Abstract
Rationale
The h5-HT7 receptor is subject to inactivation by risperidone and 9-OH-risperidone, apparently through a pseudo-irreversible complex formed between these drugs and the receptor. Although risperidone and 9-OH-risperidone (“inactivating antagonists”) completely inactivate the receptor, only 50% of the receptors form a pseudo-irreversible complex with these drugs.
Objectives
This study aims to more fully determine the mechanism(s) responsible for the novel effects of risperidone and 9-OH-risperidone and to determine if the inactivation can be reversed (reactivation).
Methods
The ability of non-inactivating drugs (competitive antagonists) to dissociate wash-resistant [3H]risperidone binding from h5-HT7 receptors was investigated. Also, the ability of non-inactivating drugs to reactivate inactivated h5-HT7 receptors was investigated, using cAMP accumulation as a functional endpoint.
Results
The competitive (non-inactivating) antagonists clozapine and mesulergine released the wash-resistant [3H]risperidone binding to the h5-HT7 receptor. The competitive antagonists clozapine, SB269970, mianserin, cyproheptadine, mesulergine, and ICI169369 reactivated the risperidone-inactivated h5-HT7 receptors in a concentration-dependent manner. The potencies for reactivation closely match the affinities of these drugs for the h5-HT7 receptor (r2=0.95), indicating that the reactivating antagonists are binding to and producing their effects through the orthosteric binding site of the h5-HT7 receptor. Bioluminescence resonance energy transfer analyses indicate that the h5-HT7 receptor forms homodimers.
Conclusions
The ability of the non-inactivating drugs to bind h5-HT7 orthosteric sites and reverse the wash-resistant effects of risperidone or 9-OH-risperidone, also bound to h5-HT7 orthosteric sites, is evidence for protomer–protomer interactions between h5-HT7 homodimers. This is the first demonstration of a non-mutated G-protein-coupled receptor homodimer engaging in protomer–protomer interactions in an intact cell preparation.
Keywords: Serotonin, Serotonin receptor, 5-HT7, Homodimer, Inactivation, Reactivation, GPCR, Risperidone, 9-OH-risperidone, Clozapine, Protomer–protomer interactions
Introduction
The possibility that G-protein-coupled receptors (GPCRs) form functional dimers is best documented for the GABABR1/GABABR2 heterodimer complex (Blein et al. 2000). The GABABR heterodimer forms in the endoplasmic reticulum (ER). The GABABR2 protomer functions by occluding the ER retention site on the GABABR1 protomer, allowing the plasma membrane expression of the GABABR1/GABABR2 functional complex. The existence of homodimers and heterodimers in recombinant cell lines has been demonstrated for many GPCRs, but the understanding of the functionality of the dimer structure has been elusive (Bouvier 2001; Milligan and Smith 2007; Minneman 2007; Devi 2001; Han et al. 2009; Ferre et al. 2009; Herrick-Davis et al. 2005, 2006; Bokoch et al. 2010). There has been a lack of convincing evidence for functional interactions between the protomers of homodimeric or heterodimeric GPCRs expressed in recombinant cell lines, despite a rather large body of data supporting the existence of dimers and higher order GPCR oligomers. The vast majority of the studies attempting to demonstrate a functional interaction between the protomers of a homo-or heterodimer involve mutated forms of the receptor expressed in recombinant cell lines, subjected to solubilization, purification, and re-constitution in an artificial environment, i.e., lipid vesicles (Ferre et al. 2009). Thus, interpreting the results of these studies has been difficult. The rhodopsin receptor has been visualized as a dimer, and possibly a tetramer, using atomic force microscopy, although this is also an artificial environment (Fotiadis et al. 2006). Furthermore, there is dispute among biophysicists as to the validity of the procedures used to produce the images indicating dimer pairs for rhodopsin (Chabre et al. 2003). In summary, while the existence of dimeric GPCRs is indicated in recombinant cell lines, the function of the dimeric structure remains a challenging but potentially critical area of GPCR research.
Recently, we have observed some unique properties of h5-HT7 receptor/drug interactions in intact cells (Smith et al. 2006; Knight et al. 2009; Toohey et al. 2009). Risperidone, 9-OH-risperidone, methiothepin, bromocriptine, metergoline, and lisuride have been demonstrated to inactivate the h5-HT7 receptor as defined by the rapid loss of the ability of 5-HT to stimulate cAMP accumulation through the receptor (Smith et al. 2006; Knight et al. 2009). This inactivation appears to be due to a pseudo-irreversible interaction between these drugs and the receptor. Furthermore, inactivating antagonists have been shown to inhibit forskolin-stimulated adenylate cyclase activity through interaction with the h5-HT7 receptor in a non-Gi/o-dependentmanner in recombinant cell lines expressing the h5-HT7 receptor (Toohey et al. 2009). This is not predicted by any model of GPCR function and indicates that the inactivating antagonists are altering the conformation of the h5-HT7 receptor and not just occluding the orthosteric binding pocket.
The complex formed by the six inactivating antagonists and the h5-HT7 receptor appears to be unique. The rapidly formed complex is a stable, inactivated state of the receptor. Preliminary studies have shown that the [3H]risperidone bound to the h5-HT7 receptor in a wash-resistant manner could be released by treatment with TCA (precipitating the receptor), indicating that [3H]risperidone binding is pseudo-irreversible (no covalent binding involved). These unusual properties of risperidone and 9-OH-risperidone on the h5-HT7 receptor are only observed in intact cells and not observed when membrane preparations from these cells are exposed to these drugs (Smith et al. 2006; unpublished observations).
Interestingly, we observed that risperidone and 9-OH-risperidone bound only 50% of the h5-HT7 binding sites in a wash-resistant manner yet fully inactivate the h5-HT7 receptor (Knight et al. 2009). Additionally, we observed that [3H]risperidone wash-resistant binding to the h5-HT7 receptor dissociated after exposure to a competitive (non-inactivating) antagonist (see “Results” section). These observations prompted us to hypothesize that h5-HT7 receptors exist in a homodimeric state, wherein the receptor protomers are capable of functional interactions. Protomer–protomer interactions conceivably allow for the inactivated conformation of a risperidone-bound protomer to inhibit the activity of the other protomer. Furthermore, binding of non-inactivating antagonists (competitive antagonists) to the unoccupied protomer of a risperidone-inactivated h5-HT7 dimer releases risperidone and allows the h5-HT7 receptor dimer to once again be stimulated by 5-HT (reactivation). This hypothesis predicts that the potencies of reactivation would match the affinities of the reactivating ligands for the h5-HT7 orthosteric binding site (Smith et al. 2006; Knight et al. 2009). This hypothesis also predicts that bioluminescence resonance energy transfer (BRET) analysis would reveal that h5-HT7 receptors exist predominantly in a homodimeric state.
Methods
Homogenate radioligand binding
Radioligand binding studies in membrane homogenates were performed as described previously with modifications (Smith et al. 2006; Purohit et al. 2005). HEK cells stably expressing h5-HT7 receptors (100-mm dish; ~100% confluent) were scraped and collected in 50 mM Tris–HCl, 0.5 mM EDTA, and 10 mM MgSO4, pH 7.7 (at 23°C), centrifuged at 14,000×g for 30 min, homogenized using a Polytron homogenizer (Kinematica, Basel, Switzerland), and centrifuged again at 14,000×g for 30 min. The membranes were resuspended in 50 mM Tris–HCl, 0.5 mM EDTA, 10 mM MgSO4, and 0.1% ascorbic acid, pH 7.5 (at 23°C). Assays were performed in triplicate 1.0 ml volumes containing 10 μg of membrane protein (added last). Assays containing 1 or 10 nM [3H]5-HT (24 Ci/mmol; Perkin-Elmer Life and Analytical Sciences, Boston, MA), 1 nM [3H]mesulergine (82 Ci/mmol; GE Healthcare, Little Chalfont, Buckinghamshire, UK), or 1 nM [3H]risperidone (22 Ci/mmol; Janssen Pharmaceutica, NewBrunswick, NJ; Gommeren et al. 1997) were performed in the absence and presence of 10 μM clozapine to detect the level of available h5-HT7 receptors. Specific binding was generally 85–95% of total binding. Tubes were incubated for 30 min at 37°C, filtered using the Brandel filtration system, and counted using liquid scintillation. Experimental results were analyzed using Prism (GraphPad Software, San Diego, CA). Protein content of the samples was determined with the use of a BCA protein assay kit (Pierce Chemical, Rockford, IL).
