SUMMARY
The natural response to itch sensation is to scratch, which relieves the itch through an unknown mechanism. Interaction between pain and itch has been frequently demonstrated, and the selectivity hypothesis of itch, based on data from electrophysiological and behavioral experiments, postulates the existence of primary pain afferents capable of repressing itch. Here, we demonstrate that deletion of vesicular glutamate transporter (VGLUT) 2 in a subpopulation of neurons partly overlapping with the vanilloid receptor (TRPV1) primary afferents resulted in a dramatic increase in itch behavior accompanied by a reduced responsiveness to thermal pain. The increased itch behavior was reduced by administration of antihistaminergic drugs and by genetic deletion of the gastrin-releasing peptide receptor, demonstrating a dependence on VGLUT2 to maintain normal levels of both histaminergic and nonhistaminergic itch. This study establishes that VGLUT2 is a major player in TRPV1 thermal nociception and also serves to regulate a normal itch response.
INTRODUCTION
Pain and itch are two critical modalities of sensory input, and improper function of nociceptive and pruriceptive transmission may have severe consequences. For example, loss of pain sensation increases the risk of exposing our bodies to harmful tissue damage, and, similarly, loss of itch sensation deprives us of a warning signal of an offending stimulus. Further, exaggerated scratching in response to increased levels of perceived itch in skin disorders can induce self-feeding itch-scratch cycles that damage the skin and worsen the condition (Ikoma et al., 2006). There is a shortage of therapeutically efficient treatments for both itch and chronic pain, calling for a deeper understanding of how the underlying circuitry is organized and how it functions.
The delivery of itch and pain sensations starts with primary afferents located in the dorsal root ganglia (DRG), which transmit their information to the brain via second-order neurons including spinothalamic tract (STT) neurons in the dorsal horn. While the pathways, mechanisms, and populations of neurons responsible for transmitting pain have been under intense investigation for several decades and are reasonably well understood, a similar level of understanding regarding pruriception is lacking.
Three theories regarding the transmission of itch have been proposed: the intensity, the specificity, and the selectivity theories (McMahon and Koltzenburg, 1992). The intensity theory, originally raised by von Frey, postulates that weak stimuli in nociceptors can transmit itch instead of pain, presumably through a common central pathway (reviewed in Carstens, 2009). In contrast, data mostly originating from electrophysiological recordings in humans have led to the specificity theory, which suggests that exclusive pruriceptors exist along with nociceptors. For instance, itch induced by focal electrical stimulation of the skin or in single neurons becomes more intense as the stimulation frequency increases, but it does not transform into pain (Ikoma et al., 2005; Tuckett, 1982). In addition, an itch-specific signaling pathway from the periphery to the spinal cord dorsal horn, mediated by the gastrin-releasing peptide (GRP) (and its receptor), has been identified (Sun and Chen, 2007; Sun et al., 2009). Furthermore, two components in a histamine-specific route to higher brain areas have been identified, a population of C fibers (Schmelz et al., 1997) and a set of spinothalamic tract neurons (Andrew and Craig, 2001), findings that support the idea of specificity. However, histamine-sensitive C fibers were later demonstrated to be selective, rather than specific, for pruritogens (Schmelz et al., 2003). This finding fits with the third theory, which suggests the presence of fibers that are pain selective along with fibers transmitting both itch and pain (McMahon and Koltzenburg, 1992), although, as previously suggested, a mixture of the specificity and selectivity theories might be more accurate (Schmelz, 2001).
The selectivity theory also addresses afferent pain fibers, which have been hypothesized to mediate reduction of itch signaling (McMahon and Koltzenburg, 1992). Interaction between pain and itch has been frequently demonstrated, where the sensation of itch can be inhibited by various painful stimuli including vigorous scratching, cooling, mustard oil, noxious heat, and electrical cutaneous field stimulation (Ward et al., 1996; Yosipovitch et al., 2007). Conversely, loss of pain sensation can lead to elevated itch sensation (Atanassoff et al., 1999), for example through intrathecal injections of the opioid agonist morphine (Raffaeli et al., 2006; Slappendel et al., 2000). While it is recognized that pruriception is mediated by small-diameter primary afferent neurons (Han et al., 2006; Schmelz et al., 1997; Sun and Chen, 2007), the neuronal subpopulations and mediators capable of restricting exaggerated itch sensation have not been identified. Recent data, based on the loss of spinal cord interneurons in the Bhlhb5 null mice, suggest a model in which disinhibition in the dorsal spinal cord results in abnormal itch. Thus, itch behavior resulting from peripheral activation of primary afferent pruriceptors can be modulated by central circuits (Ross et al., 2010). It is presently unknown whether specific transmitters that can modulate pruriceptive signals exist.
Most primary afferents are glutamatergic and use a family of solute carriers, the vesicular glutamate transporters (VGLUT1-3), to package glutamate into synaptic vesicles. Mice heterozygous for VGLUT1 do not show any changes in pain behavior (Leo et al., 2009), which may be related to the finding that VGLUT1 is predominantly expressed in nonnociceptive neurons (Brumovsky et al., 2007). VGLUT2 dominates in nociceptive primary afferents (Brumovsky et al., 2007), and mice heterozygous for Vglut2 display impaired neuropathic pain sensitivity, whereas acute and inflammatory pain behaviors are unaltered (Moechars et al., 2006). VGLUT3 is expressed in a small subset of primary afferents and was recently related to mechanical pain sensation (Seal et al., 2009). Because of the extensive expression of VGLUT2 in primary afferents (Brumovsky et al., 2007; Li et al., 2003), promoter-driven Cre lines with restricted expression may be used to study the role of different subpopulations in the DRG by removal of glutamatergic transmission. The Nav1.8 population has been associated with mechanical and inflammatory pain modalities (Abrahamsen et al., 2008), and our recent work shows that there are both VGLUT2-dependent and -independent pain modalities within the Nav1.8 population (unpublished data). Based on the presence of VGLUT in primary afferents associated with pain and itch (C fibers), we hypothesized that VGLUT2 has a role in mediating additional submodalities of sensory transmission.
To study the contribution of VGLUT2-mediated signaling in pain and itch sensations, we investigated mice lacking Vglut2 in the primary sensory subpopulations defined by the expression of tyrosine hydroxylase (Th), human tissue plasminogen (Ht-Pa), voltage-gated sodium channel 1.8 (Nav1.8), or transient receptor potential vanilloid 1 (Trpv1). This allowed for a careful encircling of a peripheral neuronal population responsible for regulating itch and thermal pain transmission.
RESULTS
Restricted Vglut2 Deficiency Results in an Elevated Itch Phenotype
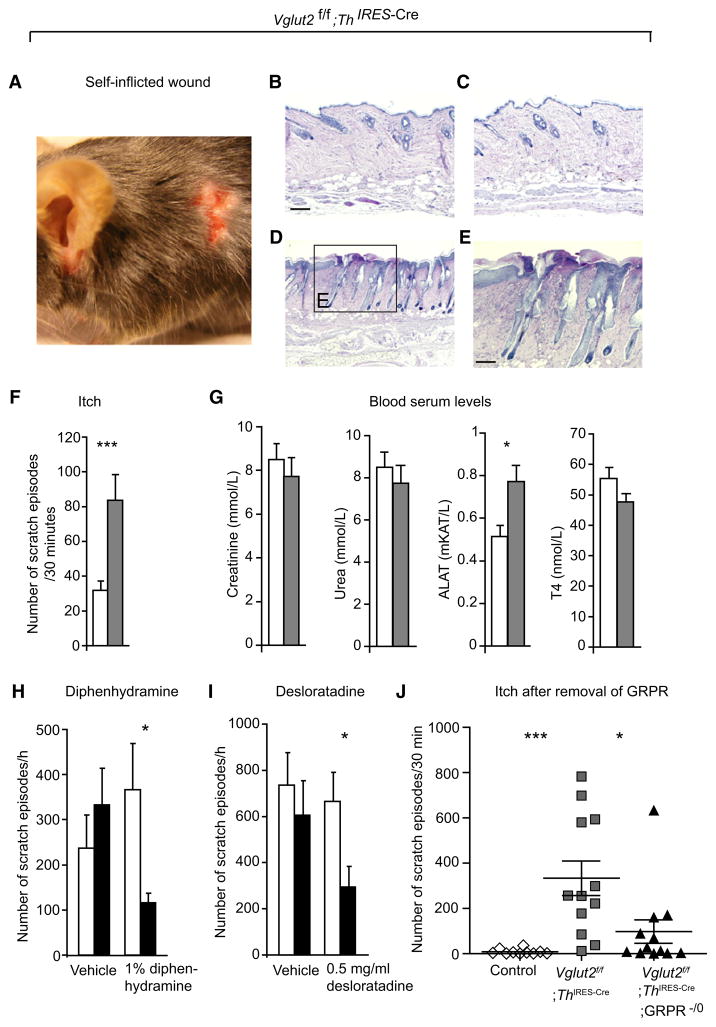
Complete deletion of Vglut2 results in a disruption of respiratory behavior after birth (Wallén-Mackenzie et al., 2006); therefore, tissue-specific promoter-driven Cre lines were needed to study the role of VGLUT2 in pain and itch circuitry. For this purpose, we generated mice lacking Vglut2 in neurons that express tyrosine hydroxylase (Th) by using Th IRES-Cre mice (Figure S1, available online). Apart from the expected Cre expression in catecholaminergic neurons, expression also comprises several noncatecholaminergic neurons, presumably because of transient expression of tyrosine hydroxylase during development (Lindeberg et al., 2004). To our benefit, the Cre activity in dorsal root ganglion neurons affected 74% ± 1% of the Vglut2 population (n = 6, 88 sections), which enabled the use of Th IRES-Cre mice in our investigation. Vglut2f/f mice that were crossed with Th IRES-Cre mice (Vglut2f/f;Th IRES-Cre) developed lesions in the neck and upper back region (Figure 1A). Histological examination of the skin showed that wounded areas in Vglut2f/f;Th IRES-Cre mice displayed thickened epidermis of uneven psoriasiform type, infiltration of immune cells in the dermis, and ulcerations, findings that are typical outcomes of prolonged scratching and rubbing. Areas without visual damage were similar to control skin (Figures 1B–1E). This observation indicated that the abrasions to the skin were the result of an external force, i.e., extensive scratching. Indeed, in 30 min recordings, Vglut2f/f;Th IRES-Cre mice displayed a chronic itch behavior, having 83.5 ± 14.9 scratch episodes/30 min compared to 31.9 ± 5.3 for controls (p = 0.0006) (Figure 1F). Chronic itch is a common symptom in systemic disorders that affect the kidneys, liver, or thyroid. To evaluate a possible inner-organ-induced itch in our mice, we analyzed plasma levels of creatinine, urea, alanine aminotransferase (ALAT), and tyroxine 4 (T4) in samples from Vglut2f/f; Th IRES-Cre mice and controls. No differences in the levels of creatinine, urea, or T4 were found, whereas the marker for liver function, ALAT, was slightly elevated but still within the normal reference interval (Loeb and Quimby, 1999), excluding a systemic disorder as the underlying cause of the elevated scratch behavior in Vglut2f/f;Th IRES-Cre mice (Figure 1G).
