Abstract
Postconditioning (PostC), or relief of myocardial ischemia in a stuttered manner, has been shown to reduce infarct size, due in part to upregulation of survival kinase signaling. Virtually all of these data have, however, been obtained in healthy adult cohorts; the question of whether PostC-induced cardioprotection is maintained in the setting of clinically relevant comorbidities has remained largely unexplored. Accordingly, our aim was to assess the consequences of a major risk factor—diabetes—on the infarct-sparing effect of stuttered reflow. Isolated buffer-perfused hearts were obtained from normoglycemic C57BL/6J mice, BKS.Cg-m+/+Leprdb/J (db/db) mice (model of type-2 diabetes), C57BL/6J mice injected with streptozotocin (model of type-1 diabetes), and streptozotocin-injected mice in which normoglycemia was re-established by islet cell transplantation. All hearts underwent 30 min of ischemia and, within each cohort, hearts received either standard (control) reperfusion or three to six 10-s cycles of stuttered reflow. PostC reduced infarct size via upregulation of extracellular signal-regulated kinase 1/2 in normoglycemic mice. In contrast, diabetic hearts were refractory to PostC-induced cardioprotection—an effect that, in the type-1 model, was reversed by restoration of normoglycemia. We provide novel evidence for a profound—but potentially reversible—diabetes-induced defect in the cardioprotective efficacy of PostC. Antioxid. Redox Signal. 14, 781–790.
Introduction
Postconditioning (PostC) is the phenomenon, first reported by Zhao et al. (50), whereby relief of sustained myocardial ischemia in a stuttered manner attenuates lethal ischemia-reperfusion injury and significantly reduces infarct size. Subsequent studies have documented PostC-induced cardioprotection in multiple models and species (1, 6, 20, 30, 34, 43, 45, 48, 49), and have identified upregulation of survival kinase signaling (i.e., extracellular signal-regulated kinase [ERK]1/2 and/or phosphatidylinositol-3-kinase/Akt) during the early minutes of reflow as playing a role in initiating this protective phenotype (6, 30, 43, 48, 49). However, the majority of these data have been obtained in healthy adult cohorts devoid of clinically relevant comorbidities (10, 44). Among the host of established independent risk factors for cardiovascular disease and acute myocardial infarction (MI), diabetes, because of its dramatic increase in prevalence over the past decade, is of particular relevance (25, 35). However, despite the escalating incidence of diabetes as a comorbid condition in patients with acute MI, there is currently no insight into the consequences of concomitant diabetes on the infarct-sparing effect of stuttered reflow.
To address this issue, we assessed the efficacy of infarct size reduction with PostC in isolated buffer-perfused hearts from healthy adult C57BL/6J mice (Protocol 1), hearts from BKS.Cg-m+/+Leprdb/J (db/db) mice (an established, genetic model of type-2 diabetes [3]; Protocol 2), and hearts from C57BL/6J mice rendered diabetic by injection of streptozotocin (STZ, a standard model of type-1 diabetes [3]; Protocol 3). We report that hearts from diabetic mice are refractory to PostC-induced cardioprotection, possibly due to failed upregulation of ERK signaling. Finally, using the type-1 diabetes model, we investigated whether restoration of normoglycemia can re-establish the infarct-sparing effect of PostC (Protocol 4). Our results revealed that the inability of stuttered reflow to limit infarct size does not reflect a permanent diabetes-associated defect in cardioprotective signaling; rather, normalization of blood glucose levels re-established the protective PostC phenotype.
Methods
This study was approved by the Institutional Animal Care and Use Committee of the University of Massachusetts Medical School, and was performed in accordance with the Guide for the Care and Use of Laboratory Animals from the Institute of Laboratory Animals Resources (NIH Publication Vol. 25 No. 28, revised 1996).
General methods
Isolated buffer-perfused heart model
For all protocols, mice were anesthetized with sodium pentobarbital (60 mg/kg intraperitoneal) and the hearts were rapidly excised and mounted on an aortic cannula for retrograde perfusion (nonrecirculating) at a constant pressure of 55 mm Hg. Standard buffer was composed of (in mM) NaCl (118), KCl (4.7), NaHCO3 (24), KH2PO4 (1.2), MgSO4-7H2O (1.2), glucose (11), and CaCl2 anhydrous (2.5) in distilled water at a pH of 7.4, and was continuously oxygenated with 95% O2/5% CO2. Care was taken to maintain both buffer temperature and heart temperature at 37°C. A balloon constructed of polyvinyl chloride plastic film was inserted into the left ventricle (LV), inflated to an end-diastolic pressure of 5 mm Hg, and used to assess cardiodynamic function throughout the experiment (30, 31). Coronary perfusion was monitored throughout each experiment using an in-line Doppler flow probe (Transonic Systems Inc.).
Blood glucose, plasma insulin levels, and plasma fatty acid concentration
Upon harvesting the hearts, blood samples were obtained for measurement of nonfasting blood glucose concentration (AccuCheck® meter and test strips; Roche Diagnostics Inc). In addition, plasma insulin levels and plasma fatty acid concentration were measured using commercially available ELISA kits according to the manufacturer's instructions (Utrasensitive Mouse Insulin ELISA kit; Crystal Chem, Inc.; Free Fatty Acid Quantification Kit; BioVision, Inc.).
Protocol 1: Efficacy of PostC in normoglycemic C57BL/6J mice
Infarct size
Infarct size was assessed in isolated buffer-perfused hearts from healthy 12–14-week-old normoglycemic C57BL/6J mice using standard methods described previously (31). After stabilization, all hearts underwent 30 min of sustained global ischemia, and were randomly assigned to receive (i) abrupt and complete reperfusion (controls); (ii) PostC with three cycles of [10 s reperfusion + 10 s reocclusion] followed by sustained restoration of flow; or (iii) PostC with six cycles of [10 s reflow + 10 s reocclusion] followed by sustained reperfusion (n = 6–10 per group). At 2 h after the onset of reperfusion, each heart was cut into 4–6 transverse slices and the extent of necrosis was delineated by triphenyltetrazolium staining. All hearts were digitally photographed, and infarct size was quantified in a blinded manner (without knowledge of the treatment group) using image analysis software (30, 31).
Kinase expression
Buffer-perfused hearts were obtained from additional C57 mice (n = 12) as described for the infarct size protocol and assigned to undergo 30 min of global ischemia followed by abrupt, complete reperfusion (controls), 30 min of ischemia followed by three 10-s cycles of stuttered reflow (PostC), or time-matched uninterrupted perfusion (nonischemic shams). At 10 min post-reflow, all hearts were frozen in liquid nitrogen and stored at −80°C until processed.
