Abstract
Objective
To determine if older women with both heart disease and diabetes experience worse physical and psychosocial functioning and higher symptom burden over an 18-month period compared with those with heart disease alone.
Methods
Data from older women with heart disease (≥60 years, n = 1008, 18% with diabetes) were used to assess the impact of diabetes on physical functioning (Sickness Impact Profile [SIP]-Physical and Six-Minute Walk test [6MWT]), psychosocial functioning (SIP-Psychosocial and depressive symptoms), and physical symptom burden (cardiac and general) at baseline and 4, 12, and 18 months later. Generalized estimating equation models compared trends in outcomes over time between groups with and without diabetes.
Results
Across all four time points, women with heart disease and diabetes had greater functional impairment, as indicated by higher SIP scores, than those without diabetes (43%–71% higher SIP-Physical scores and 32%–65% higher SIP-Pyschosocial scores; all p ≤ 0.002). 6MWT distance was 17%–30% less in the diabetes group across time points (all p ≤ 0.002). Depressive symptoms were 27%–39% higher in the diabetes group (all p < 0.03) except at month 4. Women with diabetes scored 15%–29% higher on a physical symptom index across time points (all p < 0.05) than those without diabetes; no significant differences were observed in cardiac symptoms until month 18 (diabetes group 29% higher, p = 0.02). Subgroups with and without diabetes in this sample experienced significantly different trends over time in SIP-Physical scores (p = 0.02) and 6MWT distance (p = 0.05), such that the disadvantage of the diabetes group at baseline was greater 18 months later.
Conclusions
Women with comorbid diabetes and heart disease are vulnerable to poor health-related quality of life, particularly in terms of physical functioning and symptoms, and require special efforts from clinical care providers to ameliorate a potential downward trend in these outcomes over time.
Introduction
The well-documented increase in the prevalence of type 2 diabetes is concurrent with a rise in the number of people who have cardiovascular disease (CVD), that is, heart disease or cerebrovascular disease, along with diabetes.1 In 2007, nearly one third of people with diabetes > age 35 reported a cardiovascular condition.2 Even after accounting for the metabolic syndrome factors that often accompany diabetes—dyslipidemia, hypertension, and central obesity—diabetes remains an independent risk factor for CVD.1–3
Although the presence of diabetes more than doubles the risk of developing CVD for both women and men, the excess risk is greater for women,4 and the relative risk of diabetes-associated mortality from coronary heart disease (CHD), the most common type of CVD, is 50% higher in women than in men.5 Women with diabetes and CHD or other heart-related disease may also be particularly vulnerable to impaired health-related quality of life (HRQL) for two reasons. First, decrements in physical and psychosocial functioning associated with diabetes and heart disease separately are often more pronounced in women than in men. A number of studies have shown that women with diabetes or heart disease have worse physical, mental, and social well-being than their male counterparts with these disorders.6–10 Second, accumulating evidence supports the notion of an interactive, negative influence of heart disease and diabetes on measures of HRQL and various domains of functioning.11–14
Most prior work on the effects of comorbid diabetes and heart disease on HRQL is cross-sectional in nature and has not assessed change over time in HRQL-related outcomes among people with these two conditions. In addition, the physical symptom burden, an important determinant of HRQL, of this group has not been examined. Finally, although women with diabetes and heart disease separately tend to have worse HRQL than men, only one identified study reported gender-specific data on HRQL among individuals with both disorders; in this study, women with CHD and diabetes were more likely than their male counterparts to have at least 2 weeks of physically unhealthy days/month.12
Given the growing number of women carrying the double burden of diabetes and heart disease, researchers and clinicians could benefit from more specific knowledge about how this group's functioning and symptom burden change over time. To this end, the current study makes use of longitudinal data from a large sample of women aged ≥60 with physician-diagnosed heart disease, almost one fifth of whom also report diabetes. We tested the hypothesis that women with comorbid diabetes and heart disease have worse physical and psychosocial functioning and greater physical symptom burden at each of four study time points than do their counterparts without diabetes. Additionally, given that the presence of diabetes is associated with greater mortality risk among female CHD patients,15 which implies a pattern of more steeply declining health in this group, we hypothesized that the women in our sample with diabetes would experience a greater decline in HRQL outcomes over an 18-month period than women without diabetes. To our knowledge, this is the first study to investigate the long-term trajectory of health status among women contending with both heart disease and diabetes.
Materials and Methods
The study sample is from a randomized controlled trial (RCT) of a heart disease management intervention tailored for older women. This intervention, Women take PRIDE, instructed participants in the use of behavioral self-regulation techniques as a means of making healthy lifestyle changes and reaching personal disease-management goals. The program included six units that were offered in either a group or a self-directed (at-home) format and covered such topics as medications, diet, physical activity, and stress management. All program content was tailored to the unique context—roles, responsibilities, and settings—in which women manage their heart disease. Prior publications provide additional details on study design and outcomes of this trial.16–18 All participants were community-dwelling women ≥60 years of age treated by daily heart medication for a cardiovascular condition (i.e., arrhythmia, angina, myocardial infarction [MI], congestive heart failure [CHF], or valvular disease). Names of potential study participants were generated from patient rosters at clinics in three urban areas in Michigan. A total of 1128 women were enrolled at baseline. Study procedures were approved by the University of Michigan Institutional Review Board, and informed consent was provided by all participants. Survey data were collected via telephone interviews at baseline and at 4, 12, and 18 months later. Research nurses followed a detailed protocol to obtain clinical measurements at participating hospitals at each time point.
