Summary
Background and objectives
Current assays and tests that are used to determine the degree of immunosuppression in renal transplant recipients are suboptimal. The ImmuKnowTM assay (CylexTM), a measure of intracellular CD4+ T cell ATP release proposed as a means to quantify cell-mediated immunity in transplant recipients, could be considered as a potential tool to identify patients at risk for opportunistic infections (OI) or acute rejection (AR).
Design, setting, participants, & measurements
We retrospectively analyzed 1330 ImmuKnow assay values in 583 renal transplant recipients at a single center from 2004 to 2009 and correlated these values with episodes of OI and AR in the subsequent 90 days. Assay values were compared with a control population matched for age, gender, and time post-transplantation.
Results
In patients with OI (n = 94), there were no differences in prior mean assay values compared with matched controls (386 versus 417 ng/ml, P = 0.24). In 47 patients with AR, again no differences were detected in prior assay results (390 versus 432 ng/ml, P = 0.25) when compared with controls. “Low” values (≤225 ng/ml) lacked sensitivity and specificity as a predictive test for subsequent OI, as did “strong” (≥525 ng/ml) values as a predictive test for subsequent AR.
Conclusions
Our results fail to show an association between single time point ImmuKnow assay values and the subsequent development of an adverse event in the subsequent 90 days. The optimal use of the ImmuKnow assay in kidney transplantation has yet to be determined.
Introduction
Transplant immunosuppression is a balance between the prevention of immunologic injury such as acute rejection and avoiding adverse events such as opportunistic infections, drug toxicity, and malignancy. Current methods used to determine an individual's immune status after kidney transplant include therapeutic drug monitoring, serologic markers (i.e. absolute leukocyte count, serum creatinine, and anti-HLA antibodies), and allograft biopsies. Therapeutic drug monitoring can be effective in preventing toxicity and ensuring patient adherence but does not take into account individual variations in metabolism and may not accurately predict the risk of rejection (1,2). To optimize immunosuppressive regimens and avoid untoward events, additional noninvasive methods to assess an individual's immune responsiveness in the clinical setting are necessary.
In 2002, the United States Food and Drug Administration (FDA) approved the ImmuKnowTM assay (CylexTM) as an assessment of cell-mediated immunity in immunocompromised individuals (3). The test measures increases in intracellular ATP levels released from activated CD4+ cells in response to nonspecific mitogenic stimulus. Because the cellular immune response is known to play a key role in acute rejection and in the prevention of opportunistic infections, the ImmuKnow assay may be a potentially useful predictor for the future development of such adverse events (4–7). Since the FDA review, however, few studies have clarified the value of the ImmuKnow assay in the clinical monitoring of patients after kidney transplant. Additionally, most studies have examined the assay characteristics in the presence of disease, rather than its potential as a predictive assay in patients without predefined disease.
Because many of these previous studies had small numbers of patients and events, included nonopportunistic infections, and had varying results, we performed a large retrospective analysis of ImmuKnow results obtained over a 5-year period and correlated these values specifically with subsequent clinical events of acute rejection and opportunistic infection. We hypothesized that measurements of CD4+ T cell ATP release via the ImmuKnow assay may demonstrate that individuals with high values are at increased risk for rejection, whereas those with low values are at increased risk for opportunistic infections.