Hearts were prepared and, using methods described in detail previously (6, 30, 31), were probed for expression of phospho-Akt, phospho-ERK1/2, phospho-p70S6 kinase, and phospho–glycogen synthase kinase (GSK)-3β, and then stripped and re-probed for expression of total Akt, ERK1/2, p70S6 kinase, and GSK-3β (all antibodies from Cell Signaling Technology, Inc.). Bands of interest were quantified without knowledge of the group assignment using gel analysis software, and densitometry values for phospho-Akt, phospho-ERK1/2, phospho-p70S6 kinase, and phospho-GSK-3β were normalized to the corresponding total kinase values (i.e., phospho-Akt/total Akt, phospho-ERK/total ERK1/2, phospho-p70S6 kinase/total p70S6 kinase, and phospho-GSK-3β/total GSK-3β was calculated for each sample). In addition, phospho-ERK content (in pg/μg protein) was quantified in heart lysates from all groups using a commercially available EIA kit (Assay Designs, Inc.).
Protocol 2: Efficacy of PostC in a model of type-2 diabetes
Infarct size
To investigate the consequences of type-2 diabetes on the infarct-sparing effect of PostC, hearts were isolated from 12- to 14-week-old BKS.Cg-m+/+Leprdb/J (db/db) mice (JAX® Mice; The Jackson Laboratory Inc.) and buffer-perfused as detailed in Protocol 1. The db/db model is characterized by the spontaneous development of hyperglycemia at 4–6 weeks of age, and severe, fully developed diabetes by 8 weeks of age (3). All hearts underwent 30 min of global ischemia, and were randomized to receive either control (abrupt) reflow or PostC with three or six 10-s cycles of reperfusion-reocclusion (n = 6–10 per group). Infarct size was delineated and quantified as in Protocol 1.
Kinase expression
Buffer-perfused hearts from db/db mice were assigned to sham, control, and 3-cycle PostC groups (n = 3–4 per group). Hearts were frozen at 10 min after relief of ischemia for analysis of kinase expression (immunoblot and enzyme immunometric assay) using the same methods described in Protocol 1.
Protocol 3: Efficacy of PostC in a model of type-1 diabetes
To assess the effect of type-1 diabetes on the efficacy of PostC, 10–12-week-old normoglycemic C57BL/6J mice received a single dose of STZ (150 mg/kg intraperitoneal) dissolved in 0.1 M citrate buffer (3, 14). Injection of STZ causes pancreatic β-cell toxicity and necrosis, and, as a result, rapidly leads to a deficiency in insulin production (3). Two weeks later, after confirming that the mice were diabetic [using the standard definition of blood glucose concentration >250 mg/dl (36)], STZ-injected mice were enrolled into either the infarct size or kinase expression components of Protocol 3, with group assignments identical to those described for Protocols 1 and 2 (n = 5–6 per group for the infarct size experiments; n = 3–4 per group for kinase expression).
Protocol 4: Restoration of normoglycemia in type-1 diabetes
A final series of experiments was conducted to determine whether restoration of normoglycemia would re-establish the infarct-sparing effect of PostC in diabetic mice. Accordingly, 10-week-old C57BL/6J mice were rendered diabetic by injection of STZ (150 mg/kg) as described in Protocol 3. At 2 weeks postinjection, mice were assigned to undergo either syngenic islet cell transplantation into the renal subcapsular space to restore endogenous insulin production (36) or sham surgery. Two weeks thereafter (i.e., 4 weeks after administration of STZ and 2 weeks after treatment), mice were enrolled for assessment of infarct size (control and 3-cycle PostC groups; n = 5 per group) and analysis of kinase expression (n = 3 per group).
Statistics
To confirm that our murine models displayed the hallmarks of type-2 and type-1 diabetes, values of blood glucose, plasma insulin, and plasma free fatty acid concentrations were compared among C57BL/6J, db/db, and STZ-injected cohorts enrolled in Protocols 1–3 by analysis of variance; if significant F-values were obtained, post-hoc pair-wise comparisons were made using the Newman-Keuls test. To establish that islet cell transplantation restored endogenous insulin synthesis (Protocol 4), blood glucose, insulin, and fatty acid levels were compared between STZ + transplant versus STZ + sham surgery groups by t-test. For the primary study endpoints (infarct size and kinase expression): as Protocols 1–4 were conducted consecutively rather than concurrently, separate statistical analyses were performed for each component of the study. Within each protocol, data were compared among groups by analysis of variance followed by the Newman-Keuls test. Data are reported as mean ± standard error of the mean, and p-values <0.05 were accepted as significant.
Results
Protocol 1
Values of nonfasting blood glucose concentration, plasma insulin levels, and plasma free fatty acid content in normoglycemic C57BL/6J mice averaged 178 ± 7 mg/dl, 1.40 ± 0.20 ng/ml, and 3.0 ± 0.2 nM/μl, respectively (Table 1).
Table 1.
Blood Glucose, Plasma Insulin, and Plasma-Free Fatty Acid Concentrations
| Blood glucose (nonfasting), mg/dl | Plasma insulin, ng/ml | Plasma FFA, nM/μl | |
|---|---|---|---|
| Protocol 1: C57BL/6J mice | 178 ± 7 | 1.40 ± 0.20 | 3.0 ± 0.2 |
| Protocol 2: db/db mice | 480 ± 23a | 8.50 ± 1.50a | 5.4 ± 0.4a |
| Protocol 3: STZ-injected C57 mice | 556 ± 15a | 0.18 ± 0.08a | 5.2 ± 0.7a |
| Protocol 4: STZ + Sham surgery | 558 ± 11 | 0.07 ± 0.03 | 5.4 ± 0.2 |
| Protocol 4: STZ + Islet cell transplantation | 206 ± 7b | 1.33 ± 0.09b | 2.7 ± 0.3b |
p < 0.01 versus values in C57BL/6J mice.
p < 0.01 versus values in STZ-treated rats that received sham surgery.
FFA, free fatty acid; STZ, streptozotocin.
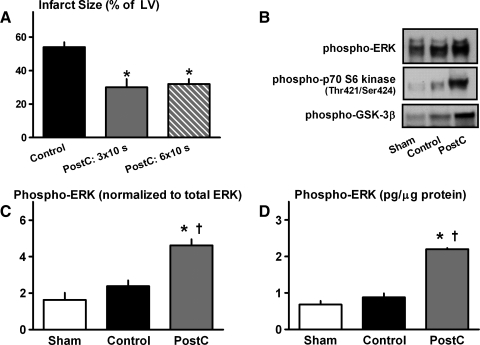
As expected (30), in hearts from healthy normoglycemic C57 mice, PostC with either 3 or 6 cycles of stuttered reflow was profoundly cardioprotective: mean infarct sizes averaged 30% ± 5% and 32% ± 3% of the total LV in the PostC groups versus 54% ± 3% in controls (p < 0.05; Figs. 1 and 2).