Whole cell radioligand binding
Whole cell radioligand binding studies were performed as described previously with modifications (Smith et al. 2006; Knight et al. 2009). Cells were lifted using 1 ml/dish of diluted trypsin-EDTA (1:3 in PBS), followed by the addition of 5 ml/dish of HEPES buffer (20 mM HEPES, 2.5 mM MgSO4, 134 mM NaCl, pH 7.5 at 23°C). Cells were gently centrifuged for 3 min at 330×g, supernatant was aspirated, and cells were resuspended in HEPES buffer. Cells were pre-treated with drug, incubated 30 min at 37°C. Cells were then washed 3× 10 min in HEPES buffer: each wash consisted of the cells being centrifuged for 3 min at 330×g, resuspended in buffer, and incubated for 10 min. The cells were then centrifuged for 3 min at 330×g, resuspended in HEPES, and added to the assay tubes. For recovery assays, cells were resuspended in the appropriate concentration of drug (or control buffer), incubated for 90 min, washed 3×10 min with HEPES, resuspended in HEPES, and added to the assay tubes. Assay tubes contained 1 nM [3H]mesulergine or 1 nM [3H]risperidone. Clozapine (10 μM) was used to define non-specific binding. Assay tubes were incubated for 30 min at 37°C, filtered using the Brandel filtration system, and counted using liquid scintillation. The Bmax values for [3H]risperidone, and [3H]mesulergine were 3,300 and 2,700 fmol/mg protein, respectively, using homologous competition curve data to estimate the Bmax.
cAMP Assay
Total cAMP accumulation was measured using the LANCE cAMP Detection kit (Perkin-Elmer). Cells were cultured for 18 h in serum-free media, then lifted using 1 ml/dish diluted Versene (1:3 in PBS), followed by the addition of 11 ml/dish HEPES buffer. Cells were centrifuged for 3 min at 330×g, supernatant was aspirated, and the cells were resuspended in HEPES buffer. Cells were incubated for 30 min with the inactivating antagonist at 37°C. The cells were then washed 2×10 min with HEPES buffer (using the wash procedure described above). After the second wash, cells were resuspended in HEPES buffer and treated with h5-HT7 receptor antagonists for 90 min at 37°C. Cells were then washed 2×10 min with HEPES buffer. Cells were resuspended in stimulation buffer (prepared according to the Perkin-Elmer LANCE cAMP Detection Kit manual) and then counted using a hematocytometer. The cells were then added to 96-well opaque plates at the appropriate density of cells per well. The cells were then exposed to 10 μM 5-HT for 30 min at room temperature. The reaction was stopped, and cells were lysed by the addition of 20 μl detection buffer (prepared according to the Perkin-Elmer LANCE cAMP Detection Kit manual). The assay plate was incubated for another 2 h at room temperature, and then time-resolved fluorescence resonance energy transfer was detected by a Victor3 1420 multilabel plate-reader (Perkin-Elmer).
Bioluminescence Resonance Energy Transfer
HEK-293 cells at 4×106 cells/100 mm dish were transiently transfected with plasmid DNA and grown overnight in serum-free MEM. Co-transfection consisted of 0.5 μg of BRET donor (h5-HT7/Renilla luciferase (Rluc), M4/Rluc) and 0.5 μg or 1 μg of BRET acceptor (h5-HT7/yellow fluorescent protein (YFP), M4/YFP). Co-transfection of 0.5 μg BRET donor and 0.5 μg pcDNA3.1 (vector that expresses YFP, no receptor) established a no BRET control and was used to calculate the CF (conversion factor) and normalize BRET readings (see below). YFP fluorescence was verified under a fluorescence microscope, and cells were counted. Media was aspirated, and cells were rinsed twice in PBS. Cells were scraped in PBS, centrifuged for 3 min at 330×g, and resuspended in PBS. Coelenterazine f (Molecular Probes, Invitrogen) and cell suspension were combined in a 96-well white opaque plate for a final concentration of 5 μM coelenterazine f and approximately 5×106 cells/ml. Luminescence was immediately detected by a Victor3 1420 multilabel plate-reader (Perkin-Elmer) under a filter set to detect light emission in the 460-nm or 535-nm wavelengths. The equation to calculate BRET ratios as previously described (Angers et al. 2000) was adapted: BRET ratio = [(emission 535−emission460×CF)]/emission 460, where CF (conversion factor) = emission 535/emission 460 for cells co-expressing the Rluc construct and pcDNA3.1.
Results
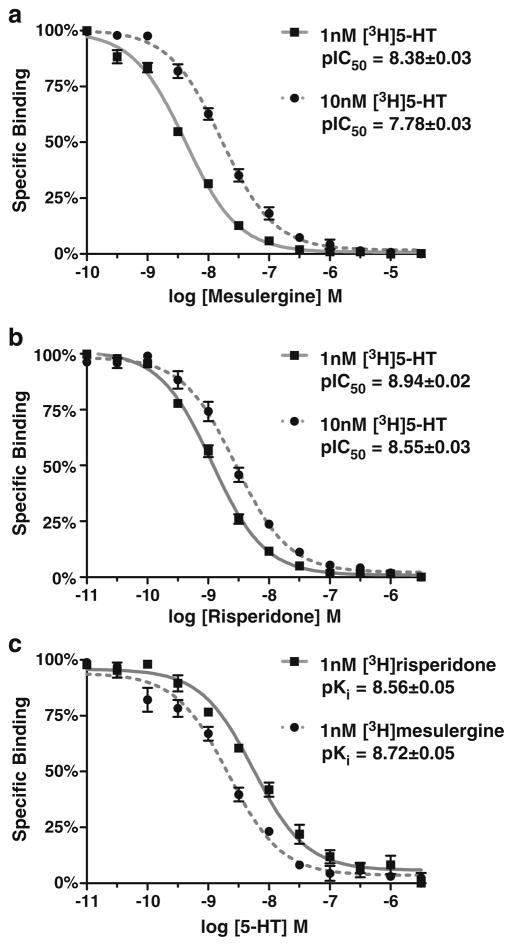
Radioligand binding studies on h5-HT7 receptors in recombinant cell lines have revealed high to moderate affinities for the drugs relevant to this study (Bard et al. 1993; Lovenberg et al. 1993; Shen et al. 1993; Smith et al. 2006; Knight et al. 2009). These previous studies determined Ki values of drugs by competition of drugs with a constant concentration of radioligand (i.e., inhibition curves). This methodology is sufficient when there is no question raised concerning the competitive nature of the inhibition. The unusual effects observed in the current investigation and our previous reports (Smith et al. 2006; Knight et al. 2009) reveal a non-competitive nature and raise the possibility of an allosteric mechanism. In order to rule out interactions of critical ligands (5-HT, risperidone, and mesulergine) at a h5-HT7 receptor allosteric site, membrane homogenate radioligand binding studies were conducted to identify orthosteric site affinities (Fig. 1). It is critical to note that the pseudo-irreversible binding properties of risperidone are not observed in membrane homogenate preparations (Smith et al. 2006; unpublished observations), hence the competitive nature of risperidone in this experiment. If the interaction is competitive at a single binding site, increasing the concentration of radioligand should increase the IC50 values for the inhibiting ligand. Therefore, we generated inhibition curves using two concentrations of the orthosteric ligand [3H]5-HT. Mesulergine and risperidone inhibit [3H]5-HT binding in membrane homogenates of h5-HT7-expressing cells, and inhibition curves are shifted rightward by increasing concentration of radioligand (Fig. 1). The use of 1 and 10 nM [3H]5-HT should produce a fourfold shift in the two competition curves (Cheng 1973). While this was observed for mesulergine, the risperidone competition curve was shifted 2.5-fold. This difference in degree of shift may be due to some unique property of risperidone’s interaction with the h5-HT7 receptor in homogenates, although it does not produce an irreversible loss of binding in homogenates (Smith et al. 2006; unpublished observations). The ability of 5-HT to potently inhibit [3H]risperidone and [3H] mesulergine binding is of particular interest (Fig. 1c). An orthosteric agonist, such as 5-HT, inhibiting the binding of an allosteric ligand to an allosteric site has not been reported for any GPCR. Thus, these results indicate that 5-HT, risperidone, and mesulergine interact with the orthosteric binding site on the h5-HT7 receptor and that the affinities of drugs determined using membrane homogenates of h5-HT7-expressing cells are those for the orthosteric site. This is critical for interpreting the data in Figs. 4 and 5 (see “Discussion” section). It should be noted that the 5-HT7 receptor does not display high and low agonist affinity states when labeled by antagonist radioligands, unlike most other monoamine GPCRs (Shen et al. 1993; Bard et al. 1993; Lovenberg et al. 1993). Consistent with these previous reports, the hill coefficients of the 5-HT competition curves for the antagonist radiolabeled h5-HT7 receptor were not significantly different than unity (Fig. 1c).