Figure 1. Vglut2f/f;Th IRES-Cre Mice Display a Chronic Itch Behavior that Is Both Dependent and Independent of Histamine.
(A) The extensive scratching results in fur loss and wounds in the neck region and around the ears.
(B–E) PAS staining of the wounded areas displayed a thickened epidermis of uneven psoriasiform type, infiltration of immune cells in dermis, and ulcerations (D and E) compared to control skin (B) and Vglut2f/f;Th IRES-Cre skin (C) from nonaffected areas. Scale bars represent 0.13 mm in (B–D) and 0.39 mm in (E).
(F) Vglut2f/f;Th IRES-Cre mice display a chronic itch behavior compared to control mice at 7 weeks of age (n = 35 for Vglut2f/f;Th IRES-Cre [grey], n = 34 for controls [white]).
(G) Markers for kidney function (creatinine and urea, p > 0.05), thyroid function (T4, p > 0.05), and liver function (ALAT, p = 0.019) did not deviate from reference values observed in different mouse strains of the same age (Loeb and Quimby, 1999) in Vglut2f/f;Th IRES-Cre mice.
(H and I) Topical antihistamine treatment reduced the level of scratch in Vglut2f/f;Th IRES-Cre mice that had developed a robust scratch phenotype (n = 12), as did oral treatment with a peripherally active antihistamine (n = 12; the white bar represents before treatment and black bar represents after treatment).
(J) Removal of the GRPR reduces the itch in Vglut2f/f;Th IRES-Cre mice (n = 11).
Mann-Whitney test, two-tailed (F–I) and Kruskal-Wallis, Dunn’s post-hoc test (J). *p < 0.05, ***p < 0.001. Data are presented as mean ± SEM.
VGLUT2 Regulates Both Histamine-Dependent and -Independent Itch
Histamine-dependent itch is caused by the release of histamine from mast cells and is involved in allergic disorders. In some pruritic conditions, for example in atopic dermatitis or psoriasis, the symptoms of itch cannot be relieved by antihistamines and are considered to have a histamine-independent origin (Ikoma et al., 2006). To investigate whether the increase in itch sensation was transmitted via the histaminergic pathway, we topically applied the histamine receptor 1 selective antagonist diphenhydramine to mice that had developed a robust scratching phenotype. This treatment significantly reduced the scratch behavior in Vglut2f/f;Th IRES-Cre mice (Figure 1H; 367 ± 102 to 116 ± 21 scratch episodes/hr, p = 0.014). To evaluate a potential central mode of action or sedating effect of diphenhydramine, we orally administered the blood-brain barrier impenetrant antihistamine desloratidine. This experiment resulted in a similar reduction in scratching behavior as the diphenhydramine treatment (Figure 1I; 666 ± 124 vs. 294 ± 89 scratch episodes/hr, p = 0.030), suggesting that the observed chronic itch is, at least partially, transmitted through histamine-sensitive primary afferents. Gastrin-releasing peptide receptor (GRPR)-mediated signaling pathway in the spinal cord dorsal horn has been shown to mediate primarily nonhistaminergic itch, although GRPR neurons are required for mediating both histamine-dependent and -independent itch (Sun and Chen, 2007; Sun et al., 2009). To examine whether VGLUT2 also regulates histamine-independent itch, we crossed GRPR mutant mice with Vglut2f/f;Th IRES-Cre mice. In control mice, scratching behavior was consistently low (8.7 ± 3.7 scratches/30 min), while in Vglut2f/f;Th IRES-Cre mice a robust scratching behavior was detected (334 ± 80 scratches/30 min). In contrast, the number of scratches decreased significantly in compound Vglut2f/f;Th IRES-Cre;GRPR-/0 mice (98 ± 52 scratches/30 min, p < 0.05 ANOVA; Figure 1J), indicating that a large part of the itch sensation was relayed via the GRP-GRPR pathway in Vglut2f/f;Th IRES-Cre mice. Hence, VGLUT2 regulates both histaminergic and nonhistaminergic itch, to a considerable extent via the GRP-GRPR signaling pathway.
The VGLUT2-Regulated Itch Behavior in Vglut2f/f;Th IRES-Cre Mice Has a Pruriceptive Origin
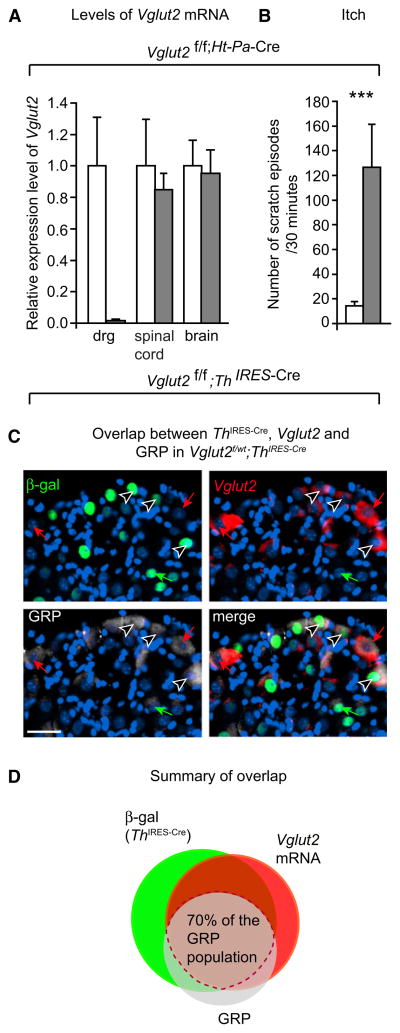
Th IRES-Cre is active both in the central nervous system (CNS) and in primary afferent neurons (Lindeberg et al., 2004) (Figure S1), and to discriminate between neurogenic (central) or pruriceptive (peripheral) itch, we crossed Vglut2f/f mice with mice expressing Cre under the peripherally active human tissue plasminogen promoter (Ht-Pa)-Cre. Ht-Pa-Cre, which affects the entire DRG population (Pietri et al., 2003), deleted Vglut2 from the primary afferent neurons as expected but did not affect the levels of Vglut2 in spinal cord or brain (Figure 2A). Such Vglut2f/f;Ht-Pa-Cre mice displayed a profoundly increased itch behavior comparable to the elevated itch in Vglut2f/f;Th IRES-Cre mice, indicating that the deletion of Vglut2 in the primary afferent neurons is sufficient to cause the itch phenotype (Figure 2B). The GRP-containing primary afferents relay pruriception to the dorsal horn, and, to investigate a possible direct role of VGLUT2 in the GRP pathway, we analyzed the overlap between GRP-, Vglut2-, and Th IRES-Cre-positive neurons in the DRG with the reporter line TaumGFP (Hippenmeyer et al., 2005). A larger part (70%) of the GRP-positive neurons also possessed Th IRES-Cre activity and expressed Vglut2 (Figures 2C and 2D and Table S1). This overlap suggests that intrinsic VGLUT2-mediated neurotransmission in the GRP population has a possible regulatory role in itch sensation.
Figure 2. The VGLUT2-Regulated Itch in Vglut2f/f;Th IRES-Cre Mice Has a Pruriceptive Origin.
(A) Vglut2 mRNA was lost in dorsal root ganglia but not in spinal cord or brain in Vglut2f/f;Ht-Pa-Cre animals as evaluated by RT-PCR. n = 3/genotype.
(B) Vglut2f/f;Ht-Pa-Cre mice (grey) scratch 127 ± 35 times/30 min compared to 14.5 ± 3.3 for controls (white) at 10 weeks of age (p = 0.0007, n = 10). Data in (A) and (B) are presented as mean ± SEM.
(C and D) The Th IRES-Cre/Vglut2-positive population overlaps with 70% of the GRP-positive population in the dorsal root ganglia. Vglut2 in situ hybridization on dorsal root ganglia cells from Th IRES-Cre;TaumGFP mice with at least one intact Vglut2 allele combined with immunohistochemistry of β-gal (Th IRES-Cre activity) and GRP (n = 3, 22 sections). The reporter line TaumGFP carries a lox-STOP-lox-mGFP-IRES-NLS-LacZ-pA allele, which enables visualization of Cre-expressing cell bodies and dendrites. Cre activity excises the lox-flanked STOP cassette and thereby initiates the expression of a membrane-bound version of GFP as well as nuclei-restricted β-gal protein (for further details see Hippenmeyer et al., 2005). Green arrows indicate a Vglut2-negative/Th IRES-Cre-positive/GRP-positive cell, red arrows indicate a Vglut2-positive/Th IRES-Cre-negative/GRP-positive cell, and black and white arrows indicate the affected GRP population where all markers colocalize. The displayed section was selected to show several stain combinations while the Venn diagram presents the averaged overlaps (for exact overlaps see Table S1). **p < 0.001, Mann-Whitney test, two-tailed. The scale bar represents 30 μm.
