye exclusion). Moreover, the minimal RATG binding to MSCs after exposure to patients' serum drawn at day 7 posttransplant did not impair their ability to inhibit MLR T cell proliferation (Figure 2E). Incubation of MSCs with methylprednisolone (MP), CsA, or MPA did not significantly affect their capability to inhibit T cell proliferation in response to anti-CD3/CD28 mAbs (Figure 2F). Of note, MPA at the highest concentration synergized with MSCs in further inhibiting T cell proliferation.
Figure 2.
In vitro pretransplant studies: 200,000 MSCs (A) or 200,000 PBMCs (B) were incubated for 30 minutes at 37°C with graded doses of RATG, washed, and then incubated with FITC-labeled goat anti-rabbit IgG secondary antibody. Percentages of RATG-positive cells were determined by FACS analysis after subtraction of nonspecific binding of secondary antibody to cells in the absence of RATG. Data are means ± SD; #P < 0.05 versus 5 and 10 μg of RATG per 106 cells (by ANOVA, n = 4 independent experiments). MSCs (C) or PBMCs (D) were also incubated with serum from patients given Sim/RATG taken pretransplant (n = 3), 7 days posttransplant (n = 3), and 14 days posttransplant (n = 3). Cells were then labeled with secondary antibody and analyzed as above. Data are means ± SD; #P < 0.05 versus basal. In (E), MLRs were conducted in the absence or in the presence of either untreated MSCs or MSCs pre-exposed to patients' serum taken at basal (n = 3) or 7 days posttransplant (n = 3). Data are means ± SD; §P < 0.05 versus −/− (by ANOVA, n = 3 independent experiments). In (F), PBMCs (100,000 per well) were also incubated with anti-CD3/CD28 conjugated beads for 3 days in the absence (white bars) or in the presence (dashed bars) of MSCs (1:2 MSCs:PBMCs). Cultures with MSCs were conducted in the absence (none) or with the indicated concentration of MP, CsA, and MPA. The percentage inhibition of anti-CD3/CD28–induced PBMC proliferation with various drug concentrations ranged from 10% to 30% for MP or CsA alone and from 60% to 87% for MPA alone. Data are means ± SD; *P < 0.05 versus allogeneic MLR, °P < 0.05 versus none (by ANOVA, n = 3 independent experiments).
On the basis of these in vitro findings, MSC infusion was set at day 7 after kidney transplantation to minimize any possible depleting effect of RATG on MSCs in vivo.
Clinical Course
In patient 1 renal function rapidly improved posttransplantation (Figure 1B). On the morning of the autologous MSC infusion, serum creatinine was 1.7 mg/dl, which fluctuated in the following days between 1.7 and 1.9 mg/dl. From day 14 onward, a mild progressive increase in serum creatinine was observed (Figure 1B). Renal ultrasound showed a slightly increased resistivity index (0.79); blood CsA trough levels were in the therapeutic range. No anti-HLA alloantibodies were detected. Graft biopsy was not performed because of a substantially prolonged bleeding time, and MP pulses were started for the clinical suspect of graft rejection (Figure 1B). Corticosteroid was then tapered and continued at the maintenance dose (8 mg/d). Renal function slowly recovered. At day 180 posttransplantation allograft function remained stable (serum creatinine 1.8 mg/dl). A protocol biopsy at 1 year posttransplantation showed no signs of acute rejection nor chronic allograft injury. Serum creatinine was 1.93 mg/dl and measured GFR (28) was 48.41 ml/min per 1.73 m2. CsA and MPA trough levels were within the anticipated ranges. Thereafter, the corticosteroid was gradually tapered and treatment discontinued. The patient is in good health with stable graft function.
In patient 2 the early postoperative course was characterized by rapid improvement of renal function (Figure 1C). Before autologous MSC infusion serum creatinine was 1.49 mg/ml and then graft function progressively deteriorated. On day 15 posttransplant a kidney biopsy was performed, which showed a slight aspecific inflammatory infiltrate not consistent with acute graft rejection. Because serum creatinine further increased up to 2.34 mg/dl, intravenous pulses of MP were started. After being tapered, corticosteroid was maintained at the dose of 8 mg/d. Renal function transiently improved, thereafter stabilizing at serum creatinine levels of 2.0 to 2.3 mg/dl. A routine abdomen ultrasonography 2 months posttransplantation disclosed two solid nodular images in the right native kidney of 1 and 2 cm, respectively (of note, small cortical cysts bilaterally on native kidneys were reported pretransplant; see Supplemental Figure 1) subsequently confirmed by angio-computed tomography (CT) scan evaluation. On the basis of the anticipated neoplastic nature of the nodular hyperdense lesions at CT scan, the patient underwent bilateral nephrectomy of native kidneys. Histologic examination showed dual renal hemangiomas in the right native kidney. Hemangioma was histologically defined by the positivity for Factor VIII, CD34, and CD31 expression and negativity for CD10, CK CAM 5.2, and CK 7 expression. Proliferative index by Ki67 staining was 10% to 15%. The surgery and the postoperative period were uneventful without worsening of kidney graft function. At 6 months posttransplantation the patient was in good health with stable graft function (serum creatinine 2.3 mg/dl, GFR 38.9 ml/min per 1.73 m2). In control recipients of a living-related kidney (n = 3) given the same induction therapy but not MSCs, serum creatinine levels at 180 and 360 days were 1.5 ± 0.3 and 1.6 ± 0.2 mg/dl, respectively (Figure 1D). No acute rejection episodes occurred in these control patients during the 1-year follow-up.