Measures
Diabetes status
Respondents were classified as having diabetes (1) if at baseline they indicated that another health condition besides heart disease was their primary health problem and named diabetes as this condition or (2) if they reported having diabetes when asked about other important health conditions besides heart disease.
Physical functioning
The Sickness Impact Profile (SIP)19 is a 136-item functional health status measure and is one of the most commonly used indicators of quality of life in heart disease patients.20 The SIP-Physical dimension score includes three subscales: ambulation, mobility, and body care and movement. Scores are derived by adding the values for the items using predetermined weights for each item within that category and dividing the sum by the maximum possible dysfunction score for that dimension or category. This yields a mean percentage score for each individual, with possible scores ranging from 0 to 100 and higher scores representing greater dysfunction or impairment because of health.
The Six-Minute Walk test (6MWT)21 (see reference 22 for a recent comprehensive review of methodological and clinical issues pertaining to this measure) is an objective indicator of physical functioning often used with chronically ill patients, including those with heart disease.23 Using a premeasured distance, participants were instructed to walk from end to end at their own pace and with stops if needed, covering as much distance as they could during the allotted 6 minutes. The distance was recorded in feet by a research nurse. This measure is highly correlated with gold standard measures of functional status.24
Psychosocial functioning
An 8-item version of the Center for Epidemiologic Studies-Depression scale (CES-D)25 was used to measure depressive symptomatology. Somatic and cognitive aspects of depression are represented by 4 items each. Possible scores range from 0 to 24, with a higher score representing more depressive symptoms. The SIP-Psychosocial dimension is made up of the following four subscales: social interaction, alertness behavior, emotional behavior, and communication. Scores are derived as per SIP-Physical dimension score.
Physical symptom burden
Physical symptom burden was indicated by the cardiac (chest pain, shortness of breath, rapid or irregular heartbeat, waking with chest pain, and waking with shortness of breath) and the general physical (nonspecific) (fatigue, dizziness, pain other than chest pain, and numbness or cramping in legs) subscales of the Symptom and Health Problem Profile.26 Respondents were asked about the frequency and bother of each symptom in the last week. Responses to the item: How much would you say the symptom bothers you? (1 = not at all to 5 = a lot; 0 = not present) for each symptom were summed within each subscale.
Covariates
Variables used for sample description or as control variables in multivariate analyses included age (continuous); race (white = 1, otherwise = 0); income (0 ≤ 20,000/year, 1 > 20,000/year); number of nondiabetes comorbidities (a continuous variable derived from the following open-ended question at baseline: Has a doctor told you that you have any other important health problems or conditions? responses were coded and counted); and number of heart-related diagnoses (angina, MI, arrhythmia, hypertension, CHF, valvular disorder). Although the larger study for which data were collected was a randomized trial of a self-management program, the current study did not involve assessing the effect of this intervention. As there were no differences in the proportion of women with diabetes in treatment vs. control conditions, we combined the groups and controlled for intervention status in multivariate analyses. Because of an innovative study design that included two program versions tested within two study arms—an RCT arm and a choice arm in which women could choose their program format—a total of 944 women were enrolled in some version of the intervention before the first follow-up point; only 184 were assigned to a no-treatment control condition.
Statistical analysis
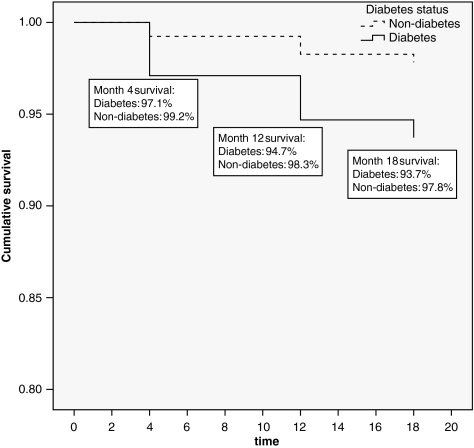
Analyses were conducted using SAS 9.2 (Cary, NC). Initially, descriptive statistics of the demographic and health attributes of the sample at baseline were computed. To examine differences in mortality between women with and without diabetes, Kaplan-Meier estimates were used to derive survival curves for each group over the 18-month follow-up period. Cox-regression models controlling for age, race, income, treatment group, number of heart diagnoses, number of comorbidities, and intervention group status were used to calculate an adjusted hazard ratio (HR).
Next, repeated-measures analysis was used to test for mean differences by diabetes status at each time point (baseline and 4, 12, and 18 months) for the six functional health status and symptom outcomes, adjusting for other factors that were expected to influence outcomes: age, race, income, treatment group, number of heart diagnoses, and number of other comorbidities. The SIP and symptom scales were log-transformed to normalize their distribution. Generalized estimating equation (GEE) techniques were used to account for within-subject correlation. In addition, GEE models were used to compare linear trends over 18 months in functioning and symptoms between women with and without diabetes. These models included the following terms: an indicator of diabetes status, the main effect of time, an interaction between time and diabetes status, and covariates as listed. Of primary interest in this analysis was the interaction of time by disease status, that is, if trajectories of outcomes over time differed between women with and without diabetes. For all analyses involving repeated measures, the subsample of respondents with baseline data and at least one other time point was used (n = 1008).