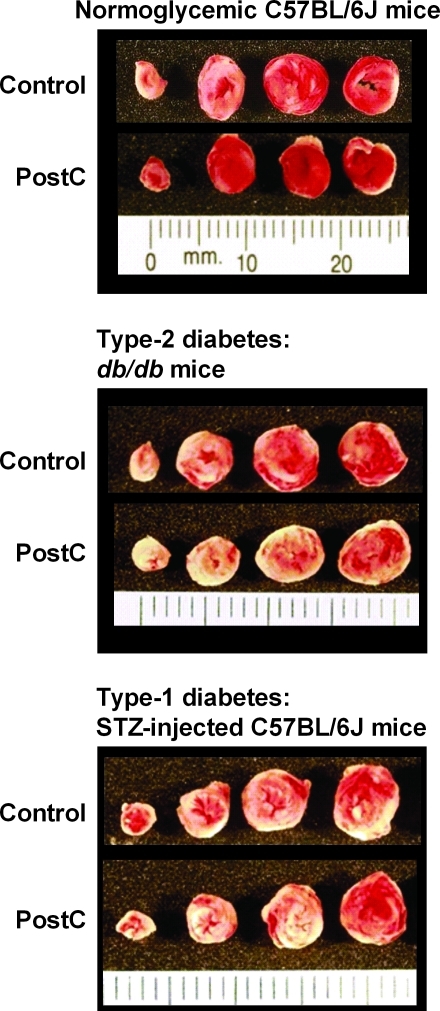
FIG. 1.
Original images of mouse hearts assigned to receive either standard, abrupt reperfusion (Control) or postconditioning (PostC) with three 10-s cycles of stuttered reflow. Hearts were cut into transverse slices and stained with triphenyltetrazolium chloride; using this method, viable tissue stained red, whereas necrotic myocardium remains unstained and so appears pale. Infarct size was reduced with PostC in normoglycemic C57BL/6J mice (Protocol 1). In contrast, in models of type-2 and type-1 diabetes (Protocols 2 and 3, respectively), infarct sizes were comparable in Control and PostC groups. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article at www.liebertonline.com/ars).
FIG. 2.
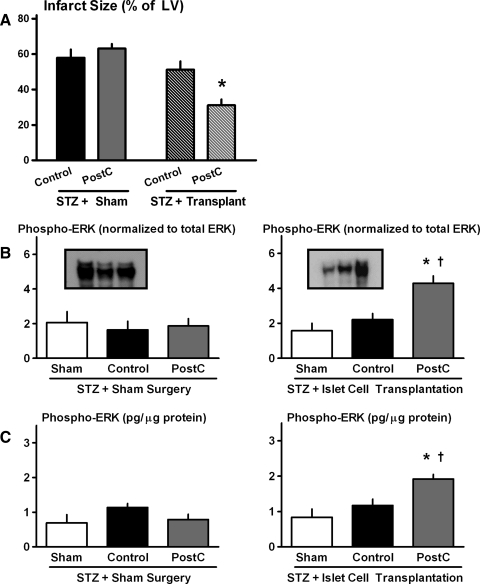
Protocol 1—Normoglycemic C57BL/6J mice. (A) Infarct size (expressed as a percentage of the total LV). *p < 0.05 versus Control. (B) Original immunoblots of phospho-ERK, p70S6 kinase, and glycogen synthase kinase–3β in sham, control, and PostC hearts. (C) Cardiac expression of phospho-ERK, assessed by immunoblotting. *p < 0.05 versus Control; †p < 0.05 versus Sham. (D) Cardiac concentration of phospho-ERK, quantified by EIA. EIA, enzyme immunometric assay; ERK, extracellular-signal regulated kinase; LV, left ventricle; PostC, postconditioned.
There were no differences in expression of total ERK, Akt, p70S6 kinase, or GSK-3β among sham hearts, control hearts, or hearts that received three cycles of stuttered reflow (data not shown). Moreover, as reported previously by our group (6, 30), no change in phospho/total Akt was seen in response to PostC (data not shown). In contrast, hearts that received interrupted reperfusion displayed significant, greater than twofold increases in expression of phospho-ERK and its downstream targets phospho-p70S6 kinase and phospho-GSK-3β, and a comparable twofold increase in phospho-ERK content as quantified by enzyme immunometric assay versus control (Fig. 2). These data corroborate the infarct-sparing effect of PostC in the murine model (1, 30) and are consistent with our previous studies identifying ERK signaling as playing a requisite role in PostC-induced cardioprotection (6, 30).
Protocol 2
As anticipated (3), db/db mice exhibited the hallmarks of type-2 diabetes: blood glucose concentration was increased to 480 ± 23 mg/dl despite augmented insulin production (plasma levels elevated at 8.5 ± 1.5 ng/ml). In addition, plasma fatty acid content was increased to 5.4 ± 0.4 nM/μl (all values p < 0.01 when compared with normoglycemic C57 mice; Table 1).
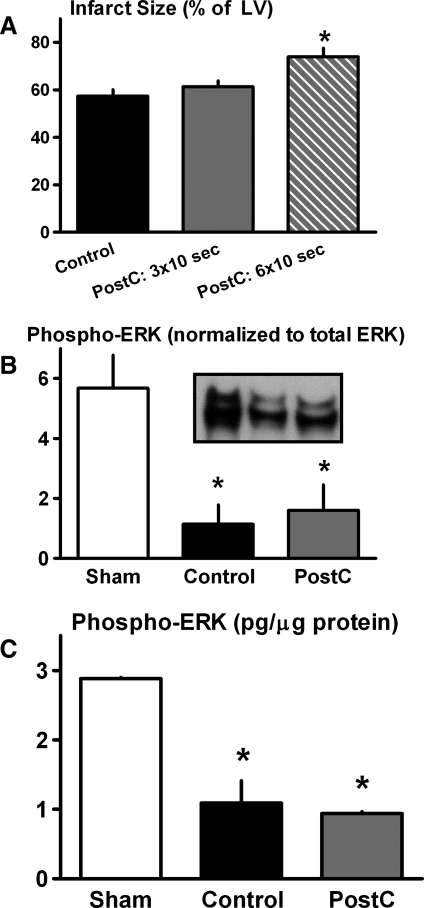
In contrast to results obtained in the normoglycemic cohort, PostC failed to initiate a cardioprotective phenotype in hearts from db/db mice. Rather, infarct size was comparable in hearts that received three cycles of stuttered reflow versus controls (61% ± 3% vs. 57% ± 3% of the LV), and exacerbated in hearts treated with the amplified, six-cycle PostC stimulus (74% ± 4% of the LV; p < 0.05 vs. controls; Figs. 1 and 3). Moreover, there was no evidence of an upregulation in ERK signaling in response to stuttered reflow; rather, both expression and content of phospho-ERK were attenuated, rather than augmented, in all hearts that received ischemia (both control and PostC groups) versus shams (Fig. 3).
FIG. 3.
Protocol 2—type-2 diabetes model. (A) Infarct size (expressed as a percentage of the total LV). *p < 0.05 versus Control. (B) Cardiac expression of phospho-ERK, assessed by immunoblotting. *p < 0.05 versus Sham. (C) Cardiac concentration of phospho-ERK, quantified by EIA. *p < 0.05 versus Sham.
Protocol 3
C57BL/6J mice injected 2 weeks previously with STZ developed type-1 diabetes (3), characterized by increased blood glucose concentrations (556 ± 15 mg/dl) together with near-total failure to produce insulin (mean plasma levels of 0.18 ± 0.08 ng/ml) and increased plasma free fatty acid concentration (5.2 ± 0.7 nM/μl: all values p < 0.01 versus healthy normoglycemic C57 mice; Table 1).
