Abstract
Objectives. We applied social network analyses to determine how hospitals within Orange County, California, are interconnected by patient sharing, a system which may have numerous public health implications.
Methods. Our analyses considered 2 general patient-sharing networks: uninterrupted patient sharing (UPS; i.e., direct interhospital transfers) and total patient sharing (TPS; i.e., all interhospital patient sharing, including patients with intervening nonhospital stays). We considered these networks at 3 thresholds of patient sharing: at least 1, at least 10, and at least 100 patients shared.
Results. Geographically proximate hospitals were somewhat more likely to share patients, but many hospitals shared patients with distant hospitals. Number of patient admissions and percentage of cancer patients were associated with greater connectivity across the system. The TPS network revealed numerous connections not seen in the UPS network, meaning that direct transfers only accounted for a fraction of total patient sharing.
Conclusions. Our analysis demonstrated that Orange County's 32 hospitals were highly and heterogeneously interconnected by patient sharing. Different hospital populations had different levels of influence over the patient-sharing network.
In many ways, hospitals in a county are analogous to individual people within a social network. Both people and hospitals have individual characteristics (e.g., height, weight, age, and gender for people; bed capacity, facility type, and payer mix for hospitals). People exhibit behaviors; hospitals implement policies and interventions. Just as people are connected by social ties and interactions, hospitals are often connected to each other through sharing patients, because patients discharged from one hospital may be admitted to other hospitals in the same region. Similar to social interactions among people, patient sharing can have significant implications for disease and patient-demographic epidemiology (e.g., hospital-acquired infections) and the impact of disease control measures, patient education and prevention programs, and fiscal policies.1–3
Social network constructs have been applied to individuals in populations to better understand the spread of behaviors, ideas,4–10 and diseases4,11–13 and their control measures.4,12,7 However, to our knowledge our study is the first to apply social network frameworks and measures to hospitals to understand how hospitals within a county are interconnected by patient sharing. We collected data from all acute care health facilities in a large United States county and constructed a social network model representing their patient-sharing connections. We used social network analyses to determine and characterize:
The amount of patient sharing occurring and the heterogeneity of this sharing among different hospitals,
Whether certain hospitals had a greater influence over the patient-sharing network and what characteristics may predict that influence,
How patient sharing correlated with geographic distance (i.e., whether hospitals that were closer together were more likely to share patients), and
The proportion of patient sharing that occurred via direct patient transfers, as opposed to patients being discharged and having an intervening stay in the community before being readmitted to another hospital.
METHODS
We used patient-level hospital discharge data from 2005 to 2006 for all 32 hospitals in Orange County, California, obtained from the California Office of Statewide Health Planning and Development.14 These hospitals serve 3.1 million people residing in 148 zip codes. The United States Census Bureau's 2006 American Community Survey provided additional descriptive data for Orange County. Of the 32 hospitals, 6 were long-term acute care facilities serving patients with prolonged high-level medical needs (e.g., mechanical ventilation).
We used UCINET for Windows version 6.0 (Analytic Technologies, Lexington, KY) to conduct social network analyses. Our analyses considered 2 general patient-sharing networks, on the basis of whether patients discharged from a hospital in 2005 were admitted to any other Orange County hospital within 365 days:
Uninterrupted patient-sharing (UPS) network: This network counted patients transferred directly from one hospital to another in the same calendar day without an intervening stay in a nonacute care setting or the community at large. Two hospitals had an uninterrupted tie if one transferred patients directly to the other.
Total patient-sharing (TPS) network: This network included both direct patient transfers (UPS) and patient sharing occurring after an intervening stay in the community or a nonhospital setting. Two hospitals had a tie if they shared a patient within 1 year of each other. The UPS network is a subset of the TPS network.
We also calculated how much patient sharing was occurring with hospitals outside Orange County.
