Abstract
Objectives
To conduct a community-based, postpartum depression (PPD) screening initiative, and recommend PPD screening practices.
Design
Descriptive correlational.
Settings
Two academic medical centers, a university research office, and participants’ homes.
Participants
Five thousand one hundred sixty nine (5,169) postpartum women aged 14–49 years.
Methods
The Agency for Healthcare Research and Quality (AHRQ) framework was implemented by identifying a cohort of mothers and conducting PPD screening followed by diagnostic evaluation of those with positive screens. Mothers in the postpartum period were recruited from two academic medical centers and screened for PPD at 4–6 weeks postpartum by telephone or mail using the Edinburgh Postnatal Depression Scale (EPDS). Mothers with EPDS scores ≥ 10 were invited to participate in the Structured Clinical Interview for DSM IV (SCID) to confirm PPD.
Results
Six hundred seventy four (674) (13%) women had EPDS scores ≥10; 185 women with elevated EPDS scores agreed to have a SCID diagnostic interview, and 144 were diagnosed with minor or major depression. A significantly higher percentage of women who self-administered and mailed in the EPDS than women who were screened via telephone had scores ≥ 10. Elevated PPD scores were not associated with age or parity. Race/ethnicity identification other than White, and having less than high school education were associated with higher PPD scores
Conclusions
The AHRQ framework was effective in guiding a large-scale PPD screening initiative by identifying mothers at risk for PPD. Results support previous findings regarding prevalence, selected risk factors, and continued use of the EPDS with SCID confirmation.
Keywords: community based screening, postpartum depression, Edinburgh Postnatal Depression Scale
Depressive disorders rank among the leading causes of disability worldwide (World Health Organization [WHO], 2010). According to the WHO, depression is the second leading contributor to global burden of disease for men and women between the ages of 15 and 44 years. When depression occurs during the first postpartum year, disabling effects are magnified, as altered mood and behavior of the affected mother influence her developing infant and possibly other family members. Despite growing recognition of postpartum depression (PPD) as a global childbirth-related problem over the past two decades (Affonso, De, Horowitz, & Mayberry, 2000), the importance of detecting and treating PPD has until recently been largely overlooked in practice (Gaynes et al., 2005). However, legislative mandates for PPD screening that began at the state level in New Jersey (Medical News Today, 2006) have culminated in 2010 with passage of H.R. 3590—the Patient Protection and Affordable Care Act—that have made specific provisions related to postpartum depression. Section 2952 of the bill outlines the actions that should be taken in regard to PPD, including further research into the cause, treatment and screening of PPD (Patient Protection and Affordable Care Act, H.R. 3590, 2010). Such mandates will further necessitate the development of effective and accurate screening methodologies.
Testing models for the identification of mothers with PPD, examining prevalence and risk factors for PPD, and validating the accuracy of screening measures used to identify women with PPD are ongoing challenges for nurses and other clinicians caring for mothers in the postpartum period (Gaynes et al., 2005). Moreover, universal screening of mothers has been advocated by the WHO (2009). Certainly determining the best screening practices is important to all clinicians caring for mothers in the postpartum period (Horowitz, Murphy, Gregory, & Wojcik, 2009). Thus, the purpose of this paper is to present results from a large-scale, community-based, PPD screening initiative designed to reach mothers early in the postpartum period. Specific aims were as follow:
To examine the feasibility of conducting community-based PPD screening to identify mothers at risk for PPD as indicated by elevated scores on the Edinburgh Postnatal Depression Scale (EPDS) (Cox Holden, & Sagovsky, 1987);
To determine the prevalence of elevated PPD symptoms and compare results from screening conducted by telephone interviews versus the mail;
To examine selected demographic variables as risk factors for PPD.
Background and Significance
Postpartum depression is a common childbirth complication with documented prevalence estimated at 13% (Gaynes et al., 2005; Gibson, Mckenzie-McNarg, Shakespeare, Price & Gray, 2009; Noorlander, Bergink, Van den Berg, 2008; O’Hara & Swain 1996). Although combinations of risk factors are likely to contribute to any individual woman's vulnerability to PPD, accumulating evidence suggests that PPD affects a cross-section of women and that factors such as prenatal depression and history of maternal depression, current stress, poor quality of relationships, young age, very low socioeconomic status or education, and possibly being African American/Black, or an immigrant may be associated with increased PPD risk (Beeghly et al., 2003; Beck, 1996a; 2001; Nielson, 2000; Mayberry, Horowitz, & Declercq, 2007; O’Hara & Swain 1996; Segre, Losch, & O’Hara, 2006; Zelkowitz et al., 2008). The negative effects of PPD are significant as PPD interferes with mothers’ cognitive processing, interpretation of information, affective expression, and efforts to interact positively and responsively with their infants (Milgrom, Ericksen, McCarthy & Gemmil, 2006; Sichel & Driscoll 2000).