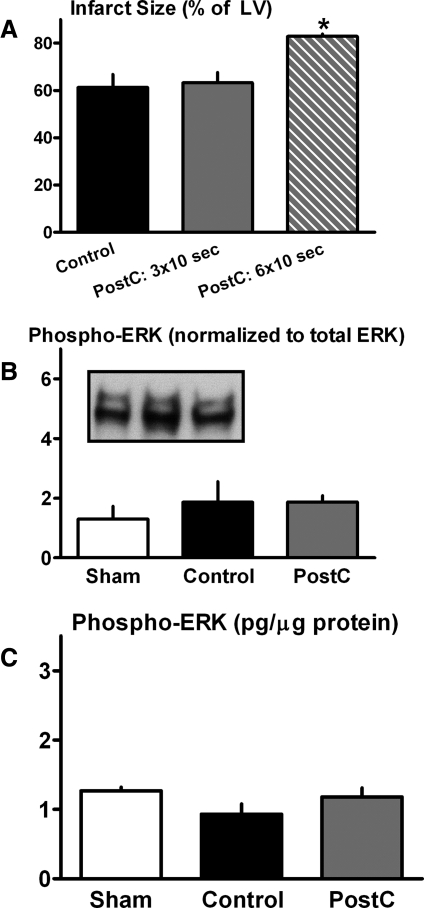
The response of hearts from type-1 diabetic mice to stuttered reflow was comparable to that observed in the type-2 model; that is, there was no evidence of a reduction in infarct size or upregulation of ERK signaling in PostC groups versus controls (Figs. 1 and 4).
FIG. 4.
Protocol 3—type-1 diabetes model. (A) Infarct size (expressed as a percentage of the total LV). *p < 0.05 versus Control. (B) Cardiac expression of phospho-ERK, assessed by immunoblotting. (C) Cardiac concentration of phospho-ERK, quantified by EIA.
Protocol 4
STZ-injected mice assigned to receive sham-transplant surgery continued to exhibit symptoms of type-1 diabetes 2 weeks postoperatively (total of 4 weeks after STZ injection), with plasma glucose, insulin and free fatty acid levels averaging 558 ± 11 mg/dl, 0.07 ± 0.03 ng/ml, and 5.4 ± 0.20 nM/μl (Table 1). In addition, as in Protocol 2, hearts from STZ-injected mice were refractory to PostC-induced cardioprotection (Fig. 5).
FIG. 5.
Protocol 4—type-1 diabetes model; restoration of normoglycemia with islet cell transplantation. (A) Infarct size (expressed as a percentage of the total LV). *p < 0.05 versus matched Control cohort. (B) Cardiac expression of phospho-ERK, assessed by immunoblotting for STZ-injected mice that received sham surgery (left panel) and islet cell transplantation (right panel). *p < 0.05 versus Control; †p < 0.05 versus Sham. (C) Cardiac concentration of phospho-ERK, quantified by EIA for STZ-injected mice that received sham surgery (left panel) and islet cell transplantation (right panel). *p < 0.05 versus Control; †p < 0.05 versus Sham. STZ, streptozotocin.
In STZ-injected mice that received islet cell transplantation, endogenous insulin production was successfully restored (plasma concentration of 1.33 ± 0.09 ng/ml); this was accompanied by a normalization of blood glucose and free fatty acid levels to 206 ± 7 mg/dl and 2.7 ± 0.3 nM/μl, respectively (Table 1). Most notably, islet cell transplantation re-established the infarct-sparing effect of PostC and restored the upregulation of ERK signaling seen with stuttered reflow in healthy normoglycemic C57BL/6J mice (Fig. 5).
Discussion
In this study, we demonstrate a loss in efficacy of PostC in murine models of type-2 and type-1 diabetes, characterized by both an apparent inability to reduce infarct size and failed upregulation of ERK phosphorylation. Moreover, we provide novel evidence that the loss in efficacy of PostC does not reflect a permanent diabetes-associated defect in cardioprotective signaling; rather, in the type-1 model, therapeutic control of insulin and blood glucose levels re-established the infarct-sparing effect of stuttered reflow.
PostC in normoglycemic C57BL/6J mice
Our observation of infarct size reduction with PostC in hearts from adult normoglycemic C57BL/6J mice is consistent with evidence of PostC-induced cardioprotection reported in multiple species (1, 6, 20, 30, 34, 43, 45, 48, 49), including the mouse (1, 30). Our specific choice of algorithm—10-s cycles of stuttered reflow—was based on pilot experiments conducted in our laboratory and previously published studies from our group and others (1, 20, 30), and, in our hands, showed greater benefit when compared with repeated 20- or 30-s episodes of brief ischemia-reperfusion [i.e., algorithms that have successfully been applied in rabbit, dog, and pig models (6, 34, 49, 50)]. With regard to our primary endpoint, we focused on infarct size, rather than acute recovery of left ventricular contractile function during the initial minutes-hours after relief of ischemia, as our index of cardioprotection. This is based on reports by our laboratory and others that reduction of infarct size with PostC is not accompanied consistent and sustained improvements in left ventricular developed pressure or rate-pressure product versus controls (6, 20, 29, 30, 48). Similar results were obtained in the current study: left ventricular developed pressure recovered to 30%–40% of baseline values during the reperfusion period, with no significant differences among control and PostC groups. Finally, with regard to cellular mechanisms, the data obtained in Protocol 1 corroborates our previous findings that in healthy adult mouse hearts, ERK signaling is upregulated (30). Moreover, the infarct-sparing effect of PostC was abrogated by administration of PD98059 (30), thereby suggesting that ERK signaling plays a requisite role in PostC-induced cardioprotection.
Cardioprotection in models of diabetes and metabolic syndrome
Postconditoning
Results of Protocols 2 and 3 revealed that in isolated buffer-perfused hearts obtained from mouse models of type-2 and type-1 diabetes, PostC with 3 or 6 cycles of stuttered reflow failed to evoke a cardioprotective phenotype. It could be argued that PostC had not been rendered ineffective in these models but, rather, that alterations in the stimulus might be required to achieve benefit. To investigate this potentially confounding issue, ancillary experiments were conducted using multiple permutations of the PostC algorithm in which we modified the number of cycles of stuttered reperfusion (ranging from 3 to 6) and duration of the episodes of brief ischemia and/or intervening reflow (including 10, 20, and 30 s). None of the algorithms yielded any evidence of a trend toward cardioprotection. It could also be argued that the inability of PostC to limit infarct size in our buffer-perfused models may reflect a mismatch between the well-documented, enhanced fatty acid utilization of diabetic hearts (3, 5) versus the absence of fatty acid in the perfusate. Accordingly, additional infarct size experiments were performed in hearts from C57BL/6J and db/db mice using buffer enriched with palmitate (final concentration: 1.5 mM) and dialyzed before perfusion (11). Comparable outcomes were obtained irrespective of the presence of fatty acid: PostC with 3 10-s cycles of stuttered reflow reduced infarct size in hearts from C57BL/6J mice (39% ± 4% vs. 57% ± 5% in matched controls; p < 0.05), whereas in hearts from db/db mice, no benefit was observed (mean infarct sizes of 59% ± 5% vs. 60% ± 3% in the PostC group vs. controls). These data strongly suggest that our observation of failed cardioprotection in diabetic models is not caused by the use of sub-optimal PostC algorithms or inappropriate perfusate composition.