We created social network diagrams (sociograms) in which each node represented a hospital and each edge (connection between 2 nodes) represented patient sharing. Node size was proportional to the hospital's bed capacity. Nodes were arranged in a circular pattern clockwise in order of decreasing bed capacity (largest hospital positioned at 6 o'clock), with the long-term acute care facilities ordered independently after the other hospitals. Edges were directional (i.e., if Hospital A sent patients to Hospital B but did not receive patients from Hospital B, then Hospital B was connected to Hospital A but not vice-versa; patient sharing is directed from A to B). Arrows indicated patient-sharing direction; a double-arrowed line implied a symmetric connection. Sociograms were binary with an edge present if patient-sharing volume (N) between 2 hospitals exceeded a threshold number (N ≥ 1, N ≥ 10, or N ≥ 100).
Social Network Measures
Our analysis calculated the following general network measures, which describe the degree of connectivity for the entire Orange County hospital network15:
Geodesic distance: smallest number of interhospital ties (edges) that connect one hospital to another.
Diameter: largest geodesic distance in the network, that is, the greatest distance between any pair of hospitals (greater diameter = network less tightly connected).
Density: number of actual existing ties divided by the total number of possible ties in a network (lower density = greater heterogeneity).
For each hospital we calculated the following centrality measures:
Degree: a hospital's total number of connections.
In-degree: total number of different hospitals that send patients to the given hospital (high in-degree = a hospital highly influenced by others = high prominence).
Out-degree: total number of different hospitals that receive patients from a given hospital (high out-degree = a hospital that highly influences others = high influence).
Betweenness: number of times a given hospital is part of the shortest path between 2 others (i.e., serves as a necessary intermediary).
Centrality measures indicate the degree of connectedness between a given member and all other network members. Highly connected hospitals may affect or be affected by more hospitals, whereas relatively isolated hospitals may have little impact. To identify hospitals with which each hospital most closely interacted, we identified each hospital's 1-step ego network, consisting of the hospital (ego) and all hospitals connected to it by an edge.
Hospital Characteristics Associated With Social Network Measures
We used Stata version 10 (StataCorp, College Station, TX) to evaluate the associations between centrality measures and various hospital characteristics, including annual admissions, mean length of stay, and proportion of patients meeting the following criteria: younger than 18 years, White race, Hispanic ethnicity, using Medicare, using Medicaid, diagnosis of certain ICD-9 coded diseases (e.g., diabetes, liver disease, renal disease, cancer, methicillin-resistant Staphylococcus aureus, and Clostridium difficile), and recent surgery. Univariable testing determined which characteristics (continuous variables) were associated with each centrality measure (each dichotomized by its median value). We entered variables associated at α less than or equal to 0.15 (on the basis of univariable testing) into a forward stepwise multivariable model, retaining variables at α less than 0.20. The small sample size motivated us to enter and retain probabilities that were greater than the typical values (0.10 and 0.15) to avoid nonsignificant association because of insufficient power.
We used a rank-based method to assess the correlation between geodesic and geographic distances by calculating the Spearman correlation coefficients (ρ) between hospitals' distances in the network (excluding hospitals without ties). We conducted additional analyses using a 30-day time horizon instead of 1 year (i.e., we determined the TPS on the basis of whether patients discharged from one hospital were admitted to any other Orange County hospital within 30 days).
RESULTS
The number of admissions over the 2005 calendar year ranged from 47 to 29 741 (median = 8532; mean = 9999; SD = 7893). The mean length of stay ranged from 4 days to 44 days (median = 5; mean = 9; SD = 10). For the hospital patient populations, the percentage of White patients ranged from 15% to 93% (median = 77%; mean = 72%; SD = 21%), the percentage of pediatric patients ranged from 0% to 99% (median = 17%; mean = 23%; SD = 27%), the percentage of Medicare patients ranged from 0% to 92% (median = 32%; mean = 35%; SD = 21%), and the percentage of Medi-Cal (California Medicaid) patients ranged from 2% to 66% (median = 15%; mean = 21%; SD = 21%).
A vast majority of all patient sharing (86.6%) occurred between Orange County hospitals. Of direct transfers for patients admitted to Orange County hospitals, 23.5% (SD = 18.4%) were to a hospital in a neighboring county, and 74.5% (SD = 20.3%) were within Orange County. Among total patient transfers (discharge from an Orange County hospital and readmission to another hospital), 86.6% were to another hospital within Orange County, and 22.7% (SD = 29.3%) were to a hospital outside Orange County.