Histology and Immunohistochemistry
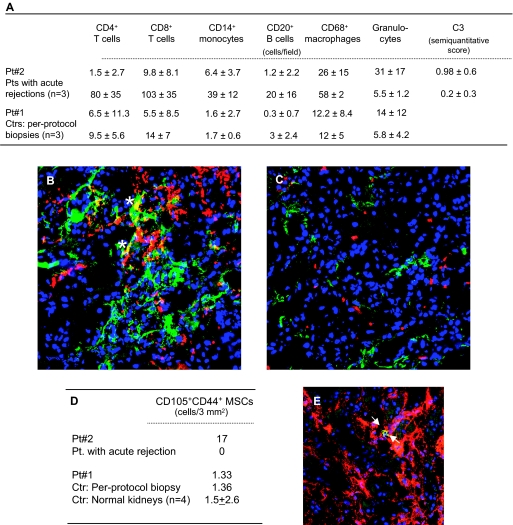
The protocol biopsy from patient 1 showed findings within normal limits, except for mild neutrophil infiltrate (Figure 3A). MSCs in the graft were negligible (Figure 3D).
Figure 3.
Characterization of infiltrating cells, complement deposition, and MSCs in kidney grafts. Kidney graft biopsies were taken at day 15 posttransplant in patient 2 because of the suspicion of graft rejection and at day 360 in patient 1 (protocol biopsy). As controls, renal biopsies from patients with acute graft rejection (n = 3) within 15 to 100 days postoperative and from patients (n = 3) undergoing protocol biopsy at 1 year posttransplant were analyzed. (A) reports counts of intragraft cell infiltrates and score of C3 complement deposition. For both immunofluorescence and immunoperoxidase analyses the number of positive cells were counted in at least 20 to 30 high-power fields. Complement deposition, analyzed by immunofluorescence technique, was scored for intensity (absent, faint, moderate, intense: 0 to 3) in at least 20 to 30 high-power fields. Data for patients 1 and 2 are the mean ± SD of cell counts in the 20 to 30 high-power fields. (B) and (C) are representative images of intragraft immunostaining for granulocytes (red) and C3 deposition (green) in patient 2 given MSCs and in a patient with acute graft rejection, respectively. In patient 2 granulocytes colocalized with C3 staining (*). Original magnification, ×400. (D) reports intragraft CD105 and CD44 double-positive cell counts in kidney graft biopsies from patients 2 and 1. As controls, renal biopsies from a patient with acute graft rejection at day 15 postoperative, a patient undergoing protocol biopsy at 1 year posttransplant, and a section of normal renal tissue from patients undergoing nephrectomy for renal carcinoma were analyzed. The total number of double-positive cells counted in 3 mm2 (corresponding to the area of about 30 high-power fields) is reported. (E) is a representative image of intragraft double-positive MSCs for CD105 and CD44 (arrows) in patient 2. Original magnification, ×400.
Early biopsy of patient 2 showed focal inflammatory cell infiltrate. Intragraft CD4+, CD8+ T cells, CD14+ monocytes, CD20+ B cells, and CD68+ macrophages were very low as compared with those in control kidney graft biopsies from patients given the same immunosuppression who experienced acute cellular rejection (Figure 3A). There were a high number of granulocytes in the peritubular inflammatory infiltrate of patient 2 (Figure 3, A through C). Granulocytes were negligible in the control graft biopsies with acute cellular rejection. Intragraft staining for C3 (mainly peritubular and interstitial localization) was higher in patient 2 than in grafts with early acute cellular rejection (Figure 3, A through C). MSCs were found in the graft interstitium of patient 2, but not in naïve untransplanted kidneys nor in a renal graft with acute cellular rejection (Figure 3, D and E).