Removal of Vglut2 Results in Decreased Thermal but Not Mechanical Pain Sensation
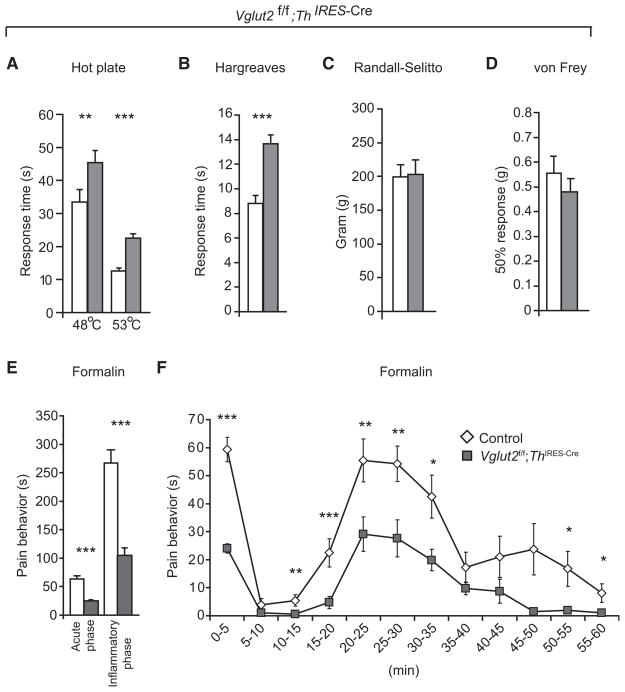
We next investigated the consequence of disturbed VGLUT2-mediated signaling for pain sensation. Vglut2f/f;Th IRES-Cre mice displayed a decreased responsiveness to radiant heat assessed by the Hargreaves test (13.7 ± 0.7 s compared to 8.7 ± 0.6 s for controls, p < 0.0001), a phenotype also shared by the Vglut2f/f;Ht-Pa-Cre mice (Figure S2). The heat pain phenotype of Vglut2f/f;Th IRES-Cre mice was further examined, and, consistent with their reduced sensitivity to radiant heat, they also responded more slowly in the hot plate test (Figures 3A and 3B). The mean latency of withdrawal of hind paw, followed by licking or biting, on a 53°C hot plate was 22.6 ± 1.3 s and 12.6 ± 1.0 s for Vglut2f/f;Th IRES-Cre and controls, respectively (p < 0.0001). To discriminate between the contribution from TRPV1 or TRPV2, we also tested Vglut2f/f;Th IRES-Cre mice and controls on a 48°C plate, which should only activate the TRPV1 receptor (Caterina et al., 1997; Tominaga and Caterina, 2004) (Figure 3A). The mean latency of hind paw withdrawal at 48°C was 45.4 ± 3.7 s and 33.4 ± 3.8 s for Vglut2f/f;Th IRES-Cre and controls, respectively (p = 0.005), which indicates that VGLUT2-mediated glutamatergic signaling also has a central role in pain signaling via the TRPV1 population. The pain measurements were carried out in mice of different ages (7–27 weeks), implying that the permanent loss of Vglut2 resulted in a chronic reduction of thermal pain sensitivity. Vglut2f/f; Th IRES-Cre mice also displayed a decreased sensitivity to inflammatory pain induced by formalin injections compared to control mice (Figures 3E and 3F). The biphasic response induced by formalin was observed both in Vglut2f/f;Th IRES-Cre mice and in littermate controls, although it was significantly lower in the Vglut2f/f;Th IRES-Cre mice (p < 0.0001 for both the first and the second phase). The sensitivity to mechanical stimuli was studied with the von Frey and Randall-Selitto tests (Figures 3C and 3D). Von Frey measurements resulted in a mean value of 0.48 ± 0.05 g and 0.56 ± 0.07 g for Vglut2f/f;Th IRES-Cre mice and littermate controls, respectively, and the mean weight that resulted in a withdrawal in the Randall-Selitto test was 203.4 ± 21.2 g and 199.3 ± 18.3 g for Vglut2f/f;Th IRES-Cre mice and littermate controls, respectively. We did not find a significant difference between the two groups in either mechanical test (p = 0.41 and p = 0.99, respectively), concluding that Vglut2f/f;Th IRES-Cre mice were less sensitive to thermal and inflammatory pain but showed a normal response to mechanical pain.
Figure 3. Vglut2f/f;Th IRES-Cre Mice Are Less Sensitive to Thermal but Not to Mechanical Pain Sensation.
(A and B) Vglut2f/f;Th IRES-Cre mice are less sensitive to thermal pain compared to control mice assessed by 53°C hot plate (n = 20/genotype), 48°C hot plate (n = 29 [Vglut2f/f;Th IRES-Cre], n = 23 [controls]), and the Hargreaves test (n = 21/genotype).
(C and D) Vglut2f/f;Th IRES-Cre mice display unaltered mechanical pain behavior compared to control mice assessed by Randall-Selitto (n = 21/genotype) and von Frey (n = 21/genotype) (p > 0.05). Von Frey measurements resulted in a mean value of 0.48 ± 0.05 g and 0.56 ± 0.07 g for Vglut2f/f;Th IRES-Cre mice and littermate controls, respectively. The mean weight that resulted in a withdrawal when the Randall-Selitto test was used 203.4 ± 21.2 g and 199.3 ± 18.3 g for Vglut2f/f;Th IRES-Cre mice and littermate controls, respectively.
(E and F) Vglut2f/f;Th IRES-Cre mice are less sensitive to inflammatory pain induced by formalin injections (n = 31 [Vglut2f/f;Th IRES-Cre], n = 25 [controls]). The mean observed pain behavior in the acute phase was 24.9 ± 1.8 s and 63.3 ± 5.6 s for Vglut2f/f;Th IRES-Cre mice and controls, respectively, whereas the mean observed pain behavior in the inflammatory phase was 99.1 ± 12.6 s and 267.0 ± 23.5 s. *p < 0.05, **p > 0.01, ***p < 0.001, Mann-Whitney test, two-tailed. Data are presented as mean ± SEM.
The Expression of Tyrosine Hydroxylase in Primary Afferent Neurons Overlaps to a Large Extent with the TRPV1 Population
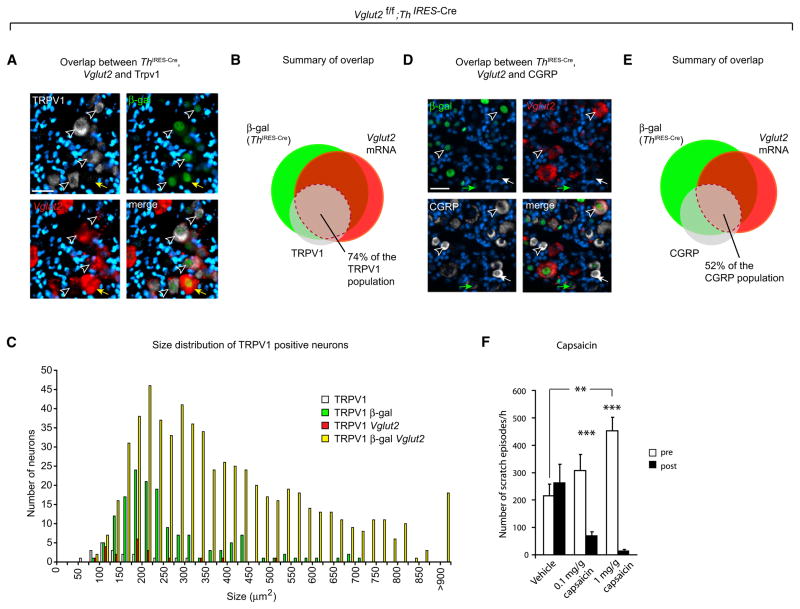
Only 10%–15% of the DRG neurons in the adult mouse express Th, and the more substantial deletion of Vglut2 from DRG neurons we report here is probably due to developmental activity of Th in other hitherto uncharacterized subpopulations during development. We next sought to determine the overlap between Th IRES-Cre, Vglut2, and two markers, calcitonin gene-related peptide (CGRP) and TRPV1, for the peptidergic primary afferents detecting noxious heat to better characterize subpopulations affected by Th IRES-Cre activity (Caterina et al., 2000; Mogil et al., 2005). Ablation of TRPV1 afferent neurons leads to a major loss of CGRP immunoreactivity, which suggests coexpression of CGRP and TRPV1 in DRG neurons (Cavanaugh et al., 2009). Th IRES-Cre activity was shown to target 93% of the TRPV1 population (Figures 4A and 4B and Table S2:1), and Vglut2 mRNA expression was observed in 77% of the TRPV1 population, which resulted in a targeting of Vglut2 in 74% of the TRPV1 primary afferent neurons in Vglut2f/f;Th IRES-Cre mice. The targeted population represented a majority of the small TRPV1 neurons (60%, <250μm2), leaving approximately one-third of the small TRPV1 population, which were devoid of Vglut2, unaffected in the Vglut2f/f;Th IRES-Cre mice (Figure 4C). Th IRES-Cre also targeted 84% of the CGRP population, which is in further support of a substantial colocalization of CGRP and TRPV1 (Figures 4D and 4E and Table S2:2). Thus, the considerable overlap between TRPV1/CGRP, Th IRES-Cre, and Vglut2 suggests that the observed thermal pain phenotype originates from VGLUT2-mediated glutamatergic release through TRPV1-positive neurons. Next, we tested the functional involvement of the TRPV1 population on the displayed scratching behavior by use of the TRPV1-selective agonist capsaicin. The result of topical administration of capsaicin was assessed by the number of scratch episodes before and 2 hr after the application of capsaicin on Vglut2f/f;Th IRES-Cre mice. The use of vehicle lotion did not result in a difference (from 216 ± 43 to 263 ± 68 scratch episodes/hr, p = 0.95) (Figure 4F). However, administration of 0.1 mg capsaicin/g vehicle induced a significant decrease in frequency of scratch episodes (from 307 ± 59 to 69 ± 16 scratches/hr, p < 0.0001). This effect was even more pronounced with 1 mg capsaicin/g vehicle (from 453 ± 50 to 13.8 ± 5.2 scratches/hr, p < 0.0001). The clear effect of capsaicin treatments on the VGLUT2-mediated induction of chronic itch further corroborated a role for the TRPV1 population in the observed sensory deficits.
Figure 4. The Expression of Tyrosine Hydroxylase in Primary Afferent Neurons Greatly Overlaps with the TRPV1 Population.
(A and B) The Th IRES-Cre/Vglut2 population overlaps with 74% of the TRPV1 population in the dorsal root ganglia. Vglut2 in situ hybridization on dorsal root ganglia cells from Th IRES-Cre;TaumGFP mice with at least one intact Vglut2 allele combined with immunohistochemistry of β-gal (Th IRES-Cre activity) and TRPV1 (n = 3, 36 sections). The yellow arrow indicates a Vglut2-positive/Th IRES-Cre-positive/TRPV1-negative cell and black and white arrows indicate the affected TRPV1 population where all markers colocalize. The scale bar represents 33 μm.