Total Patient Sharing
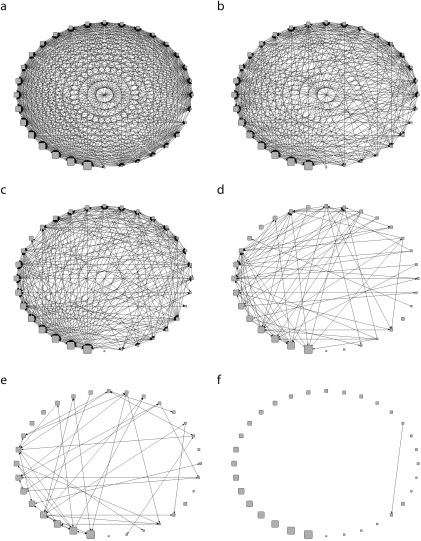
Figure 1 shows TPS network sociograms for 3 patient-sharing thresholds. All hospital pairs shared at least 1 patient over the year (network density = 81.1%). Most (88.3%) connections were reciprocal. Increasing the sharing threshold to at least 10 patients over the year still left a highly interconnected network (network density = 46.1%) with mostly reciprocated connections (82.1%). Only the smallest hospital by bed size (a long-term acute care facility) had no connections at this threshold. The threshold of at least 100 patients yielded much greater heterogeneity (network density = 7.0%). Six hospitals at this threshold had no connections, and fewer (68.3%) had reciprocal connections than were seen at lower thresholds. Eight hospitals (including 4 long-term acute care facilities) did not send at least 100 patients to other hospitals, and 7 (including 2 long-term acute care facilities) did not receive at least 100 patients that had been at other hospitals previously during the year.
FIGURE 1.
Sociograms depicting patient sharing among all hospitals within 1 year of discharge: 2005–2006, Orange County, CA.
Note. Hospital patients were admitted during the 2005 calendar year. Diagrams on the left side are binary sociograms of the total patient-sharing network at 3 different patient-sharing thresholds: ≥ 1 patient (a), ≥ 10 patients (c), and ≥ 100 patients (e). Diagrams on the right side are binary sociograms for the uninterrupted patient-sharing network at the same 3 patient-sharing thresholds (b, d, and f, respectively).
General network measures revealed the TPS network to be fairly tightly connected. When sharing at least 10 patients, the diameter (the longest distance between 2 hospitals in the network) was 4 (4 hospital pairs had this geodesic distance); 93.7% of hospital pairs had a geodesic distance of 2 or less, and 4.3% had a geodesic distance of 4 or more. Increasing the patient threshold to at least 100 resulted in the network being less connected; only 33.8% of hospital pairs had a geodesic distance of 2 or less, and 46.2% had a geodesic distance of 4 or more. The TPS diameter was 7 (3 pairs had this geodesic distance).
Uninterrupted Patient Sharing
As Figure 1 demonstrates, many hospitals connected by TPS were not connected by UPS, such that the UPS substantially underrepresented the TPS. A comparison of the sociograms in Figure 1 shows that UPS between hospitals was much less common than was TPS (UPS vs TPS network density = 45.9% vs 81.1%, at a threshold of 1). This difference was even greater when the sharing threshold was 10 patients (UPS vs TPS network density = 9.6% vs 46.1%). In fact, only 1 hospital directly transferred 100 or more patients to another over the year (UPS vs TPS network density = 0.1% vs 7.0%). Thus, the ratios of UPS to TPS network densities (proportion of patient sharing represented by direct transfers) were 56.6%, 20.8%, and 1.4% at thresholds of at least 1, at least 10, and at least 100 patients shared, respectively. Because the UPS network captured only a fraction of the TPS, the remainder of our results description focuses primarily on the TPS network.
