Abstract
Growing consensus indicates that progress in tuberculosis control in the low- and middle-income world will require not only investment in strengthening tuberculosis control programs, diagnostics, and treatment but also action on the social determinants of tuberculosis. However, practical ideas for action are scarcer than is notional support for this idea. We developed a framework based on the recent World Health Organization Commission on Social Determinants of Health and on current understanding of the social determinants of tuberculosis. Interventions from outside the health sector—specifically, in social protection and urban planning—have the potential to strengthen tuberculosis control.
In December 2009, at the International Union Against Tuberculosis (TB) and Lung Disease's World Conference in Cancun, Mexico, Mario Raviglione, director of the World Health Organization (WHO) Stop TB Department, made a clear commitment to “moving beyond the TB box.”1 His remarks echoed an emerging shift within the TB sector toward recognizing the importance of social and economic development policies and interventions in supporting TB control.2
The 5 Elements of Directly Observed Therapy—Short Course (DOTS) and 6 Components of the Stop Tuberculosis (TB) Strategy
The 5 Elements of DOTS12
Political commitment with increased and sustained financing,
Case detection through quality-assured bacteriology,
Standardized treatment with supervision and patient support,
An effective drug supply and management system, and
Monitoring and evaluation system and impact measurement.
The 6 Components of the Stop TB Strategy14b
Pursue high-quality DOTS expansion and enhancement.
- Address TB and HIV, multidrug-resistant TB, and the needs of poor and vulnerable populations.
- Scale up collaborative TB and HIV activities.
- Scale up prevention and management of multidrug-resistant TB.
- Address the needs of TB contacts and of poor and vulnerable populations.
- Contribute to health system strengthening based on primary health care.
- Help improve health policies, human resource development, financing, supplies, service delivery, and information.
- Strengthen infection control in health services, other congregate settings, and households.
- Upgrade laboratory networks and implement the Practical Approach to Lung Health.
- Adapt successful approaches from other fields and sectors, and foster action on the social determinants of health.
- Engage all care providers.
- Involve all public, voluntary, corporate, and private providers through public–private mix approaches.
- Promote use of the International Standards for Tuberculosis Care.
- Empower people with TB and communities through partnership.
- Pursue advocacy, communication, and social mobilization.
- Foster community participation in TB care.
- Promote use of the Patients’ Charter for Tuberculosis Care.
Enable and promote research.
We welcome this timely shift in emphasis but recognize too that practical ideas for action are scarcer than is notional support for the idea. In this article, we briefly described why we think this shift occurred. We developed a framework informed by the recent WHO Commission on Social Determinants of Health (CSDH)3 and WHO work on the social determinants of TB2 that guided our ideas for action in this area. We also described 2 non–health-sector domains in which we think program and policy action is feasible and could reduce the public health burden caused by TB in key settings. Finally, we call for a new era of research, action, and evaluation in this field.
RECENT INTEREST IN SOCIAL DETERMINANTS OF TUBERCULOSIS
The increased focus on addressing the social determinants of TB has been stimulated from both within and beyond the TB sector. A key driver has simply been the increasing number of TB cases and their inequitable distribution throughout the world. Not only did 2010 see more cases of TB than ever before in human history, but these cases continue to cluster among disadvantaged groups such as the poor,4,5 the hungry,6–8 and ethnic minorities.9,10 In addition, debate continues about the effectiveness of the Directly Observed Therapy—Short Course (DOTS) strategy, the central pillar of global TB control, in reducing TB incidence.11–14a As shown in the box on the following page, DOTS particularly emphasizes early case detection and successful treatment. DOTS has significantly reduced TB morbidity and mortality15 and is one of the most cost-effective public health interventions ever implemented.16,17 However, national TB incidence rates appear more closely correlated with social and economic determinants such as the human development index, access to water sanitation, and child mortality than to the success of DOTS.13,18 WHO's more recent Stop TB Strategy keeps DOTS at its heart while also reflecting a shift toward greater appreciation of the social determinants of TB (as seen in the box on the following page).
Growing awareness of the importance of social determinants of health in other areas, particularly HIV/AIDS, has stimulated interest in the role of these determinants for other communicable diseases such as TB. Major advances have been made in diagnosis and treatment of HIV/AIDS, but arguably more significant in the last 10 years has been the unparalleled emphasis on social determinants of HIV/AIDS risk and treatment access.19 An explosion of research has occurred into how gender-based and socioeconomic inequalities of opportunity undermine individual efforts to avoid HIV infection and receive effective treatment.20–24 The findings in turn have led to structural interventions for HIV prevention, including those that aim to mobilize communities and empower women, as measures to deal with distal determinants of HIV epidemiology.25–28 These developments have not gone unnoticed within the TB community, not least because HIV infection is itself a key determinant of TB risk in many settings.
