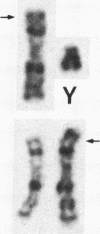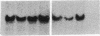Abstract
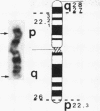
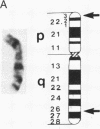
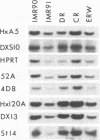
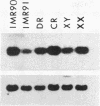
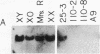
A pericentric inversion of a human X chromosome and a recombinant X chromosome [rec(X)] derived from crossing-over within the inversion was identified in a family. The rec(X) had a duplication of the segment Xq26.3----Xqter and a deletion of Xp22.3----Xpter and was interpreted to be Xqter----Xq26.3::Xp22.3----Xqter. To characterize the rec(X) chromosome, dosage blots were done on genomic DNA from carriers of this rearranged X chromosome using a number of X chromosome probes. Results showed that anonymous sequences from the distal end of the long arm to which probes 4D8, Hx120A, DX13, and St14 bind as well as the locus for glucose-6-phosphate dehydrogenase (G6PD) were duplicated on the rec(X). Mouse-human cell hybrids were constructed that retained the rec(X) in the active or inactive state. Analyses of these hybrid clones for markers from the distal short arm of the X chromosome showed that the rec(X) retained the loci for steroid sulfatase (STS) and the cell surface antigen 12E7 (MIC2); but not the pseudoautosomal sequence 113D. These molecular studies confirm that the rec(X) is a duplication-deficiency chromosome as expected. In the inactive state in cell hybrids, STS and MIC2 (which usually escape X chromosome inactivation) were expressed from the rec(X), whereas G6PD was not. Therefore, in the rec(X) X chromosome inactivation has spread through STS and MIC2 leaving these loci unaffected and has inactivated G6PD in the absence of an inactivation center in the q26.3----qter region of the human X chromosome. The mechanism of spreading of inactivation appears to operate in a sequence-specific fashion. Alternatively, STS and MIC2 may have undergone inactivation initially but could not be maintained in an inactive state.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boggs B. A., Nussbaum R. L. Two anonymous X-specific human sequences detecting restriction fragment length polymorphisms in region Xq26----qter. Somat Cell Mol Genet. 1984 Nov;10(6):607–613. doi: 10.1007/BF01535226. [DOI] [PubMed] [Google Scholar]
- Buckle V., Mondello C., Darling S., Craig I. W., Goodfellow P. N. Homologous expressed genes in the human sex chromosome pairing region. Nature. 1985 Oct 24;317(6039):739–741. doi: 10.1038/317739a0. [DOI] [PubMed] [Google Scholar]
- Caspersson T., Zech L., Johansson C. Differential binding of alkylating fluorochromes in human chromosomes. Exp Cell Res. 1970 Jun;60(3):315–319. doi: 10.1016/0014-4827(70)90523-9. [DOI] [PubMed] [Google Scholar]
- Cattanach B. M. Controlling elements in the mouse X-chromosome. 3. Influence upon both parts of an X divided by rearrangement. Genet Res. 1970 Dec;16(3):293–301. doi: 10.1017/s001667230000255x. [DOI] [PubMed] [Google Scholar]
- Chapman V. M., Kratzer P. G., Siracusa L. D., Quarantillo B. A., Evans R., Liskay R. M. Evidence for DNA modification in the maintenance of X-chromosome inactivation of adult mouse tissues. Proc Natl Acad Sci U S A. 1982 Sep;79(17):5357–5361. doi: 10.1073/pnas.79.17.5357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooke H. J., Brown W. R., Rappold G. A. Hypervariable telomeric sequences from the human sex chromosomes are pseudoautosomal. Nature. 1985 Oct 24;317(6039):687–692. doi: 10.1038/317687a0. [DOI] [PubMed] [Google Scholar]
- Drayna D., Davies K., Hartley D., Mandel J. L., Camerino G., Williamson R., White R. Genetic mapping of the human X chromosome by using restriction fragment length polymorphisms. Proc Natl Acad Sci U S A. 1984 May;81(9):2836–2839. doi: 10.1073/pnas.81.9.2836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eicher E. M. X-autosome translocations in the mouse: total inactivation versus partial inactivation of the X chromosome. Adv Genet. 1970;15:175–259. [PubMed] [Google Scholar]