Given the prevalence and negative effects of PPD on mothers and infants, developing and testing methods to detect the likely presence of PPD is an essential step in helping mothers who may be experiencing PPD to get timely mental health evaluation, treatment, and support. An assessment of the feasibility of universal PPD screening during well-child visits was deemed effective, although documentation of screening was not universal (Chaudron, Szilagyi, Kitzman, Wadkins, & Conwell, 2004). The investigators concluded that high levels of PPD are common among urban populations and that PPD screening with a standardized screening tool, such as the EPDS, during well-child visits is a feasible approach. PPD screening with the EPDS during routine postpartum clinical evaluation visits also has been shown to be feasible in a small study of 72 low-income inner-city mothers (Fergerson, Jamieson, & Lindsay, 2002). Thus studies of screening approaches using a standardized measure, specifically the EPDS, have shown promise. Nonetheless, additional demonstrations of feasibility of PPD screening of large community-based samples (i.e., not just small samples from specific clinical practices or health centers) with a standardized measure like the EPDS are needed to inform best practices and policy regarding universal PPD screening.
Theoretical Framework
The CARE screening design followed the Agency for Healthcare Research and Quality (AHRQ) framework recommended by Gaynes et al. (2005) in their evidence report on the impact of PPD screening, intervention and patient outcomes. This framework begins by identifying a cohort of mothers in the postpartum period with unknown mood state, follows with formal postpartum screening using a valid measure, proceeds with clinical diagnostic evaluation for those with positive screens, employs random group assignment for clinical trials, and concludes with follow-up evaluation.
Methods
The research reported here comprises Phase 1 of the study, CARE Intervention for Depressed Mothers and Their Infants. Phase 1 involved recruiting, screening and diagnostic evaluation of postpartum women to determine depression status and eligibility for enrollment in the randomized clinical trial (RCT). Results presented here include a description of the key features of an effective PPD screening protocol for women in the community, prevalence rates obtained for both telephone and mail-in screening using the EPDS, and risk factors that emerged from the population of over 5,000 women who were screened and from the 185 women who completed a SCID diagnostic interview to confirm the diagnosis of PPD.
Measures
Edinburgh Postnatal Depression Scale (EPDS)
The EPDS (Cox 1986; Cox, Holden & Sagovsky, l987) was designed specifically to identify symptoms of PPD and is the most widely used PPD screening instrument with community-based populations in the United States and internationally (Gaynes et al., 2005; Gibson et al., 2009). The EPDS was used in this study to identify mothers who were at risk for PPD. The EPDS consists of 10 statements describing depressive symptoms with some reverse coded items with four possible responses, each scored according to PPD symptom severity or duration. Total scores on the EPDS range from 0–30. Cutoff scores for additional screening are typically set at ≥ 10, or, ≥ 13. The authors of the EPDS have recommended using the cutoff score of ≥ 10 to reduce failed detection to less than 10% and suggested that mental health referral is indicated whenever a woman scores 13 or higher. Indicators of internal consistency reliability have been acceptable and consistent. Reported Cronbach alphas for the EPDS have usually ranged from .81 to .88 (Horowitz, et al., 1995; Horowitz, Bell, Trybulski, Munro, & Moser et al., 2001; Cox et al., 1987). For the screening sample of 5169 mothers reported here, Cronbach’s alpha was .80.
The widespread use of the EPDS with a variety of populations, its ease of administration, and free access are compelling advantages of this instrument over many other measures that may be used for PPD screening. In this study, administration over the phone took less than 10 minutes.
Mother’s Information Tool (MIT-brief)
The Mother’s Information Tool Brief (MIT-brief) measured selected demographic characteristics of the PPD screening sample and included four open-ended questions to elicit information about whether or not mothers had been asked about their emotional state by a clinician (Horowitz et al., 2009). The MIT-brief was administered by telephone or mail in conjunction with the EPDS to elicit basic demographic information including maternal age, years of education, parity, race/ethnicity, and whether or not the woman had been asked about her emotional well being by a health care provider. As the Human Subjects Committee approved collection of basic descriptive data for age, education, race/ethnicity and education only during Phase I screening for the purposes of determining preliminary study eligibility and describing the screening sample, the investigators did not collect data on more sensitive variables, such past history of depression or income during screening.
Procedures
The study protocol was approved by Partners Health Care Human Subjects Committee and the Boston College Human Subjects Committee. Following PPD screening for possible RCT eligibility, the research nurse explained the study and asked mothers with positive PPD screening scores if they were willing to have an advanced practice psychiatric-mental health registered nurse (APRN) make a home visit for a follow-up assessment interview (i.e., the SCID). For willing mothers, formal informed consent was obtained by the APRN at this home visit prior to the SCID.
