Abstract
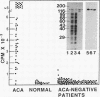
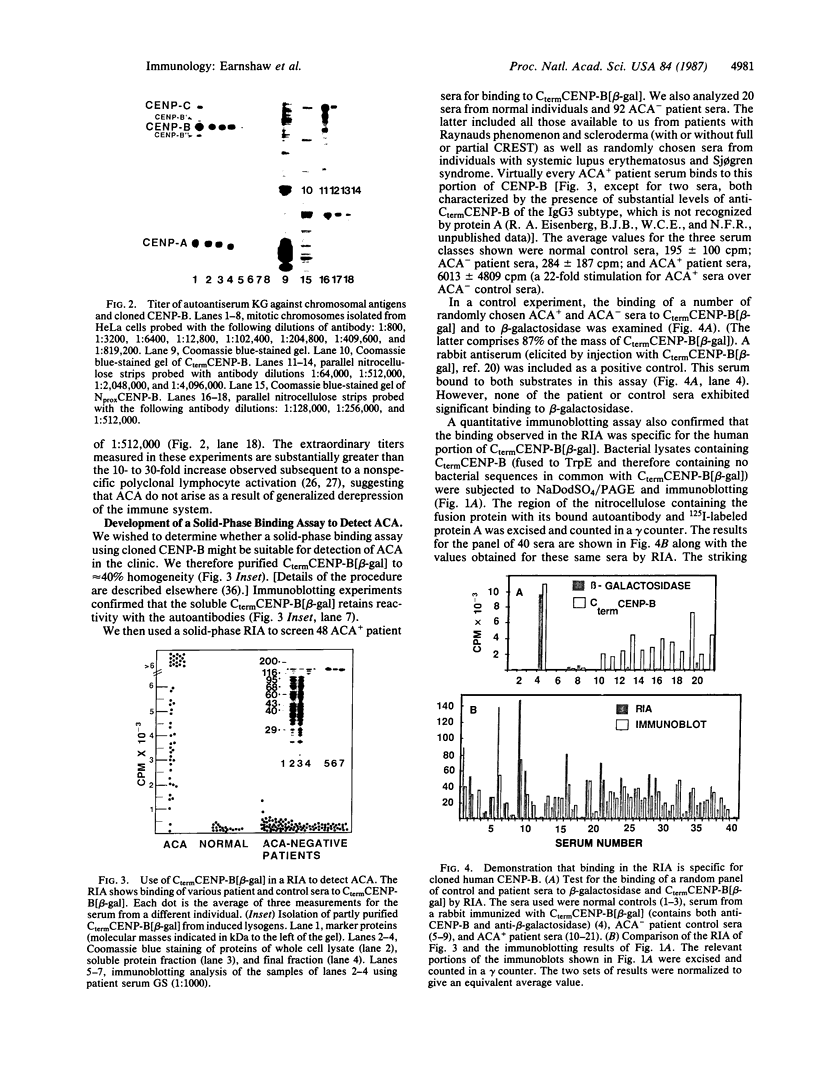
A cDNA clone encoding CENP-B, the 80-kDa human centromere autoantigen, was used to construct a panel of hybrid proteins containing four different regions of CENP-B. These have allowed us to identify three independent epitopes on CENP-B that are targets of autoantibodies. Two of these are recognized concurrently in greater than or equal to 90% of patient sera containing anticentromere autoantibodies (ACA), conclusively demonstrating that this autoimmune response is polyclonal. When present and previous data are combined, ACA are shown to recognize at least five independent epitopes on CENP-B. A radioimmunoassay based on cloned CENP-B has demonstrated that sera from greater than or equal to 96% of patients with ACA recognize the cloned antigen, thus defining a region of the protein that is recognized by virtually all patients with ACA. These findings have significant implications for models that seek to explain the origin of ACA and for the future detection of this group of autoantibodies in the clinical setting.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Budman D. R., Merchant E. B., Steinberg A. D., Doft B., Gershwin M. E., Lizzio E., Reeves J. P. Increased spontaneous activity of antibody-forming cells in the peripheral blood of patients with active SLE. Arthritis Rheum. 1977 Apr;20(3):829–833. doi: 10.1002/art.1780200312. [DOI] [PubMed] [Google Scholar]
- Cox J. V., Schenk E. A., Olmsted J. B. Human anticentromere antibodies: distribution, characterization of antigens, and effect on microtubule organization. Cell. 1983 Nov;35(1):331–339. doi: 10.1016/0092-8674(83)90236-2. [DOI] [PubMed] [Google Scholar]
- Diamond B., Scharff M. D. Somatic mutation of the T15 heavy chain gives rise to an antibody with autoantibody specificity. Proc Natl Acad Sci U S A. 1984 Sep;81(18):5841–5844. doi: 10.1073/pnas.81.18.5841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Earnshaw W. C., Rothfield N. Identification of a family of human centromere proteins using autoimmune sera from patients with scleroderma. Chromosoma. 1985;91(3-4):313–321. doi: 10.1007/BF00328227. [DOI] [PubMed] [Google Scholar]
- Earnshaw W. C., Sullivan K. F., Machlin P. S., Cooke C. A., Kaiser D. A., Pollard T. D., Rothfield N. F., Cleveland D. W. Molecular cloning of cDNA for CENP-B, the major human centromere autoantigen. J Cell Biol. 1987 Apr;104(4):817–829. doi: 10.1083/jcb.104.4.817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Earnshaw W., Bordwell B., Marino C., Rothfield N. Three human chromosomal autoantigens are recognized by sera from patients with anti-centromere antibodies. J Clin Invest. 1986 Feb;77(2):426–430. doi: 10.1172/JCI112320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eilat D., Hochberg M., Pumphrey J., Rudikoff S. Monoclonal antibodies to DNA and RNA from NZB/NZW F1 mice: antigenic specificities and NH2 terminal amino acid sequences. J Immunol. 1984 Jul;133(1):489–494. [PubMed] [Google Scholar]