Materials and Methods
We performed a single-center retrospective analysis of ImmuKnow assay (T cell assay [TCA]) results obtained in renal transplant recipients from November 2004 to July 2009. Inclusion criteria included recipients of a kidney or simultaneous pancreas-kidney transplant >18 years of age, with at least one TCA drawn during the study period. Initial baseline immunosuppression primarily included three-drug immunosuppression with the majority of regimens consisting of prednisone, a calcineurin inhibitor, and an antiproliferative agent. Rabbit antithymocyte globulin induction therapy was used for repeat transplants, African American recipients, simultaneous pancreas-kidney transplants, and patients with panel reactive antibody ≥20%. All of the patients received Pneumocystis carinii prophylaxis for a total of 6 months post-transplant. In the setting of induction therapy, cytomegalovirus (CMV) IgG+ recipients received valganciclovir for 3 months, whereas CMV IgG− recipients of a kidney from a CMV IgG+ donor received valganciclovir for 6 months. Patients were screened for BK virus (BKV) via urine and blood PCR at 1, 6, 12, and 24 months post-transplant or in the event of a clinical suspicion such as an acute rise in serum creatinine. Urine PCR levels >100 million copies/ml (high grade viruria) or a positive blood PCR (viremia) resulted in a decrease in immunosuppression with follow-up BKV blood/urine testing until urine PCR decreased to less than 100 million copies/ml, whereas patients with BKV viremia and a rise in serum creatinine underwent biopsy to confirm BKV nephropathy.
TCA were drawn in three different clinical scenarios and codified as: (1) protocol-based screening at 0, 1, 6, and 12 months post-transplant; (2) testing in the setting of clinical suspicion such as an acute rise in creatinine and/or potential opportunistic infection; and (3) testing of stable patients in the outpatient setting. The latter group included tests drawn at annual visits 2 or more years post-transplant or at any other routine clinic visit outside of the 1, 6, and 12 months in which organ transplant function was stable. TCA were performed at the University of Colorado HLA Laboratory as described previously (3). Whole blood was incubated overnight with phytohemagglutinin. CD4+ cells were selected using paramagnetic particles coated with monoclonal CD4+ antibodies, lysed to release ATP, and processed through a luminometer to calculate the numeric result, which was also categorized as “low” (≤225 ng/ml), “moderate” (226 to 524 ng/ml), or “strong” (≥525 ng/ml) per manufacturer recommendations.
These values were correlated with subsequent clinical events defined as either opportunistic infections (OI) or acute rejection (AR). TCA values drawn within 90 days before a clinical event were selected for analysis. These values were compared with TCA in patients matched for age, gender, and time of testing post-transplant but without clinical events. Further analysis included study of a subset of patients with more than one TCA value preceding the clinical event, for whom changes in TCA values over time were analyzed for the association of the future development of an adverse clinical event.
Acute rejection was defined as an acute rise in creatinine that was biopsy-proven or clinically suspected and improved with empiric intravenous corticosteroid therapy. Retrospective capture of all noninfluenza viral infections and fungal infections diagnosed clinically, serologically, and/or histologically during the study period was performed. Throughout the study period, no clinical intervention protocol was implemented based upon TCA results, and clinicians were discouraged from making interventions in immunosuppression based upon these results. An earlier analysis was performed on data collected from November 2004 to November 2007 (8). Noting no correlation between TCA results and clinical events, further data collection proceeded, again without intervention strategies based upon TCA results. This study was performed with the review and approval of the Colorado Institutional Review Board.
Statistical Analyses
TCA values were compared at baseline using t tests. Categories of TCA were compared for those with OI (or AR) versus all samples from patients with no infection or rejection events using χ2 tests. Patients with OI (or AR) versus control patients matched for age, gender, and time since transplantation were compared using McNemar's test on the matched pairs. Odds ratios were calculated using conditional logistic regression, stratified on age and time of test post-transplant. Changes in TCA over time for those with more than one value before an OI or AR event were compared with paired t tests. OI and AR were compared separately. Group sample sizes of 94 infections and 94 controls achieve 80% power to detect a difference of 74 ng/ml in TCA values, and 47 rejections and 47 controls achieve 80% power to detect a difference of 103 ng/ml, with an estimated group SD of 180 ng/ml and a significance level of 0.05 using a two-sided two-sample t test.