Fig. 1.
Radioligand binding studies of 5-HT, mesulergine, and risperidone at the h5-HT7 orthosteric binding site in membrane homogenates. a Increasing the concentration of [3H]5-HT (KD= 2.0 nM) used to radiolabel the h5-HT7 receptor from 1 to 10 nM shifts the mesulergine competition curve fourfold to the right, while the calculated affinity remains the same (Ki=2.8 nM) at 1 and 10 nM [3H]5-HT. b The risperidone competition curves are shifted 2.5-fold to the right. c 1 nM [3H]Mesulergine and [3H]risperidone were used to radiolabel the h5-HT7 receptor, and increasing concentrations of 5-HT were tested to determine its affinity for the radiolabeled receptor. The results are the mean±SEM of three experiments performed in triplicate
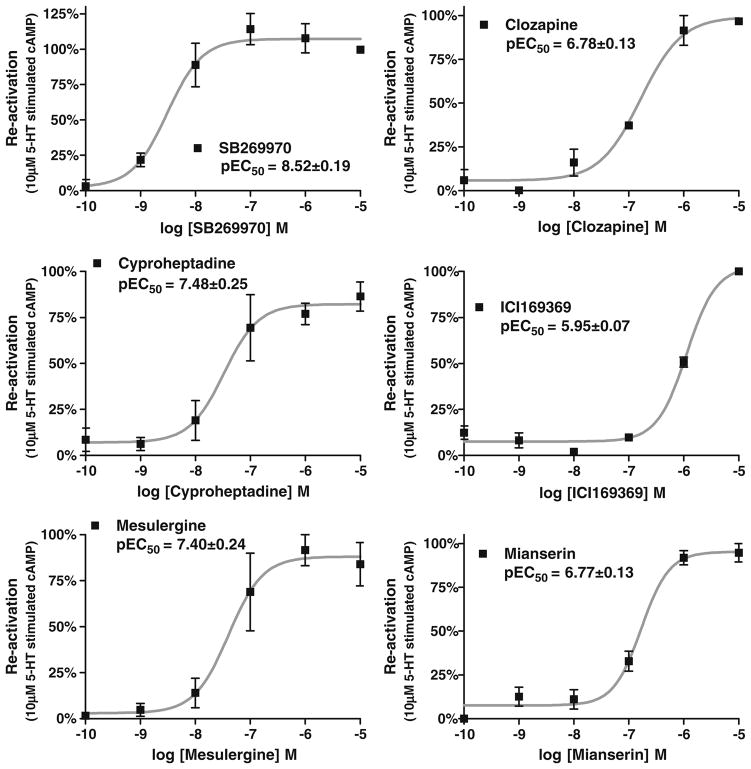
Fig. 4.
Concentration–response curves of six antagonists for the reactivation of risperidone-inactivated h5-HT7 receptors. Cells were exposed to 10 nM risperidone, and reactivation was determined as described in Fig. 3. Clozapine, SB269970, mianserin, cyproheptadine, mesulergine, and ICI169369 produced potent, concentration-dependent reactivation of the h5-HT7 receptor. The partial effect of 1 μM ICI169369 observed in Fig. 3 is due to its lower potency. The results are the mean±SEM of three experiments performed in triplicate
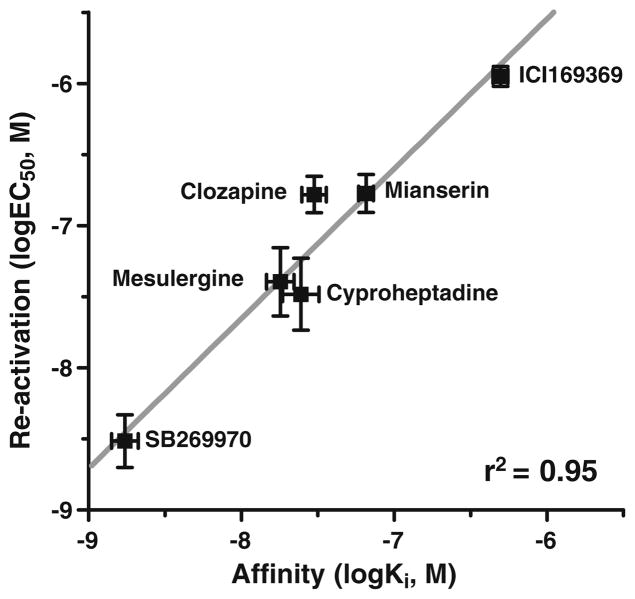
Fig. 5.