Immunophenotyping of Peripheral Blood T Lymphocytes
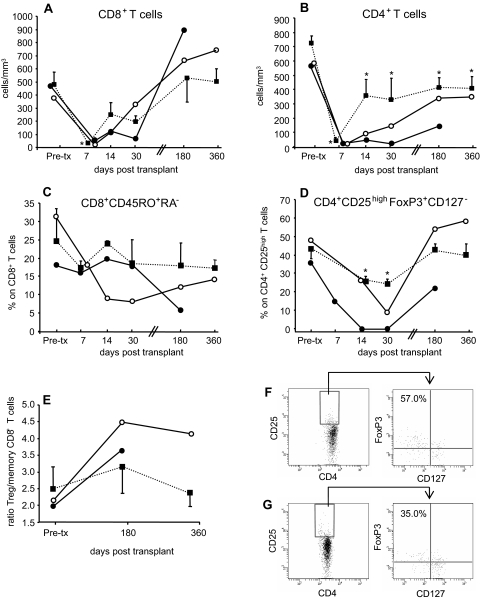
In patients 1 and 2, and in control living-related kidney transplant recipients given the same induction therapy (Simulect (Sim)/RATG patients), RATG induced profound CD8+ and CD4+ T cell depletion (Figure 4, A and B). At 180 and 360 days posttransplant, CD8+ T cells approached pretransplant levels, whereas the CD4+ T cell counts remained lower than pretransplant values both in patients given MSCs and in Sim/RATG patients (Figure 4, A and B).
Figure 4.
Kinetics of repopulating T cells in peripheral blood: (A) and (B) show the kinetics of absolute numbers of repopulating CD8+ and CD4+ T cells, respectively, in the peripheral blood of patient 1 (○) and patient 2 (●) and Sim/RATG patients (living donor-kidney transplant recipients given the same induction therapy but not MSCs, as controls, ■) from baseline (pretransplant) to day 360 posttransplant. Data are means ± SEM. *P < 0.05 versus pretransplant. Percentages of memory CD45RO+RA− T cells within CD8+ T cells (C) and of regulatory CD25highFoxP3+CD127− cells within CD4+ T cells [Treg, (D)] and the ratio of Treg/memory CD8+ T cells (E) from patient 1 (○) and patient 2 (●) and from Sim/RATG patients (■) from baseline (pretransplant) to day 360 posttransplant. Data are means ± SEM. *P < 0.05 versus pretransplant. Expression of FoxP3 and CD127 antigens (dot plots on the right) by gated CD4+ and CD25high T cells (dot plots on the left) at day 360 posttransplant from patient 1 (F) and from a living donor-kidney transplant recipient given the same induction therapy but not MSCs (G) is shown. Numbers in outlined areas indicate percentage of FoxP3+CD127− T cells.
In patient 1 the percentage of memory CD45RO+RA−CD8+ T cells within the total CD8+ T cell population progressively decreased up to day 30 posttransplant and remained lower than pretransplant values thereafter (Figure 4C). In patient 2 the percentage of memory CD45RO+RA−CD8+ T cells remained comparable to pretransplant values during the first 30 days and then decreased to a very low level at day 180 when the total CD8+ T cell counts completely recovered. Conversely, in Sim/RATG patients the percentage of CD45RO+RA−CD8+ T cells at 30, 180, and 360 days posttransplant was comparable to pretransplant values (Figure 4C).
The percentage of CD4+CD25highFoxP3+CD127− regulatory T cells (Treg) within the total CD4+ T cell population markedly decreased during the first 30 days both in patients given MSCs and in Sim/RATG patients (Figure 4D). Thereafter, in patient 1 the percentage of Treg considerably increased, reaching pretransplant values at days 180 and 360 (Figure 4, D and F). Similarly, in patient 2 a marked increase in the percentage of Treg was found from day 30 onward. In Sim/RATG recipients the percentage of Treg only marginally increased from 30 to 360 days follow-up (Figure 4, D and G). Thus, the ratio of Treg/memory CD8+ T cells was higher in patients given MSCs than in Sim/RATG recipients (Figure 4E).