Results
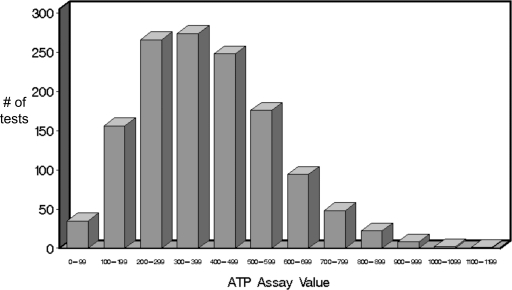
583 patients had a total of 1330 TCA results available for analysis between November 2004 and July 2009 (Table 1). 897 TCA values (67%) were drawn for protocol screening at 0, 1, 6, or 12 months post-transplant. The remaining tests were drawn for a clinical suspicion or other indication (Table 2). The frequency distribution of these results is presented in Figure 1.
Table 1.
Baseline characteristics of the 583 patients included in the study
| Variable | |
|---|---|
| Age at time of transplant (year; mean ± SD) | 46.2 ± 13.4 |
| Gender | |
| male | 349 (59.9%) |
| female | 234 (40.1%) |
| Race | |
| African American | 48 (8.2%) |
| Asian | 10 (1.7%) |
| Hispanic | 85 (14.6%) |
| Caucasian | 411 (70.5%) |
| other | 19 (3.3%) |
| unknown | 10 (1.7%) |
| Type of transplant | |
| deceased donor | 310 (53.2%) |
| living related | 153 (26.2%) |
| living unrelated | 103 (17.7%) |
| unknown | 17 (2.9%) |
| Induction therapy | |
| yes | 370 (63.4%) |
| no | 184 (31.6%) |
| unknown | 29 (5.0%) |
| Type of induction therapy | |
| thymoglobulin | 294 (79.5%) |
| basiliximab | 60 (16.2%) |
| othera | 16 (4.3%) |
| Immunosuppression regimen at 1 monthb | |
| tacrolimus and mycophenolic acid | 315 (54%) |
| tacrolimus and sirolimus | 144 (24.7%) |
| cyclosporine and mycophenolic acid | 13 (2.2%) |
| cyclosporine and sirolimus | 5 (0.9%) |
| otherc | 43 (7.4%) |
| unknown | 63 (10.8%) |
Other induction therapies included OKT3, alemtuzumab, daclizumab, and alefacept.
All of the patients were on prednisone since transplantation.
Included belatacept or everolimus in addition to calcineurin inhibitor or antiproliferative agent.
Table 2.
Conditions under which TCA (ImmuKnow) was performed for 1330 tests
| Indication for Test | Number of Tests (n = 1330) |
|---|---|
| Protocol screening at 0, 1, 6, and 12 months | 897 (67%) |
| Clinical suspiciona | 94 (7%) |
| Otherb | 339 (26%) |
Elevated creatinine, opportunistic infections, nonopportunistic infections, and malignancy.
Routine outpatient measurements outside protocol screening (see text).
Figure 1.
Distribution of TCA (ImmuKnow) results for 1330 tests performed over a 5-year period (2004 to 2009) in 583 patients.
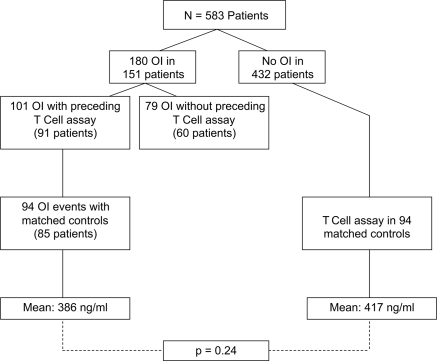
Overall, there were a total of 180 opportunistic infections in 151 patients (Table 3). There were 101 TCA values that preceded 101 OI in 91 patients. From these 101 tests, a control group could be identified for 94 TCA tests performed in patients without OI, matched for age, gender, and time post-transplant (controls). The remaining seven TCA associated with a subsequent OI did not have matched controls and were excluded from the analysis (Figure 2).
Table 3.