Correlation between the reactivation potencies (log EC50, Fig. 4) and affinities (log Ki) of non-inactivating drugs at the h5-HT7 receptor. The high correlation observed (r2=0.95) indicates that the reactivation of risperidone-inactivated h5-HT7 receptors is due to the binding of the reactivating drugs to the orthosteric binding site of the h5-HT7 receptor despite the presence of wash-resistant [3H]risperidone binding to the orthosteric site. Membrane homogenate Ki values (nanomolar): SB269970, 2±0.6; mesulergine, 18±3; cyproheptadine, 24±5; clozapine, 30±5; mianserin, 64±5, ICI169369, 393±54
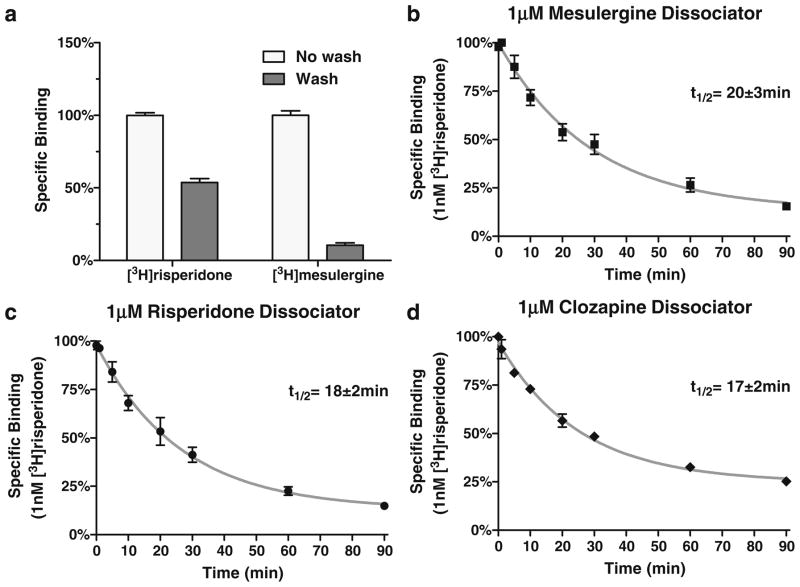
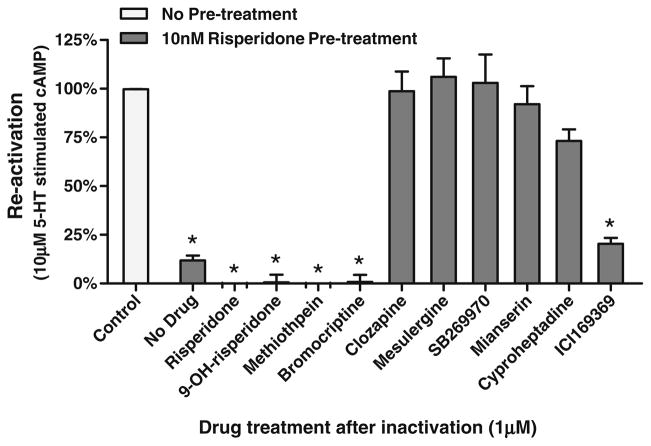
Figure 2 displays the apparently anomalous properties of [3H]risperidone specific binding to the h5-HT7 receptor in intact cells. As previously demonstrated (Smith et al. 2006; Knight et al. 2009), [3H]risperidone binds in a pseudo-irreversible manner to the h5-HT7 receptor. After exposure to 1 nM [3H]risperidone, thorough washing of recombinant cells expressing h5-HT7 receptor reveals that ~50% of the [3H]risperidone specific binding is bound pseudo-irreversibly (i.e., “wash-resistant” binding; Fig. 2a). However, when an experiment designed to detect a dissociation rate was conducted, the “wash-resistant” [3H]risperidone binding readily dissociates (Fig. 2b–d). The difference in the experimental design of Fig. 2a and of Fig. 2b–d is the presence of a second, non-radiolabeled drug in the dissociation experiments. This second drug is used to fully occupy the receptors, preventing the re-association of the radioligand to the receptor, thus revealing the radioligand’s dissociation rate. In Fig. 2b–d mesulergine, risperidone, and clozapine were used, respectively, as the receptor-occupying drugs. Surprisingly, these experiments revealed a dissociation of the “wash-resistant” [3H]risperidone specific binding. If, as shown in Fig. 2b–d, mesulergine, clozapine, and risperidone can dissociate risperidone from the h5-HT7 receptor, these drugs and others should be capable of reactivating risperidone-inactivated h5-HT7 receptors. In order to investigate this possibility, risperidone pre-treated cells were exposed to 1 μM non-inactivating and inactivating antagonists in intact cells (Fig. 3). As predicted by the results in Fig. 2b–d, exposure of the risperidone pre-treated cells to the non-inactivating antagonists mesulergine, clozapine, mianserin, cyproheptadine, SB269970, and ICI169369 followed by washout of the drugs results in reactivation of the receptor. Exposure of the risperidone pre-treated cells to the inactivating antagonists risperidone, 9-OH-risperidone, methiothepin, and bromocriptine did not reactivate the receptor. Rather, the addition of these drugs appears to produce an additive inactivating effect.
Fig. 2.
Anomalous radioligand binding properties of [3H]risperidone binding to h5-HT7 receptors on intact cells. a Fifty percent of specific [3H]risperidone binding is wash-resistant while most of the [3H] mesulergine specific binding is not. The lower level of [3H] mesulergine binding relative to [3H]risperidone binding is due to the use of 1 nM for both radioligands, although risperidone’s Ki is 1 nM and mesulergine’s Ki is 8 nM. The absolute level of binding for the radioligands under the conditions of the experiment was 1,200 fmol/mg for [3H]risperidone and 120 fmol/mg for [3H]mesulergine. b–d Adding 1 μM mesulergine, risperidone, or clozapine to the wash-resistant [3H]risperidone binding in intact cells results in the dissociation of [3H]risperidone from the receptor. The results are the mean±SEM of three experiments performed in triplicate
Fig. 3.
Reactivation of risperidone-inactivated h5-HT7 receptors. Four inactivating antagonists and six non-inactivating antagonists were tested for the ability to reverse risperidone inactivation of the h5-HT7 receptor. Cells were exposed to 10 nM risperidone, thoroughly washed, then exposed to 1 μM of drug for 90 min, thoroughly washed again, and stimulated with 10 μM 5-HT (black bars; see “Methods” section). The four inactivators (risperidone, 9-OH-risperidone, methiothepin, and bromocriptine) as well as ICI169369 did not reactivate the receptor; all other drugs reactivated the receptor as defined by sensitivity to 10 μM 5-HT. The results are the mean±SEM of three experiments performed in triplicate (*p<0.001 vs. control, one-way ANOVA with Dunnett’s post-test)
The inability of risperidone to reactivate the h5-HT7 receptor apparently contradicts the results of Fig. 2c, wherein exposure to risperidone dissociated pseudo-irreversibly bound [3H]risperidone. This apparent contradiction can be explained by the exchange of non-radioactive risperidone for [3H]risperidone. Non-radioactive risperidone appears to be binding to the same site that mesulergine and clozapine are binding to, releasing [3H]risperidone from the receptor. However, unlike mesulergine and clozapine, which interact with this site in a competitive manner, risperidone pseudo-irreversibly binds to this site, leaving receptor in an inactive state (see “Discussion” section, Fig. 10).
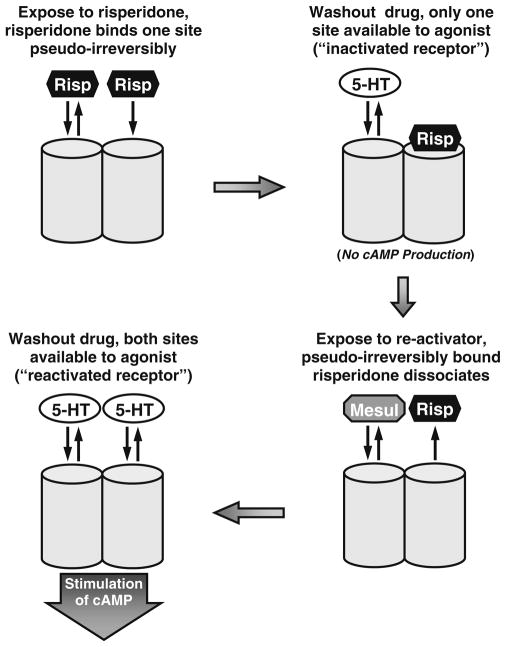
Fig. 10.