Finally, the recent WHO Commission on Social Determinants of Health showed how the “circumstances in which we grow, live, work, and age” and the “systems put in place to deal with illness” give rise to unequal, unfair distributions of population health.3 The CSDH, a comprehensive attempt to gather evidence on the social determinants of health, brought these issues to the forefront of the WHO agenda for the first time in a generation. The WHO also increasingly recognizes the links between health, human rights, and poverty reduction strategies.29 These initiatives provide a platform from which to launch a new era of action on the social determinants of TB.
SOCIAL DETERMINANTS OF TUBERCULOSIS
The CSDH defines structural determinants of health as those conditions that generate or reinforce social stratification in society. Social stratification in turn gives rise to an unequal distribution of the social determinants of health, including material living conditions and psychosocial circumstances as well as behavioral and biological risk factors.30
Key structural determinants of TB epidemiology include global socioeconomic inequalities, high levels of population mobility, and rapid urbanization and population growth. These conditions give rise to unequal distributions of key social determinants of TB, including food insecurity and malnutrition, poor housing and environmental conditions, and financial, geographic, and cultural barriers to health care access. In turn, the population distribution of TB reflects the distribution of these social determinants, which influence the 4 stages of TB pathogenesis: exposure to infection, progression to disease, late or inappropriate diagnosis and treatment, and poor treatment adherence and success.
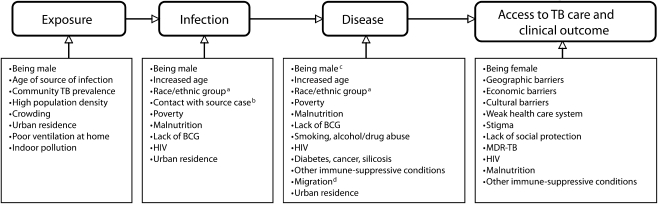
These social determinants are among the key risk factors for TB (Figure 1).31 For example, poor ventilation and overcrowding in homes, workplaces, and communities increase the likelihood of uninfected individuals being exposed to TB infection.32–34 Poverty, malnutrition, and hunger may increase susceptibility to infection,33 disease,8 and severity of clinical outcome.35 Individuals with TB symptoms such as a persistent cough often face significant social and economic barriers that delay their contact with health systems in which an appropriate diagnosis might be made, including difficulties in transport to health facilities,36 fear of stigmatization if they seek a TB diagnosis,37 and lack of social support to seek care when they fall sick.
FIGURE 1.
Risk factors for different stages of TB pathogenesis and epidemiology.
Note. BCG = bacillus Calmette-Guerin; MDR = multidrug resistant; TB = tuberculosis. a TB infection and disease rates are often reported to be higher among Black Africans and Hispanics than among Whites. b Increased TB risk associated with contact with a case of TB depends on the infectivity of the source case, the degree of exposure to the case by the susceptible person, and the degree of susceptibility of a person to infection. c It is unclear whether this observation can be explained by differences in case finding or whether it is due to different susceptibility to TB among sexes. TB disease tends to be more common among males.d Migrants’ increased risk of TB in many settings may result from higher exposure to TB in country of origin or experience of worse socioeconomic living conditions compared with residents.
Although DOTS has pioneered the use of a patient's social network to improve treatment adherence, a social determinant's framework also highlights how lack of hope for the future, driven by poverty, might also foster high rates of treatment default that undermine TB control.38,39 Finally, because of the close relationship between HIV and TB in many settings, notably sub-Saharan Africa, the key structural and social determinants of HIV infection also act as indirect determinants of TB risk. Foremost among these is widespread inequality in opportunities and expectations for men and women reinforced through cultural norms and socioeconomic systems. These opportunities and expectations create conditions that give rise to networks of concurrent sexual partnerships characterized by power inequalities between male and female partners.40
FROM ACTION ON TUBERCULOSIS TO ACTION ON ITS SOCIAL DETERMINANTS
Table 1 identifies recognized approaches to TB control that target the 4 key stages of TB pathogenesis. These interventions are generally delivered by what the CSDH refers to as “the systems put in place to deal with illness,”3 and include the use of diagnostic technologies to identify cases and medicines to treat patients and prevent infection among their close contacts.12 Existing TB control efforts also seek to increase the accessibility of health systems to the communities they serve through treatment support, along with active case-finding and outreach services for high-risk populations. Another component of TB control includes health promotion efforts that inform populations about TB and the factors that increase its risk, thereby aiming to change behaviors such as smoking or alcohol consumption. Finally, integrating HIV and TB control efforts is a major priority in many settings.