Although the current study is, to our knowledge, the first to interrogate the efficacy of PostC in animals displaying the hallmarks of fully developed type-2 and type-1 diabetes, two previous reports have concluded that PostC fails to limit lethal myocardial ischemia reperfusion injury in models of metabolic syndrome (2, 46). Specifically, in the prediabetic leptin-deficient obese (ob/ob) mouse, and in the Wistar-Ottawa-Karlsburg W rat, infarct sizes were comparable in PostC cohorts versus controls. Moreover, as in the current protocol, the inability of PostC to evoke a protective phenotype was attributed to ineffective phosphorylation of ERK (46) or failed phosphorylation of both ERK and Akt (2). Analogous results have been obtained using postconditioning mimetics (rather than stuttered reflow) in obese Zucker rats: cyclosporine A- and helium-induced cardioprotection was abolished in this prediabetic model (15, 17). Taken together, these studies provide compelling evidence that the infarct-sparing effect of PostC is undermined in the setting of diabetes as well as prediabetes/metabolic syndrome.
Preconditioning
While our current protocols focused on the ability of PostC to limit infarct size in diabetic models, similar results have been obtained when ischemic preconditioning or preconditioning mimetic agents were used as the protective stimulus [reviewed in (10)]. With the exception of one study showing persistent preconditioning-induced cardioprotection in STZ-injected rats (24), there is general agreement that the response of the diabetic heart to preconditioning stimuli is impaired (10), that is, either absent (13, 18, 19, 21), or markedly attenuated such that an augmented stimulus is required to trigger protection (42). These differences in outcomes have been attributed to both the specific model that was used (type-1 vs. type-2 diabetes), as well as the severity and duration of diabetes and hyperglycemia. In this regard, in the study documenting persistent infarct size reduction with preconditioning, the cohort of rats considered to be diabetic displayed normal fasting values of blood glucose and normal plasma insulin concentrations despite injection with STZ (24). While the mechanisms responsible for the loss in efficacy of preconditioning remain poorly defined, defects in survival kinase signaling have—as in the case of PostC—been implicated to play a role (13, 42).
Is diabetes per se cardioprotective?
It is well recognized that diabetes is an independent risk factor for cardiovascular disease and, moreover, that outcome post-MI is worsened in diabetic patients (10). Nonetheless, data obtained from animal models—in particular, STZ-induced models of type-1 diabetes—suggest that diabetes may paradoxically decrease the susceptibility of the heart to infarction. Specifically, it has been proposed that, in the early stages of diabetes (within 1–2 weeks after STZ injection), hearts from diabetic animals develop significantly smaller infarcts following sustained ischemia-reperfusion compared with healthy normoglycemic control cohorts, while, in contrast, no benefit is seen in late-stage diabetes accompanied by the development of diabetic cardiomyopathy (33, 41). Evidence in support of this concept is, however, equivocal: other studies have reported no difference, or exacerbation (rather than reduction) of infarct size, as early as 2 weeks after administration of STZ (8, 26) [reviewed in (10)]. Comparison of Protocols 1, 3, and 4 indicates that our results are consistent with these latter observations: although STZ-injected mice did not appear to develop cardiomyopathy within the timeframe of our study, infarct size was not reduced in control-STZ-treated mice versus the control C57BL/6J cohort.
Mechanism(s) for loss of in efficacy of PostC-induced protection?
The obvious question to arise from these data is: what factors contribute to the failed upregulation of ERK and loss in efficacy of PostC in these diabetic (and prediabetic) models? The fact that the inability of PostC to limit infarct size was observed in isolated, buffer-perfused hearts strongly suggests that this loss in efficacy is not due to an acute, blood-borne and humorally mediated downregulation in cardioprotective signaling. Rather, the loss of responsiveness to stuttered reflow appears to reflect a diabetes-induced defect in the cardiomocytes per se.
One potential hypothesis is that the derangement in cardioprotective signaling is triggered by previous exposure of the cardiomyocytes to increased concentrations of blood glucose. Indeed, even acute hyperglycemia, induced by short-term IV infusion of glucose or dextrose to healthy normoglycemic animals, reportedly abrogated the protective effect of pharmacologic PostC achieved by administration of anesthetics at the time of reperfusion (16, 32). This simplistic explanation is, however, unlikely, as several of the models of prediabetes/metabolic syndrome in which PostC was ineffective were normoglycemic (15, 17, 46). Rather, a constellation of factors reflecting the complex alterations in metabolic phenotype that characterize these models (3) in all likelihood triggers the apparent signaling defect.
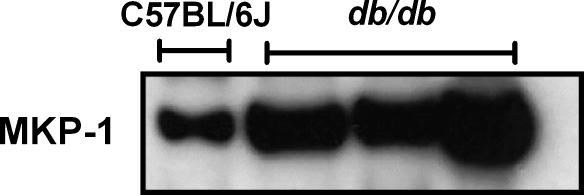
A second, as-yet unresolved question is: what is the precise nature of the diabetes-induced defect that presumably precludes the phosphorylation of ERK? Two previous studies conducted in models that were refractory to PostC-induced protection—the ob/ob mouse (2), and, from our laboratory, hearts from elderly 2-year old C57BL/6J mice (30)—concluded that the deficit in ERK phosphorylation was associated with [and possibly due to (30)] increased cardiac expression of one or more phosphatases. Although negative regulation of ERK can involve multiple phosphatases, members of the mitogen-activated protein kinase phosphatase (MKP) family, most notably MKP-1 and MKP-3, reportedly play a primary role (4, 9, 28, 39). Indeed, initial pilot experiments have revealed that hearts from db/db mice display a robust increase in expression of MKP-1 when compared with hearts from normoglycemic adult C57 mice (Fig. 6). However, further prospective studies are required to confirm this apparent increase in phosphatase expression in diabetic hearts, identify whether the increase in expression is seen in multiple phosphatases, determine whether the increased expression is associated with increased activity, and, most importantly, establish cause-and-effect and ascertain whether increased phosphatase activity is responsible for the loss in efficacy of PostC in these models.
FIG. 6.
Pilot experiments. Cardiac expression of mitogen-activated protein kinase phosphatase (MKP)-1: original immunoblots from one C57BL/6J mouse and 3 db/db mice.