Individual hospital centrality measures further demonstrated the connection heterogeneity (TPS ≥ 100 network). Hospital out-degree ranged from 0 to 7 (median = 2). Although the mode was 0 (8 hospitals), 7 hospitals had out-degrees of 3. Hospital in-degree ranged from 0 to 6 (median = 2; mode = 1; 7 hospitals). Many hospitals had unequal sharing. Eight hospitals had out-degrees greater than in-degrees—the number of patient transfers they discharged was higher than was the number of patient transfers they admitted—and 6 hospitals had greater in-degrees than out-degrees. Hospital betweenness revealed differences in different hospitals' involvement in the network, ranging from 0 to 184 (median and mode = 0). Eighteen hospitals had 0 betweenness (i.e., they were not a necessary intermediary between any pair of hospitals). Six had betweenness greater than 100. Another 6 hospitals served as key players in brokering connections between different hospital pairs.
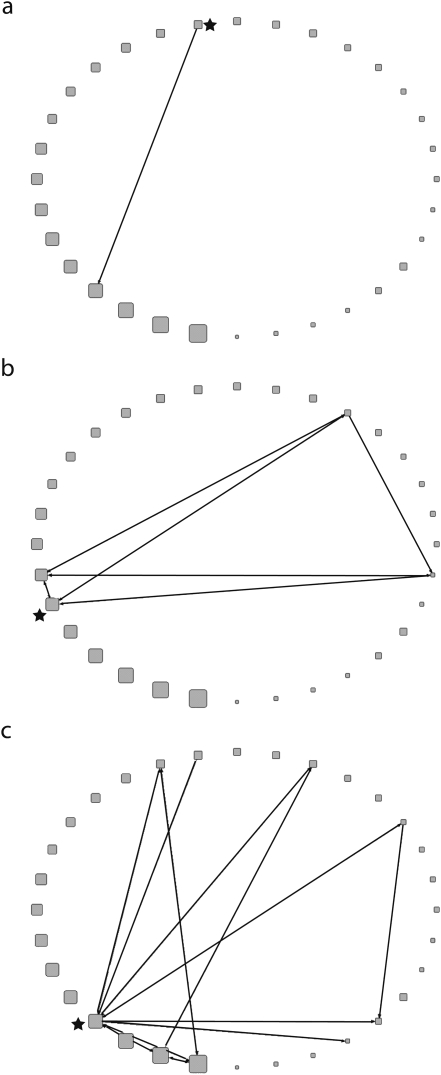
There was considerable variability in hospital ego networks, as can be seen in Figure 2. TPS (N ≥ 100) ego networks identified relatively isolated hospitals as well as highly connected hospitals. Among hospitals with more expansive ego networks, some played greater intermediary roles than others, meaning that more hospitals had to go through the ego to reach the other hospitals. The ego networks' average geodesic distances ranged from 0 to 1.33 (median = 0), density ranged from 0 to 100 (median = 29.2), and diameter ranged from 0 to 2 (median = 0).
FIGURE 2.
Ego networks depicting 1-step connections of a relatively isolated hospital (a), moderately connected hospital (b), and expansively connected hospital (c): 2005–2006, Orange County, CA.
Note. Results shown are for the total patient-sharing network at the ≥ 100 patients threshold. The star represents the ego hospital.
Hospital Characteristics Associated With Measures of High Social Networking
An evaluation of whether hospital characteristics (e.g., volume, percentage Medicare patients, or other facility-level patient descriptors) were associated with social network measures revealed that only annual volume and percentage of patients with cancer diagnoses were associated with increased network connections in multivariable testing (Table 1). Univariate analysis showed number of 2005 admissions, percentage of cancer patients, and percentage with recent surgery to be associated with in-degree, out-degree, and betweenness. Percentage of patients with diabetes was associated with in-degree; percentage of White patients was associated with out-degree. Multiple regression models (Table 1) indicated that annual admissions was significantly associated with high network connectivity using all 3 centrality measures; hospitals with higher admissions volume tended to be connected to more hospitals. Nevertheless, the strength of association between annual volume and network connectivity was modest. The only other variable associated with high connectivity was percentage of cancer patients; hospitals with higher percentages of cancer patients were more likely to receive patients that had been at other hospitals and to serve as the central point of mixing, connecting hospital pairs that otherwise would not have been connected.
TABLE 1.
