Abstract
CaV1.3 L-type channels control inner hair cell (IHC) sensory and sinoatrial node (SAN) function, and excitability in central neurons by means of their low-voltage activation and inactivation properties. In SAN cells CaV1.3 inward calcium current (ICa) inactivates rapidly whereas in IHCs inactivation is slow. A candidate suggested in slowing CaV1.3 channel inactivation is the presynaptically located ribbon-synapse protein RIM that is expressed in immature IHCs in presynaptic compartments also expressing CaV1.3 channels. CaV1.3 channel gating is also modulated by an intramolecular C-terminal mechanism. This mechanism was elicited during analysis of human C-terminal splice variants that differ in the length of their C-terminus and that modulates the channel's negative activation range and slows calcium-dependent inactivation.
Key words: CaV1.3 L-type calcium channel, channel gating, C-terminal modulation, protein-protein interaction
Voltage-gated L-type calcium channels (LTCCs) form the CaV1 channel family, which comprises the isoforms CaV1.1, CaV1.2, CaV1.3 and CaV1.4. CaV1 channels are well-known pharmacotherapeutic targets of Ca2+ channel blockers such as dihydropyridines. Among the CaV1 family, CaV1.3 L-type channels physiologically control inner hair cell (IHC) sensory and sinoatrial node (SAN) function, as well as excitability in central neurons by means of their peculiar low-voltage activation and differential inactivation properties.1 A lot of our knowledge about CaV1.3 channels comes from knock-out mouse models (CaV1.3−/−).1–3 CaV1.3−/− mice are deaf and suffer from sinoatrial node dysfunction.1,2 The deafness is due to the complete absence of L-type Ca2+ currents (ICa) in IHCs and outer hair cells of the cochlea1,4 where CaV1.3-mediated L-type currents comprise about 90% of the calcium current. CaV1.3−/− mice also exhibit an arrhythmic and bradycardic heart beat. This phenotype is due to an intrinsic defect in the SAN present at low heart rates.1
CaV1.3 channels can conduct calcium inward current in the operating range of IHCs and SAN cells (which is between −60 and −40 mV) due to their negative activation range.1,5–7 In neurons, CaV1.3 channels shape neuronal firing as for example in striatal medium spiny neurons8 and contribute to dendritic calcium oscillations in Dopamine (DA)-releasing neurons of the substantia nigra pars compacta (SNc).9 Ca2+ ions entering SNc DA neurons through LTCCs elevate cellular vulnerability to toxins used to create animal models of Parkinson's disease (PD).10 In animal models of PD, block of CaV1.3 channels appears to underlie neuroprotective therapeutic effects of dihydropyridine (DHP) LTCC blockers10 and DHPs ameliorate the development of L-DOPA-induced dyskinesias.11 In a retrospective human study, long-term use of calcium channel blockers was associated with a significantly reduced risk of a PD diagnosis.12 In this context it is important to note, that DHPs act as state-dependent blockers that need the channel's inactivated state and are thereby very likely to show activity-dependent potency.
The typical low activation threshold is intrinsic to CaV1.3 channels; however, their inactivation properties vary in a wide range and seem to be differentially modulated. Whereas CaV1.3 ICa inactivates rapidly in SAN cells1,13 its inactivation is slow in IHCs.1 CaM-like Ca2+ binding proteins (CaBPs) have been shown to eliminate calcium-dependent inactivation (CDI) of a short rat CaV1.3 channel isoform by competing CaM binding to the channel's C-terminus14,15 and also CaVβ2 was recently reported to slightly affect CDI in IHCs,16 but voltage-dependent inactivation remained largely unaltered. A candidate suggested in slowing CaV1.3 channel inactivation was a presynaptically located ribbon-synapse protein called Rab3-interacting molecule (RIM) that is co-localized with CaV1.3 in the same presynaptic compartments of IHCs.17 In tsA-201 cells, RIM proteins inhibit CaV1.3 inactivation by slowing both CDI and VDI and induce a non-inactivating current component typical for CaV1.3 currents in IHCs.17 The modulatory effects of RIM are mediated via its binding to the CaVβ-subunit of the Ca2+ channel complex (Fig. 1). Because RIM mRNA is detected in the organ of Corti in IHC preparations before the onset of hearing, RIM proteins might therefore partly account for the slow inactivation of CaV1.3 IHC currents at least in an early developmental stage.