Ex Vivo Posttransplant Immunologic Assays
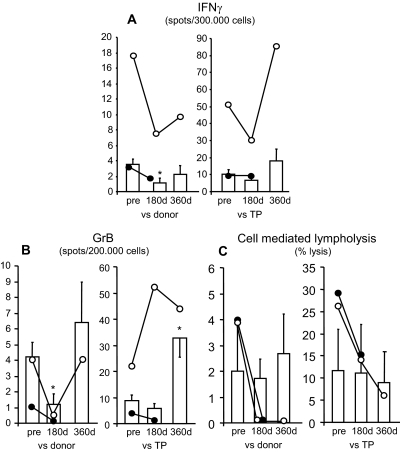
At day 180 posttransplantation, the frequencies of antidonor IFN-γ–producing memory T cells (Figure 5A) and antidonor granzyme-B–producing memory CD8+ T cells (29) (Figure 5B) in MSC-treated patients 1 and 2 were comparably lower than pretransplant values. These memory responses were less affected in Sim/RATG controls (Figure 5, A and B). At the same time posttransplant, the cytolytic function of CD8+ T cells was completely abrogated in response to donor antigens and reduced against third-party antigens in patients 1 and 2 (Figure 5C). In Sim/RATG patients the CD8+ T cell cytolytic response toward both donor and third-party antigens did not significantly change as compared with pretransplant values (Figure 5C). Both patient 1 and patient 2 did not develop anti-HLA class I and II antibodies during the entire follow-up.
Figure 5.
Immunological assays of the memory T cell response were evaluated by ELISPOT for IFN-γ (A) and Granzyme-B (B) and the CD8+ T cell function by T cell–mediated lympholysis [percentage-specific lysis at 50:1 effector-target ratio, (C)] toward donor and third-party (TP) antigens on PBMCs taken pretransplant (“pre”) and at day 180 and day 360 posttransplant from patient 1 (○) and day 180 from patient 2 (●), and from Sim/RATG patients (living donor-kidney transplant recipients given the same induction therapy but not MSCs, as controls, white bars). Data are means ± SEM. *P < 0.05 versus pretransplant.
Discussion
The main purpose of the study was to establish the safety and clinical feasibility of cell-based therapy with MSCs in the context of kidney transplantation.
Patients given autologous bone marrow–derived MSCs and kidney transplantation both developed renal insufficiency 7 to 14 days after cell infusion. In patient 1—who could not receive a kidney biopsy—normal BP, lack of fever, preserved diuresis, no graft pain or tenderness in the presence of expected blood CsA trough levels, normal size and structure of the kidney graft, and no edema of renal pelvis at ultrasound with mild increase in renal resistivity index render a diagnosis of acute rejection extremely unlikely. In patient 2 graft kidney biopsy showed a focal inflammatory infiltrate of renal interstitium, mostly granulocytes with very few T and B cells. These findings were taken to exclude a kidney graft rejection. Instead, CD44 and CD105 double-positive MSCs were found in the graft, bona fide from the autologous cells previously infused in the transplant recipient, because no MSCs were documented in untransplanted kidneys or kidney from a patient with early acute graft rejection. Despite the well known anti-inflammatory properties of MSCs (30), the various soluble factors produced by MSCs also include proinflammatory mediators (31–33), which eventually may have contributed to the intragraft recruitment of granulocytes and slow progressive deterioration of renal function. Patients 1 and 2 are in good health with stable graft function after 360 and 180 days posttransplantation, respectively, and are still on follow-up.
In the first clinical trials assessing safety of MSC infusion and possible treatment strategies for graft-versus-host disease, ischemic heart disease, spinal cord injury, and systemic lupus erythematosus, none of the patients have reported any significant adverse events associated with MSC transplantation (34–38) (Supplemental Text). Until now, however, there has been no report about the safety of MSC infusion in patients undergoing solid organ transplantation. Maldifferentiation, promotion of tumor growth, and malignant transformation have also been suggested as possible side effects after MSC injection (18,19). Furthermore, some reports showed transformation of human MSCs in culture (39), although at least in two cases a tumor was formed or initiated because of contamination of MSCs with cancer cells (40,41). This observation further underlines the need of careful phenotypic, functional, and genetic characterization of MSCs before cell administration (39). So far, however, tumor-promoting events of MSCs have never been observed in any of more than 500 patients who received this cell therapy (34–38) (Supplemental Text). The contribution of MSCs in the development of hemangiomas on native kidney in patient 2 remains ill defined. Paracrine mechanisms may favor endothelial proliferation, whereas MSC differentiation into adipocytes may contribute to adipogenesis during hemangioma involution (42). However, these lesions could also be accidental findings unrelated to MSC infusion.
In this study, we also wanted to gain initial insight into the in vivo effect of MSC infusion on homeostatic proliferation and function of T cells after peritransplant T cell depletion. We found that, in the two patients given MSCs, but not in the control transplant recipients, the percentage of memory CD8+ T cells within the overall CD8+ T cell population markedly decreased posttransplant despite the anticipated homeostatic proliferation of the remaining T cell subsets. The expansion of memory T cells that escape deletion after lymphoablation represents a major barrier to transplant tolerance. The mechanism responsible for the MSC-mediated suppression of memory CD8+ T cell proliferation remains under investigation. Because MSCs produce TGF-β (14,43), which antagonizes the effect of IL-15—relevant to memory CD8+ T cell expansion (44)—the possibility exists that MSC-induced downregulation of homeostatic repopulation of memory CD8+ T cells could be a TGF-β–mediated event.