- Eisenberg R. A., Dyer K., Craven S. Y., Fuller C. R., Yount W. J. Subclass restriction and polyclonality of the systemic lupus erythematosus marker antibody anti-Sm. J Clin Invest. 1985 Apr;75(4):1270–1277. doi: 10.1172/JCI111826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritzler M. J., Kinsella T. D. The CREST syndrome: a distinct serologic entity with anticentromere antibodies. Am J Med. 1980 Oct;69(4):520–526. doi: 10.1016/0002-9343(80)90462-3. [DOI] [PubMed] [Google Scholar]
- Guldner H. H., Lakomek H. J., Bautz F. A. Human anti-centromere sera recognise a 19.5 kD non-histone chromosomal protein from HeLa cells. Clin Exp Immunol. 1984 Oct;58(1):13–20. [PMC free article] [PubMed] [Google Scholar]
- Hardin J. A. The lupus autoantigens and the pathogenesis of systemic lupus erythematosus. Arthritis Rheum. 1986 Apr;29(4):457–460. doi: 10.1002/art.1780290401. [DOI] [PubMed] [Google Scholar]
- Izui S., McConahey P. J., Dixon F. J. Increased spontaneous polyclonal activation of B lymphocytes in mice with spontaneous autoimmune disease. J Immunol. 1978 Dec;121(6):2213–2219. [PubMed] [Google Scholar]
- Klinman D. M., Steinberg A. D. Idiotypy and autoimmunity. Arthritis Rheum. 1986 Jun;29(6):697–705. doi: 10.1002/art.1780290601. [DOI] [PubMed] [Google Scholar]
- Madaio M. P., Hodder S., Schwartz R. S., Stollar B. D. Responsiveness of autoimmune and normal mice to nucleic acid antigens. J Immunol. 1984 Feb;132(2):872–876. [PubMed] [Google Scholar]
- Maddison P. J., Reichlin M. Quantitation of precipitating antibodies to certain soluble nuclear antigens in SLE. Arthritis Rheum. 1977 Apr;20(3):819–824. doi: 10.1002/art.1780200310. [DOI] [PubMed] [Google Scholar]
- Marsden M. P., Laemmli U. K. Metaphase chromosome structure: evidence for a radial loop model. Cell. 1979 Aug;17(4):849–858. doi: 10.1016/0092-8674(79)90325-8. [DOI] [PubMed] [Google Scholar]
- McDuffie F. C., Bunch T. W. Immunologic tests in the diagnosis of rheumatic diseases. Part I. Bull Rheum Dis. 1976;27(5):900–905. [PubMed] [Google Scholar]