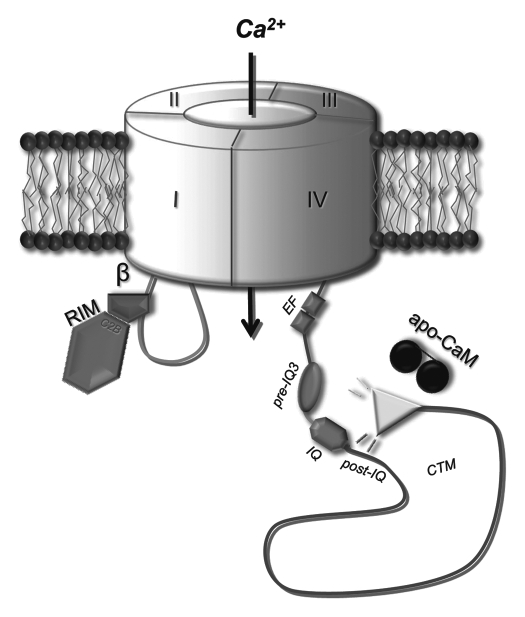
Figure 1.
Cartoon of a proposed model for differential CaV1.3 gating modulation. The CaV1.3 channel is given as transmembrane pore forming α1-subunit in grey. RIM protein (pink) via its C2B domain interacts with the CaVβ subunit (magenta), which in turn binds the I–II loop (light blue). The C-terminus (dark blue) contains the CDI maschinery (comprising the EF hand, pre-IQ and IQ domains) and a post-IQ domain that interacts with the distal C-terminus forming the CTM in CaV1.3 channels. Modulation of the channel's CDI is suggested a competitive mechanism in which the CaV1.3 CTM competes with apoCaM for binding near the channel IQ domain.18,23
Furthermore, CaV1.3 channel gating is also controlled by an intramolecular protein-protein interaction in the channel's C-terminus.18 An intrinsic C-terminal modulator (CTM) controls both activation as well as inactivation properties via binding of the distal CaV1.3 C-terminus to a more proximal domain containing the EF-hand, pre-IQ- and IQ-motif, and a regulatory domain right after the IQ-motif (Fig. 1) that has also been identified to be important in CaV1.2 channels.19 Most interestingly, alternative splicing generates CaV1.3 α1-subunits with long or short C-termini in various tissues18 and might thereby enable tight control of channel gating. In tsA-201 cells, the absence of the CTM in a human short splice variant led to a lower activation range, a negative shift of the voltage-dependence of inactivation as well as more pronounced CDI of the channel compared to the long variant.18 The physiological implications of the CaV1.3 CTM are still ambiguous. The CTM in long CaV1.3 channels may be suitable for longer lasting Ca2+ signals triggered by stronger depolarization inducing CREB phosphorylation and synaptic plasticity.20 Differences in the CTM inactivation pattern of long and short CaV1.3 channels (e.g., due a different extend of accumulation in their inactivated state) could though underlie different shapes or firing rates of action potentials as observed in different types of neurons.21 In terms of pharmacological intervention the CaV1.3 CTM should also have a strong impact on the efficiency of DHP block because this correlates with the amount of channels inactivated.5,6
Based on the emerging role of CaV1.3 for normal and potential pathological cellular function, the discovery of the CaV1.3 CTM also raises a question about its potential as an alternative concept for pharmacological modulation of CaV1.3 channels. Potential CaV1.3 selective drugs may be envisaged to have disadvantages, such as slowing of the heartbeat, as seen in CaV1.3−/− mice1 and adaptive mechanisms seem not be able to restore pace-making in cardiac cells. The pathophysiological consequences observed in mice lacking function CaV1.3 channels about ten years ago are meanwhile also reported in two consanguineous Pakistani deafness families that also show severely impaired SAN function due to a splice variant specific CaV1.3 loss-of-function mutation.22 Interference with only the CaV1.3 CTM interaction should be beneficial to alter cellular excitability by switching form the long to short channel gating mode in avoidance of complete channel block. Such shift in CaV1.3 gating properties within a limited range could provide also a novel strategy for therapeutic Ca2+ channel modulation that avoids complete state-dependent inhibition of these channels, but could nevertheless induce functional changes to obtain the desired pharmacological effects.