TABLE 1.
Key Determinants of Tuberculosis (TB) Transmission and Relevant Interventions Within the Health Care Sector
| TB Outcome |
||||
| Exposure to Infection | Susceptibility to Disease and Disease Outcome | Lack of Timely, Appropriate Treatment Initiation | Poor Treatment Adherence and Success | |
| Key determinants | Incomplete, delayed, or ineffective treatment of TB bacilli spreaders | Incomplete, delayed, or ineffective treatment | Missed diagnosis opportunities as a result of poorly trained lab staff | Complex drug regimens |
| Side effects | ||||
| Lack of chemoprophylaxis or vaccination among contacts | Imperfect diagnostic tools | Lack of patient compliance | ||
| Lack of drug availability | Lack of drug availability | |||
| Risk factors (e.g., malnutrition, diabetes, cigarette smoking, alcohol abuse, HIV infection and other immunosuppressive conditions) | Poor management of TB and HIV coinfection | |||
| Health care sector interventions | Enhanced case finding | Improved treatment adherence | Removal of health system barriers to case detection | Standardized, short-course treatment regimens |
| Improved treatment adherence | BCG vaccination among children | |||
| Infection control measures (e.g., quarantine, laboratory safety) | Chemoprophylaxis protocols for TB contacts | Improved diagnostics | Decrease of side effects | |
| Improved synergy between TB and HIV services | Treatment support (DOT) | |||
| Behavioral counseling to reduce risk behavior | Ensured drug supply | |||
| Ensured drug supply | Use of community health workers to monitor and support adherence | |||
| Linkage of HIV and TB services | ||||
Note. BCG = bacillus Calmette-Guerin; DOT = directly observed therapy.
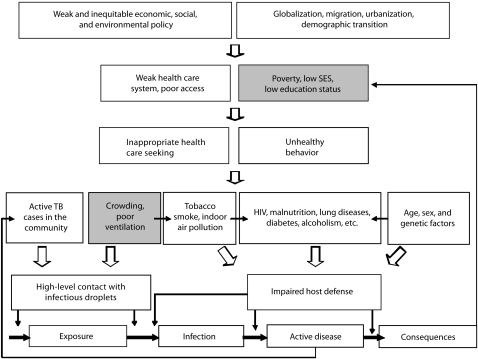
Action on the social determinants of TB will require shifting the target to “the conditions in which [populations with high levels of TB] grow, live, work, and age.”3 Although definitions vary, such approaches are sometimes referred to as structural interventions.23,41 These interventions often require leadership or significant involvement from outside the health sector. Figure 2 reproduces a recently published framework from the WHO that identifies proximate risk factors and upstream determinants of TB. Within this framework, we identified 2 strategic entry points for action that will require collaboration with stakeholders from beyond the health care sector (shown in gray in Figure 2).2
FIGURE 2.
Conceptual framework and strategic entry points for intervention outside the health care sector.
Note. SES = socioeconomic status; TB = tuberculosis. Gray boxes indicate entry points for intervention.Source. Adapted with permission from Elsevier.2
Source. Reproduced from Lonnroth et al.2
Biomedical and structural interventions are sometimes characterized as competing approaches to TB control based on different interpretations of the etiology of TB.42 This debate is unhelpful. Expanding our vision to include social determinants as targets for TB control efforts does not require us to rethink TB epidemiology nor to devote all our resources to eradicating poverty. Rather, in addition to existing TB control efforts, it may be possible (and necessary) to address selected factors in the daily living conditions of TB patients and their communities that might influence TB epidemiology. The question is not what level should be targeted in public health interventions: proximate risk factors or upstream determinants, individuals or societies, biomedical or structural factors, or pills or poverty reduction strategies.43 Rather, the opportunity for the TB community is to best use knowledge about TB, from molecular biology to social determinants, to develop effective control approaches based on strong interdisciplinary approaches that capitalize on rather than disperse the medical, microbiological, and social notions we have of the disease.