A third issue is: does the diabetes-associated defect in cardioprotective signaling extend beyond the phosophorylation of ERK and its downstream targets? Although we have focused our analysis on ERK signaling, there is evidence that, in some models, upregulation of survival kinases is not essential for PostC-induced protection (37). Indeed, even in the murine model, alternative signaling molecules—including signal transducer and activator of transcription 3—have been identified to play a pivotal role (1, 22, 23). The consequences of type-2 and type-1 diabetes on these alternative pathways are, at present, unknown.
Restoration of normoglycemia in the type-1 diabetes model
A final, novel, and potentially important contribution of the current study is the observation that in STZ-induced type-1 diabetes, restoration of endogenous insulin production and normalization of blood glucose (and plasma fatty acid) concentrations to normal values re-established the protective phenotype of PostC; that is, in the type-1 diabetes model, the defect in ERK signaling can be reversed with appropriate therapeutic management. It must, however, be emphasized that this re-establishment of PostC-induced cardioprotection was achieved when islet cell transplantation was implemented at 2 weeks after STZ injection; the outcome may not be as favorable if the duration of diabetes and hyperglycemia is prolonged. In addition, it remains to be determined whether similar restoration of the infarct-sparing effect of PostC can be evoked by clinically relevant management of hyperglycemia in the setting of type-2 diabetes.
Clinical implications
An emerging body of evidence indicates that relief of ischemia in a stuttered manner can successfully be translated to the cardiac catheterization laboratory and can significantly limit infarct size in patients undergoing primary angioplasty for the treatment of acute MI (7, 12, 27, 38, 40, 44, 47). As expected (25, 35), patients enrolled in these trials displayed the typical profile of attendant comorbidities, with the incidence of diabetes ranging from 10% to 25% (7, 38, 40, 47). Thus, the outcome of the current study raises the question: can results obtained in these relatively short-term murine models of diabetes (and, in particular, the db/db model of type-2 diabetes) be extrapolated to the clinical setting, or would the use of chronic models with attendant diabetic cardiomyopathy provide greater clinical relevance? Insight into this issue could, in theory, be gained by post-hoc subgroup analysis of infarct size in cohorts of diabetic patients. However, for the small PostC trials conducted to date [total enrollments of 30–115 patients (7, 38, 40, 47)], the n-values are too small to permit meaningful, exclusive analysis of diabetic subsets. Larger studies, with planned subgroup analyses of diabetic patients and adequate statistical power, will therefore be required to resolve this issue. In addition, if our results in Protocol 4 showing restoration of the infarct-sparing effect of PostC with appropriate therapeutic control of blood glucose are applicable to patient with type-2 diabetes, the more pertinent issue will be to discern whether stuttered reflow can initiate cardioprotection in diabetic patients who are well-managed using standard pharmacologic therapies. These important issues warrant future, prospective investigation.
Abbreviations Used
- EIA
enzyme immunometric assay
- ERK
extracellular signal-regulated kinase
- GSK
glycogen synthase kinase
- LV
left ventricle
- MI
myocardial infarction
- MKP
mitogen-activated protein kinase phosphatase
- PostC
postconditioning; postconditioned
- STZ
streptozotocin
Acknowledgment
This study was supported in part by NIH/NIDDK 5P30-DK32520 (University of Massachusetts—Diabetes and Endocrinology Pilot Research Program: to K.P.).
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Boengler K. Buechert A. Heinen Y. Roeskes C. Hilfiker-Kleiner D. Heusch G. Schulz R. Cardioprotection by ischemic postconditioning is lost in aged and STAT3-deficient mice. Circ Res. 2008;102:131–135. doi: 10.1161/CIRCRESAHA.107.164699. [DOI] [PubMed] [Google Scholar]
- 2.Bouhidel O. Pons S. Souktani R. Zini R. Berdeaux A. Ghaleh B. Myocardial ischemic postconditioning against ischemia-reperfusion is impaired in ob/ob mice. Am J Physiol Heart Circ Physiol. 2008;295:H1580–H1586. doi: 10.1152/ajpheart.00379.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bugger H. Abel ED. Rodent models of diabetic cardiomyopathy. Dis Model Mech. 2009;2:454–466. doi: 10.1242/dmm.001941. [DOI] [PubMed] [Google Scholar]
- 4.Camps M. Nichols A. Arkinstall S. Dual specificity phosphatases: a gene family for control of MAP kinase function. FASEB J. 2000;14:6–16. [PubMed] [Google Scholar]
- 5.Carley AN. Severson DL. What are the biochemical mechanisms responsible for enhanced fatty acid utilization by perfused hearts from type 2 diabetic db/db mice? Cardiovasc Drugs Ther. 2008;22:83–89. doi: 10.1007/s10557-008-6088-9. [DOI] [PubMed] [Google Scholar]
- 6.Darling CE. Jiang R. Maynard M. Whittaker P. Vinten-Johansen J. Przyklenk K. Postconditioning via stuttering reperfusion limits myocardial infarct size in rabbit hearts: role of ERK1/2. Am J Physiol Heart Circ Physiol. 2005;289:H1618–H1626. doi: 10.1152/ajpheart.00055.2005. [DOI] [PubMed] [Google Scholar]
- 7.Darling CE. Solari PB. Smith CS. Furman MI. Przyklenk K. “Postconditioning” the human heart: multiple balloon inflations during primary angioplasty may confer cardioprotection. Basic Res Cardiol. 2007;102:274–278. doi: 10.1007/s00395-007-0643-6. [DOI] [PubMed] [Google Scholar]
- 8.Di Filippo C. Marfella R. Cuzzocrea S. Piegari E. Petronella P. Giugliano D. Rossi F. D'Amico M. Hyperglycemia in streptozotocin-induced diabetic rat increases infarct size associated with low levels of myocardial HO-1 during ischemia/reperfusion. Diabetes. 2005;54:803–810. doi: 10.2337/diabetes.54.3.803. [DOI] [PubMed] [Google Scholar]