(C) Size distribution of TRPV1-positive neurons (n = 3, 829 neurons).
(D and E) The Th IRES-Cre/Vglut2-positive population overlaps with 52% of the CGRP-positive population in the dorsal root ganglia. Vglut2 in situ hybridization on primary afferent neurons from Th IRES-Cre;TaumGFP mice with at least one intact Vglut2 allele combined with immunohistochemistry of β-gal (Th IRES-Cre activity) and CGRP (n = 3, 30 sections). The green arrow indicates a Vglut2-negative/Th IRES-Cre-positive/CGRP-positive cell, the white arrow indicates a Vglut2-negative/Th IRES-Cre-negative/CGRP-positive cell, and black and white arrows indicate the affected CGRP population where all markers colocalize. The scale bar represents 60 μm (for exact overlap see Tables S2:1 and S2:2).
(F) Topical application of capsaicin reduced the itch phenotype in Vglut2f/f;Th IRES-Cre mice (n = 14). Moreover, one topical application of capsaicin resulted in an increase in the level of chronic itch the following day (p = 0.005), from 216 ± 43 scratch episodes/hr to 453 ± 50. Preapplication levels are in white and postapplication are in black. Data are presented as mean ± SEM. **p < 0.01, ***p < 0.001, Mann-Whitney test, two-tailed.
Removal of Vglut2 from Trpv1-Cre-Positive Neurons Results in Enhanced Itch and Decreased Thermal Pain Sensitivity
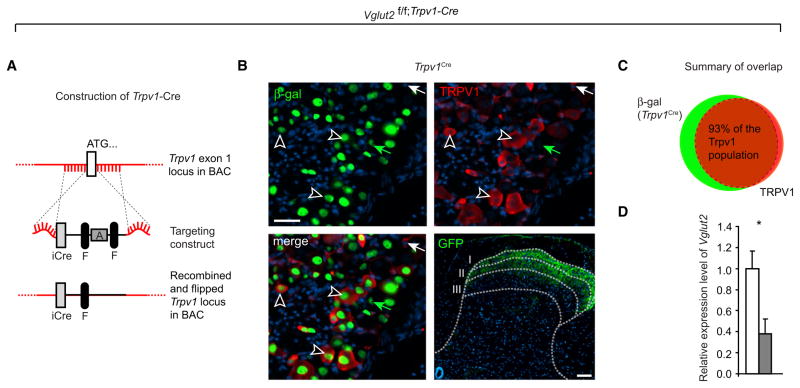
TRPV1 is the receptor for capsaicin, the pungent component in chili pepper, and has also been shown to respond to thermal heat (Caterina et al., 1997). The role of the TRPV1 population has previously been related to acute thermal pain sensitivity and inflammation-induced thermal hyperalgesia (Caterina et al., 1997; Cavanaugh et al., 2009). However, a large proportion of capsaicin-sensitive neurons also respond to the pruritogens histamine and chloroquine (Liu et al., 2009), which suggests a dual role for this population in sensory transmission. The large overlap between TRPV1 and Vglut2, together with the reduced thermal pain phenotype and the elevated itch, prompted us to further explore the role of VGLUT2 in this population of neurons. For this purpose, we generated Trpv1-Cre transgenic mice by using a bacterial artificial chromosome (BAC)-cloning strategy to insert a codon-improved Cre into the locus of the first exon of Trpv1 (Figure 5A). Trpv1-Cre activity, visualized as β-gal expression with the reporter line TaumGFP, was found to largely overlap (93%) with TRPV1 immunoreactivity in the primary afferent neurons (Figures 5B and 5C) and was also detected in a small number of scattered neurons in spinal cord and brain (Figure S3 and Table S5). To validate the specific removal of Vglut2 in response to the detected Trpv1-Cre activity, we measured Vglut2 mRNA expression in the dorsal root ganglia by RT-PCR. This resulted in a significant decrease of Vglut2 mRNA to approximately 40% of control levels (Figure 5D).
Figure 5. Generation and Characterization of a Mouse Expressing Cre Driven by the Trpv1 Promoter.
(A) Schematic drawing of the BAC cloning for the generation of Trpv1-Cre transgenic mice. The following abbreviations are used: iCre, codon-improved Cre; F, frt site; A, ampicillin resistance gene.
(B and C) Trpv1-Cre overlapped with 93% of the TRPV1-immunoreactive cells, which corresponds to 67% of the Trpv1-Cre-positive population (scale bar represents 33 μm); the white arrow denotes TRPV1-immunoreactive cell without Trpv1-Cre activity, the green arrow indicates Trpv1-Cre active cell without TRPV1-immunmoreactivity, and black and white arrows show some of the overlapping cells (n = 3, 20 sections). Fibers from DRG neurons with TrpV1-Cre activity were visualized with the TaumGFP reporter line and were predominantly found in the most dorsal lamina of the spinal cord (scale bar represents 60 μm).
(D) Dorsal root ganglia from Vglut2f/f;Trpv1-Cre mice (grey) displayed decreased expression of Vglut2 mRNA compared to controls (white) (p = 0.048, Student’s two-tailed t test, n = 3/genotype). Data are presented as mean ± SEM.
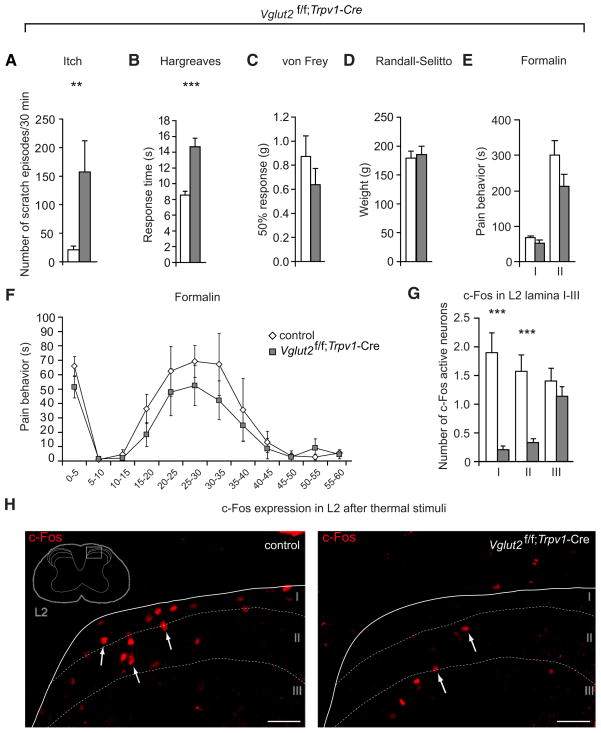
Consistent with the overlap between Th IRES-Cre and TRPV1 populations, the deletion of Vglut2 from Trpv1-Cre-positive neurons resulted in an increase of scratch episodes (157.1 ± 54.8 scratches/30 min for Vglut2f/f;Trpv1-Cre mice compared to 21.0 ± 6.4 scratches/30 min for controls; Figure 6A and Movie S1). Similarly, decreased thermal pain sensitivity in the Hargreaves test (13.7 ± 1.5 s for Vglut2f/f;Trpv1-Cre mice compared to 7.7 ± 0.7 s for controls) was also observed (Figure 6B). Mechanical pain sensitivity, assessed by Randall-Selitto and von Frey tests, did not differ between Vglut2f/f;Trpv1-Cre mice and controls (Figures 6C and 6D) nor did inflammatory pain sensitivity induced by formalin (Figures 6E and 6F). Thus, all investigated sensory modalities, except thermal pain and itch, were unaffected. In light of our data from the Vglut2f/f; Th IRES-Cre analysis, our examination of Vglut2f/f;Trpv1-Cre mice supports the interpretation that the reduced thermal pain sensitivity and the increased scratch behavior were due to the loss of VGLUT2-mediated glutamatergic neurotransmission in the Trpv1-Cre population. In order to strengthen this conclusion, we investigated the downstream effect of an expected impaired glutamate release in the spinal cord dorsal horn by counting the number of c-Fos neurons after thermal heat stimulation using the Hargreaves test. The c-Fos immediate early gene is induced by neural excitation and can be used as an activation marker for neurons in the superficial dorsal horn of the spinal cord downstream of primary afferent stimulation (Hunt et al., 1987). The most prominent c-Fos response was detected in lamina I/II in lumbar level 2 (Figure S4), consistent with the location of Trpv1-Cre;TaumGFP-labeled central projections (Figure 5B). The number of c-Fos-positive nuclei detected in Vglut2f/f;Trpv1-Cre mice was significantly decreased when compared to control mice (Figures 6G and 6H; p < 0.001). The decrease was profound in lamina I (10-fold) and II (5-fold), while the decrease detected in lamina III was small and not significant. Thus, the Trpv1-Cre-driven Vglut2 deletion significantly decreased the number of c-Fos-responding dorsal horn interneurons, suggesting that the reduced Vglut2 levels led to decreased downstream activation.
Figure 6. Removal of Vglut2 from Trpv1-Cre-Positive Neurons Results in Enhanced Itch and Decreased Thermal Pain Sensitivity.
(A) Vglut2f/f;Trpv1-Cre mice display an increased scratch behavior compared to control mice (p = 0.0015, n = 10/genotype, age 7–9 weeks).
(B) Vglut2f/f;Trpv1-Cre mice are less sensitive to thermal pain compared to control mice (p = 0.0002, n = 16/genotype).
(C and D) Vglut2f/f;Trpv1-Cre mice do not display a mechanical pain phenotype compared to control mice in von Frey (p = 0.24, n = 8/genotype) or Randall-Selitto tests (p = 0.71, n = 8/genotype). The mean weight that resulted in a withdrawal was 0.64 ± 0.13 g and 0.87 ± 0.17 g in the von Frey test and 179.3 ± 12.0 g and 185.0 ± 14.5 g in the Randall-Selitto test for Vglut2f/f;Trpv1-Cre mice and littermate controls, respectively.
(E and F) Vglut2f/f;Trpv1-Cre mice display no difference in formalin-induced inflammatory pain behavior compared to control mice (n = 8/genotype). The mean observed pain behavior in the acute phase I was 52.8 ± 7.6 s and 67.0 ± 6.4 s (p = 0.16), whereas the mean observed pain behavior in the inflammatory phase II was 212.8 ± 34.5 s and 300.1 ± 40.1 s (p = 0.16) for Vglut2f/f;Trpv1-Cre mice and controls, respectively. Data are presented as mean ± SEM. **p < 0.01, ***p < 0.001, Mann-Whitney test, two-tailed.