Study recruitment was initiated by nurses on the postpartum units of two academic medical centers in Boston after identifying women who met the following study eligibility criteria: being English-speaking, medically stable and sufficiently recovered from labor and birth to participate, having stable maternal and infant health status, and living within a 90-miniute drive of the hospital. Exclusion criteria were being unable to understand and converse in English well enough to respond to the items in the instruments; having a history of schizophrenia, psychotic, or bipolar disorder; giving birth to multiple infants; and having a premature or ill infant that required extended hospital care after the mother’s discharge. Mothers with a history of mental health disorders other than schizophrenia, psychotic or bipolar disorders were not excluded. Inclusion/Exclusion criteria were based on practical, research, and clinical concerns regarding resources for conducting screening interviews and the clinical trial. For example, non-English speakers were excluded because it was not feasible to have study staff who were fluent in the many languages spoken in the greater Boston area to conduct PPD screening and then interact with mothers who could be enrolled in the RCT. Mothers of infants requiring extended stay in the neonatal intensive care unit (NiCU) were excluded because an extended NICU stay would decrease opportunity for mothers to interact with their infants, thereby, possibly affecting RCT outcomes. Mothers of premature or ill infants were excluded because their infants’ capacity to interact would likely be compromised. Mothers who birthed multiples were excluded because maternal-infant interaction could be affected by having more than one infant. Geographic limitation concerning distance of residence from the hospital was necessary due to the home visits involved in the RCT design and realistic limits for research nurses to make home visits.
Staff nurses provided potential participants with study brochures and verbal information about the study, and asked them if they were willing to be contacted at home 4 weeks after the birth for depression symptom PPD screening. CARE research nurses then approached the interested mothers to discuss the study further, respond to questions and request mothers’ signatures and contact information (e.g., telephone number and address), on the Permission to Contact Form (PCF). Prior to discharge, women signed the form to indicate agreement to be contacted by phone at home at 4 weeks postpartum, or took the information home to consider it further.
Mothers who signed the form to indicate willingness to be contacted, provided contact information. These mothers, except for those who returned the opt-out card to decline, were contacted by a research nurse by telephone for PPD screening at 4 weeks postpartum. At this time, the nurse administered the EPDS screening questionnaire and the MIT-brief. Because postpartum blues typically resolve by 2 weeks postpartum, and PPD is defined as beginning by 4 weeks postpartum (American Psychiatric Association, 2000), this timeframe was deemed appropriate for depression screening. If after three attempts, the screening nurse failed to reach a woman by phone at the end of the 4th postpartum week, the screening materials were sent by mail with a self-addressed postage-paid envelope. If screening was not conducted by telephone or mail by 6–8 weeks postpartum, the woman was eliminated from the screening population.
Structured clinical interview for DSM IV (SCID)
The Structured Clinical Interview for DSM-IV Axis Disorders (SCID-1) (First, Spitzer, Gibbon & Williams, 1997) was offered to all mothers who scored ≥ 10 on the EPDS. All who were willing to have an APRN make a home visit were asked to provide informed consent for possible inclusion in the RCT. The purpose of administering the SCID was to determine whether or not participants with elevated EPDS scores also met diagnostic criteria for PPD and were qualified to participate in the RCT. Mothers with positive PPD screens were contacted by telephone and given an explanation about Phase II of the CARE Study including details about informed consent and aspects of the protocol such as the required 4–6 home visits over a 9-month period and videotaping. If the mother agreed that she could participate in all aspects of the protocol, a visit to her home was scheduled. Due to the logistics of making home visits within a large geographical area, the informed consent followed by the SCID diagnostic interview were conducted during the same home visit by a certified APRN when the mother was approximately 6 weeks postpartum. Due to the comprehensive nature of the Phase II protocol and logistics, the investigators were not able to offer a diagnostic interview to women who screened positive but declined to participate in the RCT. However, all women with EPDS scores ≥ 13 were contacted by an APRN to assess for safety and provide assistance with seeking additional mental health evaluation.
The SCID assessment classified participants into the following categories: not being depressed (i.e., negligible presence of depression symptoms), mildly depressed (i.e., having minor depression or the presence of symptoms without meeting diagnostic criteria), or being depressed (i.e., meeting diagnostic criteria for a major depression). The SCID interview also identified mothers who met study exclusion criteria for bipolar or psychotic disorders. A diagnostic interview at 6-weeks postpartum provided additional protection for consented, willing participants by insuring that mothers with moderate to severe depression, and/or suicidal ideation or intent, were identified and referred for additional evaluation and treatment. The diagnostic interview conducted by a qualified clinician such as an APRN is the “gold standard” for confirming a depression diagnosis (Gibson et al., 2009). Inclusion of a diagnostic interview further strengthened the study design because reliance on self-report to diagnose PPD is a major limitation of many studies (Beck, 1999).
Follow-up Procedures
During screening, and at any point in the 9-month clinical trial, whenever a mother's depression score was in the moderate to severe symptom range (i.e., EPDS ≥ 13) or her response to EPDS item 10 (that indicated thoughts of self-harm) was positive (i.e., hardly ever, sometimes, or yes quite often), the research nurse determined whether or not the mother was currently receiving any mental health treatment and subsequently encouraged the mother to contact her clinician immediately. If the mother was not receiving mental health treatment, the nurse encouraged her to contact her Primary Care Provider (PCP) and/or the Department of Psychiatry at the delivery hospital for follow-up evaluation. The research nurse also offered to assist the mother in contacting a clinician. In addition, one of the study APRNs contacted all mothers whose EPDS scores placed them at a high risk for PPD (i.e. EPDS score ≥13), conducted a brief telephone evaluation, and assisted them to seek follow-up with their PCP or other clinician. In the event that nurses were not able to contact a mother with an EPDS score ≥13 by phone, a note advising the woman to contact her doctor, midwife or other health care professional, and the telephone number of the CARE study, was mailed to the address listed on the woman’s PCF.