References
- 1.Platzer J, Engel J, Schrott-Fischer A, Stephan K, Bova S, Chen H, et al. Congenital deafness and sinoatrial node dysfunction in mice lacking class D L-type Ca2+ channels. Cell. 2000;102:89–97. doi: 10.1016/s0092-8674(00)00013-1. [DOI] [PubMed] [Google Scholar]
- 2.Namkung Y, Skrypnyk N, Jeong MJ, Lee T, Lee MS, Kim HL, et al. Requirement for the L-type Ca2+ channel α1D subunit in postnatal pancreatic b cell generation. J Clin Invest. 2001;108:1015–1022. doi: 10.1172/JCI13310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Striessnig J, Koschak A, Sinnegger-Brauns MJ, Hetzenauer A, Nguyen NK, Busquet P, et al. Role of voltage-gated L-type Ca2+ channel isoforms for brain function. Biochem Soc Trans. 2006;34:903–909. doi: 10.1042/BST0340903. [DOI] [PubMed] [Google Scholar]
- 4.Michna M, Knirsch M, Hoda JC, Muenkner S, Langer P, Platzer J, et al. CaV1.3 (α1D) Ca2+ currents in neonatal outer hair cells of mice. J Physiol. 2003;553:747–758. doi: 10.1113/jphysiol.2003.053256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Koschak A, Reimer D, Huber I, Grabner M, Glossmann H, Engel J, et al. Alpha1D (CaV1.3) subunits can form L-type Ca2+ channels activating at negative voltages. J Biol Chem. 2001;276:22100–22106. doi: 10.1074/jbc.M101469200. [DOI] [PubMed] [Google Scholar]
- 6.Xu W, Lipscombe D. Neuronal CaV1.3α1 L-type channels activate at relatively hyperpolarized membrane potentials and are incompletely inhibited by dihydropyridines. J Neurosci. 2001;21:5944–5951. doi: 10.1523/JNEUROSCI.21-16-05944.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lipscombe D, Helton TD, Xu W. L-type calcium channels: the low down. J Neurophysiol. 2004;92:2633–2641. doi: 10.1152/jn.00486.2004. [DOI] [PubMed] [Google Scholar]
- 8.Olson PA, Tkatch T, Hernandez-Lopez S, Ulrich S, Ilijic E, Mugnaini E, et al. G-protein-coupled receptor modulation of striatal CaV1.3 L-type Ca2+ channels is dependent on a Shank-binding domain. J Neurosci. 2005;25:1050–1062. doi: 10.1523/JNEUROSCI.3327-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Guzman JN, Sanchez-Padilla J, Chan CS, Surmeier DJ. Robust pacemaking in substantia nigra dopaminergic neurons. J Neurosci. 2009;29:11011–11019. doi: 10.1523/JNEUROSCI.2519-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chan CS, Guzman JN, Ilijic E, Mercer JN, Rick C, Tkatch T, et al. ‘Rejuvenation’ protects neurons in mouse models of Parkinson's disease. Nature. 2007;447:1081–1086. doi: 10.1038/nature05865. [DOI] [PubMed] [Google Scholar]
- 11.Schuster S, Doudnikoff E, Rylander D, Berthet A, Aubert I, Ittrich C, et al. Antagonizing L-type Ca2+ channel reduces development of abnormal involuntary movement in the rat model of L-3,4-dihydroxyphenylalanine-induced dyskinesia. Biol Psychiatry. 2009;65:518–526. doi: 10.1016/j.biopsych.2008.09.008. [DOI] [PubMed] [Google Scholar]
- 12.Becker C, Jick SS, Meier CR. Use of antihypertensives and the risk of Parkinson disease. Neurology. 2008;70:1438–1444. doi: 10.1212/01.wnl.0000303818.38960.44. [DOI] [PubMed] [Google Scholar]