Results
Of this sample of women with diagnosed heart disease, 18% (n = 207) also reported diabetes. Table 1 reports demographic and health characteristics for the total sample and by diabetes status. Women with diabetes compared with those without were significantly younger (mean 71 vs. 73 years) and less likely to be white (76% vs. 84%; nearly all nonwhite respondents were African American). Overall, women with diabetes had a greater number of cardiac-related diagnoses (2.9 vs. 2.5) but no difference in number of nondiabetes comorbidities. Between-group comparisons of unadjusted study outcomes at baseline revealed that women with diabetes had significantly higher SIP-Physical scores than those without diabetes (13.3 vs. 8.3), indicating greater impairment due to illness; a shorter 6MWT distance (603 vs. 778 feet); higher SIP-Psychosocial scores (10.6 vs. 6.7); a greater number of depressive symptoms (4.6 vs. 3.3); and a greater general physical symptom burden (6.5 vs. 5.5). The difference in cardiac symptom burden (3.9 vs. 3.3) did not reach significance.
Table 1.
Sociodemographic and Health Characteristics of Study Sample at Baseline (n = 1128)
| Variable |
Total sample (n = 1128) Mean (SD) or % (n) |
Diabetes present (n = 207) Mean (SD) or % (n) |
No diabetes present (n = 921) Mean (SD) or % (n) |
|---|---|---|---|
| Age in years (range 60–90) | 72.5 (6.3) | 70.8 (6.1) | 72.8 (6.3)*** |
| Education | |||
| Less than high school | 17.1 (193) | 19.8 (41) | 16.5 (152) |
| High school | 40.0 (451) | 41.1 (85) | 39.7 (366) |
| More than high school | 42.9 (484) | 39.1 (81) | 43.8 (403) |
| Race (% white) | 82.7 (933) | 76.3 (158) | 84.1 (775)*** |
| Number of nondiabetes comorbidities | 1.2 (1.1) | 1.1 (1.0) | 1.2 (1.0) |
| Heart-related diagnosis | |||
| Angina | 36.5 (412) | 45.9 (95) | 34.4 (317)** |
| Myocardial infarction | 40.3 (455) | 49.8 (103) | 38.2 (352)** |
| Arrhythmia | 54.7 (617) | 49.3 (102) | 55.9 (515) |
| CHF | 23.7 (267) | 37.7 (78) | 20.5 (189)*** |
| Valvular disorder | 27.4 (309) | 21.3 (44) | 28.8 (265)* |
| Hypertension | 72.6 (819) | 81.2 (168) | 70.7 (651)** |
| Number of heart-related diagnoses | 2.6 (1.3) | 2.9 (1.4) | 2.5 (1.2)*** |
| Unadjusted baseline values of outcomes | |||
| SIP-physical (observed range 0–66) | 9.3 (10.1) | 13.3 (12.2) | 8.3 (9.4)*** |
| Six-Minute Walk distance (ft) (12-2070) | 748 (460) | 603 (401) | 778 (466)*** |
| SIP-psychosocial (0–68) | 7.4 (10.3) | 10.6 (12.2) | 6.7 (9.7)*** |
| Depressive symptoms (0–24) | 3.5 (4.3) | 4.6 (5.0) | 3.3 (4.1)*** |
| Cardiac symptom burden (0–25) | 3.4 (4.3) | 3.9 (4.5) | 3.3 (4.2) |
| General physical symptom burden (0–20) | 5.7 (4.9) | 6.5 (5.0) | 5.5 (4.8)** |
p < 0.001, **p < 0.01, and *p < 0.05 for difference between diabetes and nondiabetes groups based on independent-samples t test or chi-square test.
CHF, congestive heart failure; SD, standard deviation; SIP, Sickness Impact Profile.
During the study period, 33 women died and 147 withdrew. A greater proportion of women with diabetes died compared to those without diabetes (6.3 vs. 2.2%, p = 0.004). Figure 1 shows the Kaplan-Meier survival curves for the diabetes and nondiabetes groups. Cox-regression analysis shows that women with diabetes were 2.3 times more likely than women without diabetes to die during the 18-month interval. Of the sociodemographic and health covariates initially included in these models, only count of heart-related diagnoses was significant and included in the final model. The log-rank test was used to test for differences between diabetes and nondiabetes groups (p = 0.001). The difference in withdrawals by diabetes status was not significant. In terms of study outcomes, women who died had worse baseline SIP-Physical and SIP-Psychological (p = 0.01) scores, higher depression scores (p = 0.003), and greater cardiac symptom burden (p = 0.04) compared with completers. Women who withdrew had worse SIP-Physical (p = 0.04), SIP-Psychological (p = 0.001), and depression (p = 0.04) scores than those who remained in the study.
FIG. 1.
Kaplan-Meier survival curves by diabetes status at 4, 12, and 18 months. The Cox-regression model was used to derive the hazard ratio, adjusting for count of heart-related diagnoses. The log-rank test was used to test for differences between diabetes and nondiabetes groups (p = 0.001).
Table 2 shows the results of the repeated-measures analysis comparing the means of women with and without diabetes on outcomes at four time points, adjusted for health and demographic characteristics. Women with diabetes reported significantly worse physical functioning (i.e., shorter 6MWT distances and higher SIP-Physical scores) than their counterparts without diabetes at each time point. Among women with diabetes, SIP-Physical scores were 51% higher at baseline, 43% higher at month 4, 68% higher at month 12, and 71% higher at month 18. (Adjusted means, but not percentage differences, are shown in Table 2.) The 6MWT distance was 23% less among the diabetes group at baseline, 17% less at month 4, 28% less at month 12, and 30% less at month 18. For psychosocial functioning, women with diabetes also reported significantly greater impairment at each time point as measured by the SIP-Psychosocial; these scores were 40% higher at baseline, 32% higher at month 4, 65% higher at month 12, and 63% higher at month 18. Depression scale scores were higher in the diabetes group at baseline (27% higher), month 12 (30% higher), and month 18 (39% higher). At every time point, women with diabetes had significantly greater general physical symptom burden: 15% higher at baseline, 19% higher at month 4 and month 12, and 27% higher at month 18. Women with diabetes had significantly greater cardiac symptom burden (29% greater) than those without at month 18 only.