Results
PPD Screening and Prevalence
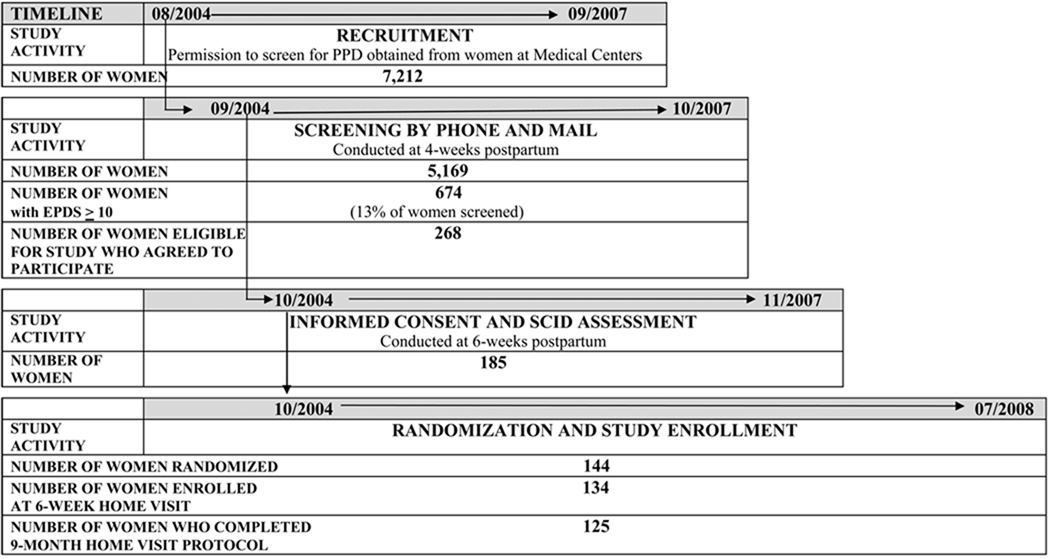
CARE study nurses received signed PCFs from 7,212 mothers that indicated permission to contact these mothers at 4 weeks postpartum. Figure 1 details the disposition of the 7,212 mothers. Of these 7,212 mothers, 3,018 (42%) mothers received their questionnaires in the mail and 3806 (53%) were screened by telephone. Of those who received mailed forms, 1,363 (19% of the total population) sent them back and 1,655 (23% of the total population) did not. In addition, 88 (1.2%) women returned opt out cards, and 57 (.8%) of mailed questionnaires were returned by the post office. Other reasons for failure to screen mothers who provided permission to be contacted included disconnected phones, incorrect addresses, and failure of mothers to meet inclusion criteria.
Fig. 1.
Screening women for postpartum depression (PPD)
EPDS: Edinburgh Postnatal Depression Scale
SCID: Structured Clinical Interview for DSM IV
Consequently, research nurses screened a total of 5,169 mothers for PPD with the EPDS by telephone, or scored mothers’ mailed-in questionnaires. Of the 5,169 women screened, 13% met criteria for PPD risk with scores ≥10 on the EPDS. Ethnicity/race of mothers of the screening sample was representative of the greater Boston area. Table 1 displays demographic profiles of the screening and SCID study samples.
Table 1.
Demographic Characteristics of CARE Study Samples
| Sample | n | Age | Years Education |
Race/Ethnicity | First Baby | ||||
|---|---|---|---|---|---|---|---|---|---|
| x̄ | sd | x̄ | sd | ||||||
| White | 66% | ||||||||
| Hispanic | 12% | ||||||||
| Screening sample | 5169 | 32.2 | 5.4 | 16.3 | 2.9 | African Am | 9% | 48% | |
| Asian/PI | 8% | ||||||||
| Other | 5% | ||||||||
| White | 56% | ||||||||
| Hispanic | 21% | ||||||||
| DSM IV sample | 185 | 31.5 | 5.7 | 15.8 | 3.3 | African Am | 12% | 56% | |
| Asian/PI | 7% | ||||||||
| Other | 4% | ||||||||
| White | 54% | ||||||||
| Hispanic | 22% | ||||||||
| Enrolled sample | 134 | 31 | 5.7 | 15.6 | 3.4 | African Am | 12% | 56% | |
| Asian/PI | 8% | ||||||||
| Other | 4% | ||||||||
Mail versus telephone screening
When the EPDS scores of women who self-administered and mailed in their questionnaires (n=1,363) were compared with the scores of mothers who were screened by a nurse on the telephone (n = 3,806) the PPD risk prevalence (i.e., those with scores ≥10 on the EPDS) differed. Results revealed that 420 (i.e., a rate of 11% positive screens were obtained via telephone and 258 (i.e., a rate of 19% for elevated EPDS scores) positive screens were obtained via mail. These findings were examined further by a separate variance t-test that indicated that mothers who filled out their questionnaires at home and returned them by mail had a significantly higher EPDS mean score (x = 6.04) than did women screened by a nurse on the telephone (x = 5.14) (t (2146) = 7.031, p ≤ .001). Further examination of the data revealed significant demographic differences between the mothers who were screened by phone and mail. White mothers (74%) (X2 (4, N = 5135) = 88.96, p ≤ .001), mothers who completed 4 or more years of college (48%) (X2 (3, N = 5135) = 46.83, p ≤ .001), and mothers in the 30–39 age group (74%) (X2 (3, N = 5135) = 63.44, p ≤ .001) were more likely to have been screened by mail than were other mothers. No differences were noted for parity groups.