(G and H) The downstream effect on spinal cord central interneurons was tested by determining the number of c-Fos activated neurons (white arrows) in response to thermal stimulation. Deletion of Vglut2 from Trpv1-Cre primary afferents results in a reduced number of c-Fos-activated interneurons in lamina I and II in the L2 segment after Hargreaves stimulation (n = 2/genotype, 150 sections). The scale bar represents 30 μm.
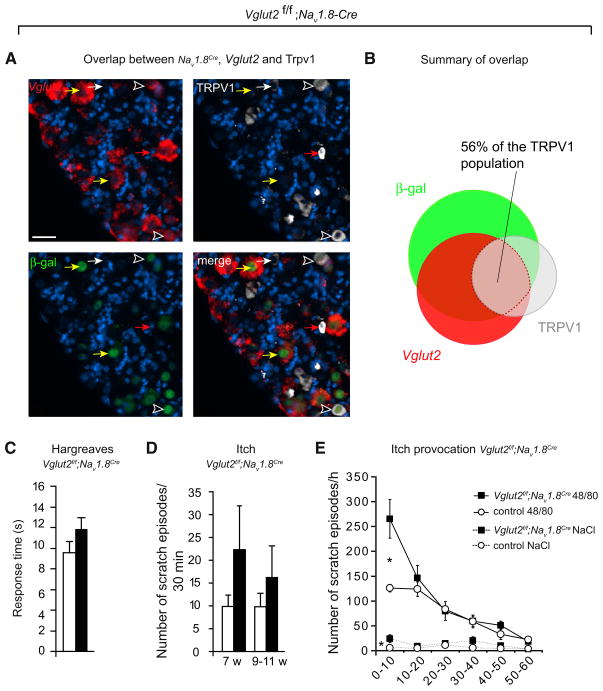
Loss of Vglut2 in the Nav1.8 Population, Partially Overlapping with the TRPV1 Population, Affected Neither Spontaneous Itch nor Thermal Pain
So far, our data have demonstrated that removal of Vglut2 from Trpv1-Cre-positive neurons results in an elevated itch phenotype accompanied by a decrease in thermal pain sensitivity. A population partially overlapping with TRPV1 is the Nav1.8 population, which, in addition to the pain-mediating role, has also been suggested to provide a basal level of activity to balance itch pathways (Roza et al., 2003). TRPV1 and Nav1.8Cre populations positive for Vglut2 overlap with 56% (Figures 7A and 7B and Table S3), indicating that these populations might overlap in their functionalities. However, deletion of Vglut2 in Nav1.8Cre-positive neurons did not result in a significant increase in spontaneous scratching behavior or in an altered thermal pain response (Figures 7C and 7D). A tendency toward a spontaneous increase in scratching behavior was noted at both ages investigated (Figure 7C), and, similarly, a tendency to decreased thermal pain response could be noted and possibly be explained by a partial loss of Vglut2 in the TRPV1 population. To further investigate potential defects in pruriception, we provoked itch sensation with the pruritogen 48/80. This irritant led to a significantly higher number of scratching bouts in Vglut2f/f;Nav1.8Cre mice compared to control mice (Figure 7E). Notably, the difference in provoked sensitivity rapidly subsided, suggesting that mice lacking Vglut2 in the Nav1.8Cre population, affecting only 56% of the TRPV1 population, retained the ability to regulate itch sensation, albeit with a disturbed and delayed response.
Figure 7. Vglut2f/f;Nav1.8Cre Mice Do Not Display Spontaneous Itch or Altered Thermal Pain Response.
(A) Overlap between Vglut2 mRNA, Nav1.8Cre activity, and TRPV1 immunoreactivity was detected using cryo in situ hybridization combined with immunohistochemistry. Nav1.8Cre activity was assessed, through β-gal immunoreactivity using the reporter mouse TaumGFP. Yellow arrows denote Vglut2 mRNA-positive/Nav1.8Cre-positive/TrpV1-negative cells. Red arrows denote a Vglut2 mRNA-positive/Nav1.8Cre-negative/TrpV1-positive cell. White arrows display a Vglut2-negative/Th IRES-Cre-negative/TrpV1-positive cell. Black and white arrowheads denote triple-labeled cells. The scale bar represents 33 μm.
(B) Venn diagram displaying the overlap between Nav1.8Cre, Vglut2, and TRPV1 (n = 2, 35 sections), for exact overlap see Table S3.
(C) Thermal heat pain was assessed by the Hargreaves test (n = 14 [controls], n = 13 [Vglut2f/f;Nav1.8Cre]) and did not differ between the groups (p = 0.09).
(D) Vglut2f/f;Nav1.8Cre mice displayed an unaltered spontaneous itch behavior compared to control mice at 7 weeks (n = 10/genotype, p = 0.26, Mann-Whitney test, two-tailed) and at 9–11 weeks of age (n = 10/genotype, p = 0.94). (E) Vglut2f/f;Nav1.8Cre mice initially displayed an increased response to intradermal injections of the pruritogen 48/80 (100 μg) or NaCl compared to control mice (n = 8/genotype). *p < 0.05, Mann-Whitney test, two-tailed.
Data in (C), (D), and (E) are presented as mean ± SEM.
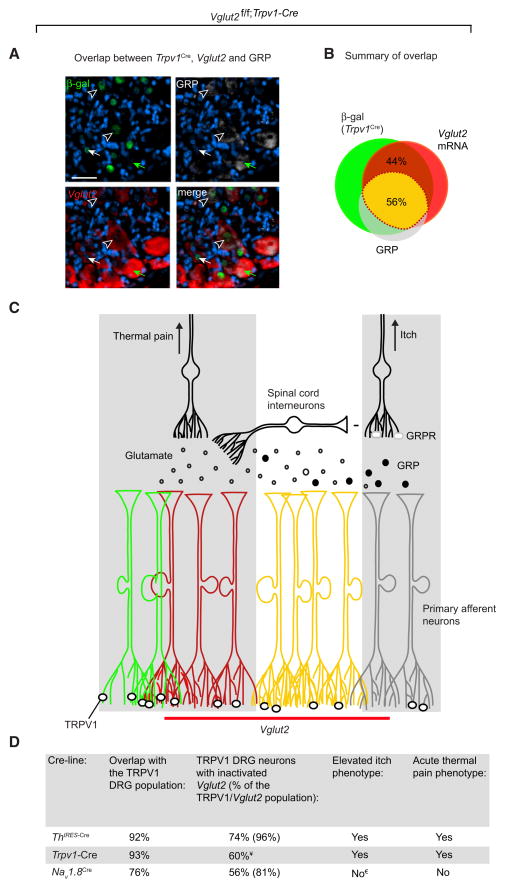
Expression of Vglut2, Trpv1-Cre, and GRP Suggests the Presence of Dual Modality Afferents as well as Afferents Selective for Thermal Pain or Itch
To test whether the presence of the itch-selective GRP transmitter in Trpv1-Cre neurons could shed light on the perceived dual role of the TRPV1 subpopulation, we performed fluorescent Vglut2 in situ combined with GRP and β-gal immunohistochemistry on DRG sections from Trpv1-Cre;TaumGFP mice. The analyses defined one small GRP population negative for Trpv1-Cre (9% of the GRP population), which is likely to mediate itch sensation independent of TRPV1 (Figures 8A and 8B). Further, we found a large Trpv1-Cre population negative for GRP (56% of the Trpv1-Cre population), which, in the absence of other known primary afferent itch transmitters, suggests the presence of thermal-pain-selective fibers. Finally, we defined two different subpopulations involving Vglut2 in the Trpv1-Cre neurons, which could provide an explanation for the observed pain and itch phenotypes in our Vglut2-deficient mice: a GRP-negative population (44%) that represents a VGLUT2-dependent thermal-pain-selective population and a GRP-positive population (56%), which, considering the demonstrated roles of GRP in itch and TRPV1 in thermal pain (Caterina et al., 1997, 2000; Sun and Chen, 2007; Sun et al., 2009), suggests the presence of dual modality fibers (Figure 8C and Table S4).
Figure 8. Overlap Analysis of Vglut2, Trpv1-Cre and GRP Suggests the Presence of Dual Modality Afferents as well as Afferents Selective for Thermal Pain or Itch.
(A and B) GRP overlap in the Trpv1-Cre/Vglut2-positive population defines two subpopulations: one Trpv1-Cre/Vglut2 population (44%) and one Trpv1-Cre/Vglut2/GRP (56%) population in the dorsal root ganglia. Vglut2 in situ hybridization on primary afferent neurons from TrpV1-Cre;TaumGFP mice with at least one intact Vglut2 allele combined with immunohistochemistry of β-gal (Trpv1-Cre activity) and GRP (n = 2, 20 sections) (scale bar represents 80 μm), for exact overlap see Table S4.
(C) Schematic drawing of possible Vglut2-, GRP-, and/or TRPV1-mediated neuronal interactions regulating itch. TRPV1-positive thermal nociceptive primary afferents (green) transmit pain, for example by scratching, and GRP-positive pruriceptors (grey) transmit itch from a pruritogen-induced stimuli. VGLUT2-mediated release of glutamate from a TRPV1 subpopulation (dark red) of neurons may prevent excessive scratching by activating inhibitory interneurons projecting to GRPR-positive neurons. Additionally, fibers capable of transmitting both pain, through glutamate, and itch, through GRP, are present (yellow). The two gray boxes represent the thermal pain and the itch pathways, respectively.
(D) Summary of thermal pain and itch phenotypes, in relation to the TRPV1 population, after conditional ablation of Vglut2 with denoted Cre lines. ¥Based on the overlap between Trpv1-Cre and Vglut2 (Table S4). €Provocable itch.