Table 2.
Adjusted Means of Functioning Indicators at Baseline and 4, 12, and 18 Months: Comparison Between Women Heart Disease Patients With and Without Diabetes (n = 1008)
| |
Cross-sectional: adjusted means (SE) |
|||
|---|---|---|---|---|
| Baseline | Month 4 | Month 12 | Month 18 | |
| SIP physical (0–100) | ||||
| Diabetes | 12.5 (0.89) | 11.0 (0.84) | 12.9 (0.95) | 13.2 (1.0) |
| No diabetes | 8.3 (0.31) | 7.7 (0.33) | 7.7 (0.32) | 7.7 (0.33) |
| p value | <0.001 | <0.001 | <0.001 | <0.001 |
| Six-Minute Walk (feet) | ||||
| Diabetes | 604.2 (35.3) | 672.1 (41.4) | 592.0 (40.4) | 567.6 (41.2) |
| No diabetes | 787.1 (16.8) | 812.7 (19.1) | 825.0 (18.4) | 809.9 (18.5) |
| p value | <0.001 | 0.002 | <0.001 | <0.001 |
| SIP psychosocial (0–100) | ||||
| Diabetes | 9.1 (0.86) | 7.5 (0.82) | 9.4 (0.93) | 9.6 (1.0) |
| No diabetes | 6.5 (0.32) | 5.7 (0.36) | 5.7 (0.31) | 5.9 (0.33) |
| p value | <0.001 | 0.002 | <0.001 | <0.001 |
| Depression (0–24) | ||||
| Diabetes | 4.2 (0.35) | 3.6 (0.35) | 4.3 (0.40) | 4.6 (0.42) |
| No diabetes | 3.3 (0.14) | 3.2 (0.16) | 3.3 (0.17) | 3.3 (0.17) |
| p value | 0.02 | 0.30 | 0.03 | 0.004 |
| Cardiac symptoms (0–25) | ||||
| Diabetes | 3.7 (0.34) | 2.8 (0.39) | 3.3 (0.40) | 3.6 (0.37) |
| No diabetes | 3.3 (0.14) | 2.9 (0.16) | 2.8 (0.15) | 2.8 (0.15) |
| p value | 0.27 | 0.55 | 0.37 | 0.02 |
| General physical symptoms (0–20) | ||||
| Diabetes | 6.3 (0.38) | 6.2 (0.41) | 6.2 (0.41) | 6.5 (0.44) |
| No diabetes | 5.5 (0.16) | 5.2 (0.18) | 5.2 (0.18) | 5.1 (0.17) |
| p value | 0.05 | 0.003 | 0.003 | 0.013 |
Adjusted for count of heart diagnoses, age, baseline comorbidities, income, treatment group status, and race. Includes respondents with data at baseline and at least one other time point.
All outcomes, except depression and 6MWT, were log-transformed to normalize their distribution. Adjusted means are in original scale for clearer interpretation, but p values are based on log-transformed variables.
SE, standard error; 6MWT, Six-Minute Walk test.
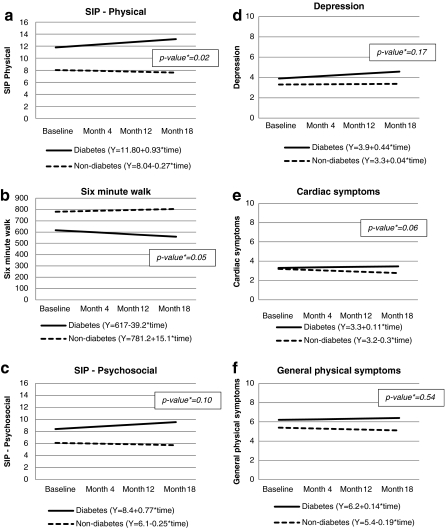
The GEE longitudinal analysis assessed the significance of differences in trends over time between women with diabetes and those without. Figure 2 depicts the trajectories of these two groups for each outcome of interest, adjusting for age, race, income, baseline count of heart-related diagnoses, baseline count of comorbidities, and intervention group status. These graphs also display the equations for the regression lines for each group, where the intercept represents the main effect of diabetes on the outcome at baseline, and the slope represents the trend over time. The p values displayed within the graph for each outcome indicate the significance of the difference in trends over time for the diabetes and nondiabetes groups (i.e., interaction between time and diabetes status). The coefficient indicating the presence of a linear trend in outcomes among the subgroup with diabetes was not significant for any outcome. Trend differences (i.e., interactions between time and group) were significant for SIP-Physical (p = 0.02) (Fig. 2a) and 6MWT (p = 0.05) (Fig. 2b). For these outcomes, women with heart disease and diabetes began the study period with worse scores than their counterparts without diabetes, and the magnitude of this difference increased over the study period. In terms of covariates, in all models, number of heart diagnoses, number of comorbidities, and income significantly (p < 0.01) predicted outcomes, as did age for all models except those with depression and cardiac symptoms as outcomes. Race was a significant predictor of 6MWT score. Treatment group status was not a predictor of any outcome. The effect of diabetes at baseline was significant (p < 0.01) in all models, except for the depression and cardiac symptom models (coefficients of covariates not shown in Fig. 2).
