Abstract
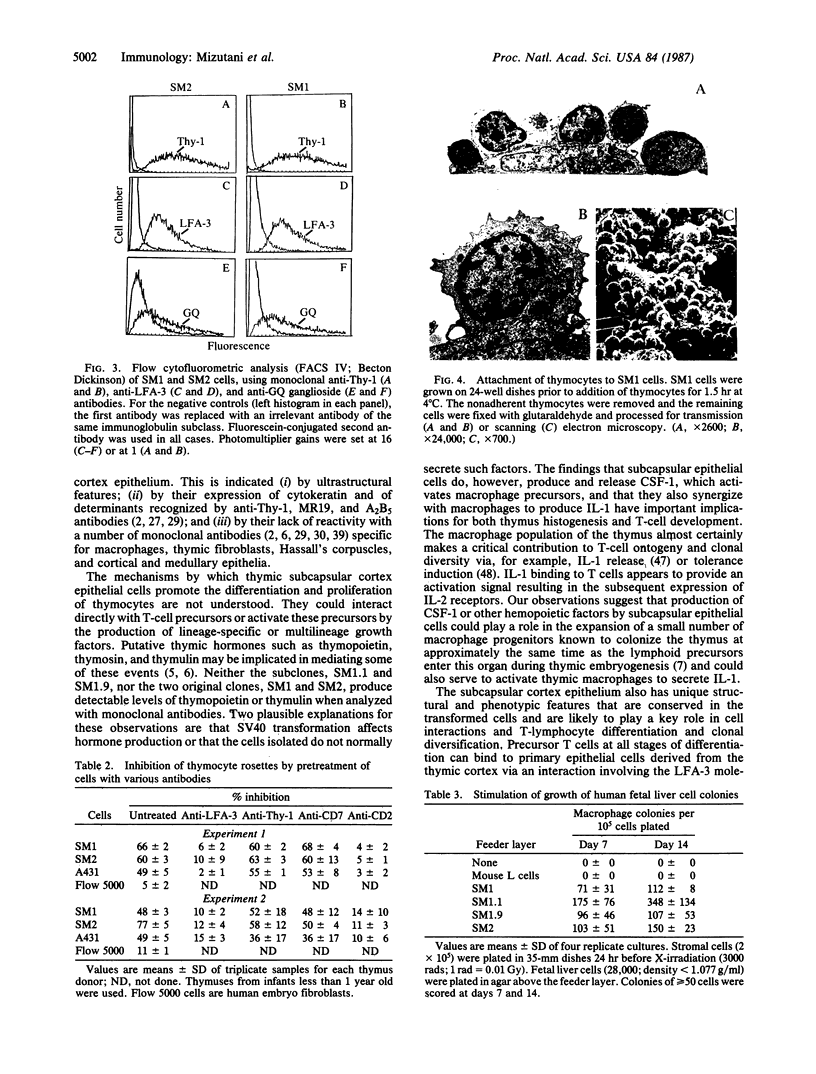
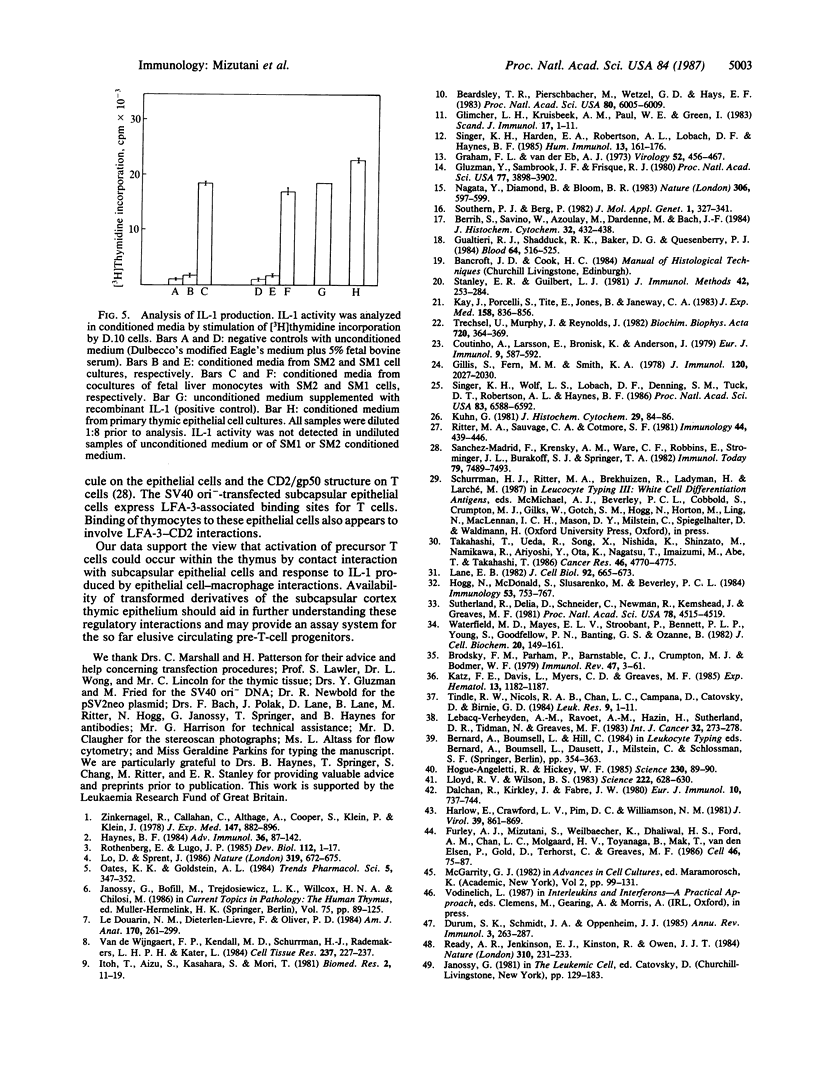
The thymic microenvironment involves complex cell interactions among different types of epithelial cells, macrophages, tissue histiocytes, and immature and maturing T cells. We describe the isolation of a subset of thymic epithelial cells by selective primary culture followed by cotransfection with a simian virus 40 replication-origin-defective mutant and pSV2neo plasmid. The cloned cells have the composite immunophenotype that is unique to thymic subcapsular epithelial cells, suggesting that they may provide a model system in vitro for analyzing the earliest steps in T-cell differentiation. This possibility is supported by the finding that these epithelial cells express LFA-3-associated binding sites for T cells, secrete a macrophage hemopoietic growth factor, and synergize with macrophages in the production of interleukin 1.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Angeletti R. H., Hickey W. F. A neuroendocrine marker in tissues of the immune system. Science. 1985 Oct 4;230(4721):89–90. doi: 10.1126/science.3898368. [DOI] [PubMed] [Google Scholar]
- Beardsley T. R., Pierschbacher M., Wetzel G. D., Hays E. F. Induction of T-cell maturation by a cloned line of thymic epithelium (TEPI). Proc Natl Acad Sci U S A. 1983 Oct;80(19):6005–6009. doi: 10.1073/pnas.80.19.6005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berrih S., Savino W., Azoulay M., Dardenne M., Bach J. F. Production of anti-thymulin (FTS) monoclonal antibodies by immunization against human thymic epithelial cells. J Histochem Cytochem. 1984 Apr;32(4):432–438. doi: 10.1177/32.4.6200531. [DOI] [PubMed] [Google Scholar]
- Brodsky F. M., Parham P., Barnstable C. J., Crumpton M. J., Bodmer W. F. Monoclonal antibodies for analysis of the HLA system. Immunol Rev. 1979;47:3–61. doi: 10.1111/j.1600-065x.1979.tb00288.x. [DOI] [PubMed] [Google Scholar]
- Coutinho A., Larsson E. L., Grönvik K. O., Andersson J. Studies on T lymphocyte activation II. The target cells for concanavalin A-induced growth factors. Eur J Immunol. 1979 Aug;9(8):587–592. doi: 10.1002/eji.1830090803. [DOI] [PubMed] [Google Scholar]
- Dalchau R., Kirkley J., Fabre J. W. Monoclonal antibody to a human leukocyte-specific membrane glycoprotein probably homologous to the leukocyte-common (L-C) antigen of the rat. Eur J Immunol. 1980 Oct;10(10):737–744. doi: 10.1002/eji.1830101003. [DOI] [PubMed] [Google Scholar]
- Durum S. K., Schmidt J. A., Oppenheim J. J. Interleukin 1: an immunological perspective. Annu Rev Immunol. 1985;3:263–287. doi: 10.1146/annurev.iy.03.040185.001403. [DOI] [PubMed] [Google Scholar]
- Furley A. J., Mizutani S., Weilbaecher K., Dhaliwal H. S., Ford A. M., Chan L. C., Molgaard H. V., Toyonaga B., Mak T., van den Elsen P. Developmentally regulated rearrangement and expression of genes encoding the T cell receptor-T3 complex. Cell. 1986 Jul 4;46(1):75–87. doi: 10.1016/0092-8674(86)90861-5. [DOI] [PubMed] [Google Scholar]