Opportunistic Infections 2004 to 2009 with and without preceding TCA (ImmuKnow)
| Type of Infection | TCA in the 90 Days before OI | No TCA in the 90 Days before OI | Total |
|---|---|---|---|
| CMV | 18 (17.8%)b | 30 (38.0%) | 48 (26.4%) |
| BKV | 58 (57.4%)c | 25 (31.6%) | 83 (45.6%) |
| HSV | 6 (5.9%) | 5 (6.3%) | 11 (6.1%) |
| VZV | 5 (4.9%) | 4 (5.0%) | 9 (4.9%) |
| EBV | 6 (5.9%)d | 7 (8.9%) | 13 (7.1%) |
| Candida | 3 (3.0%) | 7 (8.9%) | 12 (6.6%) |
| Othera | 5 (4.9%) | 1 (1.3%) | 6 (3.3%) |
| Total | 101 | 79 | 180 |
HSV, herpes simplex virus; VZV, varicella-zoster virus.
Rhizopus, nocardia, pneumocystis pneumonia, and cryptococcal infections.
CMV infections were identified in the evaluation of gastrointestinal symptoms (n = 9), leukopenia (n = 4), or other viral presentations (n = 5).
The 58 BKV infections included in the analysis were subclassified as BKV nephropathy (n = 7), BKV viremia (n = 36), and high-grade BKV viruria (n = 15) at the time of diagnosis.
All of the EBV infections were identified in the setting of clinical symptoms, none of which were related to post-transplant lymphoproliferative disorder.
Figure 2.
TCA (ImmuKnow) results in patients with subsequent OI compared with controls. TCA results with a subsequent OI within 90 days were compared with a control group comprised of patients without OI, matched for age and gender and with similar TCA testing within ±90 days from transplant date as patients with OI. The mean TCA values were then compared for each group.
There was no statistically significant difference noted when comparing the mean TCA in those associated with a subsequent OI versus controls (386 versus 417 ng/ml respectively, P = 0.24; Figure 2). Similar distributions in TCA values were present in both groups (Table 4). Notably, low values (≤225 ng/ml) were not associated with the future development of OI (P = 0.58). Within our overall study sample, 35 tests were <100 ng/ml, three of which were associated with a subsequent OI. To determine whether changes in TCA values over time may be more predictive of an OI event, we analyzed 49 of the 91 patients with an OI who had more than one preceding TCA value. In these patients, there was no significant difference between prior and subsequent mean values (395 ng/ml versus 354 ng/ml, P = 0.15). Twelve of 49 (25%) of these patients had a TCA decrease of ≥150 ng/ml, seven of 49 patients (14%) had an increase of ≥150 ng/ml, whereas the remainder (30 of 49 patients, 61%) had a change in TCA of <150 ng/ml. When compared with controls, the calculated sensitivity and specificity of TCA values <226 ng/ml for OI were 21.3 and 83.0%, respectively. The odds ratio (OR) for a low value, stratifying on age and time post-transplant, was NS at 1.34 (95% confidence interval [CI] 0.64 to 2.82, P = 0.43) (Table 5). The results were similar when the relationship between low values and OI were compared with all other samples (OR 1.29, 95% CI 0.79 to 2.09, P = 0.31, an uncontrolled comparison).
Table 4.
Distribution of T cell assay (TCA, ImmuKnow) values in the setting of subsequent opportunistic infection (OI) versus controls without OI
| TCA Result | OI (n = 94) | No OI (n = 94) |
|---|---|---|
| Low (≤225 ng/ml) | 20 (21%) | 16 (17%) |
| Moderate (226 to 524 ng/ml) | 53 (57%) | 52 (55%) |
| Strong (≥525 ng/ml) | 21 (22%) | 26 (28%) |
The values are the percentages of tests.
Table 5.