Schematic presentation of a homodimeric model consistent with the anomalous effects of drugs on the h5-HT7 receptor. Risperidone (Risp) binds to a protomer of an h5-HT7 receptor dimer resulting in a pseudo-irreversible complex between the drug and the protomer, the inactivation of the h5-HT7 receptor, and the inability of the second protomer to bind risperidone irreversibly. The second protomer retains reversible drug binding properties. The binding of a non-inactivating antagonist, such as mesulergine (Mesul) to the second protomer releases the pseudo-irreversibly bound risperidone from the first protomer, leaving a reactivated receptor. Presumably methiothepin and bromocriptine bind in a wash-resistant manner to both protomers, resulting in an inactivated receptor that cannot be reactivated (not shown in model)
The homodimer hypothesis predicts that both binding sites should have similar, if not identical, affinities for drugs, which can be assessed by further characterizing the reactivation process. Increasing concentrations of reactivating antagonists were used to produce concentration–response curves for the reactivation of risperidone-inactivated h5-HT7 receptors (Fig. 4). SB269970, clozapine, cyproheptadine, ICI169369, mesulergine, and mianserin produced a concentration-dependent reactivation. The potencies of these drugs in reactivating the h5-HT7 receptor correlated closely with their affinities for the receptor (Fig. 5). The partial effect of 1 μM ICI169369 observed in Fig. 3 is consistent with the low potency of this drug, presumably due to its relatively low affinity for the h5-HT7 receptor. Of primary importance is that the correlation indicates the reactivating drugs are interacting with the orthosteric binding site of the h5-HT7 receptor. This, in turn, indicates that an unoccupied h5-HT7 orthosteric site is binding a reactivating antagonist despite the presence of a pseudo-irreversibly bound inactivator (risperidone) at ~50% of the sites. This is consistent with the homodimer model proposed (see Discussion section; Fig. 10).
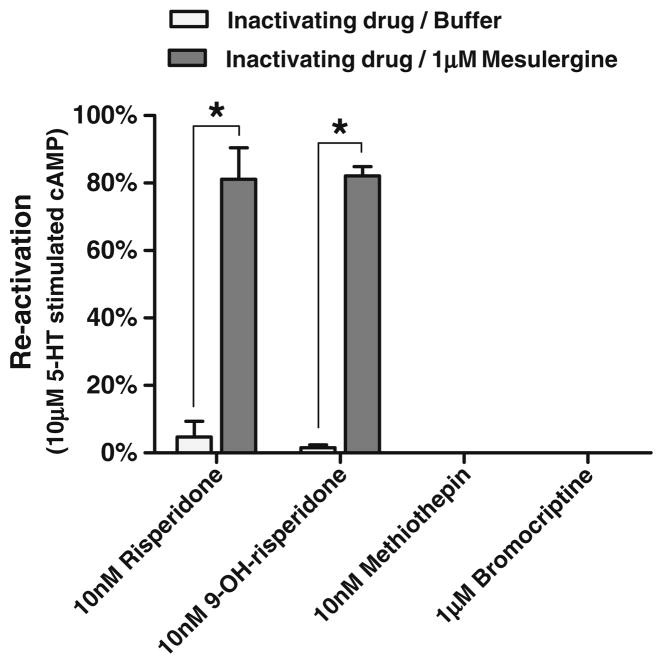
In order to determine if competitive antagonists could reverse the effects of other inactivators, cells were pre-treated with risperidone, 9-OH-risperidone, methiothepin, and bro-mocriptine, thoroughly washed, then treated with mesulergine (Fig. 6). While risperidone and 9-OH-risperidone-inactivated cells were reactivated by mesulergine, methiothepin- and bromocriptine-inactivated cells were not reactivated by mesulergine.
Fig. 6.
Mesulergine reactivation of inactivated h5-HT7 receptors. Risperidone, 9-OH-risperidone, methiothepin, and bromocriptine were used to inactivate the h5-HT7 receptor; cells were thoroughly washed and were subsequently exposed to 1 μM mesulergine. After removal of mesulergine, cells were exposed to 10 μM 5-HT and assayed for stimulation of cAMP accumulation (as described above, Figs. 3 and 4). The four inactivating antagonists fully inactivated the h5-HT7 receptor. Mesulergine was able to reactivate the receptors treated with risperidone and 9-OH risperidone but was not able to reactivate those treated with methiothepin and bromocriptine. The results are the mean±SEM of three experiments performed in triplicate (*p<0.001, two-way ANOVA with Bonferroni’s post-tests)
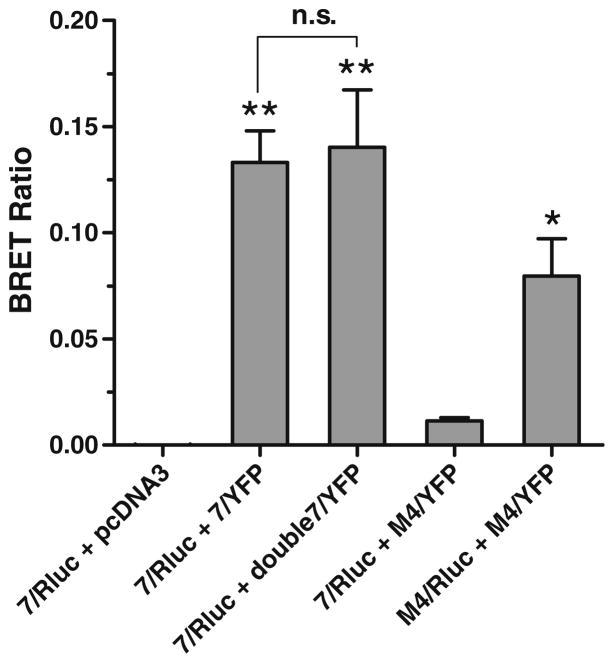
The hypothesis asserts that the h5-HT7 receptor should exist predominantly in a homodimeric state. Using BRET methodology, we demonstrated that h5-HT7 receptors have a high propensity to form homodimers in recombinant cells. cDNA coding for the h5-HT7 receptor tagged with either Rluc or YFP were synthesized, incorporated into a plasmid (pcDNA3.1), and co-transfected into HEK-293 cells (Fig. 7). Previous confocal microscopy studies have shown that GFP-tagged h5-HT7 receptors are predominantly expressed on the cell surface when transiently expressed in HEK-293 cells at levels similar to Fig. 7 (Smith et al. 2006). A robust BRET ratio signal was observed in cells co-expressing Rluc and YFP forms of the h5-HT7 receptor. Doubling the h5-HT7-YFP construct did not increase the BRET signal, indicating the h5-HT7-Rluc receptors were saturated with h5-HT7-YFP receptors and that artifactual BRET is not generated by receptor packing and other non-specific interactions (Pfleger and Eidne 2005). Significant BRET signals were not detected in cells transfected with the Rluc construct and an empty plasmid or in cells co-expressing h5-HT7-Rluc cDNA and M4-YFP muscarinic receptor constructs. We confirmed that co-transfection with the Rluc and YFP forms of the M4 muscarinic receptor also produces a robust BRET signal. These BRET results strongly indicate that h5-HT7 receptors exist predominantly as homodimers in the HEK-293 cells. Treatment of the cells expressing the Rluc and YFP forms of the h5-HT7 receptor with either 1 μM risperidone or 1 μM 5-HT did not produce a significant change in the BRET signal (data not shown). This is not surprising as the BRET signal has been found to be sufficiently sensitive so as to provide strong support for the dimer structure of various GPCRs but not sufficiently sensitive to detect the interaction of drugs with the GPCR dimer structure (Ayoub and Pfleger 2010; Pfleger and Eidne 2005).
Fig. 7.