Risk factors for PPD
For the 5,169 mothers in the screening sample, only parity (r = .048; p ≤ .001) was significantly related to EPDS scores. Given the small magnitude of the correlation and the large sample size, these results were interpreted cautiously.
Contingency analyses for age, parity, education and racial/ethnic groupings by EPDS ≥ 10 and EPDS < 10 were also conducted for the screening sample. Results revealed that fewer than expected White women (11%) met criteria for a positive EPDS when compared to Hispanic (18%), Asian American (17%), women in the Other Category (16%), and African American women (14.5%) (X2 (4, N = 5135) = 32.47, p ≤ .001). When educational groupings were compared, women who were not high school graduates (26%) met criteria more often than women who were high school graduates (14%), women who completed 1–4 years of college (13%) and women who completed college and beyond (12%) (X2 (3, N = 5131) = 32.62, p ≤ .001) No such differences were noted for age or parity groups.
SCID Diagnostic Interview Results
Of the 674 mothers with positive screens, 268 (39%) agreed to be considered for the Phase II 9-month RCT and 267 (39%) declined; 74 (11%) mothers were deemed to be too late with respect to study timelines; 52 (9%) mothers were not reached by phone or mail; and 16 (2%) mothers did not meet inclusion criteria. The available data elicited from mothers indicated that reasons for declining to participate and/or not completing the diagnostic interview visit included being unable to schedule the home visit (n = 39, 14%), changing their mind about participating (n = 18, 7%), being unable to be reached by phone or mail (n = 11, 4%), not meeting study criteria (n = 9, 3%), refusing videorecording (n = 3, 1%), not having time (n = 2, .7%), not being interested (n = 1, .4%) and moving (n = 1, .4%). Ultimately, 185 mothers provided informed consent via signed consent forms and participated in a SCID diagnostic interview to confirm major or minor depression and 144 mothers were deemed eligible for enrollment in the RCT.
Risk factors for mothers with PPD confirmed by SCID
Contingency analyses for age, parity, education, racial/ethnic groupings and history of depression by SCID depression diagnosis (i.e., depressed/not depressed) were also conducted for the sample of mothers who participated in the diagnostic interview. Women in this group provided informed consent and provided more extensive health history data than did the screening sample, including data concerning depression during pregnancy and history of previous depression In contrast to results for the screening sample, results for the 185 women who completed diagnostic interviews revealed that there were no significant differences for women who did/did not meet depression criteria with respect to age, education, race/ethnicity or parity. However, when women who did/did not meet depression diagnostic criteria were compared with those who did/did not have a history of depression, 100% of women with a history of depression met SCID depression criteria and 50% of women who did not have a history of depression met SCID depression criteria. (X2 (1, N = 185) = 30.40, p ≤ .001). Thus history of depression emerged as an important risk factor among women with confirmed PPD.
Discussion and Implications
In the screening (Phase I) of the CARE study mothers with unknown mood state were screened for PPD and those with positive screens who agreed then participated in diagnostic evaluation. Willing mothers who qualified were subsequently randomly assigned for participation in the RCT (Phase II), and referred for treatment options as necessary during follow-up. Feasibility of the AHRQ framework was validated, as over 5,000 mothers were willingly screened for PPD with the EPDS. Because mothers in this study had interacted with a research nurse in the delivery hospital and had signed PCFs, they anticipated calls or mailings from the CARE study research nurses and were, therefore, receptive to being screened.
Both telephone and mailed screening approaches were effective. Consistent with other research studies (Seehusen & Clark, 2007) there were more positive PPD screens for women who filled out the EPDS at home and returned it by mail than for those screened by a nurse via telephone. One possible explanation for this finding is that the nurse may have clarified questions, thus helping to reduce false positive responses. It is also possible that mothers who self–administered the EPDS in the privacy of their homes were more honest in their responses. To balance the pros and cons of each approach in the practice setting, we suggest that mothers complete the EPDS on their own, and that a clinician should then discuss the responses with mothers to confirm answers and follow-up as needed for positive screens.