- Gillis S., Ferm M. M., Ou W., Smith K. A. T cell growth factor: parameters of production and a quantitative microassay for activity. J Immunol. 1978 Jun;120(6):2027–2032. [PubMed] [Google Scholar]
- Glimcher L. H., Kruisbeek A. M., Paul W. E., Green I. Functional activity of a transformed thymic epithelial cell line. Scand J Immunol. 1983 Jan;17(1):1–11. doi: 10.1111/j.1365-3083.1983.tb00759.x. [DOI] [PubMed] [Google Scholar]
- Gluzman Y., Sambrook J. F., Frisque R. J. Expression of early genes of origin-defective mutants of simian virus 40. Proc Natl Acad Sci U S A. 1980 Jul;77(7):3898–3902. doi: 10.1073/pnas.77.7.3898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham F. L., van der Eb A. J. A new technique for the assay of infectivity of human adenovirus 5 DNA. Virology. 1973 Apr;52(2):456–467. doi: 10.1016/0042-6822(73)90341-3. [DOI] [PubMed] [Google Scholar]
- Gualtieri R. J., Shadduck R. K., Baker D. G., Quesenberry P. J. Hematopoietic regulatory factors produced in long-term murine bone marrow cultures and the effect of in vitro irradiation. Blood. 1984 Aug;64(2):516–525. [PubMed] [Google Scholar]
- Harlow E., Crawford L. V., Pim D. C., Williamson N. M. Monoclonal antibodies specific for simian virus 40 tumor antigens. J Virol. 1981 Sep;39(3):861–869. doi: 10.1128/jvi.39.3.861-869.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haynes B. F. The human thymic microenvironment. Adv Immunol. 1984;36:87–142. doi: 10.1016/s0065-2776(08)60900-1. [DOI] [PubMed] [Google Scholar]
- Hogg N., MacDonald S., Slusarenko M., Beverley P. C. Monoclonal antibodies specific for human monocytes, granulocytes and endothelium. Immunology. 1984 Dec;53(4):753–767. [PMC free article] [PubMed] [Google Scholar]
- Janossy G., Bofill M., Trejdosiewicz L. K., Willcox H. N., Chilosi M. Cellular differentiation of lymphoid subpopulations and their microenvironments in the human thymus. Curr Top Pathol. 1986;75:89–125. doi: 10.1007/978-3-642-82480-7_3. [DOI] [PubMed] [Google Scholar]
- Katz F. E., Davis L., Myers C. D., Greaves M. F. Selective expression of class-II MHC antigens during hemopoietic differentiation. Exp Hematol. 1985 Dec;13(11):1182–1187. [PubMed] [Google Scholar]
- Kaye J., Porcelli S., Tite J., Jones B., Janeway C. A., Jr Both a monoclonal antibody and antisera specific for determinants unique to individual cloned helper T cell lines can substitute for antigen and antigen-presenting cells in the activation of T cells. J Exp Med. 1983 Sep 1;158(3):836–856. doi: 10.1084/jem.158.3.836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhn H. A simple method for the preparation of cell cultures for ultrastructural investigation. J Histochem Cytochem. 1981 Jan;29(1):84–86. doi: 10.1177/29.1.7009731. [DOI] [PubMed] [Google Scholar]