We described 2 approaches to socioeconomic development that have been shown to be feasible in a range of settings and target important social determinants of TB. However, access to these approaches remains restricted to a minority of those who might benefit from them. Whether policymakers expand access to such programs will be influenced by many factors, including cost, feasibility, and politics. However, because these approaches to development may have powerful impacts on TB epidemiology, we believe that discussion about their adoption should be brought to the center of the TB control debate. Key questions include the following: How might these initiatives be designed to best contribute to TB control? In what settings might they be most relevant to TB? What are the basic health system conditions necessary for these approaches to impact TB epidemiology? And, where implemented, what are their impacts on TB? We believe that the TB sector must lead research in this area, pulling in expertise from beyond its borders to strengthen this research, and in turn pushing out its conclusions to debates across sectors when decisions are to be made about the adoption or refinement of programs in these domains.
ADDRESSING THE SOCIAL DETERMINANTS OF TUBERCULOSIS
The social determinants of TB might be addressed by strengthening social protection and livelihood-strengthening interventions or through urban regeneration.
Social Protection and Livelihood-Strengthening Interventions
A significant TB burden falls among populations with high levels of chronic poverty and malnutrition.44 In turn, TB illness can further exacerbate poverty, food insecurity, and malnutrition. Alleviating the poverty and improving the food security of these populations may reduce their TB burden.
Social protection initiatives reduce vulnerability to poverty, mitigate the impact of economic shocks such as illness or loss of employment, and support people who suffer from chronic incapacities as a result of age, illness, disability, or discrimination to secure basic livelihoods.45 Recently, social protection has emerged as a possibility for poor countries owing to growing political and financial support from their governments, bilateral donors, and other global and regional institutions. Part of the impetus for social protection is that poverty reduces investment in the health, nutrition, and education of children, which leads to reduced earnings later in life and thus perpetuates intergenerational cycles of poverty.46 Social protection initiatives can enable households to move structurally out of poverty by protecting and building their financial, physical, and human capital assets, thereby contributing to long-term productivity and economic growth.47 Two key components of social protection include providing direct transfers of food or money to poor households, with the receipt of these transfers sometimes conditional on other actions, and increasing access to microfinance opportunities to support business development. Training activities often run in parallel with both components to maximize their impact.
Cash transfer programs are currently reaching large numbers of poor people in Latin America and sub-Saharan Africa. Evidence of impacts on children's health, education, and nutrition is emerging from a diverse range of settings.48 For example, South Africa has a number of schemes that collectively reach nearly 12 million beneficiaries, from cash grants for poor households with children to grants for the severely ill. Data from 6 cash transfer programs in southern Africa show that the vast majority of the transfer is spent on food, but other expenditures include education, health care, clothing, and transportation.48
Throughout Latin America and in several countries in Asia, conditional cash transfer schemes provide money to poor families who meet certain behavioral requirements. In well-described government-led programs, conditions include sending children to school and attending health services for prenatal checkups, immunization, and growth monitoring.49 The conditions are designed to ensure that the programs act as instruments for long-term human capital development as well as short-term assistance. Conditional cash transfer programs have been demonstrated to increase household consumption,50,51 reduce vulnerability to economic shocks,49 improve food security and quality,52,53 increase participation in health services,54,55 and, although evidence remains sparse, improve health outcomes.56
Microfinance initiatives provide a complementary approach to social protection often delivered by the nongovernmental sector. They provide the poor with access to credit to improve their opportunities to engage in productive activities.57 Many microfinance initiatives deliver loans through the creation of neighborhood-based associations of women that meet regularly. These programs are widespread: the Microcredit Summit Campaign reports that, by the end of December 2007, approximately 3600 microfinance initiatives were reaching about 155 million clients in 134 countries with loans.58 A major strength of the microfinance sector has been its move away from donor dependency, with successful initiatives creating sustainable systems for credit delivery that pay for themselves through interest on loans.59
Training to support human capital development is central to livelihood programs. Training may support skills development toward productive activities. For example, programs in rural areas might provide seeds, fertilizer, and training to people who have been weakened by illness so that they can maintain kitchen gardens.60 Many microfinance programs provide clients with business development training and mentoring to support the productive use of loans. Alternatively, training may target health-related goals directly. For example, attendance at health training has been a key requirement of many conditional cash transfer programs in Latin America, whereas microfinance initiatives have used client meetings to engage women on health-related matters, including malnutrition, vaccination coverage, contraceptive use, breastfeeding practices, intimate partner violence, HIV prevention, HIV impact mitigation, and child care.61
Social protection strategies such as cash transfers, microcredit, and training might be harnessed to improve prevention and mitigation of TB in at least 4 ways. First, these programs might be made more widely available in communities with high levels of TB. Where they reach high coverage in these settings, they would improve material conditions for many households and help reduce financial barriers to diagnosis often experienced by individuals with TB symptoms. Second, efforts to increase community action and education about TB could be linked to participation in these programs. Participation in health education could be mandatory or voluntary. Benefit distribution points have been used as a site for information dissemination and outreach.62a
Third, these interventions may target TB patients and their close contacts, who likely share a risk environment with index cases and could also be at direct risk of infection from them. Cash transfers could be provided to TB patients conditional on treatment adherence or other relevant health behaviors such as stopping smoking. Transfers might also be offered to contacts of diagnosed TB patients to support goals such as sputum sample collection and latent TB infection diagnosis or successful provision of preventive medication to children, all of which should reduce further the morbidity associated with an index case.