Multivariate Associations Between Social Network Measures and Hospital Characteristics: Orange County, CA, 2005–2006
| Social Network Measure | Covariate | Coefficient (SD; 95% CI) | P (P > t ) | Spearman Correlation Coefficient |
| In-degree | 2005 admissions | 0.000096 (0.000034; 0.000027, 0.00017) | .008 | 0.56 |
| In-degree | % cancer | 21.98 (9.55; 2.44, 41.51) | .029 | 0.61 |
| Out-degree | 2005 admissions | 0.00014 (0.000029; 0.000085, 0.00020) | .001 | 0.72 |
| Betweenness | 2005 admissions | 0.0033 (0.0019; –0.00059, 0.0073) | .093 | 0.61 |
| Betweenness | % cancer | 1061.03 (607.72; –181.91, 2303.96) | .091 | 0.53 |
Note. CI = confidence interval. Results shown are for the total patient-sharing network at the ≥ 100 patients threshold.
Although some correlation existed between geographic and geodesic distances, this correlation was well below 100%. Hospitals in close geographic proximity were somewhat more likely to share patients, but much patient sharing occurred between distant hospitals. For the TPS network (N ≥ 10), correlations were only modest (sending patients: ρ = 0.42; receiving patients: ρ = 0.44), meaning that geographic proximity accounted for less than half of the patient-sharing likelihood. This likelihood increased somewhat for higher thresholds (for ≥ 100 patients, sending: ρ = 0.57; receiving: ρ = 0.61).
Reducing the time horizon had some effects, demonstrating that significant proportions of patient sharing occurred in time frames that were longer than 30 days. The 30-day TPS network was less dense (had fewer connections) than was the year-long TPS network (74.1% vs 81.1% at patient threshold ≥ 1). The 30-day to 1-year network density ratios were 91.4%, 70.9%, and 21.4% at patient thresholds of at least 1, at least 10, and at least 100 patients, respectively. The diameter for the threshold of 10 or more patients (4) was the same for the 30-day TPS network and for the 1-year TPS network. However, increasing the threshold to at least 100 showed considerable difference in diameter (2 compared with 7). Centrality measures also varied. Hospital out-degree ranged from 0 to 2 (mode = 0), in-degree ranged from 0 to 4 (mode = 0), and betweenness ranged from 0 to 1 (mode = 0). Despite these significant differences, the same number of hospitals had greater out-degrees than in-degrees (8) and in-degrees than out-degrees (6) as in the year-long network. The ego networks of the 30-day TPS were also smaller at the patient threshold of 100 or more. The largest ego network had only 4 connections (compared with 8 in the 1-year network); its network density ranged from 0 to 50 (median = 0), compared with the 0 to 100 seen in the year-long network.
DISCUSSION
Researchers have used social network analysis to characterize the transmission of infectious diseases, information (e.g., gossip), social preferences (e.g., clothing styles), and habits (e.g., alcoholic beverage consumption) across populations of people.7 Social network analysis can identify which players within a network may be more influential and how changing players may affect others.4,12,7 Analysis and understanding of a social network can facilitate predictions of the ramifications of different policies and interventions.12,7,16 Social network analysis can also help identify closely associated groups, helping researchers and policymakers tailor policies and interventions.12
For readers unfamiliar with the US health care system, it is important to realize that US hospital patient sharing may be driven by either the patient or the health care provider. Many patients can choose which hospital they use; some may select services and providers at different locations.17,18 A patient's health insurance policy may be purchased individually, purchased through or provided by employers, or purchased through or provided by the state or federal government (for low-income people or those aged 65 years or older). The patient's insurance coverage determines the patient's options for hospital admission, with some policies being more restrictive.17,18 Each hospital accepts reimbursement from a certain range of insurance policies; changes in a patient's policy may cause changes in the hospitals available to that patient. Uninsured patients have no such restrictions, as long as they can pay their own expenses. Alternatively, one hospital's health care providers may refer or transfer patients to another hospital. Hospitals that only provide basic services may send patients with more complicated medical problems to another hospital that offers more comprehensive specialty care.19,20 A hospital (or providers in that hospital) may arrange to send patients to or receive patients from specific hospitals or providers.18,21
Our analysis demonstrated that Orange County hospitals are, like people, highly and heterogeneously interconnected. Different hospitals may influence the network in different ways. In particular:
Many hospital pairs shared more than 100 patients throughout the year (high network density), and very few went “untouched” by patients from other hospitals (low network diameter).