FIG. 2.
Longitudinal effect of diabetes over 18 months among women with heart disease and difference in trends by diabetes status on functioning and symptom outcomes (n = 1008). All models control for age, race, income, baseline count of heart-related diagnoses, baseline count of comorbidities, and intervention group status. p value is for the difference in trend between the diabetes and nondiabetes groups. SIP, Sickness Impact Profile.
Discussion
In our sample of older women with diagnosed heart disease, 18% also reported having diabetes. This may underestimate the actual diabetes prevalence in our sample; a study of over 30,000 women (mean age 66 years) with CHD enrolled in 14 international trials showed that 23% had diabetes.27 As expected, given the excess diabetes burden among nonwhites, women of color were more likely than white women to report diabetes. Women with diabetes were 2.3 times more likely to die during the 18-month study period. In addition to higher mortality rates, using both objective (6MWT) and self-report (SIP-Physical) measures, sample women with the combination of diabetes and heart disease demonstrated poorer physical functioning than those without diabetes. Past research has also found an adverse effect of diabetes on various indicators of self-reported physical functioning in older samples, including mobility, activities of daily living (ADLs), and instrumental activities of daily living (IADLs).28–31 Our study reinforces these findings by adding an objective measure of physical functioning. Like previous studies, our data suggest that cardiovascular and unrelated comorbidities, such as arthritis, cannot fully account for the negative effect of diabetes on functioning. Other factors, not measured in the current study, are likely to link diabetes to functional impairment, for example, peripheral neuropathy, visual impairment, and cognitive decline.28,30
We found evidence of substantial physical impairment among the group of women with both heart disease and diabetes. The unadjusted mean 6MWT distance among women heart disease patients with diabetes at baseline (603 feet) (data not shown) compares unfavorably with that of similar-age samples with mobility limitations (764 feet) and history of stroke (748 feet).32 In terms of the SIP-Physical score, women with diabetes at baseline had an unadjusted mean score of 13.3 (data not shown), indicating moderate dysfunction, whereas the mean of 8.4 for women without diabetes suggests mild dysfunction.33 In contrast, a study of older patients with diabetes in an outpatient clinic reported a mean SIP-Physical score of only 5.6.14
Our study also provides evidence that the gap in physical functioning by diabetes status in this population of older women with heart disease diverges further over time. Although a significant linear trend toward decreased functioning was not evident among women with diabetes, this group tended to walk shorter distances over the 18-month period, whereas women without diabetes tended to walk longer distances, such that the between-group difference in trends was statistically significant. This pattern was mirrored in the SIP-Physical score, a self-report scale indicating how women experience health limitations in physical functioning in daily life (e.g., walking less or more slowly, staying closer to home).
The presence of diabetes was associated with worse psychosocial functioning at multiple points, as measured by the SIP-Psychosocial and an abridged version of the CES-D depression scale, although trends over time in psychosocial functioning were not significantly different between the two groups. Other studies have found stronger effects of diabetes on physical domains compared to psychosocial domains.11,14 Because women with heart disease are already at high risk for depression and psychosocial impairment, any additional contribution of diabetes to poor psychosocial health is of concern. Depression is predictive of poor health-related outcomes, including mortality, in both diabetes34 and heart disease8 patients and may also contribute to poor physical functioning, for example, through reduced motivation to be physically active.
Throughout the study period, women with diabetes were more bothered by general physical symptoms (e.g., dizziness, pain other than chest pain) than women without diabetes. Although the difference in trends by diabetes status was not significant for either symptom measure, by month 18, the general physical symptom burden of women without diabetes had slightly decreased since baseline, whereas that of women with diabetes had slightly increased. These findings suggest that general physical symptoms may play a role in the physical functioning deficits observed among women with both heart disease and diabetes, for example, by discouraging physical activity. It is also notable that physical symptoms have been shown to predict future hospitalization and mortality, independent of comorbidities, in an older population.35 Although differences by diabetes status in cardiac-specific symptoms were smaller, women with diabetes nonetheless had greater cardiac symptom burden by long-term follow-up.
Our findings have important implications for clinicians. Older women with both diabetes and heart disease, a comorbidity for which women of color are at especially high risk, are vulnerable to poor functioning and HRQL. Such patients would benefit from special efforts from clinical providers regarding treatment, counseling, and education in order to deter or ameliorate a potential downward trend in functioning and symptom burden over time. Ideally, such intervention would take place as early in the disease course as possible, with additional ongoing resources and support provided as needed.