Sensitivity, specificity of a TCA ≤225 ng/ml, and opportunistic infection
| Versus Controls | Versus All Samples | |
|---|---|---|
| Sensitivity | 21.3 | 22.8 |
| Specificity | 83.0 | 81.4 |
| Odds ratio | 1.34 | 1.29 |
| 95% confidence interval | 0.64 to 2.82 | 0.79 to 2.09 |
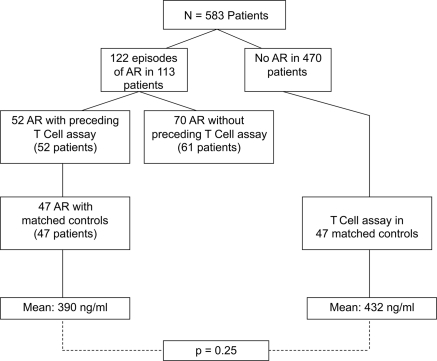
There were a total of 122 episodes of acute rejection in 113 patients overall (Table 6). Of 113 patients with 122 episodes of acute rejection, 52 episodes in 52 patients had a TCA in the prior 90 days available for analysis and were able to be matched with 47 age, gender, and time post-transplant controls. Five patients without controls available were excluded from the analysis.
Table 6.
Classification of acute rejection 2004 to 2009 with and without preceding TCA (ImmuKnow)
| Classification of Acute Rejection | TCA in the 90 Days before AR | No TCA in the 90 Days before AR | Total (%) |
|---|---|---|---|
| Clinical suspicion | 18 | 30 | 48 (39%) |
| Biopsy proven | 34 | 40 | 74 (61%) |
| cellular | 14 | 30 | 44 |
| humoral | 8 | 6 | 14 |
| cellular and humoral | 12 | 4 | 16 |
| Total | 52 | 70 | 122 |
When comparing TCA values in those with subsequent acute rejection versus controls, the mean values between the two groups were not statistically different (390 ng/ml in AR versus 432 ng/ml in controls, P = 0.25; Figure 3). In patients with acute rejection, 51% of the preceding TCA values were classified as moderate (226 to 524 ng/ml), 26% as low (≤225 ng/ml), and 23% as strong (≥525 ng/ml) (Table 7). The proportion of those TCA values classified as strong was not significantly different between patients with AR versus controls (P = 0.77). There were significantly more low values in individuals with AR when compared with controls (26% versus 7%, P = 0.01; Table 7). Within our overall study sample, of 81 tests ≥700 ng/ml only two were associated with subsequent AR. Of the 47 patients with AR, 11 had more than one preceding ImmuKnow assay value. In two patients the TCA decreased by ≥150 ng/ml, eight patients had a change in TCA of <150 ng/ml, and one patient increased by ≥150 ng/ml before an AR event. High TCA values lacked sensitivity and specificity for predicting the development of acute rejection when compared with controls (23.4 and 80.9%, respectively). The OR was not statistically significant compared with controls (OR 1.87, 95% CI 0.47 to 8.38, P = 0.48) or compared with all samples (OR 1.15, 95% CI 0.61 to 2.19, P = 0.67) (Table 8).
Figure 3.
TCA (ImmuKnow) results in patients with subsequent AR compared with controls. TCA results with a subsequent AR within 90 days were compared with a control group comprised of patients without AR, matched for age and gender and with similar TCA testing within ±90 days from transplant date as patients with AR. The mean TCA values were then compared for each group.
Table 7.
Distribution of TCA (Immuknow) values in the setting of subsequent AR versus controls without AR
| TCA Results | AR (n = 47) | No AR (n = 47) |
|---|---|---|
| Low (≤225) | 12 (26%)a | 3 (6%) |
| Moderate (226 to 524) | 24 (51%) | 35 (75%) |
| Strong (≥525) | 11 (23%) | 9 (19%) |
The values are the percentages of tests.
P = 0.01 versus control.
Table 8.
Sensitivity, specificity of a TCA ≥525, and acute rejection
| Versus Controls | Versus All Samples | |
|---|---|---|
| Sensitivity | 23.4 | 25.0 |
| Specificity | 80.9 | 77.5 |
| Odds ratio | 1.87 | 1.15 |
| 95% confidence interval | 0.47 to 8.38 | 0.61 to 2.19 |
Discussion
To our knowledge, this is the most comprehensive study to analyze the utility of a single ImmuKnow assay (CylexTM) measure in its predictive value for clinical events such as opportunistic infection and/or acute rejection in renal transplant recipients. We found no association between preceding TCA levels and the future development of either an OI or AR in the subsequent 3 months. Within our study population, low TCA values (≤225 ng/ml) lacked both sensitivity and specificity for the prediction of OI, as did high values (≥525 ng/ml) for the prediction of AR. The distribution of test values preceding both clinical outcomes was quite variable between low, moderate, and strong values, rendering the interpretation of a single test result of little clinical value for the purposes of defining patients at risk for OI or AR.