Patient sharing was often unequal: hospitals sometimes received patients from many other hospitals (high in-degree) but sent patients to few hospitals (low out-degree).
Some hospitals were heavily involved (high betweenness) in patient sharing.
Some shared patients with many hospitals (extensive ego network), and others shared patients with few hospitals (limited ego network).
These findings have numerous implications. First, a hospital is not an island when making plans that could affect its patient population, such as disease control measures, patient education and prevention programs, and fiscal policies. A hospital's decisions may affect other connected hospitals and vice versa.7,20 Second, such an analysis could help public health officials forecast disease epidemiology, as in the spread of hospital-acquired infection,7,12,22 or patient demographics, such as predicting whether an increase in intravenous drug use or chronic disease prevalence will eventually be seen in other hospitals. For example, changes in a hospital's patient population may immediately affect hospitals with which it has close ties.23,24
Third, when regional public health policies such as patient education or disease control measures are implemented in a situation during which scarce resources preclude blanket policy changes, highly interconnected hospitals (e.g., high betweenness) may be initial targets.25 Fourth, such an analysis may enable better understanding of legal and fiscal policy change ramifications.26,27 For example, closing a highly interconnected hospital or reducing its services could affect the region more than doing the same for a relatively isolated hospital.26,28–30 Finally, focusing only on direct transfers overlooks a large proportion of total patient sharing. A hospital administrator may know where a hospital directly transfers patients to or receives them from but may lack records of where a hospital's patients have been if they had intervening stays at home. In this way, hospitals may be unknowingly linked to each other.
Additionally, our study identified hospital characteristics that may predict interconnectivity and, in turn, potential public health intervention targets. High patient sharing was not limited to the only major academic center in Orange County. As our study shows, hospital patient volume was a predictor of interconnectivity, but not a powerful one; having more patients did not necessarily mean connections to more hospitals. Not surprisingly, hospitals with high percentages of cancer patients also tended to receive patients from many other hospitals, although this too was not limited to the academic center. This finding likely reflects 2 facts: cancer treatment facilities are tertiary care hospitals that receive many referrals, and a wide range of private-sector hospitals are increasingly delivering specialty care.
These findings suggest that patient volume and cancer referrals are part (but not all) of the patient-sharing picture. Future studies can explore other potential predictors of high patient sharing, such as hospital financial relationships. Nevertheless, without identifying hospital-based characteristics that strongly and consistently predict patient sharing, regional data sources that evince actual patient sharing among hospitals may provide invaluable information about how patient sharing affects transmission of pathogens, behaviors, and (mis)conceptions.
Although studies have evaluated posttransfer patient outcomes, few have identified factors leading to patient transfer and sharing. Some have shown that hospitals transfer patients when they lack the necessary medical expertise, services, or equipment, or when patients lack adequate insurance.31–36 Others have shown that a dearth of intensive care unit beds, renal support services, or other resources (e.g., limited staffing and expertise, equipment, supplies, and technological capabilities) drives interhospital transfers.32,37 Hospitals that tended to send patients instead of receiving them were more likely to be smaller and less likely to have a medical school affiliation or advanced surgical capacity.19
In addition to annual volume and cancer patient proportion, we found that geographic proximity was somewhat correlated with 2 hospitals being closely connected, but many hospitals shared patients with more distant hospitals (correlation between geodesic and geographic < 100%). Several factors could be contributing to this state of affairs, such as referral patterns or fiscal relationships between hospitals, insurance coverage, and patients traveling substantial distances to procure different health care services (i.e., hospital shop).
Limitations
Our study has several limitations. First, data were from a limited time period; it is unclear how patient sharing may change over longer periods of time. Second, the population was limited to patients with a unique identifier that enabled tracking across hospitals, but 25% of patients (half of whom were newborns) lacked this identifier, potentially leading to underestimations of patient sharing. Third, this study only assessed a limited number of hospital characteristics. Data on other characteristics and factors that may affect patient sharing (e.g., financial and legal data; greater details on health care services offered, such as types of cancer treatments; and size and composition of hospital staff) were unavailable. Further work may examine how well these and other characteristics can predict patient sharing and serve as surrogates for more complicated social networking measures. Finally, the generalizability of our findings to other counties is unclear, although a large diversity of hospital types and sizes were represented.