BRET assessment of h5-HT7 receptor homodimers in HEK-293 cells. Receptor constructs fusing Rluc or YFP onto the C-terminal tails of the h5-HT7 and muscarinic M4 receptors were synthesized, and combinations of these constructs were transiently transfected into HEK-293 cells. BRET ratios were calculated as described in the “Methods” section. A significant BRET signal was observed in cells co-transfected with equal quantities of h5-HT7/Rluc and h5-HT7/YFP receptor constructs. Doubling the expression of the BRET acceptor (h5-HT7/YFP) did not increase the BRET signal, which is consistent with a specific interaction between the h5- HT7 receptor constructs. Co-transfection of equal quantities of h5-HT7/Rluc and M4/YFP receptor constructs did not yield a significant BRET signal. This is consistent with the lack of specific interaction between these receptors. A significant BRET signal is observed between M4/Rluc and M4/YFP receptor constructs, indicating that the M4 receptor constructs can produce BRET. Taken together, these results indicate that h5-HT7 receptor exists as a homodimer in the h5-HT7 receptor-expressing HEK-293 cells. Results are the means±SEM of three independent experiments (*p<0.01, **p<0.001, one-way ANOVA with Dunnett’s post-test and “7/Rluc+pcDNA3” as control; n.s.p>0.05, unpaired t test)
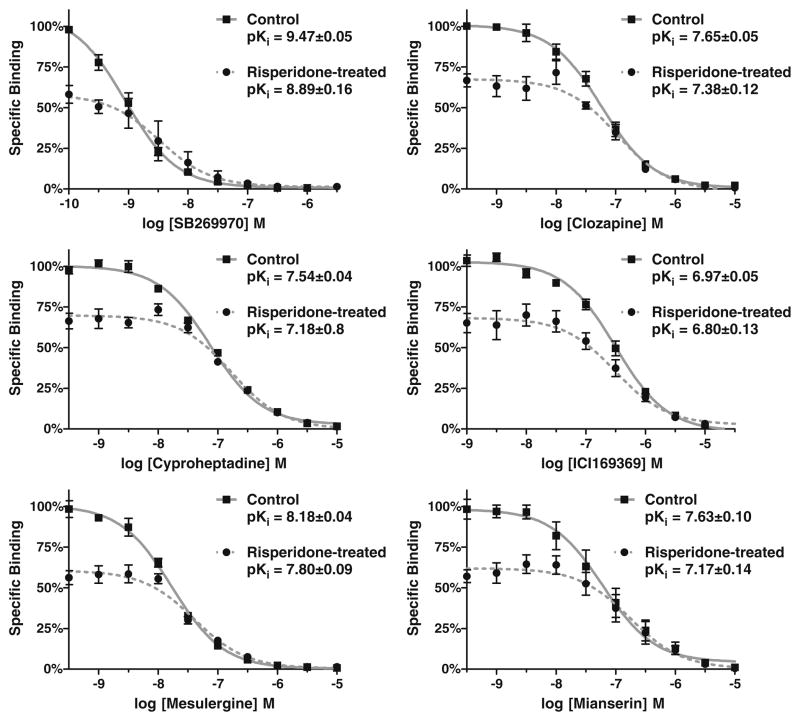
In order to determine the effect of pseudo-irreversibly bound risperidone on the 50% of h5-HT7 receptors not blocked in a wash-resistant manner, competition studies with six non-inactivating antagonists were performed (Fig. 8). The affinities of the six drugs for the remaining unoccupied h5-HT7 receptors are similar to the affinities for the h5-HT7 receptor not exposed to risperidone. A slight, but statistically significant, shift to lower affinities was detected for mianserin and SB269970 (legend, Fig. 8). This indicates the major effect of risperidone on the unoccupied site is to render it incapable of irreversible binding risperidone: the affinities for drugs appears only slightly altered.
Fig. 8.
Intact cell affinities of six antagonists for the h5-HT7 receptors not pseudo-irreversibly blocked by risperidone. Cells were exposed to 1 μM risperidone or buffer and washed, and radioligand binding studies were conducted on the untreated and risperidone-exposed h5-HT7 receptors on intact cells. [3H] 5-HT (2 nM) binding was used to radiolabel intact cells. Results are the means±SEM of three independent experiments performed in triplicate. Two-way ANOVA with Bonferroni’s post-tests shows significant differences (p<0.05) between the control and risperidone-treated groups for SB269970 and mianserin only, but not for clozapine, mesulergine, cyproheptadine, or ICI169369
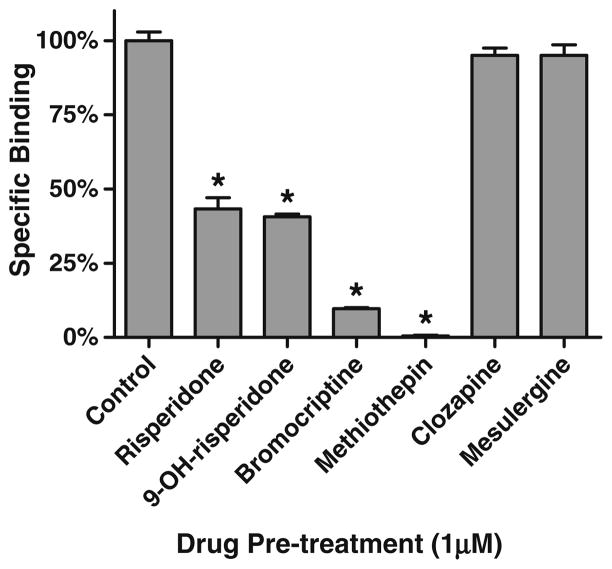
In order to determine the stability of the inactivating antagonist/h5-HT7 receptor complex, h5-HT7 receptor- expressing cells were exposed to the inactivating antagonists risperidone, 9-OH-risperidone, bromocriptine, and methiothepin, then washed, homogenized, and centrifuged (Fig. 9). Radioligand binding assays were performed on the resuspended membrane preparations. The loss of 50% of the h5-HT7 receptor binding sites due to risperidone or 9- OH-risperidone treatment was retained in the membrane homogenates as was the complete loss of binding due to bromocriptine or methiothepin treatment. The inactivating antagonist/h5-HT7 receptor complex appears to be resistant to the physical disruption of homogenization.
Fig. 9.
Wash-resistant inhibition of h5-HT7 radioligand binding after exposure of intact cells to drugs followed by homogenization. h5-HT7 receptor-expressing cells were exposed to either 1 μM drug or buffer (control) for 30 min. Cells were washed, homogenized, and assayed for radioligand binding using 2 nM [3H]5-HT. Risperidone and 9-OH-risperidone inhibit 50% of the binding while methiothepin and bromocriptine inhibit all of the binding; exposure to clozapine and mesulergine did not affect binding. Results are the means±SEM of three independent experiments performed in triplicate.*p<0.001, determined by one-way ANOVA with Dunnett’s post-test
Discussion
In order to understand the necessity of invoking a homodimer model of h5-HT7 receptor function, it is necessary to focus on four observations:
Risperidone and 9-OH-risperidone fully inactivate the h5-HT7 receptor (Fig. 3; Smith et al. 2006; Knight et al. 2009).
Risperidone and 9-OH-risperidone pseudo-irreversibly occlude only 50% of the h5-HT7 receptor orthosteric sites (Fig. 2; Knight et al. 2009).
Non-inactivating (competitive) antagonists act through the h5-HT7 receptor orthosteric binding site to release pseudo-irreversibly bound [3H]risperidone from the h5-HT7 receptor (Fig. 2).
Non-inactivating antagonists act through the h5-HT7 receptor orthosteric binding site to reverse the inactivating effects of risperidone and 9-OH-risperidone (Figs. 4 and 5).