Prior reports have suggested that low TCA values are associated with both opportunistic infections and nonopportunistic infections. For example, Batal et al. (9) found significantly lower median TCA values in 25 patients with BK viremia when compared with patients with BK viruria and controls during the first 6 months post-transplant. Another study of 12 renal transplant patients with active viral infections (BKV and Epstein-Barr virus [EBV] most common) noted a mean TCA value of 56.8 ± 58.2 ng/ml at the time of diagnosis, and with a reduction in immunosuppression and treatment of the viral infection, the mean TCA value increased to 194.5 ± 118.9 (10). Importantly, these studies address the TCA at the time of disease rather than before the onset of disease. Other small studies have attempted to assess the ImmuKnow assay as a predictive test and have reported that low TCA values are associated with an increased incidence of infections in stable transplant recipients on maintenance immunosuppression (11–14). However, these studies were small with relatively few infection episodes compared with our study and included many nonopportunistic or undefined infections. Unlike these prior studies, we specifically defined and included only opportunistic infections, included tests performed both as screening tests in the asymptomatic patient as well as under conditions of clinical suspicion, and compared these results with a control group matched for age/gender/time of testing post-transplant.
There is less evidence in the literature correlating ATP release values and acute rejection. In a study of 509 heart, liver, kidney, and small bowel transplant recipients, patients with TCA values >700 ng/ml were 30 times more likely to develop cellular rejection. However, within the cohort of kidney transplant recipients with AR (n = 22), the median TCA value was 462 ng/ml (12). Reinsmoen et al. (15) reported that from a 126-patient cohort, pretransplant TCA levels were higher in 18 patients with subsequent acute rejection (occurring at any time after transplant) compared with 57 patients without subsequent rejection (pretransplant TCA 414.3 ng/ml versus 285.3 ng/ml, P = 0.04). Additional smaller studies are contradictory, with some suggesting that TCA may have predictive value for AR (14,16,17), while others have not (9).
Unlike these previous studies, our study had 2.5 to 10-fold more AR events (a total of 47 rejection episodes in 47 patients), a defined time of event from TCA (3 months), and a comparison with a gender/age/time match from the transplant control group. In our study the mean ATP release values for patients with AR was classified as moderate, with significantly more patients with AR and a low TCA when compared with controls. These findings demonstrate that patients with moderate or low ImmuKnow assay values still have a strong intact cellular immune response and can experience acute rejection.
A number of limitations in our study must be acknowledged. We included ImmuKnow assay values that were drawn within the preceding 90 days before an event for this analysis. This cutoff was selected as a reasonable time frame (quarterly) that transplant centers and care providers outside of a transplant center may consider utilizing the test, particularly after the first year post-transplant. Tests obtained nearer an event (e.g. 2 weeks or 1 months) may be more representative of an individual patient's risk for future clinical events (9,12) but would require significantly more sampling. Another limitation is that, despite standard immunosuppression and use of depleting antibody induction therapy in nearly half of all patients, TCA values within our population were rarely less than 100 ng/ml. Individuals with TCA values <100 ng/ml may indeed be at higher risk of developing an OI as discussed above (11,12). However, of 35 TCA <100 ng/ml, only three were associated with OI in the subsequent 90 days, indicating that extremely low values are not consistently associated with untoward events. Our lack of data in this regard may be due to differences in immunosuppression regimens compared with other studies (not well defined in other reports). Data regarding changes in immunosuppression after TCA measures, which could potentially influence the incidence of end points, were not collected. However, our immunosuppression regimens and protocols are not inconsistent with those commonly implemented for kidney transplantation in the United States and thus represent a reasonable “real world” experience with TCA interpretation. One cannot entirely exclude the possibility of clinical decision making that may have occurred on the basis of test results. However, this practice was discouraged, and an interim analysis in 2007 that suggested a lack of predictive value reinforced this policy (8). We did not perform serial screening for asymptomatic EBV, CMV, or hepatitis C viremia; thus we cannot comment upon the value of ImmuKnow monitoring for these conditions. Finally, it should be reiterated that the sensitivity and specificity as reported in Tables 5 and 8 are calculated based upon the features of our patient population, immunosuppression protocols, and testing protocol and thus may not be applicable to other transplant populations.