The change in the memory CD8+ T cell profile in the peripheral blood of the patients given MSCs was associated with a profound reduction in CD8+ T cell activity. These effects were less pronounced in control kidney transplant recipients given the same induction therapy but not MSCs. These findings can be interpreted as to indicate that MSCs have an additional effect beyond that of immunosuppressive drug therapy on the inhibition of memory CD8+ T cell expansion and function in the transplant setting.
Evidence from experimental models of solid organ transplantation suggests that the mechanisms of MSC-induced tolerance include regulatory T cells (17,45,46). Despite the two patients given MSCs were receiving CsA, known to prevent the development of Treg by the inhibition of IL-2 (10,47), a progressive expansion of Treg was documented posttransplant. This skewed the Treg/memory CD8+ T cell ratio toward the regulatory cells, favoring a protolerogenic environment.
Our findings in the two patients show, for the first time, that MSC infusion in kidney transplant recipients is feasible and restricts memory T cell expansion while enlarging the Treg population. However, infusion of MSCs after kidney transplantation induced graft dysfunction. The safety concern of posttransplant MSCs anticipated the need to modify the study protocol moving cell infusion pretransplant, an approach being effective in experimental models of solid organ transplantation (17,48). Moreover, we advise for future clinical trials with MSCs to look with the greatest care for the unexpected, especially concerning unwanted side effects.
Disclosures
None.
Supplementary Material
Acknowledgments
This study has been partially supported by grants from Fondazione ART per la Ricerca sui Trapianti (Milan, Italy) and from AIRC (Associazione Italiana Ricerca sul Cancro), Piano Regionale Sangue-Lombardia, AIL Bergamo sez “Paolo Belli.” Dr. Monica Cortinovis is a recipient of a fellowship from Fondazione Aiuti per la Ricerca sulle Malattie Rare (ARMR), Bergamo, Italy. We are grateful to Dr. Mario Bontempelli for the lymphocyte subset profiling in the peripheral blood and Dr. Aurelio Sonzogni and Mr. Franco Marchetti for histologic processing and analysis of biopsy specimens. The authors are members of the Mesenchymal Stem Cells in Solid Organ Transplantation (MISOT) study group, www.misot.de.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
Supplemental information for this article is available online at www.cjasn.org.
References
- 1. Sayegh MH, Carpenter CB: Transplantation 50 years later–progress, challenges, and promises. N Engl J Med 351: 2761–2766, 2004 [DOI] [PubMed] [Google Scholar]
- 2. Sayegh MH, Remuzzi G: Clinical update: Immunosuppression minimisation. Lancet 369: 1676–1678, 2007 [DOI] [PubMed] [Google Scholar]
- 3. Millan MT, Shizuru JA, Hoffmann P, Dejbakhsh-Jones S, Scandling JD, Grumet FC, Tan JC, Salvatierra O, Hoppe RT, Strober S: Mixed chimerism and immunosuppressive drug withdrawal after HLA-mismatched kidney and hematopoietic progenitor transplantation. Transplantation 73: 1386–1391, 2002 [DOI] [PubMed] [Google Scholar]
- 4. Shapiro R, BasGray E, Kahn A, Randhawa P, Basu A, Tan H, Murase N, Zeevi A, Girnita A, Metes D, Ness R, Bass DC, Demetris AJ, Fung JJ, Marcos A, Starzl TE: Kidney transplantation under minimal immunosuppression after pretransplant lymphoid depletion with Thymoglobulin or Campath. J Am Coll Surg 200: 505–515, quiz A559–A561, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kirk AD, Mannon RB, Kleiner DE, Swanson JS, Kampen RL, Cendales LK, Elster EA, Wakefield T, Chamberlain C, Hoffmann SC, Hale DA: Results from a human renal allograft tolerance trial evaluating T-cell depletion with alemtuzumab combined with deoxyspergualin. Transplantation 80: 1051–1059, 2005 [DOI] [PubMed] [Google Scholar]