- 9.Dickinson RJ. Keyse SM. Diverse physiological functions for dual-specificity MAP kinase phosphatases. J Cell Sci. 2006;119:4607–4615. doi: 10.1242/jcs.03266. [DOI] [PubMed] [Google Scholar]
- 10.Ferdinandy P. Schulz R. Baxter GF. Interaction of cardiovascular risk factors with myocardial ischemia/reperfusion injury, preconditioning, and postconditioning. Pharmacol Rev. 2007;59:418–458. doi: 10.1124/pr.107.06002. [DOI] [PubMed] [Google Scholar]
- 11.Gambert S. Vergely C. Filomenko R. Moreau D. Bettaieb A. Opie LH. Rochette L. Adverse effects of free fatty acid associated with increased oxidative stress in postischemic isolated rat hearts. Mol Cell Biochem. 2006;283:147–152. doi: 10.1007/s11010-006-2518-9. [DOI] [PubMed] [Google Scholar]
- 12.Granfeldt A. Lefer DJ. Vinten-Johansen J. Protective ischaemia in patients: preconditioning and postconditioning. Cardiovasc Res. 2009;83:234–246. doi: 10.1093/cvr/cvp129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gross ER. Hsu AK. Gross GJ. Diabetes abolishes morphine-induced cardioprotection via multiple pathways upstream of glycogen synthase kinase-3{beta} Diabetes. 2007;56:127–136. doi: 10.2337/db06-0907. [DOI] [PubMed] [Google Scholar]
- 14.Hayashi K. Kojima R. Ito M. Strain differences in the diabetogenic activity of streptozotocin in mice. Biol Pharm Bull. 2006;29:1110–1119. doi: 10.1248/bpb.29.1110. [DOI] [PubMed] [Google Scholar]
- 15.Huhn R. Heinen A. Hollmann MW. Schlack W. Preckel B. Weber NC. Cyclosporine A administered during reperfusion fails to restore cardioprotection in prediabetic Zucker obese rats in vivo. Nutr Metab Cardiovasc Dis. 2009 doi: 10.1016/j.numecd.2009.06.010. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 16.Huhn R. Heinen A. Weber NC. Hollmann MW. Schlack W. Preckel B. Hyperglycaemia blocks sevoflurane-induced postconditioning in the rat heart in vivo: cardioprotection can be restored by blocking the mitochondrial permeability transition pore. Br J Anaesth. 2008;100:465–471. doi: 10.1093/bja/aen022. [DOI] [PubMed] [Google Scholar]
- 17.Huhn R. Heinen A. Weber NC. Kerindongo RP. Oei GT. Hollmann MW. Schlack W. Preckel B. Helium-induced early preconditioning and postconditioning are abolished in obese Zucker rats in vivo. J Pharmacol Exp Ther. 2009;329:600–607. doi: 10.1124/jpet.108.149971. [DOI] [PubMed] [Google Scholar]
- 18.Kersten JR. Toller WG. Gross ER. Pagel PS. Warltier DC. Diabetes abolishes ischemic preconditioning: role of glucose, insulin, and osmolality. Am J Physiol Heart Circ Physiol. 2000;278:H1218–H1224. doi: 10.1152/ajpheart.2000.278.4.H1218. [DOI] [PubMed] [Google Scholar]
- 19.Kersten JR. Toller WG. Tessmer JP. Pagel PS. Warltier DC. Hyperglycemia reduces coronary collateral blood flow through a nitric oxide-mediated mechanism. Am J Physiol Heart Circ Physiol. 2001;281:H2097–H2104. doi: 10.1152/ajpheart.2001.281.5.H2097. [DOI] [PubMed] [Google Scholar]
- 20.Kin H. Zatta AJ. Lofye MT. Amerson BS. Halkos ME. Kerendi F. Zhao Z-Q. Guyton RA. Headrick JP. Vinten-Johansen J. Postconditioning reduces infarct size via adenosine receptor activation by endogenous adenosine. Cardiovasc Res. 2005;67:124–133. doi: 10.1016/j.cardiores.2005.02.015. [DOI] [PubMed] [Google Scholar]
- 21.Kristiansen SB. Lofgren B. Stottrup NB. Khatir D. Nielsen-Kudsk JE. Nielsen TT. Botker HE. Flyvbjerg A. Ischaemic preconditioning does not protect the heart in obese and lean animal models of type 2 diabetes. Diabetologia. 2004;47:1716–1721. doi: 10.1007/s00125-004-1514-4. [DOI] [PubMed] [Google Scholar]
- 22.Lacerda L. Somers S. Opie LH. Lecour S. Ischaemic postconditioning protects against reperfusion injury via the SAFE pathway. Cardiovasc Res. 2009;84:201–208. doi: 10.1093/cvr/cvp274. [DOI] [PubMed] [Google Scholar]
- 23.Lecour S. Activation of the protective Survivor Activating Factor Enhancement (SAFE) pathway against reperfusion injury: does it go beyond the RISK pathway? J Mol Cell Cardiol. 2009;47:32–40. doi: 10.1016/j.yjmcc.2009.03.019. [DOI] [PubMed] [Google Scholar]
- 24.Liu Y. Thornton JD. Cohen MV. Downey JM. Schaffer SW. Streptozotocin-induced non-insulin-dependent diabetes protects the heart from infarction. Circulation. 1993;88:1273–1278. doi: 10.1161/01.cir.88.3.1273. [DOI] [PubMed] [Google Scholar]
- 25.Lloyd-Jones D. Adams RJ. Brown TM. Carnethon M. Dai S. De Simone G. Ferguson TB. Ford E. Furie K. Gillespie C. Go A. Greenlund K. Haase N. Hailpern S. Ho PM. Howard V. Kissela B. Kittner S. Lackland D. Lisabeth L. Marelli A. McDermott MM. Meigs J. Mozaffarian D. Mussolino M. Nichol G. Roger VL. Rosamond W. Sacco R. Sorlie P. Stafford R. Thom T. Wasserthiel-Smoller S. Wong ND. Wylie-Rosett J. Heart disease and stroke statistics—2010 update: a report from the american heart association. Circulation. 2010;121:e46–e215. doi: 10.1161/CIRCULATIONAHA.109.192667. [DOI] [PubMed] [Google Scholar]
- 26.Marfella R. Di Filippo C. Esposito K. Nappo F. Piegari E. Cuzzocrea S. Berrino L. Rossi F. Giugliano D. D'Amico M. Absence of inducible nitric oxide synthase reduces myocardial damage during ischemia reperfusion in streptozotocin-induced hyperglycemic mice. Diabetes. 2004;53:454–462. doi: 10.2337/diabetes.53.2.454. [DOI] [PubMed] [Google Scholar]
- 27.Mewton N. Ivanes F. Cour M. Ovize M. Postconditioning: from experimental proof to clinical concept. Dis Model Mech. 2010;3:39–44. doi: 10.1242/dmm.004309. [DOI] [PubMed] [Google Scholar]
- 28.Olson AK. Protheroe KN. Segar JL. Scholz TD. Mitogen-activated protein kinase activation and regulation in the pressure-loaded fetal ovine heart. Am J Physiol. 2006;290:H1587–H1595. doi: 10.1152/ajpheart.00984.2005. [DOI] [PubMed] [Google Scholar]
- 29.Penna C. Tullio F. Merlino A. Moro F. Raimondo S. Rastaldo R. Perrelli MG. Mancardi D. Pagliaro P. Postconditioning cardioprotection against infarct size and post-ischemic systolic dysfunction is influenced by gender. Basic Res Cardiol. 2009;104:390–402. doi: 10.1007/s00395-008-0762-8. [DOI] [PubMed] [Google Scholar]