- Moroi Y., Peebles C., Fritzler M. J., Steigerwald J., Tan E. M. Autoantibody to centromere (kinetochore) in scleroderma sera. Proc Natl Acad Sci U S A. 1980 Mar;77(3):1627–1631. doi: 10.1073/pnas.77.3.1627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyman U., Hallman H., Hadlaczky G., Pettersson I., Sharp G., Ringertz N. R. Intranuclear localization of snRNP antigens. J Cell Biol. 1986 Jan;102(1):137–144. doi: 10.1083/jcb.102.1.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plotz P. H. Autoantibodies are anti-idiotype antibodies to antiviral antibodies. Lancet. 1983 Oct 8;2(8354):824–826. doi: 10.1016/s0140-6736(83)90740-7. [DOI] [PubMed] [Google Scholar]
- Schwartz R. S., Stollar B. D. Origins of anti-DNA autoantibodies. J Clin Invest. 1985 Feb;75(2):321–327. doi: 10.1172/JCI111704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shero J. H., Bordwell B., Rothfield N. F., Earnshaw W. C. High titers of autoantibodies to topoisomerase I (Scl-70) in sera from scleroderma patients. Science. 1986 Feb 14;231(4739):737–740. doi: 10.1126/science.3003910. [DOI] [PubMed] [Google Scholar]
- Shoenfeld Y., Schwartz R. S. Immunologic and genetic factors in autoimmune diseases. N Engl J Med. 1984 Oct 18;311(16):1019–1029. doi: 10.1056/NEJM198410183111605. [DOI] [PubMed] [Google Scholar]
- Shores E. W., Eisenberg R. A., Cohen P. L. Role of the Sm antigen in the generation of anti-Sm autoantibodies in the SLE-prone MRL mouse. J Immunol. 1986 May 15;136(10):3662–3667. [PubMed] [Google Scholar]
- Tan E. M. Autoantibodies to nuclear antigens (ANA): their immunobiology and medicine. Adv Immunol. 1982;33:167–240. doi: 10.1016/s0065-2776(08)60836-6. [DOI] [PubMed] [Google Scholar]
- Tan E. M., Rodnan G. P., Garcia I., Moroi Y., Fritzler M. J., Peebles C. Diversity of antinuclear antibodies in progressive systemic sclerosis. Anti-centromere antibody and its relationship to CREST syndrome. Arthritis Rheum. 1980 Jun;23(6):617–625. doi: 10.1002/art.1780230602. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valdivia M. M., Brinkley B. R. Fractionation and initial characterization of the kinetochore from mammalian metaphase chromosomes. J Cell Biol. 1985 Sep;101(3):1124–1134. doi: 10.1083/jcb.101.3.1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young R. A., Davis R. W. Efficient isolation of genes by using antibody probes. Proc Natl Acad Sci U S A. 1983 Mar;80(5):1194–1198. doi: 10.1073/pnas.80.5.1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young R. A., Davis R. W. Yeast RNA polymerase II genes: isolation with antibody probes. Science. 1983 Nov 18;222(4625):778–782. doi: 10.1126/science.6356359. [DOI] [PubMed] [Google Scholar]
- van Venrooij W. J., Stapel S. O., Houben H., Habets W. J., Kallenberg C. G., Penner E., van de Putte L. B. Scl-86, a marker antigen for diffuse scleroderma. J Clin Invest. 1985 Mar;75(3):1053–1060. doi: 10.1172/JCI111767. [DOI] [PMC free article] [PubMed] [Google Scholar]