Available evidence suggests that regular physical activity can prevent decline in physical functioning among older adults.36 Therefore, this behavior should be strongly encouraged among women with dual heart disease/diabetes morbidity. The importance of physical activity to optimal management of both diabetes and heart disease is well recognized, and encouraging exercise is an integral part of both traditional diabetes self-management interventions and cardiac rehabilitation programs. Participation rates in these formal programs are low,37,38 however, and gender-based and race-based disparities have been identified in cardiac rehabilitation referrals and participation, such that women, and especially women of color, are least likely to take part.39 Because women of color are disproportionately affected by diabetes, which increases the risk of poor functioning and other adverse health outcomes, eliminating race-based disparities in cardiac rehabilitation rates is one strategy for improving the functioning of this group. Similarly, older women with diabetes and heart disease should receive particular encouragement and motivation to take part in formal diabetes education. These programs should maximize participation by vulnerable groups; for example, they should fit criteria for being Medicare reimbursable40 and offered at settings, for example, community-based settings, that are acceptable and appropriate for the intended target audience. In order to maximize participation and retention, these programs must be responsive to the needs of older women and women of color. The literature offers several examples of tailored diabetes management interventions for African American and older women that have been successful in increasing physical activity.41,42
Our results suggest the possible value of routine clinical assessment of functioning as well as physical activity level in older women who have diabetes/heart disease morbidity. Encouragement and support for engaging in appropriate levels of activity should be provided on an ongoing basis. Our data showed greater physical symptomatology among the subgroup of women with diabetes. These symptoms may present barriers to exercise, and patients should receive support in their management and interpretation. To date, no studies have been conducted that examine unique barriers to physical activity among patients with heart disease/diabetes comorbidity, nor have any interventions been tailored to address identified barriers for this group. This represents a potentially fruitful area for additional research and program development.
Special assistance to help women with comorbid diabetes/heart disease manage negative psychological states may also help alleviate their disease burden. Routine screening for depression in this population and initiation of appropriate treatment are critical. Among both diabetes and heart disease patients, interventions have been developed to identify and address depression.43,44 Notably, a new study of a nurse-managed intervention that addresses depression along with medical disease control for patients with diabetes or CHD or both with comorbid depression is currently underway.45
There are a number of limitations to this study. The first concerns the nature of our data about diabetes status. Participants reported having diabetes in an open-ended question about the presence of other important health conditions besides heart disease. It is likely, therefore, that some of the women in our nondiabetes group actually had diabetes, a misclassification that would bias between-group comparisons to the null, resulting in an underestimate of the differences between women with and without diabetes. Second, we did not collect additional data about the diabetes diagnosis, such as duration, complications, or treatment. Such data may have been useful in further characterizing the sample, examining differential outcomes in diabetes subgroups, or explaining functioning deficits among those with diabetes. Third, we were not able to directly test an interactive effect of diabetes and heart disease on our HRQL outcomes, as all respondents had heart disease at baseline. Future studies should attempt to quantify the extent to which functioning and symptoms among older women are impacted by diabetes and heart disease together compared with each condition separately. Because all sample members agreed to participate in an RCT of a disease management intervention study, they may be at a higher level of functioning than the general population of older women with heart disease or be unrepresentative of this population in other ways. This is unlikely to bias the comparisons between women with and without diabetes within the sample; however, our results cannot be generalized to a larger population, and it is possible that they underestimate functional impairment and symptom burden in this population.
Conclusions
The findings from this study add to a growing body of research on the effects of specific comorbidities in women by showing that the combination of diabetes and heart disease has substantial negative effects on certain aspects of clinical health status. Implementing treatment plans and supportive services that address the particular needs of women who are managing both heart disease and diabetes may help clinicians to ameliorate negative outcomes for these patients.
Acknowledgments
The study was supported by grant 5-R01-HL58611 from the Heart Division of the U.S. National Heart, Lung, and Blood Institute. Special thanks to Margaret K. Wilkin, M.P.H., for her assistance with graph preparation.
Disclosure Statement
No competing financial interests exist.
References