- 30.Przyklenk K. Maynard M. Darling CE. Whittaker P. Aging mouse hearts are refractory to infarct size reduction with post-conditioning. J Am Coll Cardiol. 2008;51:1393–1398. doi: 10.1016/j.jacc.2007.11.070. [DOI] [PubMed] [Google Scholar]
- 31.Przyklenk K. Maynard M. Whittaker P. First molecular evidence that inositol trisphosphate signaling contributes to infarct size reduction with preconditioning. Am J Physiol. 2006;291:H2008–H2012. doi: 10.1152/ajpheart.00313.2006. [DOI] [PubMed] [Google Scholar]
- 32.Raphael J. Gozal Y. Navot N. Zuo Z. Hyperglycemia inhibits anesthetic-induced postconditioning in the rabbit heart via modulation of phosphatidylinositol-3-kinase/Akt and endothelial nitric oxide synthase signaling. J Cardiovasc Pharmacol. 2010;55:348–357. doi: 10.1097/FJC.0b013e3181d26583. [DOI] [PubMed] [Google Scholar]
- 33.Ravingerova T. Neckar J. Kolar F. Ischemic tolerance of rat hearts in acute and chronic phases of experimental diabetes. Mol Cell Biochem. 2003;249:167–174. doi: 10.1023/a:1024751109196. [DOI] [PubMed] [Google Scholar]
- 34.Rodriguez-Sinovas A. Cabestrero A. Garcia del Blanco B. Inserte J. Garcia A. Garcia-Dorado D. Intracoronary acid infusion as an alternative to ischemic postconditioning in pigs. Basic Res Cardiol. 2009;104:761–771. doi: 10.1007/s00395-009-0032-4. [DOI] [PubMed] [Google Scholar]
- 35.Ryan JG. Cost and policy implications from the increasing prevalence of obesity and diabetes mellitus. Gend Med. 2009;6(Suppl 1):86–108. doi: 10.1016/j.genm.2009.01.002. [DOI] [PubMed] [Google Scholar]
- 36.Seung E. Iwakoshi N. Woda BA. Markees TG. Mordes JP. Rossini AA. Greiner DL. Allogeneic hematopoietic chimerism in mice treated with sublethal myeloablation and anti-CD154 antibody: absence of graft-versus-host disease, induction of skin allograft tolerance, and prevention of recurrent autoimmunity in islet-allografted NOD/Lt mice. Blood. 2000;95:2175–2182. [PubMed] [Google Scholar]
- 37.Skyschally A. van Caster P. Boengler K. Gres P. Musiolik J. Schilawa D. Schulz R. Heusch G. Ischemic postconditioning in pigs: no causal role for RISK activation. Circ Res. 2009;104:15–18. doi: 10.1161/CIRCRESAHA.108.186429. [DOI] [PubMed] [Google Scholar]
- 38.Staat P. Rioufol G. Piot C. Cottin Y. Cung TT. L'Huillier I. Aupetit JF. Bonnefoy E. Finet G. Andre-Fouet X. Ovize M. Postconditioning the human heart. Circulation. 2005;112:2143–2148. doi: 10.1161/CIRCULATIONAHA.105.558122. [DOI] [PubMed] [Google Scholar]
- 39.Tamura S. Hanada M. Ohnishi M. Katsura K. Sasaki M. Kobayashi T. Regulation of stress-activated protein kinase signaling pathways by protein phosphatases. Eur J Biochem. 2002;269:1060–1066. doi: 10.1046/j.0014-2956.2002.02754.x. [DOI] [PubMed] [Google Scholar]
- 40.Thibault H. Piot C. Staat P. Bontemps L. Sportouch C. Rioufol G. Cung TT. Bonnefoy E. Angoulvant D. Aupetit JF. Finet G. Andre-Fouet X. Macia JC. Raczka F. Rossi R. Itti R. Kirkorian G. Derumeaux G. Ovize M. Long-term benefit of postconditioning. Circulation. 2008;117:1037–1044. doi: 10.1161/CIRCULATIONAHA.107.729780. [DOI] [PubMed] [Google Scholar]
- 41.Tosaki A. Engelman DT. Engelman RM. Das DK. The evolution of diabetic response to ischemia/reperfusion and preconditioning in isolated working rat hearts. Cardiovasc Res. 1996;31:526–536. [PubMed] [Google Scholar]
- 42.Tsang A. Hausenloy DJ. Mocanu MM. Carr RD. Yellon DM. Preconditioning the diabetic heart: the importance of Akt phosphorylation. Diabetes. 2005;54:2360–2364. doi: 10.2337/diabetes.54.8.2360. [DOI] [PubMed] [Google Scholar]
- 43.Tsang A. Hausenloy DJ. Mocanu MM. Yellon DM. Postconditioning: a form of “modified reperfusion” protects the myocardium by activating the phosphatidylinositol 3-kinase-Akt pathway. Circ Res. 2004;95:230–232. doi: 10.1161/01.RES.0000138303.76488.fe. [DOI] [PubMed] [Google Scholar]
- 44.Vinten-Johansen J. Granfeldt A. Mykytenko J. Undyala VV. Dong Y. Przyklenk K. The multidimensional physiological responses to postconditioning. Antioxid Redox Signal. 2011;14:791–810. doi: 10.1089/ars.2010.3396. [DOI] [PubMed] [Google Scholar]
- 45.Vinten-Johansen J. Zhao ZQ. Zatta AJ. Kin H. Halkos ME. Kerendi F. Postconditioning—a new link in nature's armor against myocardial ischemia-reperfusion injury. Basic Res Cardiol. 2005;100:295–310. doi: 10.1007/s00395-005-0523-x. [DOI] [PubMed] [Google Scholar]
- 46.Wagner C. Kloeting I. Strasser RH. Weinbrenner C. Cardioprotection by postconditioning is lost in WOKW rats with metabolic syndrome: role of glycogen synthase kinase 3beta. J Cardiovasc Pharmacol. 2008;52:430–437. doi: 10.1097/FJC.0b013e31818c12a7. [DOI] [PubMed] [Google Scholar]
- 47.Yang XC. Liu Y. Wang LF. Cui L. Wang T. Ge YG. Wang HS. Li WM. Xu L. Ni ZH. Liu SH. Zhang L. Jia HM. Vinten-Johansen J. Zhao ZQ. Reduction in myocardial infarct size by postconditioning in patients after percutaneous coronary intervention. J Invasive Cardiol. 2007;19:424–430. [PubMed] [Google Scholar]
- 48.Yang XM. Philipp S. Downey JM. Cohen MV. Postconditioning's protection is not dependent on circulating blood factors or cells but involves adenosine receptors and requires PI3-kinase and guanylyl cyclase activation. Basic Res Cardiol. 2005;100:57–63. doi: 10.1007/s00395-004-0498-4. [DOI] [PubMed] [Google Scholar]
- 49.Yang XM. Proctor JB. Cui L. Krieg T. Downey JM. Cohen MV. Multiple, brief coronary occlusions during early reperfusion protect rabbit hearts by targeting cell signaling pathways. J Am Coll Cardiol. 2004;44:1103–1110. doi: 10.1016/j.jacc.2004.05.060. [DOI] [PubMed] [Google Scholar]
- 50.Zhao ZQ. Corvera JS. Halkos ME. Kerendi F. Wang NP. Guyton RA. Vinten-Johansen J. Inhibition of myocardial injury by ischemic postconditioning during reperfusion: comparison with ischemic preconditioning. Am J Physiol. 2003;285:H579–H588. doi: 10.1152/ajpheart.01064.2002. [DOI] [PubMed] [Google Scholar]