- 6. Kawai T, Cosimi AB, Spitzer TR, Tolkoff-Rubin N, Suthanthiran M, Saidman SL, Shaffer J, Preffer FI, Ding R, Sharma V, Fishman JA, Dey B, Ko DS, Hertl M, Goes NB, Wong W, Williams WW, Jr., Colvin RB, Sykes M, Sachs DH: HLA-mismatched renal transplantation without maintenance immunosuppression. N Engl J Med 358: 353–361, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wood KJ, Jones ND, Bushell AR, Morris PJ: Alloantigen-induced specific immunological unresponsiveness. Philos Trans R Soc Lond B Biol Sci 356: 665–680, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Goldrath AW, Luckey CJ, Park R, Benoist C, Mathis D: The molecular program induced in T cells undergoing homeostatic proliferation. Proc Natl Acad Sci U S A 101: 16885–16890, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ruggenenti P, Perico N, Gotti E, Cravedi P, D'Agati V, Gagliardini E, Abbate M, Gaspari F, Cattaneo D, Noris M, Casiraghi F, Todeschini M, Cugini D, Conti S, Remuzzi G: Sirolimus versus cyclosporine therapy increases circulating regulatory T cells, but does not protect renal transplant patients given alemtuzumab induction from chronic allograft injury. Transplantation 84: 956–964, 2007 [DOI] [PubMed] [Google Scholar]
- 10. Noris M, Casiraghi F, Todeschini M, Cravedi P, Cugini D, Monteferrante G, Aiello S, Cassis L, Gotti E, Gaspari F, Cattaneo D, Perico N, Remuzzi G: Regulatory T cells and T cell depletion: Role of immunosuppressive drugs. J Am Soc Nephrol 18: 1007–1018, 2007 [DOI] [PubMed] [Google Scholar]
- 11. Sewgobind VD, Kho MM, van der Laan LJ, Hendrikx TK, van Dam T, Tilanus HW, Ijzermans JN, Weimar W, Baan CC: The effect of rabbit anti-thymocyte globulin induction therapy on regulatory T cells in kidney transplant patients. Nephrol Dial Transplant 24: 1635–1644, 2009 [DOI] [PubMed] [Google Scholar]
- 12. Valujskikh A, Li XC: Frontiers in nephrology: T cell memory as a barrier to transplant tolerance. J Am Soc Nephrol 18: 2252–2261, 2007 [DOI] [PubMed] [Google Scholar]
- 13. Crop M, Baan C, Weimar W, Hoogduijn M: Potential of mesenchymal stem cells as immune therapy in solid-organ transplantation. Transpl Int 22: 365–376, 2009 [DOI] [PubMed] [Google Scholar]
- 14. English K, Ryan JM, Tobin L, Murphy MJ, Barry FP, Mahon BP: Cell contact, prostaglandin E(2) and transforming growth factor beta 1 play non-redundant roles in human mesenchymal stem cell induction of CD4+CD25(High) forkhead box P3+ regulatory T cells. Clin Exp Immunol 156: 149–160, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Krampera M, Glennie S, Dyson J, Scott D, Laylor R, Simpson E, Dazzi F: Bone marrow mesenchymal stem cells inhibit the response of naive and memory antigen-specific T cells to their cognate peptide. Blood 101: 3722–3729, 2003 [DOI] [PubMed] [Google Scholar]
- 16. Karlsson H, Samarasinghe S, Ball LM, Sundberg B, Lankester AC, Dazzi F, Uzunel M, Rao K, Veys P, Le Blanc K, Ringden O, Amrolia PJ: Mesenchymal stem cells exert differential effects on alloantigen and virus-specific T-cell responses. Blood 112: 532–541, 2008 [DOI] [PubMed] [Google Scholar]
- 17. Casiraghi F, Azzollini N, Cassis P, Imberti B, Morigi M, Cugini D, Cavinato RA, Todeschini M, Solini S, Sonzogni A, Perico N, Remuzzi G, Noris M: Pretransplant infusion of mesenchymal stem cells prolongs the survival of a semiallogeneic heart transplant through the generation of regulatory T cells. J Immunol 181: 3933–3946, 2008 [DOI] [PubMed] [Google Scholar]
- 18. Feng B, Chen L: Review of mesenchymal stem cells and tumors: executioner or coconspirator? Cancer Biother Radiopharm 24: 717–721, 2009 [DOI] [PubMed] [Google Scholar]
- 19. Motaln H, Schichor C, Lah TT: Human mesenchymal stem cells and their use in cell-based therapies. Cancer 116: 2519–2530, 2010 [DOI] [PubMed] [Google Scholar]