- 1.Lloyd-Jones D. Adams R. Carnethon M, et al. Heart disease and stroke statistics—2009 update: A report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2009;119:480–486. doi: 10.1161/CIRCULATIONAHA.108.191259. [DOI] [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention, National Center for Health Statistics, Division of Health Interview Statistics. Age-adjusted percentage of persons with diabetes aged 35 years and older reporting any cardiovascular disease condition, by sex, United States, 1997–2007. www.cdc.gov/diabetes/statistics/cvd/fig5.htm. [Nov 30;2009 ]. www.cdc.gov/diabetes/statistics/cvd/fig5.htm
- 3.Almdal T. Scharling H. Jensen JS. Vestergaard H. The independent effect of type 2 diabetes mellitus on ischemic heart disease, stroke, and death: A population-based study of 13,000 men and women with 20 years of follow-up. Arch Intern Med. 2004;164:1422–1426. doi: 10.1001/archinte.164.13.1422. [DOI] [PubMed] [Google Scholar]
- 4.Franco OH. Steyerberg EW. Hu FB. Mackenbach J. Nusselder W. Associations of diabetes mellitus with total life expectancy and life expectancy with and without cardiovascular disease. Arch Intern Med. 2007;167:1145–1151. doi: 10.1001/archinte.167.11.1145. [DOI] [PubMed] [Google Scholar]
- 5.Huxley R. Barzi F. Woodward M. Excess risk of fatal coronary heart disease associated with diabetes in men and women: Meta-analysis of 37 prospective cohort studies. BMJ. 2006;332:73–78. doi: 10.1136/bmj.38678.389583.7C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Unden AL. Elofsson S. Andreasson A. Hillered E. Eriksson I. Brismar K. Gender differences in self-rated health, quality of life, quality of care, and metabolic control in patients with diabetes. Gend Med. 2008;5:162–180. doi: 10.1016/j.genm.2008.05.003. [DOI] [PubMed] [Google Scholar]
- 7.Davidson PM. Daly J. Hancock K. Moser D. Chang E. Cockburn J. Perceptions and experiences of heart disease: A literature review and identification of a research agenda in older women. Eur J Cardiovasc Nurs. 2003;2:255–264. doi: 10.1016/S1474-5151(03)00056-2. [DOI] [PubMed] [Google Scholar]
- 8.Frasure-Smith N. Lesperance F. Juneau M. Talajic M. Bourassa MG. Gender, depression, and one-year prognosis after myocardial infarction. Psychosom Med. 1999;61:26–37. doi: 10.1097/00006842-199901000-00006. [DOI] [PubMed] [Google Scholar]
- 9.Li C. Ford ES. Strine TW. Mokdad AH. Prevalence of depression among U.S. adults with diabetes: Findings from the 2006 Behavioral Risk Factor Surveillance System. Diabetes Care. 2008;31:105–107. doi: 10.2337/dc07-1154. [DOI] [PubMed] [Google Scholar]
- 10.Burnette D. Mui AC. Zodikoff BD. Gender, self-care and functional status among older persons with coronary heart disease: A national perspective. Women Health. 2004;39:65–84. doi: 10.1300/J013v39n01_04. [DOI] [PubMed] [Google Scholar]
- 11.Brown DW. Balluz LS. Giles WH, et al. Diabetes mellitus and health-related quality of life among older adults: Findings from the Behavioral Risk Factor Surveillance System (BRFSS) Diabetes Res Clin Pract. 2004;65:105–115. doi: 10.1016/j.diabres.2003.11.014. [DOI] [PubMed] [Google Scholar]
- 12.Ford ES. Mokdad AH. Li C, et al. Gender differences in coronary heart disease and health-related quality of life: Findings from 10 states from the 2004 Behavioral Risk Factor Surveillance System. J Womens Health. 2008;17:757–768. doi: 10.1089/jwh.2007.0468. [DOI] [PubMed] [Google Scholar]
- 13.Oldridge NB. Stump TE. Nothwehr FK. Clark DO. Prevalence and outcomes of comorbid metabolic and cardiovascular conditions in middle- and older-age adults. J Clin Epidemiol. 2001;54:928–934. doi: 10.1016/s0895-4356(01)00350-x. [DOI] [PubMed] [Google Scholar]
- 14.de Grauw WJ. van de Lisdonk EH. Behr RR. van Gerwen WH. van den Hoogen HJ. van Weel C. The impact of type 2 diabetes mellitus on daily functioning. Fam Pract. 1999;16:133–139. doi: 10.1093/fampra/16.2.133. [DOI] [PubMed] [Google Scholar]
- 15.Hu FB. Stampfer MJ. Solomon CG, et al. The impact of diabetes mellitus on mortality from all causes and coronary heart disease in women: 20 years of follow-up. Arch Intern Med. 2001;161:1717–1723. doi: 10.1001/archinte.161.14.1717. [DOI] [PubMed] [Google Scholar]
- 16.Janevic MR. Janz NK. Dodge JA, et al. The role of choice in health education intervention trials: A review and case study. Soc Sci Med. 2003;56:1581–1594. doi: 10.1016/s0277-9536(02)00158-2. [DOI] [PubMed] [Google Scholar]
- 17.Clark NM. Janz NK. Dodge JA, et al. Heart disease management by women: Does intervention format matter? Health Educ Behav. 2009;36:394–409. doi: 10.1177/1090198107309458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Clark NM. Janz NK. Dodge JA. Mosca L. Lin X. Long Q. The effect of patient choice of intervention on health outcomes. Contemp Clin Trials. 2008;29:679–686. doi: 10.1016/j.cct.2008.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bergner M. Bobbitt RA. Carter WB. Gilson BS. The Sickness Impact Profile: Development and final revision of a health status measure. Med Care. 1981;19:787–805. doi: 10.1097/00005650-198108000-00001. [DOI] [PubMed] [Google Scholar]
- 20.Thompson DR. Yu CM. Quality of life in patients with coronary heart disease-I: Assessment tools. Health Qual Life Outcomes. 2003;1:42. doi: 10.1186/1477-7525-1-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Guyatt GH. Sullivan MJ. Thompson PJ, et al. The 6-Minute Walk: A new measure of exercise capacity in patients with chronic heart failure. Can Med Assoc J. 1985;132:919–923. [PMC free article] [PubMed] [Google Scholar]
- 22.Du H. Newton PJ. Salamonson Y. Carrieri-Kohlman VL. Davidson PM. A review of the Six-Minute Walk test: Its implication as a self-administered assessment tool. Eur J Cardiovasc Nurs. 2009;8:2–8. doi: 10.1016/j.ejcnurse.2008.07.001. [DOI] [PubMed] [Google Scholar]