Our study was designed to test the hypothesis that the ImmuKnow assay could identify patients at risk for acute rejection or opportunistic infection under routine clinical management of renal transplant recipients. It should be clarified that there is no indication for use of the ImmuKnow assay for this purpose and that its FDA approved indication is for the “detection of cell-mediated immunity in an immunosuppressed population.” Earlier studies have suggested that this test should be used to determine the strength of a patient's immune system and provide guidance in individualizing and minimizing immunosuppression regimens to avoid adverse events (12). Others have suggested that changes in TCA value over time are more predictive of events, a hypothesis that is supported by uncontrolled studies but could not be tested in our series given relatively small numbers of patients with events who had more than one preceding TCA performed (17,18). On the basis of our data and interpreted within the context of our testing protocol, immunosuppression regimens, and 3-month assessment window described, we suggest that immunosuppressive medications not be adjusted solely on the basis of a single TCA measure.
In conclusion, ImmuKnow assay measures at a single time point did not identify individuals at risk for the future development of clinically significant events. Further studies are required, perhaps examining changes in ImmuKnow assay values over time, to clarify the role of this test in immune monitoring of kidney transplant recipients.
Disclosures
None.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1. Keown PA. on behalf of the International Neoral Renal Transplantation Study Group: Randomized, international study of cyclosporine microemulsion absorption profiling in renal transplantation with basiliximab immunoprophylaxis. Am J Transplant 2: 157–166, 2002 [DOI] [PubMed] [Google Scholar]
- 2. Mahalati K, Belitsky P, Sketris I, West K, Panek R: Neoral monitoring by simplified sparse sampling area under the concentration-time curve: Its relationship to acute rejection and cyclosporine nephrotoxicity early after kidney transplantation. Transplantation 68: 55–62, 1999 [DOI] [PubMed] [Google Scholar]
- 3. Kowalski R, Post D, Schneider MC, Britz J, Thomas J, Deierhoi M, Lobashevsky A, Redfield R, Schweitzer E, Heredia A, Reardon E, Davis C, Bentlejewski C, Fung J, Shapiro R, Zeevi A: Immune cell function testing: An adjunct to therapeutic drug monitoring in transplant patient management. Clin Transplant 17: 77–88, 2003 [DOI] [PubMed] [Google Scholar]
- 4. Rowshani AT, Bemelman FJ, van Leeuwen EM, van Lier RA, ten Berge IJ: Clinical and immunologic aspects of cytomegalovirus infection in solid organ transplant recipients. Transplantation 79: 381–386, 2005 [DOI] [PubMed] [Google Scholar]
- 5. Joosten SA, Ottenhoff TH: Human CD4 and CD8 regulatory T cells in infectious diseases and vaccination. Hum Immunol 69: 760–770, 2008 [DOI] [PubMed] [Google Scholar]
- 6. Chen Y, Trofe J, Gordon J, Du Pasquier RA, Roy-Chaudhury P, Kuroda MJ, Woodle ES, Khalili K, Koralnik IJ: Interplay of cellular and humoral immune responses against BK virus in kidney transplant recipients with polyomavirus nephropathy. J Virol 80: 3495–3505, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Le Moine A, Goldman M, Abramowicz D: Multiple pathways to allograft rejection. Transplantation 73: 1373–1381, 2002 [DOI] [PubMed] [Google Scholar]