DISCUSSION
Using several genetic mouse models, which lack Vglut2 in various subpopulations of DRG neurons, we provide evidence that self-inflicted wounds in the skin of these mutant mice were caused by an incessant spontaneous scratching behavior. The increased scratching behavior was significantly reduced by antihistamine treatments or loss of the GRP receptor, which suggests that the chronic scratch behavior originated in an elevated itch transmission comprising both histamine-dependent and -independent components. Using extensive colocalization studies of Vglut2 expression with markers for primary afferent subpopulations, we demonstrate a major overlap with the thermal-pain-responsive TRPV1-positive population. This analysis also provides evidence of two separate TRPV1 subpopulations of neurons based on their differential expression of GRP. Further, we show that the permanent loss of Vglut2 in the investigated mouse lines leads to decreased thermal pain perception. The use of the Ht-Pa-Cre mouse line helped determine that the increased VGLUT2-dependent itch originated from the periphery while the analyses from Th IRES-Cre and Trpv1-Cre mouse lines both include most of the TRPV1-positive neurons, strengthening the involvement of the TRPV1 population in the regulation of itch. Moreover, we have demonstrated that loss of Vglut2 in the Nav1.8 population did not affect spontaneous itch or thermal pain sensitivity. Our data lead us to conclude that both thermal pain sensation and regulation of itch transmission are dependent on VGLUT2-mediated glutamatergic neurotransmission in TRPV1-positive neurons and that thermal, but not mechanical, pain and itch transmission might be connected.
Caveats in this study that need further discussion include the complex interpretation of the reduced itch in response to capsaicin (Figure 4F). Capsaicin is known to acutely induce pain through the TRPV1 receptor but has also been reported to evoke itch (Sikand et al., 2009). In addition, prolonged treatment with capsaicin can desensitize pain fibers; for example, intrathecal administration of capsaicin has been used to desensitize the central branch of primary afferents of TRPV1 neurons (Cavanaugh et al., 2009). Thus, the sensory response to capsaicin can vary depending on dosage, location, and route of administration. While the reduced scratching behavior in our mice produced by capsaicin treatment corroborates a role for the TRPV1 population in VGLUT2-mediated itch regulation, further experiments are needed to determine the particular mechanism behind this behavioral change. Second, while the reduced c-fos response clearly indicates aberrant signaling in the dorsal horn of Vglut2f/f;Trpv1-Cre mice, it will ultimately be important to assess glutamatergic transmission with electrophysiological methods. A third caveat is the possible confounding factor of an increased scratch behavior in the analysis of pain behavior. To address this issue, we analyzed a possible association between scratching intensity and pain response. We decided to analyze the response to formalin since it contains two analyzable phases and has the longest duration. The excessive scratch behavior in the neck and upper back region would have the largest impact in a pain test occupying the longest space of time. However, no correlation between an increased itch and a pain response was found in either the acute or the inflammatory phase (Figure S2).
Previous studies have shown that capsaicin-induced ablation of TRPV1 afferents causes a partial loss of heat pain sensitivity (Jessell et al., 1978). The role of TRPV1 for transmission of heat nociception has been further substantiated by investigating TRPV1 knockout mice (Caterina et al., 1997; Davis et al., 2000), and we here report that normal heat pain sensation transmitted via the TRPV1 population requires VGLUT2 (Figure 6). In addition to its role in thermal pain, transmitted by the TRPV1 population, VGLUT2 is critical for a normal response to mechanical pain transmitted by the Nav1.8 subpopulation (unpublished data). Notably, Vglut2f/f;Nav1.8-Cre mice have a normal response to thermal pain and do not display increased spontaneous scratching (Figure 7). Likewise, Vglut2f/f;Th IRES-Cre or Vglut2f/f;Trpv1-Cre mice have a normal response to mechanical pain (Figures 3 and 6). The TRPV1 and the nonpeptidergic C fiber Mas-related gene D (MrgD) neurons are predominantly nonoverlapping populations while the MrgD and Nav1.8 neurons are largely overlapping (82%; unpublished data). Mice that had lost the MrgD-positive population, through acute diphtheria toxin ablation, show a decreased sensitivity to mechanical sensation (Cavanaugh et al., 2009). In a similar experiment, except for the nonacute administration of diphtheria toxin, MrgD cell-ablated mice do not show different responses to pruritogens, suggesting, if the deletion of the MrgD population has not been compensated for during development, that these neurons are not involved in the regulation of itch (Imamachi et al., 2009). Thus, our data together with previous observations argue for a link between itch and thermal, but not mechanical, pain through the TRPV1 population.
We here establish that VGLUT2 in the TRPV1 population is required to restrain itch sensation and for normal thermal pain sensitivity. The observed increase in itch behavior implies that VGLUT2 expression in Trpv1-Cre-positive sensory neurons is dispensable for itch sensation. How can the reduction of VGLUT2-mediated transmission within the TRPV1 afferents lead to increased itch and decreased pain, either dependent or independent of each other? The opposite responses to reduced VGLUT2 transmission rule out the intensity theory. Rather, our data suggest the copresence of itch/pain dual modality fibers with pain-selective fibers (selectivity theory), where reduced VGLUT2 transmission would lead to reduced activity in pain-selective afferent fibers. In support of this, 44% of the Trpv1-Cre/Vglut2 population does not contain GRP (Figure 8 and Table S4), which includes the possibility of a thermal-nociceptive-selective population. Consequently, a larger part, 56%, of the Trpv1-Cre- and Vglut2-positive neurons is GRP positive and this extensive overlap between GRP, Vglut2, and TRPV1 indicates a possible cotransmission of glutamate and GRP. As such, these neurons represent possible dual modality fibers where the pruriceptive signal could primarily be mediated by GRP while it is regulated by VGLUT2-dependent glutamatergic signaling. It is also possible that the observed coincident increase in itch sensation is mediated by a TRPV1-independent population of GRP neurons (9%). It was recently shown that neurons sensitive to capsaicin overlap extensively with histaminergic, as well as nonhistaminergic, pruriceptive neurons (Liu et al., 2009) and that the TRPV1 population responds to various pruritogens through several mechanisms (Imamachi et al., 2009). In humans, capsaicin has been shown to acutely elicit both itch and pain when administered via spicules in a single dose (Sikand et al., 2009). Further, the majority of the primary afferents investigated in humans responding to histamine also responded to intracutaneous injection of capsaicin (Schmelz et al., 1997), suggesting that the TRPV1 population can transmit both modalities. Our data provide evidence that VGLUT2-mediated glutamatergic neurotransmission is critical for thermal pain sensation in the TRPV1 population. Taking all of the above into account, the evidence for the presence of itch-mediating neurons within the TRPV1 population is overwhelming. Therefore, we here suggest that the pruritic capacity within the TRPV1 population is, most likely, transmitted by GRP and that primary afferents exist that can regulate itch through VGLUT2-mediated glutamatergic release.
How can the increased itch sensation be explained by a decrease in excitatory activation? A heightened activity in the itch pathway could be due to reduced glutamatergic excitation of spinal cord dorsal horn inhibitory interneurons. In this scenario, excessive pruriception would be suppressed by parallel inputs via pain-sensitive fibers activated by scratching. A small pruritic skin abrasion would under normal circumstances induce a scratch response to sufficiently reduce the itch sensation, allowing for periods of healing. However, when the parallel input mediated by VGLUT2 is reduced, this causes the initiation of injurious skin-scratch cycles, resulting in the severe skin wounds observed in the Vglut2-deficient mice. This hypothesis assumes the presence of a mechanism, or interneuron population, capable of transforming the excitatory glutamatergic activity released by the TRPV1 population to an inhibitory influence on pruriception. Until very recently, no such mechanism or population of interneurons had been identified. The revelation of dorsal inhibitory interneurons capable of regulating itch behavior (Ross et al., 2010) supplies evidence for a component in itch regulation necessary for recognizing a model of disinhibition (Figure 8D). The itch phenotype of the mutant mice presented in this study was similar to the increased itch displayed by the Bhlhb5 mouse mutant, in which, once a very small skin irritation appeared, the persistent licking and scratching very soon thereafter resulted in a skin lesion (Ross et al., 2010).
In summary, removal of VGLUT2-mediated glutamatergic signaling in a subset of primary afferent neurons coinciding with the TRPV1 and GRP populations results in a decrease in thermal pain while mechanical pain is unaffected. We conclude that thermal pain, as opposed to mechanical pain, could be part of an intricate interplay with itch sensation within the TRPV1 population. The present study provides a basis for a mechanism involved in regulating itch and defines a neuronal population and a neurotransmitter that can mediate this effect and, as such, is a starting point for further experiments of somatosensory interactions through tissue-specific or molecule-specific ablation studies. For example, cell-specific ablation of the TRPV1 population will provide information to dissociate the roles of itch-selective and itch/pain polymodal fibers in the regulation of pruriception. In a more clinical perspective, the rescue of chronic itch by interfering with the GRP-GRPR pathway suggests that GRPR antagonists could be useful for treating chronic itch conditions.
EXPERIMENTAL PROCEDURES
Generation of Transgenic Vglut2 Animals
Mice homozygous for the floxed allele for Vglut2 (Vglut2f/f) (Wallén-Mackenzie et al., 2006) were crossed with ThIRES-Cre mice (Lindeberg et al., 2004) to generate mice lacking Vglut2 in Th-expressing neurons (Vglut2f/f;ThIRES-Cre) and controls (Vglut2f/f). Male Vglut2f/f;ThIRES-Cre mice were crossed with the reporter mouse TaumGFP (Hippenmeyer et al., 2005) to identify ThIRES-Cre-positive neurons. Male Vglut2f/f;ThIRES-Cre mice were also crossed with GRPR mutant females (Sun and Chen, 2007) to generate Vglut2f/f;ThIRES-Cre mice lacking the GRPR receptor (Vglut2f/f;ThIRES-Cre;GRPR−/0, GRPR is X-bound). Vglut2f/f females were also crossed with the periphery-specific Cre line Ht-Pa-Cre (kindly provided by professor Sylvie Dufour) (Pietri et al., 2003) or the primary afferent-specific Cre line Nav1.8-Cre (Stirling et al., 2005) to generate Vglut2f/f;Ht-Pa-Cre or Vglut2f/f;Nav1.8Cre mice, respectively. Trpv1-Cre mice were generated by recombineering of BAC DNA (Supplemental Experimental Procedures).
Multiplex Single-Cell RT-PCR
Dorsal root ganglia were dissected and dissociated from Vglut2f/f;Th−IRES-Cre; TaumGFP P2 pups, and GFP-expressing cells were individually collected. The content of each cell was analyzed by a first round of PCR, followed by a second round with 10% of the first PCR reaction. Primers were designed to bind in different exons; therefore, they detect only mRNA and do not interact with each of the other primers in the multiplex PCR (see Supplemental Experimental Procedures for further details).
qPCR
Quantitative real-time PCR on homogenate from dissected dorsal root ganglia (Vglut2f/f;Trpv1-Cre, Vglut2f/f;Ht-Pa-Cre), spinal cord (Vglut2f/f;Ht-Pa-Cre), and brain (Vglut2f/f;Ht-Pa-Cre) and from control mice was performed as described previously (Olszewski et al., 2009) with some exceptions (see Supplemental Experimental Procedures).