- 20. Fiorina P, Jurewicz M, Augello A, Vergani A, Dada S, La Rosa S, Selig M, Godwin J, Law K, Placidi C, Smith RN, Capella C, Rodig S, Adra CN, Atkinson M, Sayegh MH, Abdi R: Immunomodulatory function of bone marrow-derived mesenchymal stem cells in experimental autoimmune type 1 diabetes. J Immunol 183: 993–1004, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Tolar J, Nauta AJ, Osborn MJ, Panoskaltsis Mortari A, McElmurry RT, Bell S, Xia L, Zhou N, Riddle M, Schroeder TM, Westendorf JJ, McIvor RS, Hogendoorn PC, Szuhai K, Oseth L, Hirsch B, Yant SR, Kay MA, Peister A, Prockop DJ, Fibbe WE, Blazar BR: Sarcoma derived from cultured mesenchymal stem cells. Stem Cells 25: 371–379, 2007 [DOI] [PubMed] [Google Scholar]
- 22. Li H, Fan X, Kovi RC, Jo Y, Moquin B, Konz R, Stoicov C, Kurt-Jones E, Grossman SR, Lyle S, Rogers AB, Montrose M, Houghton J: Spontaneous expression of embryonic factors and p53 point mutations in aged mesenchymal stem cells: A model of age-related tumorigenesis in mice. Cancer Res 67: 10889–10898, 2007 [DOI] [PubMed] [Google Scholar]
- 23. Capelli C, Domenghini M, Borleri G, Bellavita P, Poma R, Carobbio A, Mico C, Rambaldi A, Golay J, Introna M: Human platelet lysate allows expansion and clinical grade production of mesenchymal stromal cells from small samples of bone marrow aspirates or marrow filter washouts. Bone Marrow Transplant 40: 785–791, 2007 [DOI] [PubMed] [Google Scholar]
- 24. Capelli C, Salvade A, Pedrini O, Barbui V, Gotti E, Borleri G, Cabiati B, Belotti D, Perseghin P, Bellavita P, Biondi A, Biagi E, Rambaldi, Golay J, Introna M: The washouts of discarded bone marrow collection bags and filters are a very abundant source of hMSCs. Cytotherapy 11: 403–413, 2009 [DOI] [PubMed] [Google Scholar]
- 25. Ruggenenti P, Codreanu I, Cravedi P, Perna A, Gotti E, Remuzzi G: Basiliximab combined with low-dose rabbit anti-human thymocyte globulin: a possible further step toward effective and minimally toxic T cell-targeted therapy in kidney transplantation. Clin J Am Soc Nephrol 1: 546–554, 2006 [DOI] [PubMed] [Google Scholar]
- 26. Baldelli S, Merlini S, Perico N, Nicastri A, Cortinovis M, Gotti E, Remuzzi G, Cattaneo D: C-440T/T-331C polymorphisms in the UGT1A9 gene affect the pharmacokinetics of mycophenolic acid in kidney transplantation. Pharmacogenomics 8: 1127–1141, 2007 [DOI] [PubMed] [Google Scholar]
- 27. Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, Deans R, Keating A, Prockop D, Horwitz E: Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 8: 315–317, 2006 [DOI] [PubMed] [Google Scholar]
- 28. Gaspari F, Perico N, Ruggenenti P, Mosconi L, Amuchastegui CS, Guerini E, Daina E, Remuzzi G: Plasma clearance of nonradioactive iohexol as a measure of glomerular filtration rate. J Am Soc Nephrol 6: 257–263, 1995 [DOI] [PubMed] [Google Scholar]
- 29. Nowacki TM, Kuerten S, Zhang W, Shive CL, Kreher CR, Boehm BO, Lehmann PV, Tary-Lehmann M: Granzyme B production distinguishes recently activated CD8(+) memory cells from resting memory cells. Cell Immunol 247: 36–48, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Uccelli A, Moretta L, Pistoia V: Mesenchymal stem cells in health and disease. Nat Rev Immunol 8: 726–736, 2008 [DOI] [PubMed] [Google Scholar]
- 31. Bischoff DS, Zhu JH, Makhijani NS, Yamaguchi DT: Acidic pH stimulates the production of the angiogenic CXC chemokine, CXCL8 (interleukin-8), in human adult mesenchymal stem cells via the extracellular signal-regulated kinase, p38 mitogen-activated protein kinase, and NF-kappaB pathways. J Cell Biochem 104: 1378–1392, 2008 [DOI] [PubMed] [Google Scholar]
- 32. Dahl SR, Kleiveland CR, Kassem M, Lea T, Lundanes E, Greibrokk T: Determination of thromboxanes, leukotrienes and lipoxins using high-temperature capillary liquid chromatography-tandem mass spectrometry and on-line sample preparation. J Chromatogr A 1216: 4648–4654, 2009 [DOI] [PubMed] [Google Scholar]
- 33. Huang H, Kim HJ, Chang EJ, Lee ZH, Hwang SJ, Kim HM, Lee Y, Kim HH: IL-17 stimulates the proliferation and differentiation of human mesenchymal stem cells: Implications for bone remodeling. Cell Death Differ 16: 1332–1343, 2009 [DOI] [PubMed] [Google Scholar]