The validity of the screening approach described here was further supported by the rate of positive EPDS screens obtained (13%), which is consistent with previously reported PPD prevalence (Gaynes et al., 2005; O’Hara & Swain, 1996). Although other researchers have identified specific demographic and situational risk factors for PPD, such as very young maternal age, low education and income, race/ethnicity (i.e., African American race), very low infant birth weight, prior depression history and onset of depression during pregnancy (Beeghly et al., 2003; Mayberry et al., 2007; Segre et al., 2006), data from this study support only two of these risk factors. While ethnicity and race have not been consistent significant predictors of PPD in studies conducted in the United States (Yonkers et al., 2001), in this study, significantly fewer than expected White women met criteria for a positive EPDS screen than did Hispanic, Asian American, women in the Other category, and African American women. These outcomes partially confirm some of the findings of other investigators (Beeghly at al., 2003; Segre et al., 2006) who found that, in the United States, race/ethnicity, specifically being African/American, increases PPD risk. Nonetheless, it is important to note our finding that Hispanic women in the screening sample had higher percentages of elevated PPD symptom scores than did White women. This finding contradicts previous research outcomes that suggested that Hispanic women have lower PPD prevalence than other mothers (Beeghly et al., 2003; Segre et al., 2006; Wei et al., 2007). Certainly, continued examination of race/ethnicity, and other socio-demographic variables in relation to PPD, is appropriate given that no group can be definitively eliminated from PPD risk. Nonetheless, our findings in concert with previous research outcomes suggest that African American/Black and Hispanic mothers may face elevated PPD risk.
Our findings support those of Mayberry et al., (2007), which indicated that limited education may be associated with increased maternal depression risk. No such differences were found for age. The small percentage of very young mothers in the CARE study sample likely explains why age was not related to PPD symptom severity as measured by the EPDS. When young age has emerged as a predictor of PPD or young mothers have had higher rates of PPD, samples have included higher proportions of young and adolescent mothers (Mayberry et al.; Troutman & Cutrona 1990). However, in the large community-based screening sample in this study, the mean age was 32.2 years and the mean age of women who had a diagnostic interview and who subsequently enrolled in the RCT was 31 years. It may well be that only very young age is associated with increased PPD risk due to developmental and social factors that make motherhood a particularly stressful event. Parity was not related to PPD symptom severity in this study underscoring the importance of screening and supporting all postpartum women, not just first-time mothers.
Given the findings about risk factors, clinicians and researchers alike are charged to continue to examine risk profiles, consider preventive interventions, educate childbearing women about relative risk for depression, and test PPD screening models in primary care settings for translation to practice. In addition, this study confirms the importance of identifying all mothers with EPDS scores ≥ 13 and/or who indicate that they have thoughts of self-harm, for immediate mental health referral and safety evaluation. Follow-up phone assessment by one of the study APRNs to invite the mother to have a diagnostic interview and possible enrollment in the RCT, created yet another opportunity to discuss PPD symptoms and need for mental health care.
By creating the CARE protocol to guide follow-up for every woman with probable PPD or risk of self-harm based on EPDS-10 scores, study nurses were able to contact all at-risk women to assist them in contacting their primary care providers or the psychiatric service at the delivery hospital. Systematic mental health follow-up is currently lacking in postpartum health care in the United States, and in many other countries (Gibson et al., 2009). The mental health referral plan provided in this study was a major strength of this screening initiative. While screening alone has yet to demonstrate an improvement in mental health outcomes (Gaynes et al., 2005), implementing follow-up by the clinician or psychiatric services has the potential to bridge this gap. However, to show effects on health outcomes, more is needed than measuring changes in PPD symptoms following psychosocial or psychopharmacologic treatment. Models are needed that demonstrate the feasibility of referral efforts for mental health evaluation and follow-up for large-scale PPD screening efforts. Our experience shows that introducing a formal PPD screening measure, such as the EPDS, begins the conversation about how a woman is feeling emotionally. Women in our study were receptive to feedback about their EPDS responses and to suggestions that they contact their PCPs, as evidenced by the willingness of the 185 women with positive screens who agreed to a diagnostic interview.
Although resistance to PPD screening by PCPs and postpartum women has been reported (Tam et al., 2002), we can only conclude that failure to provide universal PPD screening for early case identification and mental health treatment referral is due in large measure to a sense of inadequacy on the part of providers, rather than resistance to PPD screening on the part of mothers. Our ability to screen more than 5,000 women for PPD via telephone and mail challenges primary care and other providers who care for mothers in the postpartum period and their infants to discard assumptions or fears that mothers will resist PPD screening, and to forge ahead to incorporate universal PPD screening into their practice. It is imperative that current knowledge about PPD prevalence and associated risks for infants, along with training in the use of standardized screening measures like the EPD S and mental health referral practices, be integrated into physician and nurse preparation programs by the current generation of clinical educators.
Testing screening procedures is an important public health goal. One of the strengths of this study is the size of the screening sample, which was significantly larger than samples from all studies reviewed by Gibson et al. (2009). In this recent comprehensive review, the largest sample reported was 876. Additionally, our sample was representative of the geographic area served by the academic medical centers and included diagnostic interview data from a subset of mothers.
We also echo the recommendation of Gaynes et al. (2005) that the EPDS or Postpartum Screening Scale (PDSS) (Beck & Gable, 2000) be used at present as the standard PPD screening measures due to their demonstrated substantive and psychometric properties. Widespread use of the EPDS as the most commonly employed PPD screening measure also enables users to compare results from PPD studies from the United States and around the world.