Histology and Blood Analyses
Adult mice (>7 weeks old) were perfused as previously described (Gezelius et al., 2006), and skin was isolated. Sections were stained with hematoxylin and eosin (H&E) and periodic acid-Schiff (PAS) for a histopathological evaluation (see Supplemental Experimental Procedures for further details).
Probes
The generation of the in situ probes is described elsewhere (Gezelius et al., 2006). The Vglut2 probe covers nucleotides 1616–2203 (NM_080853.2) and was used at a concentration of 1000 ng/ml. Vesicular acetylcholine transporter (Vacht) probe covers nucleotides 1534–2413 (NM_021712) and vesicular inhibitory amino acid transporter (Viaat) probe covers nucleotides 1578–1889 (NM_009508.1).
In Situ Hybridization and Immunohistochemistry
Primary antibodies used were chicken anti-β-galactosidase (β-gal) 1:5000 (Abcam), rabbit anti-CGRP 1:1000 (Peninsula Labs), rabbit anti-TRPV1 1:1000 (Abcam), and rabbit anti-GRP 1:1000 (ImmunoStar). Secondary antibodies were goat anti-chicken Alexa 488 1:400 (Invitrogen) and goat anti-rabbit Alexa 647 1:400 (Invitrogen). Cryo in situ was performed as described previously (Schaeren-Wiemers and Gerfin-Moser, 1993) with a few exceptions. For staining of lumbar spinal cord sections, primary antibodies used were anti-GFP 1:1000 rabbit (Invitrogen) or chick (Abcam), NeuN 1:400 mouse (Millipore), and goat anti-c-Fos 1:200 (Santa Cruz) and secondary antibodies were goat anti-chicken Alexa 488 1:400 (Invitrogen), goat anti-rabbit Alexa 488 1:200 (Invitrogen), goat Cy3 anti-mouse 1:500 (Invitrogen), and donkey anti-goat 488 Alexa 1:150 (Invitrogen) (see Supplemental Experimental Procedures for further details).
Imaging
Fluorescent images were viewed in an Olympus BX61WI microscope and analyzed with Volocity software (Improvision).
Behavior Analyses
General Behavior
All behavioral tests were performed on adult (>7 weeks old) female and male mice. Control mice were littermates and gender matched. All behavior analyses were performed in a controlled environment of 20–24°C, 45%–65% humidity, and 12 hr day/night cycle. All animal procedures were approved by the local ethical committee in Uppsala, Sweden (permits C12/9, C79/9, C98/7, C125/8, C147/7, C195/8, C198/7, C156/4, C210/7, C251/6, C256/9, and C269/6) and followed the guidelines of European Communities Council Directive (86/609/EEC).
Pain Behavior
Hot Plate Test
The latency of withdrawal of hind paw followed by licking or biting of the paw at 48.0–48.2°C (warm plate) or 52.5–53.0°C (hot plate) was measured two times for each animal, separated by at least 15 s. Results were expressed as mean withdrawal latency for each animal and group ± the standard mean error (SEM) (see Supplemental Experimental Procedures for further details).
Hargreaves Test
The Hargreaves heat source (IITC Life Science) was placed with the guide light pointing toward the plantar surface of the left hind paw and the thermal beam was started. A paw withdrawal would stop the test and the time was monitored. The result was expressed as the mean withdrawal latency time for each animal and group ± SEM. For assessment of c-Fos induction, both hind paws were exposed to the thermal beam until pain behavior was observed (3–5 min between each paw). The mice were then left in the setup for 1 hr before perfusion (previously described in Gezelius et al., 2006). Spinal cords were dissected out and prepared for cryosectioning (see above).
Von Frey Test
The Von Frey filaments (Scientific Marketing Associates) were applied with the Chaplan “up-and-down paradigm” (Chaplan et al., 1994). The 50% threshold was calculated with the Dixon method and the result expressed as the mean value for each animal and group ± S.E.M.
Randall-Selitto Test
The tail was gently placed under the Randal-Selitto arm (Ugo Basile) before an increasing weight was applied to the tail. The weight for which the mouse would withdraw its tail was monitored. The result was expressed as the mean withdrawal latency in grams for each animal and group ± SEM.
Formalin Test
Twenty microliters of 5% formalin (35% formaldehyde [Sigma] in 0.9% saline [Fresenius Kabi]) was injected subcutaneously into the plantar surface of the right hind paw with a Hamilton microsyringe (1710 TLL 100 μl) with a 30G needle (BD Microlance). The mouse was observed in a transparent cage for the following 60 min to monitor pain behavior, licking, and biting of the injected paw.
Itch Behavior
The behavior was recorded with a digital video camera for 30 min (chronic itch) or 1 hr (treatment of itch behavior). One bout of scratching by either hind paw was defined as a scratching episode. The result was expressed as the mean number of scratch episodes for each group/30 min ± SEM (see Supplemental Experimental Procedures for further details).
Treatment of Itch Behavior
Topical Treatments
For the treatment with vehicle and diphenhydramine (1%), mice were recorded for 1 hr prior to the first topical application. Each administration was performed twice/day with a 4 hr interval and repeated for 5 days. The treatment ended with a 1 hr recording of each mouse 1 hr after the last application (see Supplemental Experimental Procedures for further details).
Oral Treatment
Mice were recorded for 1 hr prior to the first oral administration of NaCl or desloratadin (Aerius0.5 mg/ml [Schering-Plough]). Each administration of 5 mg/kg was performed once/day with a 40 mm straight feeding needle (Agnthos) and repeated for 5 days (the volume NaCl used corresponded to the volume Aerius for each animal). The treatment ended with a 1 hr recording of each mouse 1 hr after the last application.
Provocation of Itch
Vglut2f/f;Nav1.8Cre and control mice were recorded for 1 hr before they were injected intradermally with 50 μl NaCl with a 100 μl Hamilton syringe supplied with a 30G1/2 needle. The animals were then rerecorded for 1 hr. The following day, the animals were again recorded for 1 hr before they were injected intradermally with 100 μg 48/80 dissolved in 50 μl NaCl. The protocol ended with a 1 hr recording of each mouse directly after the injection.
Statistics
Nonparametric calculations of p values (≤ 2 groups) were conducted with a Mann-Whitney test, two-tailed (Prism version 5.01, GraphPad Software). Nonparametric calculations of p values (≥ 2 groups) were conducted with Kruskal-Wallis (1-way ANOVA) followed by Dunn’s post-hoc test (Prism version 5.01, GraphPad Software).
Supplementary Material
Acknowledgments
We thank H. Wootz, J. Dahlbom, C. Ingman, and T. Jansson for technical assistance; Drs. T. Ebendal, S. Arber, and S. Dufour for providing Th-Cre, TaumGFP and Ht-Pa-Cre mice. This work was supported by grants from the Swedish Medical Research Council (2003-2258, 2004-5567, and 2007-3630/4479), the Swedish Brain Foundation, and the foundations of Knut and Alice Wallenberg, Å. Wiberg, M. Bergwall, Åhlén, Hedlund, and Uppsala University. K.K. is a Royal Swedish Academy of Sciences Research Fellow supported by a grant from the Knut and Alice Wallenberg Foundation.
Footnotes
Supplemental Information includes Supplemental Experimental Procedures, four figures, five tables, and one movie and can be found with this article online at doi:10.1016/j.neuron.2010.09.016.
The authors declare no competing financial interests.