- 23.Verrill DE. Barton C. Beasley W. Lippard M. King CN. Six-Minute Walk performance and quality of life comparisons in North Carolina cardiac rehabilitation programs. Heart Lung. 2003;32:41–45. doi: 10.1067/mhl.2003.7. [DOI] [PubMed] [Google Scholar]
- 24.Hamilton DM. Haennel RG. Validity and reliability of the 6-Minute Walk test in a cardiac rehabilitation population. J Cardiopulm Rehabil. 2000;20:156–164. doi: 10.1097/00008483-200005000-00003. [DOI] [PubMed] [Google Scholar]
- 25.Krause N. Stress, alcohol use, and depressive symptoms in later life. Gerontologist. 1995;35:296–307. doi: 10.1093/geront/35.3.296. [DOI] [PubMed] [Google Scholar]
- 26.Janz NK. Clark NM. Dodge JA. Schork MA. Mosca L. Fingerlin TE. The impact of a disease-management program on the symptom experience of older women with heart disease. Women Health. 1999;30:1–24. doi: 10.1300/j013v30n02_01. [DOI] [PubMed] [Google Scholar]
- 27.Khot UN. Khot MB. Bajzer CT, et al. Prevalence of conventional risk factors in patients with coronary heart disease. JAMA. 2003;290:898–904. doi: 10.1001/jama.290.7.898. [DOI] [PubMed] [Google Scholar]
- 28.Gregg EW. Mangione CM. Cauley JA, et al. Diabetes and incidence of functional disability in older women. Diabetes Care. 2002;25:61–67. doi: 10.2337/diacare.25.1.61. [DOI] [PubMed] [Google Scholar]
- 29.Sinclair AJ. Conroy SP. Bayer AJ. Impact of diabetes on physical function in older people. Diabetes Care. 2008;31:233–235. doi: 10.2337/dc07-1784. [DOI] [PubMed] [Google Scholar]
- 30.Maty SC. Fried LP. Volpato S. Williamson J. Brancati FL. Blaum CS. Patterns of disability related to diabetes mellitus in older women. J Gerontol A Biol Sci Med Sci. 2004;59:148–153. doi: 10.1093/gerona/59.2.m148. [DOI] [PubMed] [Google Scholar]
- 31.Wray LA. Ofstedal MB. Langa KM. Blaum CS. The effect of diabetes on disability in middle-aged and older adults. J Gerontol A Biol Sci Med Sci. 2005;60:1206–1211. doi: 10.1093/gerona/60.9.1206. [DOI] [PubMed] [Google Scholar]
- 32.Perera S. Mody SH. Woodman RC. Studenski SA. Meaningful change and responsiveness in common physical performance measures in older adults. J Am Geriatr Soc. 2006;54:743–749. doi: 10.1111/j.1532-5415.2006.00701.x. [DOI] [PubMed] [Google Scholar]
- 33.Patrick DL. Kinne S. Engelberg RA. Pearlman RA. Functional status and perceived quality of life in adults with and without chronic conditions. J Clin Epidemiol. 2000;53:779–785. doi: 10.1016/s0895-4356(00)00205-5. [DOI] [PubMed] [Google Scholar]
- 34.Katon W. Fan MY. Unutzer J. Taylor J. Pincus H. Schoenbaum M. Depression and diabetes: A potentially lethal combination. J Gen Intern Med. 2008;23:1571–1575. doi: 10.1007/s11606-008-0731-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sha MC. Callahan CM. Counsell SR. Westmoreland GR. Stump TE. Kroenke K. Physical symptoms as a predictor of health care use and mortality among older adults. Am J Med. 2005;118:301–306. doi: 10.1016/j.amjmed.2004.12.015. [DOI] [PubMed] [Google Scholar]
- 36.Nelson ME. Rejeski WJ. Blair SN, et al. Physical activity and public health in older adults: Recommendation from the American College of Sports Medicine and the American Heart Association. Circulation. 2007;116:1094–1105. doi: 10.1161/CIRCULATIONAHA.107.185650. [DOI] [PubMed] [Google Scholar]
- 37.Funnell MM. Brown TL. Childs BP, et al. National standards for diabetes self-management education. Diabetes Care. 2010;33(Suppl 1):S89–96. doi: 10.2337/dc10-S089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cooper AF. Jackson G. Weinman J. Horne R. Factors associated with cardiac rehabilitation attendance: A systematic review of the literature. Clin Rehabil. 2002;16:541–552. doi: 10.1191/0269215502cr524oa. [DOI] [PubMed] [Google Scholar]
- 39.Allen JK. Scott LB. Stewart KJ. Young DR. Disparities in women's referral to and enrollment in outpatient cardiac rehabilitation. J Gen Intern Med. 2004;19:747–753. doi: 10.1111/j.1525-1497.2004.30300.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.American Diabetes Association. Third-party reimbursement for diabetes care, self-management education, and supplies. Diabetes Care. 2007;30:S86–87. doi: 10.2337/dc07-S086. [DOI] [PubMed] [Google Scholar]
- 41.Keyserling TC. Samuel-Hodge CD. Ammerman AS, et al. A randomized trial of an intervention to improve self-care behaviors of African-American women with type 2 diabetes: Impact on physical activity. Diabetes Care. 2002;25:1576–1583. doi: 10.2337/diacare.25.9.1576. [DOI] [PubMed] [Google Scholar]
- 42.Toobert DJ. Glasgow RE. Strycker LA. Barrera M., Jr Ritzwoller DP. Weidner G. Long-term effects of the Mediterranean Lifestyle Program: A randomized clinical trial for postmenopausal women with type 2 diabetes. Int J Behav Nutr Phys Act. 2007;4:1. doi: 10.1186/1479-5868-4-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Katon WJ. Von Korff M. Lin EH, et al. The pathways study: A randomized trial of collaborative care in patients with diabetes and depression. Arch Gen Psychiatry. 2004;61:1042–1049. doi: 10.1001/archpsyc.61.10.1042. [DOI] [PubMed] [Google Scholar]
- 44.Kang-Yi CD. Gellis ZD. A systematic review of community-based health interventions on depression for older adults with heart disease. Aging Ment Health. 2010;14:1–19. doi: 10.1080/13607860903421003. [DOI] [PubMed] [Google Scholar]
- 45.Katon W. Lin EH. Von Korff M, et al. Integrating depression and chronic disease care among patients with diabetes and/or coronary heart disease: The design of the TEAMcare study. Contemp Clin Trials. 2010;31:312–322. doi: 10.1016/j.cct.2010.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]