Conclusions
Hospitals in Orange County, California, were highly and heterogeneously interconnected with each other, such that different hospitals influenced the network in different ways. Applying social network principles and constructs to a region's hospitals may assist public health officials, policymakers, hospital administrators, and researchers in designing interventions to monitor and control diseases.
Acknowledgments
This study was supported by grants from the National Institute General Medical Sciences Models of Infectious Disease Agent Study (1U54GM088491-0109 and 1U01 GM076672) and the National Institutes of Health (K23AI64161).
The funders had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the article.
Human Participant Protection
No protocol approval was required because the study used publicly available secondary data.
References
- 1.Haley RW, Hightower AW, Kabbaz RG, et al. The emergence of methicillin-resistant Staphyoloccus aureus infections in United States hospitals. Ann Intern Med. 1982;97(3):297–308 [DOI] [PubMed] [Google Scholar]
- 2.Pittet D. Infection control and quality health care in the new millenium. Am J Infect Control. 2005;33(5):258–267 [DOI] [PubMed] [Google Scholar]
- 3.Page D. Patient care: spread of infectious diseases may run high with patient sharing. Hosp Health Netw. 2009;83(9):12. [PubMed] [Google Scholar]
- 4.Drewe JA. Who infects whom? Social networks and tuberculosis transmission in wild meerkats. Proc Biol Sci. 2009;277(1681):633–642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dube C, Ribble C, Kelton D, McNab B. A review of network analysis terminology and its application to foot-and-mouth disease modelling and policy development. Transbound Emerg Dis. 2009;56(3):73–85 [DOI] [PubMed] [Google Scholar]
- 6.Martínéz-López B, Perez AM, Sánchez-Vizcaíno JM. Social network analysis: review of general concepts and use in preventive veterinary medicine. Transbound Emerg Dis. 2009;56(4):109–120 [DOI] [PubMed] [Google Scholar]
- 7.Luke DA, Harris JK. Network analysis in public health: history, methods, and applications. Annu Rev Public Health. 2007;28:69–93 [DOI] [PubMed] [Google Scholar]
- 8.Mercken L, Snijders TA, Steglich C, de Vries H. Dynamics of adolescent friendship networks and smoking behavior: social network analyses in six European countries. Soc Sci Med. 2009;69(10):1506–1514 [DOI] [PubMed] [Google Scholar]
- 9.Vaananen A, Kouvonen A, Kivimaki M, Pentti J, Vahtera J. Social support, network heterogeneity, and smoking behavior in women: the 10-town study. Am J Health Promot. 2008;22(4):246–255 [DOI] [PubMed] [Google Scholar]
- 10.Lurie SJ, Fogg TT, Dozier AM. Social network analysis as a method of assessing institutional culture: three case studies. Acad Med. 2009;84(8):1029–1035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Boots M, Childs D, Reuman DC, Mealor M. Local interactions lead to pathogen-driven change to host population dynamics. Curr Biol. 2009;19(19):1660–1664 [DOI] [PubMed] [Google Scholar]
- 12.Koopman JS. Infection transmission science and models. Jpn J Infect Dis. 2005;58(6):S3–S8 [PubMed] [Google Scholar]
- 13.Smieszek T. A mechanistic model of infection: why duration and intensity of contacts should be included in models of disease spread. Theor Biol Med Model. 2009;6:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Office of Statewide Health Planning and Development. MIRCal - Inpatient Data Reporting Manual, 7th Edition (11/10). Available at: http://www.oshpd.ca.gov/HID/MIRCal/IPManual.html. Accessed December 14, 2010.