- Lane E. B. Monoclonal antibodies provide specific intramolecular markers for the study of epithelial tonofilament organization. J Cell Biol. 1982 Mar;92(3):665–673. doi: 10.1083/jcb.92.3.665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Douarin N. M., Dieterlen-Lièvre F., Oliver P. D. Ontogeny of primary lymphoid organs and lymphoid stem cells. Am J Anat. 1984 Jul;170(3):261–299. doi: 10.1002/aja.1001700305. [DOI] [PubMed] [Google Scholar]
- Lebacq-Verheyden A. M., Ravoet A. M., Bazin H., Sutherland D. R., Tidman N., Greaves M. F. Rat AL2, AL3, AL4 and AL5 monoclonal antibodies bind to the common acute lymphoblastic leukaemia antigen (CALLA gp 100). Int J Cancer. 1983 Sep 15;32(3):273–279. doi: 10.1002/ijc.2910320303. [DOI] [PubMed] [Google Scholar]
- Lloyd R. V., Wilson B. S. Specific endocrine tissue marker defined by a monoclonal antibody. Science. 1983 Nov 11;222(4624):628–630. doi: 10.1126/science.6635661. [DOI] [PubMed] [Google Scholar]
- Lo D., Sprent J. Identity of cells that imprint H-2-restricted T-cell specificity in the thymus. Nature. 1986 Feb 20;319(6055):672–675. doi: 10.1038/319672a0. [DOI] [PubMed] [Google Scholar]
- Nagata Y., Diamond B., Bloom B. R. The generation of human monocyte/macrophage cell lines. Nature. 1983 Dec 8;306(5943):597–599. doi: 10.1038/306597a0. [DOI] [PubMed] [Google Scholar]
- Ready A. R., Jenkinson E. J., Kingston R., Owen J. J. Successful transplantation across major histocompatibility barrier of deoxyguanosine-treated embryonic thymus expressing class II antigens. Nature. 1984 Jul 19;310(5974):231–233. doi: 10.1038/310231a0. [DOI] [PubMed] [Google Scholar]
- Ritter M. A., Sauvage C. A., Cotmore S. F. The human thymus microenvironment: in vivo identification of thymic nurse cells and other antigenically-distinct subpopulations of epithelial cells. Immunology. 1981 Nov;44(3):439–446. [PMC free article] [PubMed] [Google Scholar]
- Rothenberg E., Lugo J. P. Differentiation and cell division in the mammalian thymus. Dev Biol. 1985 Nov;112(1):1–17. doi: 10.1016/0012-1606(85)90114-9. [DOI] [PubMed] [Google Scholar]
- Sanchez-Madrid F., Krensky A. M., Ware C. F., Robbins E., Strominger J. L., Burakoff S. J., Springer T. A. Three distinct antigens associated with human T-lymphocyte-mediated cytolysis: LFA-1, LFA-2, and LFA-3. Proc Natl Acad Sci U S A. 1982 Dec;79(23):7489–7493. doi: 10.1073/pnas.79.23.7489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer K. H., Harden E. A., Robertson A. L., Lobach D. F., Haynes B. F. In vitro growth and phenotypic characterization of mesodermal-derived and epithelial components of normal and abnormal human thymus. Hum Immunol. 1985 Jul;13(3):161–176. doi: 10.1016/0198-8859(85)90009-6. [DOI] [PubMed] [Google Scholar]