A fundamental concept necessary for interpreting our results is that drugs are generally designed to interact with empty binding sites. [3H]risperidone clearly binds in a wash-resistant, and apparently pseudo-irreversible manner to the orthosteric binding site of the h5-HT7 receptor. Therefore, any theory to explain the “displacement” of risperidone from the h5-HT7 receptor by other drugs must explain the apparent access of these drugs to an empty binding site that causes risperidone to dissociate from the occupied orthosteric site of the h5-HT7 receptor.
Classical allosterism is one mechanism by which drugs bind to a non-orthosteric binding site on a receptor (an allosteric site) and alter the behavior of the drug–receptor interaction at the orthosteric site. Thus, classical allosterism could explain the ability of mesulergine, clozapine, mianserin, cyproheptadine, SB269970, and ICI169369 to dissociate risperidone from the h5-HT7 receptor. Allosterism is a well-established phenomenon in both the ligand-gated ion channel receptor family and GPCR families. However, the pharmacological profiles of allosteric binding sites are very different than the pharmacological profiles of orthosteric sites (Conn et al. 2009). Drugs that allosterically modulate the activity of orthosteric sites do not have significant affinities for the orthosteric site. The results in Figs. 4 and 5 strongly indicate that an allosteric mechanism is highly unlikely. Otherwise, one would be forced to hypothesize that both an allosteric and an orthosteric site exist on the h5-HT7 receptor monomer that are essentially identical in their binding affinities for the six drugs used as reactivators. Such a situation has never been reported in the GPCR literature.
Taken together, a homodimeric structure for the h5-HT7 receptor involving a form of protomer–protomer interaction appears to explain the results of the present study as well as previous studies (Smith et al. 2006; Knight et al. 2009; Fig. 10). The active, unoccupied receptor is capable of binding one molecule of risperidone or 9-OH-risperidone in a pseudo-irreversible manner. This binding produces two subsequent results: (1) the receptor is inactivated and incapable of activating Gs, and (2) the other protomer is capable of binding another molecule of risperidone or 9-OH-risperidone in a reversible manner with high affinity. The binding of a non-inactivator to the second protomer of a risperidone or 9-OH-risperidone-inactivated h5-HT7 dimer results in the dissociation of risperidone or 9-OH-risperidone from the other protomer. After washout of these drugs, the h5-HT7 receptor will be in its initial activated, drug-unoccupied state. Thus, the homodimeric structure involving protomer–protomer interaction predicts the observed reactivation of the h5-HT7 receptor by competitive antagonists as well as the maximal 50% irreversible binding of risperidone or 9-OH-risperidone.
In order to learn more about the state of the unblocked receptors remaining after risperidone or 9-OH-risperidone treatment, radioligand binding studies were performed on the intact cells after treatment with 1 μM risperidone (Fig. 8). The affinities of the six drugs studied for the risperidone-resistant sites appear slightly lower than their affinities for the h5-HT7 receptors on cells not exposed to risperidone, although statistical significance was observed for only mianserin and SB269970 (legend, Fig. 8). These results indicate the major effect of risperidone on the non-pseudo-irreversibly labeled h5-HT7 receptors is to render them incapable of binding risperidone (or 9-OH-risperidone) irreversibly, without producing a profound alteration in the affinities of drugs for the orthosteric site. The ability of risperidone (and 9-OH-risperidone) to produce this effect is in contrast to the effects of methiothepin and bromocriptine, which appear to pseudo-irreversibly bind to all the h5-HT7 receptors (Knight et al. 2009). Thus, the radioligand binding studies on risperidone or 9-OH-risperidone-treated cells are consistent with protomer–protomer interactions (Fig. 10).
We have noted that the novel pseudo-irreversible binding of risperidone and 9-OH-risperidone are lost when membrane homogenates from the h5-HT7 receptor-expressing cells are used (Knight et al. 2009; unpublished observations). In order to determine the role the membrane environment might play in the complex formed between the inactivators and the h5-HT7 receptor, we exposed the cells to the drugs, washed out the drugs, and assayed membrane homogenates from the drug-exposed cells for receptor binding (Fig. 9). The ability of risperidone and 9-OH-risperidone to inhibit 50% of the receptors and the ability of bromocriptine and methiothepin to inhibit all of the receptors is retained even if the cells are homogenized, so long as the cells were intact when first exposed to these drugs. This indicates that there is a key factor in the intact cell that allows the novel drug–receptor interaction. Apparently, this factor is lost when the cells are first homogenized and then exposed to the inactivating drugs. Determining the properties of this factor may prove to be an interesting avenue of investigation, although beyond the scope of the current studies.
The inactivators methiothepin and bromocriptine appear capable of binding both protomers of the h5-HT7 receptor pseudo-irreversibly, producing an inactivated receptor but, unlike risperidone and 9-OH-risperidone, leaves no unoccupied orthosteric sites. When both protomers are occupied by these other inactivators, competitive antagonists are unable to disassociate the inactivators and reactivate the receptor (Fig. 6). Alternatively, the data do not rule out the possibility that the binding of methiothepin or bromocriptine to one protomer results in a type of protomer–protomer interaction in which the second protomer is rendered incapable of binding 5-HT or any drugs. Further investigations will be necessary to determine which of these models is correct, yet both models are consistent with the dimer hypothesis.
The existence of h5-HT7 receptor homodimers was demonstrated biophysically using BRET in order to support this unique receptor model (Fig. 7). The significant BRET signal observed when Rluc and YFP-conjugated forms of the h5-HT7 receptor are co-expressed in HEK-293 cells is consistent with the robust formation of h5-HT7 receptor dimers. Control transfections involving plasmids lacking the h5-HT7-YFP construct or substituting the M4-muscarinic-YFP construct for the h5-HT7-YFP construct demonstrate the specificity of the BRET signal seen from the Rluc and YFP forms of the h5-HT7 receptor. The lack of increased BRET signal when doubling the h5-HT7-YFP construct relative to the h5-HT7-Rluc construct indicates that random receptor interaction is not responsible for the signal, as a 1:1 stoichiometry of these receptor constructs is sufficient in order to observe maximum BRET.
In summary, the model presented in Fig. 10 appears to explain the results observed. While the existence of dimers is certainly not a novel observation, the readily observed functional interaction between the protomers of a native, human form of a GPCR is a rarity (Pin et al. 2007). It will be of interest to use the strategies developed in these studies to determine the possibility that other receptor homodimers can display the pseudo-irreversible and/or reactivating properties observed with the h5-HT7 receptor. Other than the confirmatory BRET experiment, our ability to observe the dimer-related GPCR properties described herein was achieved using native h5-HT7 receptors and methodologies that are not limited to mutated or tagged receptors expressed in recombinant cell lines. This suggests that the existence of native 5-HT7 homodimers can be determined in tissues that endogenously express the 5-HT7 receptor by demonstrating the unique functional properties of the 5-HT7 homodimer. Investigation of GPCR dimers in primary cells and isolated tissue preparations is critical to establishing the physiological relevance of the GPCR dimer (Pin et al. 2007; manuscript submitted). Also, the role that dimer protomer–protomer interactions may play in the regulation of synaptic activity is an unexplored area of investigation. Neurological disorders may be traced to dysfunction in the protomer–protomer interactions of GPCRs, producing synaptic dysfunctional states. Drugs that take advantage of these newly discovered properties of GPCRs may produce novel therapeutic effects.
Acknowledgments
This work was supported by the United States Public Health Service [Grant MH56650].