- 15.Hanneman RA, Riddle M. Introduction to Social Network Methods. Riverside, CA: University of California, Riverside; 2005 [Google Scholar]
- 16.Koopman J. Modeling infection transmission. Annu Rev Public Health. 2004;25:303–326 [DOI] [PubMed] [Google Scholar]
- 17.Chernew M, Scanlon D, Hayward R. Insurance type and choice of hospital by coronary artery bypass graft surgery. Health Serv Res. 1998;33(3):447–466 [PMC free article] [PubMed] [Google Scholar]
- 18.Forrest CB. Primary care gatekeeping and referrals: effective filter or failed experiment? BMJ. 2003;326(7391):692–695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Iwashyna TJ, Christie JD, Moody J, Kahn JM, Asch DA. The structure of critical care transfer networks. Med Care. 2009;47(7):787–793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bolland JM, Wilson JV. Three faces of integrative coordination: a model of interorganizational relations in community-based health and human services. Health Serv Res. 1994;29(3):341–366 [PMC free article] [PubMed] [Google Scholar]
- 21.Shea D, Stuart B, Vasey J, Nag S. Medicare physican referral patterns. Health Serv Res. 1999;34(1):331–348 [PMC free article] [PubMed] [Google Scholar]
- 22.Cooper BS, Medley GF, Stone SP, et al. Methicillin-resistant Staphylococcus aureus in hopsitals and the community: stealth dynamics and control catastrophes. Proc Natl Acad Sci USA. 2004;101(27):10223–10228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Donker T, Wallinga J, Grundmann H. Patient referral patterns and the spread of hospital-aquired infections through national health care networks. PLOS Comput Biol. 2010;6(3):e1000715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Smith DL, Levin SA, Laxminarayan R. Strategic interactions in multi-dimensional epidemics of antibiotic resistance. Proc Natl Acad Sci USA. 2005;102(8):3153–3158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gibbons DE. Interorganizational network structures and diffusion of information through a health system. Am J Public Health. 2007;97(9):1684–1692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Luke RD. Spatial competition and cooperation in local hospital markets. Med Care Res Rev. 1991;48(2):207–237 [DOI] [PubMed] [Google Scholar]
- 27.Hsiao WC, Heller PS. What Macroeconomists Should Know About Health Care Policy. Washington, DC: International Monetary Fund; 2007 [Google Scholar]
- 28.Iwashyna TJ, Christie JD, Kahn JM, Asch DA. Uncharted paths: hospital networks in critical care. Chest. 2009;135(3):827–833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ho V, Hamilton BH. Hospital mergers and aquisitions: does market consolidation harm patients? J Health Econ. 2000;19(5):767–791 [DOI] [PubMed] [Google Scholar]
- 30.White C, Seagrave S. What happens when hospital-based skilled nursing facilities close? A propensity score analysis. Health Serv Res. 2005;40(6, pt 1):1883–1897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Himmelstein DU, Woolhandler S, Harnly M, et al. Patient transfers: medical practice as social triage. Am J Public Health. 1984;74(5):494–497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mackenzie PA, Smith EA, Wallace PGM. Transfer of adults between intensive care units in the United Kingdom: postal survey. BMJ. 1997;314(7092):1455–1456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Avitzour M, Abaronson-Daniel L, Peleg K. Secondary transfer of trauma patients: rationale and characteristics. Isr Med Assoc J. 2006;8(8):539–542 [PubMed] [Google Scholar]
- 34.Bertazzoni G, Cristogani M, Ponzanetti A, et al. Scant justification for interhospital transfers: a cause of reduced efficiency in the emergency department. Emerg Med J. 2008;25(9):558–561 [DOI] [PubMed] [Google Scholar]
- 35.Durairaj L, Will JG, Torner JC, Doebbeling BN. Prognostic factors for mortality following interhospital transfers to the medical intensive care unit of a tertiary referral center. Crit Care Med. 2003;31(7):1981–1986 [DOI] [PubMed] [Google Scholar]
- 36.Teresi JA, Holmes D, Bloom HG, Monaco C, Rosen S. Factors differentiating hospital transfers from long-term care facilities with high and low transfer rates. Gerontologist. 1991;31(6):795–806 [DOI] [PubMed] [Google Scholar]
- 37.Wakefield DS, Ward M, Miller T, et al. Intensive care unit utilization and interhospital transfers as potential indicators of rural hospital quality. J Rural Health. 2004;20(4):394–400 [DOI] [PubMed] [Google Scholar]