Limitations include our inability to follow up with mothers with positive PPD screens on the EPDS of ≥ 13 who declined to have a diagnostic interview. Although we did encourage these mothers to seek additional mental health evaluation, we could not force such follow-up or track outcomes. We also acknowledge that some eligible mothers may not have received study information during their postpartum stay. Thus given that PPD screening was not universal practice at the time of the study, our efforts to recruit a population of mothers after delivery could only result in a population of convenience.
Conclusions
In summary, results presented here demonstrate that mothers were receptive to PPD screening, both by telephone and by mail. The risk factors identified add to the growing knowledge about mothers who may be at increased risk for PPD. Our findings reinforce the recommendations of Gaynes et al. (2005) for continued research to test the effectiveness of methods for routine PPD screening, including examination of whether or not health outcomes are improved through such screening efforts. The success of our PPD screening approach suggests that it may be adapted to other PPD research studies and methods such as online screening, and tested in practice settings. Once developed and widely implemented, initiatives such as the plan described here will assist clinicians to conduct universal PPD screening, support PPD screening across practice settings, and implement treatment and support to improve the mental health and emotional well being of mothers and their infants through early PPD detection and treatment follow-up.
Acknowledgement
Funded by CARE Intervention for depressed mothers and their infants, National Institutes of Health, National Institute for Nursing Research Grant# R01 NR008033 (2004–2010).
Footnotes
Disclosure, The authors report no conflict of interest or relevant financial relationships.
Call out #1
This community-based postpartum depression screening initiative demonstrated the feasibility of large scale postpartum depression screening.
Call out #2
Identified risk factors for postpartum depression were race/ethnicity other than White, very low educational level, and history of depression.
Call out # 3
Screening by mail yielded a significantly higher percentage of mothers with elevated postpartum depression symptoms than screening by telephone.
References
- Affonso DD, De A, Horowitz JA, Mayberry LJ. An international comparative study exploring postpartum depression symptoms. Journal of Psychosomatic Research. 2000;49:207–216. doi: 10.1016/s0022-3999(00)00176-8. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4th ed. Washington DC: Author; 2000. text revision. [Google Scholar]
- Beck CT. A meta-analysis of predictors of postpartum depression. Nursing Research. 1996;45:297–303. doi: 10.1097/00006199-199609000-00008. [DOI] [PubMed] [Google Scholar]
- Beck CT. Maternal depression and child behavior problems: A meta-analysis. Journal of Advanced Nursing. 1999;29:623–629. doi: 10.1046/j.1365-2648.1999.00943.x. [DOI] [PubMed] [Google Scholar]
- Beck CT. Predictors of postpartum depression: An update. Nursing Research. 2001;50:275–285. doi: 10.1097/00006199-200109000-00004. [DOI] [PubMed] [Google Scholar]
- Beck CT, Gable RK. Postpartum depression screening scale: Development and psychometric testing. Nursing Research. 2000;49(5):272–282. doi: 10.1097/00006199-200009000-00006. [DOI] [PubMed] [Google Scholar]
- Beeghly M, Olson KL, Weinberg MK, Pierre SC, Downey N, Tronick EZ. Prevalence, stability, and socio-demographic correlates of depressive symptoms in black mothers during the first 18 months postpartum. Maternal Child Health. 2003;7:157–168. doi: 10.1023/a:1025132320321. [DOI] [PubMed] [Google Scholar]
- Chaudron LH, Szilagyi PG, Kitzman HJ, Wadkins HIM, Conwell Y. Detection of postpartum depression at well-child visits. Pediatrics. 2004;113:551–558. doi: 10.1542/peds.113.3.551. [DOI] [PubMed] [Google Scholar]
- Cox JL. Postnatal depression: A guide for health professionals. Edinburgh, Scotland: Churchill Livingston; 1986. [Google Scholar]
- Cox JL, Holden JM, Sagovsky R. Detection of postnatal depression: Development of the 10-item Edinburgh postnatal depression scale. British Journal of Psychiatry. 1987;150:782–786. doi: 10.1192/bjp.150.6.782. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured clinical interview for DSM-IV axis disorders (SCID-1): Clinician version administration booklet: Biometrics Research Department. New York: Columbia University; 1997. [Google Scholar]
- Gaynes BN, Gavin N, Meltzer-Brody S, Lohr KN, Swinson T, Gartlehner G, Miller WC. Perinatal depression: Prevalence, screening accuracy, and screening outcomes. Agency for Healthcare Research and Quality. 2005;119:1–8. doi: 10.1037/e439372005-001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson J, McKenzie-McHarg K, Shakespeare J, Price J. A systematic review of studies validating the Edinburgh Postnatal Depression Scale in antepartum and postpartum women. Acta Psychiatrica Scandinavica. 2009;119:350–364. doi: 10.1111/j.1600-0447.2009.01363.x. [DOI] [PubMed] [Google Scholar]
- Horowitz JA, Bell M, Trybulski JA, Munro BH, Moser D, Hartz SA, Sokol ES. Promoting responsiveness between mothers with depressive symptoms and their infants. Journal of Nursing Scholarship. 2001;33:323–329. doi: 10.1111/j.1547-5069.2001.00323.x. [DOI] [PubMed] [Google Scholar]
- Horowitz JA, Murphy CA, Gregory KE, Wojcik J. Community-based postpartum depression screening: Results from the CARE project. Psychiatric Services. 2009;60:432–434. doi: 10.1176/appi.ps.60.11.1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horowitz JA, Damato E, Solon L, von Metzsch G, Gill V. Postpartum depression: Issues in clinical assessment. Journal of Perinatology. 1995;15:268–278. [PubMed] [Google Scholar]
- Fergerson SS, Jamieson DJ, Lindsay M. Diagnosing postpartum depression: Can we do better? American Journal of Obstetrics & Gynecology. 2002;186:899–902. doi: 10.1067/mob.2002.123404. [DOI] [PubMed] [Google Scholar]
- Mayberry LJ, Horowitz JA, Declercq E. Depression symptom prevalence and demographic risk factors among U.S. women during the first 2 years postpartum. Journal of Obstetric, Gynecologic and Neonatal Nursing. 2007;36:542–548. doi: 10.1111/j.1552-6909.2007.00191.x. [DOI] [PubMed] [Google Scholar]
- Medical News Today. New Jersey Law Requiring Postpartum Depression Screening Goes Into Effect. 2006 October 27; Retrieved from http://www.medicalnewstoday.com/articles/54096.php.