- 13.Mangoni ME, Couette B, Bourinet E, Platzer J, Reimer D, Striessnig J, et al. Functional role of L-type CaV1.3 Ca2+ channels in cardiac pacemaker activity. Proc Natl Acad Sci USA. 2003;100:5543–5548. doi: 10.1073/pnas.0935295100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yang PS, Alseikhan BA, Hiel H, Grant L, Mori MX, Yang W, et al. Switching of Ca2+-dependent inactivation of CaV1.3 channels by calcium binding proteins of auditory hair cells. J Neurosci. 2006;26:10677–10689. doi: 10.1523/JNEUROSCI.3236-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cui G, Meyer AC, Calin-Jageman I, Neef J, Haeseleer F, Moser T, et al. Ca2+-binding proteins tune Ca2+-feedback to CaV1.3 channels in mouse auditory hair cells. J Physiol. 2007;585:791–803. doi: 10.1113/jphysiol.2007.142307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Neef J, Gehrt A, Bulankina AV, Meyer AC, Riedel D, Gregg RG, et al. The Ca2+ channel subunit β2 regulates Ca2+ channel abundance and function in inner hair cells and is required for hearing. J Neurosci. 2009;29:10730–10740. doi: 10.1523/JNEUROSCI.1577-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gebhart M, Juhasz-Vedres G, Zuccotti A, Brandt N, Engel J, Trockenbacher A, et al. Modulation of CaV1.3 Ca2+ channel gating by Rab3 interacting molecule. Mol Cell Neurosci. 2010;44:246–259. doi: 10.1016/j.mcn.2010.03.011. [DOI] [PubMed] [Google Scholar]
- 18.Singh A, Gebhart M, Fritsch R, Sinnegger-Brauns MJ, Poggiani C, Hoda JC, et al. Modulation of voltage- and Ca2+-dependent gating of CaV1.3 L-type calcium channels by alternative splicing of a C-terminal regulatory domain. J Biol Chem. 2008;283:20733–20744. doi: 10.1074/jbc.M802254200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hulme JT, Yarov-Yarovoy V, Lin TW, Scheuer T, Catterall WA. Autoinhibitory control of the CaV1.2 channel by its proteolytically processed distal C-terminal domain. J Physiol. 2006;576:87–102. doi: 10.1113/jphysiol.2006.111799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang H, Fu Y, Altier C, Platzer J, Surmeier DJ, Bezprozvanny I. CaV1.2 and CaV1.3 neuronal L-type calcium channels: differential targeting and signaling to pCREB. Eur J Neurosci. 2006;23:2297–2310. doi: 10.1111/j.1460-9568.2006.04734.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bean BP. The action potential in mammalian central neurons. Nat Rev Neurosci. 2007;8:451–465. doi: 10.1038/nrn2148. [DOI] [PubMed] [Google Scholar]
- 22.Baig SM, Koschak A, Lieb A, Gebhart M, Dafinger C, Nürnberg G, et al. Loss of CaV1.3 (CACNA1D) function in a human channelopathy with bradycardia and congenital deafness. Nat Neurosci. 2011;14:77–84. doi: 10.1038/nn.2694. [DOI] [PubMed] [Google Scholar]
- 23.Liu X, Yang PS, Yang W, Yue DT. Enzyme-inhibitor-like tuning of Ca2+ channel connectivity with calmodulin. Nature. 2010;463:968–972. doi: 10.1038/nature08766. [DOI] [PMC free article] [PubMed] [Google Scholar]