- 8. Huskey J, Gralla J, Wiseman AC. Use of the Cylex ImmunoKnow assay in the prediction of acute rejection or opportunistic infection following kidney transplantation. J Am Soc Nephrol 19: 9A, 2008 [Google Scholar]
- 9. Batal I, Zeevi A, Heider A, Girnita A, Basu A, Tan H, Shapiro R, Randhawa P: Measurements of global cell-mediated immunity in renal transplant recipients with BK virus reactivation. Am J Clin Pathol 129: 587–591, 2008 [DOI] [PubMed] [Google Scholar]
- 10. Gautam A, Fischer SA, Yango AF, Gohh RY, Morrissey PE, Monaco AP: Cell mediated immunity (CMI) and post transplant viral infections: Role of a functional immune assay to titrate immunosuppression. Int Immunopharmacol 6: 2023–2026, 2006 [DOI] [PubMed] [Google Scholar]
- 11. Millan O, Sanchez-Fueyo A, Rimola A, Guillen D, Hidalgo S, Benitez C, Campistol JM, Brunet M: Is the intracellular ATP concentration of CD4+ T-Cells a predictive biomarker of immune status in stable transplant recipients? Transplantation 88[Suppl 3]: S78–-S84, 2009 [DOI] [PubMed] [Google Scholar]
- 12. Kowalski RJ, Post DR, Mannon RB, Sebastian A, Wright HI, Sigle G, Burdick J, Elmagd KA, Zeevi A, Lopez-Cepero M, Daller JA, Gritsch HA, Reed EF, Jonsson J, Hawkins D, Britz JA: Assessing relative risks of infection and rejection: A meta-analysis using an immune function assay. Transplantation 82: 663–668, 2006 [DOI] [PubMed] [Google Scholar]
- 13. Sanchez-Velasco P, Rodrigo E, Valero R, Ruiz JC, Fernandez-Fresnedo G, Lopez-Hoyos M, Pinera C, Palomar R, Layva-Cobian F, Arias M: Intracellular ATP concentrations of CD4 cells in kidney transplant patients with and without infection. Clin Transplant 22: 55–60, 2008 [DOI] [PubMed] [Google Scholar]
- 14. Perez-Flores I, Sanchez-Fructuoso A, Santiago JL, Fernandez-Arquero M, Calvo N, de la Concha EG, Barrientos A: Intracellular ATP levels in CD4+ lymphocytes are a risk marker of rejection and infection in renal graft recipients. Transplant Proc 41: 2106–2108, 2009 [DOI] [PubMed] [Google Scholar]
- 15. Reinsmoen NL, Cornett KM, Kloehn R, Burnette AD, McHugh L, Flewellen BK, Matas A, Savik K.: Pretransplant donor-specific and non-specific immune parameters associated with early acute rejection. Transplantation 85: 462–470, 2008 [DOI] [PubMed] [Google Scholar]
- 16. Cadillo-Chavez R, de Echegaray S, Santiago-Delpin EA, Rodriguez-Trinidad AT, Camacho-Carrazo B, Alfaro T, Saavedra-Pozo M, Carrasquillo L, González-Caraballo ZA, Morales-Otero LA: Assessing the risk of infection and rejection in Hispanic renal transplant recipients by means of an adenosine triphosphate release assay. Transplant Proc 38: 918–920, 2006 [DOI] [PubMed] [Google Scholar]
- 17. Israeli M, Yussim A, Mor E, Sredni B, Klein T: Preceeding the rejection: in search for a comprehensive post-transplant immune monitoring platform. Transpl Immunol 18: 7–12, 2007 [DOI] [PubMed] [Google Scholar]
- 18. Israeli M, Ben-Gal T, Yaari V, Valdman A, Matz I, Medalion B, Battler A, Sredni B, Kristt D, Klein T.: Individualized immune monitoring of cardiac transplant recipients by noninvasive longitudinal cellular immunity tests. Transplantation 89: 968–976, 2010 [DOI] [PubMed] [Google Scholar]