- Geller R. L., Shapiro L. J., Mohandas T. K. Fine mapping of the distal short arm of the human X chromosome using X/Y translocations. Am J Hum Genet. 1986 Jun;38(6):884–890. [PMC free article] [PubMed] [Google Scholar]
- Goodfellow P., Banting G., Levy R., Povey S., McMichael A. A human X-linked antigen defined by a monoclonal antibody. Somatic Cell Genet. 1980 Nov;6(6):777–787. doi: 10.1007/BF01538976. [DOI] [PubMed] [Google Scholar]
- Hsu L. C., Tani K., Fujiyoshi T., Kurachi K., Yoshida A. Cloning of cDNAs for human aldehyde dehydrogenases 1 and 2. Proc Natl Acad Sci U S A. 1985 Jun;82(11):3771–3775. doi: 10.1073/pnas.82.11.3771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu L. C., Yoshida A., Mohandas T. Chromosomal assignment of the genes for human aldehyde dehydrogenase-1 and aldehyde dehydrogenase-2. Am J Hum Genet. 1986 May;38(5):641–648. [PMC free article] [PubMed] [Google Scholar]
- Immken L., Mohandas T., Sparkes R. S., Shapiro L. J. The steroid sulfatase locus on structurally abnormal inactive X chromosomes is expressed. Am J Hum Genet. 1984 Sep;36(5):979–986. [PMC free article] [PubMed] [Google Scholar]
- Lester S. C., Korn N. J., DeMars R. Derepression of genes on the human inactive X chromosome: evidence for differences in locus-specific rates of derepression and rates of transfer of active and inactive genes after DNA-mediated transformation. Somatic Cell Genet. 1982 Mar;8(2):265–284. doi: 10.1007/BF01538681. [DOI] [PubMed] [Google Scholar]
- Liskay R. M., Evans R. J. Inactive X chromosome DNA does not function in DNA-mediated cell transformation for the hypoxanthine phosphoribosyltransferase gene. Proc Natl Acad Sci U S A. 1980 Aug;77(8):4895–4898. doi: 10.1073/pnas.77.8.4895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattei M. G., Mattei J. F., Vidal I., Giraud F. Structural anomalies of the X chromosome and inactivation center. Hum Genet. 1981;56(3):401–408. doi: 10.1007/BF00274702. [DOI] [PubMed] [Google Scholar]
- Mohandas T., Heinzmann C., Sparkes R. S., Wasmuth J., Edwards P., Lusis A. J. Assignment of human 3-hydroxy-3-methylglutaryl coenzyme A reductase gene to q13----q23 region of chromosome 5. Somat Cell Mol Genet. 1986 Jan;12(1):89–94. doi: 10.1007/BF01560731. [DOI] [PubMed] [Google Scholar]
- Mohandas T., Shapiro L. J., Sparkes R. S., Sparkes M. C. Regional assignment of the steroid sulfatase-X-linked ichthyosis locus: implications for a noninactivated region on the short arm of human X chromosome. Proc Natl Acad Sci U S A. 1979 Nov;76(11):5779–5783. doi: 10.1073/pnas.76.11.5779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohandas T., Sparkes R. S., Bishop D. F., Desnick R. J., Shapiro L. J. Frequency of reactivation and variability in expression of X-linked enzyme loci. Am J Hum Genet. 1984 Jul;36(4):916–925. [PMC free article] [PubMed] [Google Scholar]
- Mohandas T., Sparkes R. S., Shapiro L. J. Reactivation of an inactive human X chromosome: evidence for X inactivation by DNA methylation. Science. 1981 Jan 23;211(4480):393–396. doi: 10.1126/science.6164095. [DOI] [PubMed] [Google Scholar]
- Oberlé I., Drayna D., Camerino G., White R., Mandel J. L. The telomeric region of the human X chromosome long arm: presence of a highly polymorphic DNA marker and analysis of recombination frequency. Proc Natl Acad Sci U S A. 1985 May;82(9):2824–2828. doi: 10.1073/pnas.82.9.2824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parada L. F., Tabin C. J., Shih C., Weinberg R. A. Human EJ bladder carcinoma oncogene is homologue of Harvey sarcoma virus ras gene. Nature. 1982 Jun 10;297(5866):474–478. doi: 10.1038/297474a0. [DOI] [PubMed] [Google Scholar]