- 34. Battiwalla M, Hematti P: Mesenchymal stem cells in hematopoietic stem cell transplantation. Cytotherapy 11: 503–515, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Lucchini G, Introna M, Dander E, Rovelli A, Balduzzi A, Bonanomi S, Salvadè A, Capelli C, Belotti D, Gaipa G, Perseghin P, Vinci P, Lanino E, Chiusolo P, Rofino MG, Marktel S, Golay J, Rambaldi A, Biondi A, D'Amico G, Biagi E: Platelet-lysate-expanded mesenchymal stromal cells as a salvage therapy for severe resistant graft versus host disease in a pediatric population. Biol Blood Marrow Transplant 16: 1293–1301, 2010 [DOI] [PubMed] [Google Scholar]
- 36. Giordano A, Galderisi U, Marino IR: From the laboratory bench to the patient's bedside: An update on clinical trials with mesenchymal stem cells. J Cell Physiol 211: 27–35, 2007 [DOI] [PubMed] [Google Scholar]
- 37. Pal R, Venkataramana NK, Bansal A, Balaraju S, Jan M, Chandra R, Dixit A, Rauthan A, Murgod U, Totey S: Ex vivo-expanded autologous bone marrow-derived mesenchymal stromal cells in human spinal cord injury/paraplegia: A pilot clinical study. Cytotherapy 11: 897–911, 2009 [DOI] [PubMed] [Google Scholar]
- 38. Sun L, Akiyama K, Zhang H, Yamaza T, Hou Y, Zhao S, Xu T, Le A, Shi S: Mesenchymal stem cell transplantation reverses multiorgan dysfunction in systemic lupus erythematosus mice and humans. Stem Cells 27: 1421–1432, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Prockop DJ: Defining the probability that a cell therapy will produce a malignancy. Mol Ther 18: 1249–1250, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Garcia S, Bernad A, Martin MC, Cigudosa JC, Garcia-Castro J, de la Fuente R: Pitfalls in spontaneous in vitro transformation of human mesenchymal stem cells. Exp Cell Res 316: 1648–1650, 2010 [DOI] [PubMed] [Google Scholar]
- 41. Torsvik A, Rosland GV, Svendsen A, Molven A, Immervoll H, McCormack E, Lonning PE, Primon M, Sobala E, Tonn JC, Goldbrunner R, Schichor C, Mysliwietz J, Lah TT, Motaln H, Knappskog S, Bjerkvig R: Spontaneous malignant transformation of human mesenchymal stem cells reflects cross-contamination: Putting the research field on track – letter. Cancer Res 70: 6393–6396, 2010 [DOI] [PubMed] [Google Scholar]
- 42. Yu Y, Fuhr J, Boye E, Gyorffy S, Soker S, Atala A, Mulliken JB, Bischoff J: Mesenchymal stem cells and adipogenesis in hemangioma involution. Stem Cells 24: 1605–1612, 2006 [DOI] [PubMed] [Google Scholar]
- 43. Di Nicola M, Carlo-Stella C, Magni M, Milanesi M, Longoni PD, Matteucci P, Grisanti S, Gianni AM: Human bone marrow stromal cells suppress T-lymphocyte proliferation induced by cellular or nonspecific mitogenic stimuli. Blood 99: 3838–3843, 2002 [DOI] [PubMed] [Google Scholar]
- 44. Williams KM, Hakim FT, Gress RE: T cell immune reconstitution following lymphodepletion. Semin Immunol 19: 318–330, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Ge W, Jiang J, Baroja ML, Arp J, Zassoko R, Liu W, Bartholomew A, Garcia B, Wang H: Infusion of mesenchymal stem cells and rapamycin synergize to attenuate alloimmune responses and promote cardiac allograft tolerance. Am J Transplant 9: 1760–1772, 2009 [DOI] [PubMed] [Google Scholar]
- 46. Wang Y, Zhang A, Ye Z, Xie H, Zheng S: Bone marrow-derived mesenchymal stem cells inhibit acute rejection of rat liver allografts in association with regulatory T-cell expansion. Transplant Proc 41: 4352–4356, 2009 [DOI] [PubMed] [Google Scholar]
- 47. Zeiser R, Nguyen VH, Beilhack A, Buess M, Schulz S, Baker J, Contag CH, Negrin RS: Inhibition of CD4+CD25+ regulatory T-cell function by calcineurin-dependent interleukin-2 production. Blood 108: 390–399, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Popp FC, Eggenhofer E, Renner P, Slowik P, Lang SA, Kaspar H, Geissler EK, Piso P, Schlitt HJ, Dahlke MH: Mesenchymal stem cells can induce long-term acceptance of solid organ allografts in synergy with low-dose mycophenolate. Transpl Immunol 20: 55–60, 2008 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.