- Milgrom J, Erickson J, McCarthy R, Gemmil A. Stressful impact of depression on early mother-infant relations. Stress and Health. 2006;22:229–238. [Google Scholar]
- Nielson FD, Videbech P, Hedegaard M, Dalby SJ, Secher NJ. Postpartum depression: Identification of women at risk. British Journal of Obstetrics and Gynecology. 2000;107:1210–1217. doi: 10.1111/j.1471-0528.2000.tb11609.x. [DOI] [PubMed] [Google Scholar]
- Noorlander Y, Bergink V, Van den Berg MP. Perceived and observed mother-child interaction at time of hospitalization and release in postpartum depression and psychosis. Archives of Women's Mental Health. 2008;11:49–56. doi: 10.1007/s00737-008-0217-0. [DOI] [PubMed] [Google Scholar]
- O'Hara MW, Swain AM. Rates and risk of postpartum depression: A meta-analysis. International Review of Psychiatry. 1996;8:37–54. [Google Scholar]
- Patient Protection and Affordable Care Act, HR 3590. United States Government Printing Office. 2010 Retrieved from http://thomas.loc.gov/cgibin/query/F?c111:7:./temp/~c111lgUnM1:e730003.
- Seehusen DA, Clark G. Barriers to postpartum depression screening, diagnosis and treatment. In: Rosenfield AI, editor. New research on postpartum depression. Nova Science Publishers; 2007. pp. 59–68. [Google Scholar]
- Segre LS, Losch ME, O’Hara MW. Race/ethnicity and perinatal depressed mood. Journal of Reproductive & Infant Psychology. 2006;24:99–106. [Google Scholar]
- Sichel D, Driscoll JW. Women’s moods: What every woman must know about hormones, the brain, and emotional health. New York: Quill/Harper Collins; 2000. [Google Scholar]
- Tam LW, Newton M, Dern M, Parry BL. Screening women for postpartum depression at well baby visits: Resistance encountered and recommendations. Archives of Women’s Mental Health. 2002;5:79–82. doi: 10.1007/s00737-002-0143-5. [DOI] [PubMed] [Google Scholar]
- Troutman B, Cutrona C. Nonpsychotic postpartum depression among adolescent mothers. Journal of Abnormal Psychology. 1990;99:69–78. doi: 10.1037//0021-843x.99.1.69. [DOI] [PubMed] [Google Scholar]
- Wei G, Greaver LB, Marson SM, Herndon CH, Rogers J Robeson Healthcare Corp. Postpartum depression: Racial differences and ethnic disparities in a tri racial and bi ethnic population. Maternal & Child Health Journal. 2007;12:699–707. doi: 10.1007/s10995-007-0287-z. [DOI] [PubMed] [Google Scholar]
- World Health Organization. Integrated management of pregnancy and childbirth: WHO recommended interventions for improving maternal and newborn health 2nd ed. Geneva, Switzerland: Author; 2009 WHO/MPSO7.05, Retrieved from http://www.who.int/making_pregnancy_safer/en.
- World Health Organization. Mental health. Geneva, Switzerland: Author; 2010 Retrieved from http://www.who.int/mental_health/management/depression/definition/en/
- Yonkers KA, Ramin SM, Rush AJ, Navarette AJ, Carmody T, March D, Leveno KJ. Onset and persistence of postpartum depression in an inner-city maternal health clinic system. American Journal of Psychiatry. 2001;158:1856–1863. doi: 10.1176/appi.ajp.158.11.1856. [DOI] [PubMed] [Google Scholar]
- Zelkowitz P, Saucier JF, Wang T, Katofsky L, Valenzuela M, Westrich R. Stability and change in depressive symptoms from pregnancy to two months postpartum in childbearing immigrant women. Archives of Women’s Mental Health. 2008;11:1–11. doi: 10.1007/s00737-008-0219-y. doi: 10.1007/s00737-008-0219-y. [DOI] [PubMed] [Google Scholar]