- Patel P. I., Framson P. E., Caskey C. T., Chinault A. C. Fine structure of the human hypoxanthine phosphoribosyltransferase gene. Mol Cell Biol. 1986 Feb;6(2):393–403. doi: 10.1128/mcb.6.2.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polani P. E., Angell R., Giannelli F., De la Chapelle A., Race R. R., Sanger R. Evidence that the Xg locus is inactivated in structurally abnormal X chromosomes. Nature. 1970 Aug 8;227(5258):613–616. doi: 10.1038/227613a0. [DOI] [PubMed] [Google Scholar]
- Rastan S. Non-random X-chromosome inactivation in mouse X-autosome translocation embryos--location of the inactivation centre. J Embryol Exp Morphol. 1983 Dec;78:1–22. [PubMed] [Google Scholar]
- Rouyer F., Simmler M. C., Johnsson C., Vergnaud G., Cooke H. J., Weissenbach J. A gradient of sex linkage in the pseudoautosomal region of the human sex chromosomes. Nature. 1986 Jan 23;319(6051):291–295. doi: 10.1038/319291a0. [DOI] [PubMed] [Google Scholar]
- Shapiro L. J. Steroid sulfatase deficiency and the genetics of the short arm of the human X chromosome. Adv Hum Genet. 1985;14:331-81, 388-9. doi: 10.1007/978-1-4615-9400-0_5. [DOI] [PubMed] [Google Scholar]
- Shapiro L. J., Weiss R., Buxman M. M., Vidgoff J., Dimond R. L., Roller J. A., Wells R. S. Enzymatic basis of typical X-linked icthyosis. Lancet. 1978 Oct 7;2(8093):756–757. doi: 10.1016/s0140-6736(78)92646-6. [DOI] [PubMed] [Google Scholar]
- Simmler M. C., Rouyer F., Vergnaud G., Nyström-Lahti M., Ngo K. Y., de la Chapelle A., Weissenbach J. Pseudoautosomal DNA sequences in the pairing region of the human sex chromosomes. Nature. 1985 Oct 24;317(6039):692–697. doi: 10.1038/317692a0. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Summitt R. L., Tipton R. E., Wilroy R. S., Jr, Martens P. R., Phelan J. P. X-autosome translocations: a review. Birth Defects Orig Artic Ser. 1978;14(6C):219–247. [PubMed] [Google Scholar]
- Takagi N. Primary and secondary nonrandom X chromosome inactivation in early female mouse embryos carrying Searle's translocation T(X; 16)16H. Chromosoma. 1980;81(3):439–459. doi: 10.1007/BF00368155. [DOI] [PubMed] [Google Scholar]
- Takizawa T., Huang I. Y., Ikuta T., Yoshida A. Human glucose-6-phosphate dehydrogenase: primary structure and cDNA cloning. Proc Natl Acad Sci U S A. 1986 Jun;83(12):4157–4161. doi: 10.1073/pnas.83.12.4157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Therman E., Patau K. Abnormal X chromosomes in man: origin, behavior and effects. Humangenetik. 1974;25(1):1–16. doi: 10.1007/BF00281002. [DOI] [PubMed] [Google Scholar]
- Venolia L., Gartler S. M. Comparison of transformation efficiency of human active and inactive X-chromosomal DNA. Nature. 1983 Mar 3;302(5903):82–83. doi: 10.1038/302082a0. [DOI] [PubMed] [Google Scholar]
- Venolia L., Gartler S. M., Wassman E. R., Yen P., Mohandas T., Shapiro L. J. Transformation with DNA from 5-azacytidine-reactivated X chromosomes. Proc Natl Acad Sci U S A. 1982 Apr;79(7):2352–2354. doi: 10.1073/pnas.79.7.2352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willard H. F., Skolnick M. H., Pearson P. L., Mandel J. L. Report of the Committee on Human Gene Mapping by Recombinant DNA Techniques. Cytogenet Cell Genet. 1985;40(1-4):360–489. doi: 10.1159/000132180. [DOI] [PubMed] [Google Scholar]
- Yen P. H., Marsh B., Mohandas T. K., Shapiro L. J. Isolation of genomic clones homologous to transcribed sequences from human X chromosome. Somat Cell Mol Genet. 1984 Nov;10(6):561–571. doi: 10.1007/BF01535221. [DOI] [PubMed] [Google Scholar]
- Yen P. H., Patel P., Chinault A. C., Mohandas T., Shapiro L. J. Differential methylation of hypoxanthine phosphoribosyltransferase genes on active and inactive human X chromosomes. Proc Natl Acad Sci U S A. 1984 Mar;81(6):1759–1763. doi: 10.1073/pnas.81.6.1759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida A., Lebo R. V. Existence of glucose-6-phosphate dehydrogenase-like locus on chromosome 17. Am J Hum Genet. 1986 Aug;39(2):203–206. [PMC free article] [PubMed] [Google Scholar]