References
- Abrahamsen B, Zhao J, Asante CO, Cendan CM, Marsh S, Martinez-Barbera JP, Nassar MA, Dickenson AH, Wood JN. The cell and molecular basis of mechanical, cold, and inflammatory pain. Science. 2008;321:702–705. doi: 10.1126/science.1156916. [DOI] [PubMed] [Google Scholar]
- Andrew D, Craig AD. Spinothalamic lamina I neurons selectively sensitive to histamine: a central neural pathway for itch. Nat Neurosci. 2001;4:72–77. doi: 10.1038/82924. [DOI] [PubMed] [Google Scholar]
- Atanassoff PG, Brull SJ, Zhang J, Greenquist K, Silverman DG, Lamotte RH. Enhancement of experimental pruritus and mechanically evoked dysesthesiae with local anesthesia. Somatosens Mot Res. 1999;16:291–298. doi: 10.1080/08990229970357. [DOI] [PubMed] [Google Scholar]
- Brumovsky P, Watanabe M, Hökfelt T. Expression of the vesicular glutamate transporters-1 and -2 in adult mouse dorsal root ganglia and spinal cord and their regulation by nerve injury. Neuroscience. 2007;147:469–490. doi: 10.1016/j.neuroscience.2007.02.068. [DOI] [PubMed] [Google Scholar]
- Carstens E. Itch. In: Basbaum A, Bushnell M, editors. Science of Pain. Oxford: Elsevier; 2009. pp. 115–126. [Google Scholar]
- Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD, Julius D. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature. 1997;389:816–824. doi: 10.1038/39807. [DOI] [PubMed] [Google Scholar]
- Caterina MJ, Leffler A, Malmberg AB, Martin WJ, Trafton J, Petersen-Zeitz KR, Koltzenburg M, Basbaum AI, Julius D. Impaired nociception and pain sensation in mice lacking the capsaicin receptor. Science. 2000;288:306–313. doi: 10.1126/science.288.5464.306. [DOI] [PubMed] [Google Scholar]
- Cavanaugh DJ, Lee H, Lo L, Shields SD, Zylka MJ, Basbaum AI, Anderson DJ. Distinct subsets of unmyelinated primary sensory fibers mediate behavioral responses to noxious thermal and mechanical stimuli. Proc Natl Acad Sci USA. 2009;106:9075–9080. doi: 10.1073/pnas.0901507106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL. Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods. 1994;53:55–63. doi: 10.1016/0165-0270(94)90144-9. [DOI] [PubMed] [Google Scholar]
- Davis JB, Gray J, Gunthorpe MJ, Hatcher JP, Davey PT, Overend P, Harries MH, Latcham J, Clapham C, Atkinson K, et al. Vanilloid receptor-1 is essential for inflammatory thermal hyperalgesia. Nature. 2000;405:183–187. doi: 10.1038/35012076. [DOI] [PubMed] [Google Scholar]
- Gezelius H, Wallén-Mackenzie A, Enjin A, Lagerström M, Kullander K. Role of glutamate in locomotor rhythm generating neuronal circuitry. J Physiol (Paris) 2006;100:297–303. doi: 10.1016/j.jphysparis.2007.05.001. [DOI] [PubMed] [Google Scholar]
- Han SK, Mancino V, Simon MI. Phospholipase Cbeta 3 mediates the scratching response activated by the histamine H1 receptor on C-fiber nociceptive neurons. Neuron. 2006;52:691–703. doi: 10.1016/j.neuron.2006.09.036. [DOI] [PubMed] [Google Scholar]
- Hippenmeyer S, Vrieseling E, Sigrist M, Portmann T, Laengle C, Ladle DR, Arber S. A developmental switch in the response of DRG neurons to ETS transcription factor signaling. PLoS Biol. 2005;3:e159. doi: 10.1371/journal.pbio.0030159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt SP, Pini A, Evan G. Induction of c-fos-like protein in spinal cord neurons following sensory stimulation. Nature. 1987;328:632–634. doi: 10.1038/328632a0. [DOI] [PubMed] [Google Scholar]
- Ikoma A, Handwerker H, Miyachi Y, Schmelz M. Electrically evoked itch in humans. Pain. 2005;113:148–154. doi: 10.1016/j.pain.2004.10.003. [DOI] [PubMed] [Google Scholar]
- Ikoma A, Steinhoff M, Ständer S, Yosipovitch G, Schmelz M. The neurobiology of itch. Nat Rev Neurosci. 2006;7:535–547. doi: 10.1038/nrn1950. [DOI] [PubMed] [Google Scholar]
- Imamachi N, Park GH, Lee H, Anderson DJ, Simon MI, Basbaum AI, Han SK. TRPV1-expressing primary afferents generate behavioral responses to pruritogens via multiple mechanisms. Proc Natl Acad Sci USA. 2009;106:11330–11335. doi: 10.1073/pnas.0905605106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jessell TM, Iversen LL, Cuello AC. Capsaicin-induced depletion of substance P from primary sensory neurones. Brain Res. 1978;152:183–188. doi: 10.1016/0006-8993(78)90146-4. [DOI] [PubMed] [Google Scholar]
- Leo S, Moechars D, Callaerts-Vegh Z, D’Hooge R, Meert T. Impairment of VGLUT2 but not VGLUT1 signaling reduces neuropathy-induced hypersensitivity. Eur J Pain. 2009;13:1008–1017. doi: 10.1016/j.ejpain.2008.12.001. [DOI] [PubMed] [Google Scholar]
- Li JL, Fujiyama F, Kaneko T, Mizuno N. Expression of vesicular glutamate transporters, VGluT1 and VGluT2, in axon terminals of nociceptive primary afferent fibers in the superficial layers of the medullary and spinal dorsal horns of the rat. J Comp Neurol. 2003;457:236–249. doi: 10.1002/cne.10556. [DOI] [PubMed] [Google Scholar]
- Lindeberg J, Usoskin D, Bengtsson H, Gustafsson A, Kylberg A, Söderström S, Ebendal T. Transgenic expression of Cre recombinase from the tyrosine hydroxylase locus. Genesis. 2004;40:67–73. doi: 10.1002/gene.20065. [DOI] [PubMed] [Google Scholar]
- Liu Q, Tang Z, Surdenikova L, Kim S, Patel KN, Kim A, Ru F, Guan Y, Weng HJ, Geng Y, et al. Sensory neuron-specific GPCR Mrgprs are itch receptors mediating chloroquine-induced pruritus. Cell. 2009;139:1353–1365. doi: 10.1016/j.cell.2009.11.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loeb W, Quimby F. Clinical Chemistry of Laboratory Animals. 2. Philadelphia, PA: CRC; 1999. [Google Scholar]
- McMahon SB, Koltzenburg M. Itching for an explanation. Trends Neurosci. 1992;15:497–501. doi: 10.1016/0166-2236(92)90102-e. [DOI] [PubMed] [Google Scholar]
- Moechars D, Weston MC, Leo S, Callaerts-Vegh Z, Goris I, Daneels G, Buist A, Cik M, van der Spek P, Kass S, et al. Vesicular glutamate transporter VGLUT2 expression levels control quantal size and neuropathic pain. J Neurosci. 2006;26:12055–12066. doi: 10.1523/JNEUROSCI.2556-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mogil JS, Miermeister F, Seifert F, Strasburg K, Zimmermann K, Reinold H, Austin JS, Bernardini N, Chesler EJ, Hofmann HA, et al. Variable sensitivity to noxious heat is mediated by differential expression of the CGRP gene. Proc Natl Acad Sci USA. 2005;102:12938–12943. doi: 10.1073/pnas.0503264102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olszewski PK, Fredriksson R, Olszewska AM, Stephansson O, Alsiö J, Radomska KJ, Levine AS, Schiöth HB. Hypothalamic FTO is associated with the regulation of energy intake not feeding reward. BMC Neurosci. 2009;10:129. doi: 10.1186/1471-2202-10-129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pietri T, Eder O, Blanche M, Thiery JP, Dufour S. The human tissue plasminogen activator-Cre mouse: a new tool for targeting specifically neural crest cells and their derivatives in vivo. Dev Biol. 2003;259:176–187. doi: 10.1016/s0012-1606(03)00175-1. [DOI] [PubMed] [Google Scholar]
- Raffaeli W, Marconi G, Fanelli G, Taddei S, Borghi GB, Casati A. Opioid-related side-effects after intrathecal morphine: a prospective, randomized, double-blind dose-response study. Eur J Anaesthesiol. 2006;23:605–610. doi: 10.1017/S026502150600038X. [DOI] [PubMed] [Google Scholar]
- Ross SE, Mardinly AR, McCord AE, Zurawski J, Cohen S, Jung C, Hu L, Mok SI, Shah A, Savner EM, et al. Loss of inhibitory inter-neurons in the dorsal spinal cord and elevated itch in Bhlhb5 mutant mice. Neuron. 2010;65:886–898. doi: 10.1016/j.neuron.2010.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roza C, Laird JM, Souslova V, Wood JN, Cervero F. The tetrodotoxin-resistant Na+ channel Nav1.8 is essential for the expression of spontaneous activity in damaged sensory axons of mice. J Physiol. 2003;550:921–926. doi: 10.1113/jphysiol.2003.046110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaeren-Wiemers N, Gerfin-Moser A. A single protocol to detect transcripts of various types and expression levels in neural tissue and cultured cells: in situ hybridization using digoxigenin-labelled cRNA probes. Histochemistry. 1993;100:431–440. doi: 10.1007/BF00267823. [DOI] [PubMed] [Google Scholar]
- Schmelz M. A neural pathway for itch. Nat Neurosci. 2001;4:9–10. doi: 10.1038/82956. [DOI] [PubMed] [Google Scholar]
- Schmelz M, Schmidt R, Bickel A, Handwerker HO, Torebjörk HE. Specific C-receptors for itch in human skin. J Neurosci. 1997;17:8003–8008. doi: 10.1523/JNEUROSCI.17-20-08003.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmelz M, Schmidt R, Weidner C, Hilliges M, Torebjork HE, Hand-werker HO. Chemical response pattern of different classes of C-nociceptors to pruritogens and algogens. J Neurophysiol. 2003;89:2441–2448. doi: 10.1152/jn.01139.2002. [DOI] [PubMed] [Google Scholar]
- Seal RP, Wang X, Guan Y, Raja SN, Woodbury CJ, Basbaum AI, Edwards RH. Injury-induced mechanical hypersensitivity requires C-low threshold mechanoreceptors. Nature. 2009;462:651–655. doi: 10.1038/nature08505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sikand P, Shimada SG, Green BG, LaMotte RH. Similar itch and nociceptive sensations evoked by punctate cutaneous application of capsaicin, histamine and cowhage. Pain. 2009;144:66–75. doi: 10.1016/j.pain.2009.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slappendel R, Weber EW, Benraad B, van Limbeek J, Dirksen R. Itching after intrathecal morphine. Incidence and treatment. Eur J Anaesthesiol. 2000;17:616–621. doi: 10.1046/j.1365-2346.2000.00727.x. [DOI] [PubMed] [Google Scholar]
- Stirling LC, Forlani G, Baker MD, Wood JN, Matthews EA, Dickenson AH, Nassar MA. Nociceptor-specific gene deletion using heterozygous NaV1.8-Cre recombinase mice. Pain. 2005;113:27–36. doi: 10.1016/j.pain.2004.08.015. [DOI] [PubMed] [Google Scholar]
- Sun YG, Chen ZF. A gastrin-releasing peptide receptor mediates the itch sensation in the spinal cord. Nature. 2007;448:700–703. doi: 10.1038/nature06029. [DOI] [PubMed] [Google Scholar]
- Sun YG, Zhao ZQ, Meng XL, Yin J, Liu XY, Chen ZF. Cellular basis of itch sensation. Science. 2009;325:1531–1534. doi: 10.1126/science.1174868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tominaga M, Caterina MJ. Thermosensation and pain. J Neurobiol. 2004;61:3–12. doi: 10.1002/neu.20079. [DOI] [PubMed] [Google Scholar]
- Tuckett RP. Itch evoked by electrical stimulation of the skin. J Invest Dermatol. 1982;79:368–373. doi: 10.1111/1523-1747.ep12529734. [DOI] [PubMed] [Google Scholar]
- Wallén-Mackenzie A, Gezelius H, Thoby-Brisson M, Nygård A, Enjin A, Fujiyama F, Fortin G, Kullander K. Vesicular glutamate transporter 2 is required for central respiratory rhythm generation but not for locomotor central pattern generation. J Neurosci. 2006;26:12294–12307. doi: 10.1523/JNEUROSCI.3855-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward L, Wright E, McMahon SB. A comparison of the effects of noxious and innocuous counterstimuli on experimentally induced itch and pain. Pain. 1996;64:129–138. doi: 10.1016/0304-3959(95)00080-1. [DOI] [PubMed] [Google Scholar]
- Yosipovitch G, Duque MI, Fast K, Dawn AG, Coghill RC. Scratching and noxious heat stimuli inhibit itch in humans: a psychophysical study. Br J Dermatol. 2007;156:629–634. doi: 10.1111/j.1365-2133.2006.07711.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.