References
- Angers S, Salahpour A, Joly E, Hilairet S, Chelsky D, Dennis M, Bouvier M. Detection of beta 2-adrenergic receptor dimerization in living cells using bioluminescence resonance energy transfer (BRET) Proc Natl Acad Sci USA. 2000;97:3684–3689. doi: 10.1073/pnas.060590697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayoub MA, Pfleger KD. Recent advances in bioluminescence resonance energy transfer technologies to study GPCR heteromerization. Curr Opin Pharmacol. 2010;10:44–52. doi: 10.1016/j.coph.2009.09.012. [DOI] [PubMed] [Google Scholar]
- Bard JA, Zgombick J, Adham N, Vaysse P, Branchek TA, Weinshank RL. Cloning of a novel human serotonin receptor (5-HT7) positively linked to adenylate cyclase. J Biol Chem. 1993;268:23422–23426. [PubMed] [Google Scholar]
- Blein S, Hawrot E, Barlow P. The metabotropic GABA receptor: molecular insights and their functional consequences. Cell Mol Life Sci. 2000;57:635–650. doi: 10.1007/PL00000725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bokoch MP, Zou Y, Rasmussen SG, Liu CW, Nygaard R, Rosenbaum DM, Fung JJ, Choi HJ, Thian FS, Kobilka TS, Puglisi JD, Weis WI, Pardo L, Prosser RS, Mueller L, Kobilka BK. Ligand-specific regulation of the extracellular surface of a G-protein-coupled receptor. Nature. 2010;463:108–112. doi: 10.1038/nature08650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouvier M. Oligomerization of G-protein-coupled transmitter receptors. Nat Rev Neurosci. 2001;2:274–286. doi: 10.1038/35067575. [DOI] [PubMed] [Google Scholar]
- Chabre M, Cone R, Saibil H. Biophysics: is rhodopsin dimeric in native retinal rods? Nature. 2003;426:30–31. doi: 10.1038/426030b. [DOI] [PubMed] [Google Scholar]
- Cheng Y, Prusoff WH. Relationship between the inhibition constant (K1) and the concentration of inhibitor which causes 50 per cent inhibition (I50) of an enzymatic reaction. Biochem Pharmacol. 1973;22:3099–3108. doi: 10.1016/0006-2952(73)90196-2. [DOI] [PubMed] [Google Scholar]
- Conn PJ, Christopoulos A, Lindsley CW. Allosteric modulators of GPCRs: a novel approach for the treatment of CNS disorders. Nat Rev Drug Discov. 2009;8:41–54. doi: 10.1038/nrd2760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devi LA. Heterodimerization of G-protein-coupled receptors: pharmacology, signaling and trafficking. Trends Pharmacol Sci. 2001;22:532–537. doi: 10.1016/s0165-6147(00)01799-5. [DOI] [PubMed] [Google Scholar]
- Ferre S, Baler R, Bouvier M, Caron MG, Devi LA, Durroux T, Fuxe K, George SR, Javitch JA, Lohse MJ, Mackie K, Milligan G, Pfleger KD, Pin JP, Volkow ND, Waldhoer M, Woods AS, Franco R. Building a new conceptual framework for receptor heteromers. Nat Chem Biol. 2009;5:131–134. doi: 10.1038/nchembio0309-131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fotiadis D, Jastrzebska B, Philippsen A, Muller DJ, Palczewski K, Engel A. Structure of the rhodopsin dimer: a working model for G-protein-coupled receptors. Curr Opin Struct Biol. 2006;16:252–259. doi: 10.1016/j.sbi.2006.03.013. [DOI] [PubMed] [Google Scholar]
- Gommeren W, Janssen PFM, Schotte A, Leysen JE. Detection of 5HT7 receptors using 3H-risperidone: a brain regional distribution. Psychotic Disorders and Antipsychotics. 1997;S4:120. [Google Scholar]
- Han Y, Moreira IS, Urizar E, Weinstein H, Javitch JA. Allosteric communication between protomers of dopamine class A GPCR dimers modulates activation. Nat Chem Biol. 2009;5:688–695. doi: 10.1038/nchembio.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrick-Davis K, Grinde E, Harrigan TJ, Mazurkiewicz JE. Inhibition of serotonin 5-hydroxytryptamine2c receptor function through heterodimerization: receptor dimers bind two molecules of ligand and one G-protein. J Biol Chem. 2005;280:40144–40151. doi: 10.1074/jbc.M507396200. [DOI] [PubMed] [Google Scholar]
- Herrick-Davis K, Weaver BA, Grinde E, Mazurkiewicz JE. Serotonin 5-HT2C receptor homodimer biogenesis in the endoplasmic reticulum: real-time visualization with confocal fluorescence resonance energy transfer. J Biol Chem. 2006;281:27109–27116. doi: 10.1074/jbc.M604390200. [DOI] [PubMed] [Google Scholar]
- Knight JA, Smith C, Toohey N, Klein MT, Teitler M. Pharmacological analysis of the novel, rapid, and potent inactivation of the human 5-hydroxytryptamine7 receptor by risperidone, 9-OH-risperidone, and other inactivating antagonists. Mol Pharmacol. 2009;75:374–380. doi: 10.1124/mol.108.052084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovenberg TW, Baron BM, De Lecea L, Miller JD, Prosser RA, Rea MA, Foye PE, Racke M, Slone AL, Siegel BW. A novel adenylyl cyclase-activating serotonin receptor (5-HT7) implicated in the regulation of mammalian circadian rhythms. Neuron. 1993;11:449–458. doi: 10.1016/0896-6273(93)90149-l. [DOI] [PubMed] [Google Scholar]
- Milligan G, Smith NJ. Allosteric modulation of heterodimeric G-protein-coupled receptors. Trends Pharmacol Sci. 2007;28:615–620. doi: 10.1016/j.tips.2007.11.001. [DOI] [PubMed] [Google Scholar]
- Minneman KP. Heterodimerization and surface localization of G protein coupled receptors. Biochem Pharmacol. 2007;73:1043–1050. doi: 10.1016/j.bcp.2006.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfleger KD, Eidne KA. Monitoring the formation of dynamic G-protein-coupled receptor–protein complexes in living cells. Biochem J. 2005;385:625–637. doi: 10.1042/BJ20041361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pin JP, Neubig R, Bouvier M, Devi L, Filizola M, Javitch JA, Lohse MJ, Milligan G, Palczewski K, Parmentier M, Spedding M. International union of basic and clinical pharmacology. LXVII. Recommendations for the recognition and nomenclature of G protein-coupled receptor heteromultimers. Pharmacol Rev. 2007;59:5–13. doi: 10.1124/pr.59.1.5. [DOI] [PubMed] [Google Scholar]
- Purohit A, Smith C, Herrick-Davis K, Teitler M. Stable expression of constitutively activated mutant h5HT6 and h5HT7 serotonin receptors: inverse agonist activity of antipsychotic drugs. Psychopharmacology (Berl) 2005;179:461–469. doi: 10.1007/s00213-004-2057-6. [DOI] [PubMed] [Google Scholar]
- Shen Y, Monsma FJ, Jr, Metcalf MA, Jose PA, Hamblin MW, Sibley DR. Molecular cloning and expression of a 5-hydroxytryptamine7 serotonin receptor subtype. J Biol Chem. 1993;268:18200–18204. [PubMed] [Google Scholar]
- Smith C, Rahman T, Toohey N, Mazurkiewicz J, Herrick-Davis K, Teitler M. Risperidone irreversibly binds to and inactivates the h5-HT7 serotonin receptor. Mol Pharmacol. 2006;70:1264–1270. doi: 10.1124/mol.106.024612. [DOI] [PubMed] [Google Scholar]
- Toohey N, Klein MT, Knight J, Smith C, Teitler M. Human 5-HT7 receptor-induced inactivation of forskolin-stimulated adenylate cyclase by risperidone, 9-OH-risperidone and other “inactivating antagonists”. Mol Pharmacol. 2009;76:552–559. doi: 10.1124/mol.109.056283. [DOI] [PMC free article] [PubMed] [Google Scholar]