- Singer K. H., Wolf L. S., Lobach D. F., Denning S. M., Tuck D. T., Robertson A. L., Haynes B. F. Human thymocytes bind to autologous and allogeneic thymic epithelial cells in vitro. Proc Natl Acad Sci U S A. 1986 Sep;83(17):6588–6592. doi: 10.1073/pnas.83.17.6588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern P. J., Berg P. Transformation of mammalian cells to antibiotic resistance with a bacterial gene under control of the SV40 early region promoter. J Mol Appl Genet. 1982;1(4):327–341. [PubMed] [Google Scholar]
- Stanley E. R., Guilbert L. J. Methods for the purification, assay, characterization and target cell binding of a colony stimulating factor (CSF-1). J Immunol Methods. 1981;42(3):253–284. doi: 10.1016/0022-1759(81)90156-3. [DOI] [PubMed] [Google Scholar]
- Sutherland R., Delia D., Schneider C., Newman R., Kemshead J., Greaves M. Ubiquitous cell-surface glycoprotein on tumor cells is proliferation-associated receptor for transferrin. Proc Natl Acad Sci U S A. 1981 Jul;78(7):4515–4519. doi: 10.1073/pnas.78.7.4515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi T., Ueda R., Song X., Nishida K., Shinzato M., Namikawa R., Ariyoshi Y., Ota K., Kato K., Nagatsu T. Two novel cell surface antigens on small cell lung carcinoma defined by mouse monoclonal antibodies NE-25 and PE-35. Cancer Res. 1986 Sep;46(9):4770–4775. [PubMed] [Google Scholar]
- Tindle R. W., Nichols R. A., Chan L., Campana D., Catovsky D., Birnie G. D. A novel monoclonal antibody BI-3C5 recognises myeloblasts and non-B non-T lymphoblasts in acute leukaemias and CGL blast crises, and reacts with immature cells in normal bone marrow. Leuk Res. 1985;9(1):1–9. doi: 10.1016/0145-2126(85)90016-5. [DOI] [PubMed] [Google Scholar]
- Trechsel U., Dew G., Murphy G., Reynolds J. J. Effects of products from macrophages, blood mononuclear cells and or retinol on collagenase secretion and collagen synthesis in chondrocyte culture. Biochim Biophys Acta. 1982 Jul 22;720(4):364–370. doi: 10.1016/0167-4889(82)90113-6. [DOI] [PubMed] [Google Scholar]
- Waterfield M. D., Mayes E. L., Stroobant P., Bennet P. L., Young S., Goodfellow P. N., Banting G. S., Ozanne B. A monoclonal antibody to the human epidermal growth factor receptor. J Cell Biochem. 1982;20(2):149–161. doi: 10.1002/jcb.240200207. [DOI] [PubMed] [Google Scholar]
- Zinkernagel R. M., Callahan G. N., Althage A., Cooper S., Klein P. A., Klein J. On the thymus in the differentiation of "H-2 self-recognition" by T cells: evidence for dual recognition? J Exp Med. 1978 Mar 1;147(3):882–896. doi: 10.1084/jem.147.3.882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van de Wijngaert F. P., Kendall M. D., Schuurman H. J., Rademakers L. H., Kater L. Heterogeneity of epithelial cells in the human thymus. An ultrastructural study. Cell Tissue Res. 1984;237(2):227–237. doi: 10.1007/BF00217140. [DOI] [PubMed] [Google Scholar]