Fourth, to support longer-term developmental aims, opportunities for training and business development might be provided to TB sufferers or members of their household who are not suffering with TB. This provision may create further incentive to maximize treatment adherence as well as to provide the building blocks for longer-term livelihood strengthening. In time, these actions might also support more rapid case finding in communities as overlapping fears about the costs and stigma associated with a TB diagnosis are replaced by awareness of TB and an incentive to get diagnosed and treated quickly. Indeed, fostering economic empowerment and a greater sense of self-efficacy among TB-affected families engaged in social protection programs might also ultimately lead to stronger confidence and greater voice to influence political decisions about TB care rights and TB care-related education.
Adapting social protection initiatives to support TB control aims will be complex. For example, direct targeting of TB sufferers for social protection raises potential problems. Cash transfer interventions are generally targeted to poor communities or poor households in localities with high illness burdens, not toward ill people or their households. Extensive research on cash transfers to support families affected by HIV/AIDS has concluded that direct targeting of AIDS-affected families or orphans poses significant challenges. These challenges arise from the perspective of equity, since poor households not affected by illness may be equally in need of assistance, and stigma, because households with illness are singled out and identified.45
One approach may be to establish targeting criteria that capture both the very poor as well as TB sufferers—for example, by including a requirement that eligible households have no or few adults able to work, an approach used by community-based selection committees in cash transfer programs in Zambia and Malawi.62b Setting conditions for grant receipt also adds complexity to programs requiring careful consideration. Many very poor countries where social protection programs may be most relevant to TB have weak health systems. Making cash benefits conditional on health system access may be impractical or can result in withholding assistance from those who most need it. More generally, whether setting conditions improves human capital outcomes better than unconditional transfers is an unresolved debate. The evidence from Latin America supports conditionality,63 whereas the only evidence thus far from Africa, from a small program in Malawi, demonstrated no added impact.64 Finally, complex issues exist related to linking training activities to other aspects of social protection. For example, many within the microfinance sector feel that microfinance initiatives should not compromise their central credit delivery aim by trying to do too much.38 Resolving these issues will be key to maximizing the benefits of these types of approach for TB control.
Urban Regeneration
TB is more common in urban than in rural areas, and consequently, it is a greater problem in more rapidly urbanizing societies.65 With almost 1 billion people living in urban slums in developing countries and annual population growth in Asia and Africa projected to be 2.4%, urbanization is one of the largest obstacles to the full implementation of current TB control strategies.66,67 The poor living conditions of many urban communities that suffer a high burden of TB are not coincidental to this outcome but rather a fundamental driver of the problem. In particular, overcrowding and poor ventilation in homes, workplaces, recreational spaces, and health facilities foster high rates of transmission,68,69 and inefficient health services delay diagnosis and treatment onset and reduce adherence.70
Undiagnosed smear-positive patients are the main source of secondary infection in most communities.71–74 TB can therefore be considered an environmental problem in which TB bacilli, spread by undiagnosed or ineffectively treated contagious cases, represent a pollutant that spoils the quality of the air and makes it unhealthy for all community members. Housing design, urban regeneration, and slum upgrading programs might therefore have a role to play in TB control. In addition, they are consistent with the Millennium Development Goal of “by 2020, to have achieved a significant improvement in the lives of at least 100 million slum dwellers.”75
Urban regeneration and slum upgrading projects may affect TB epidemiology through 2 major pathways. First, they may influence TB transmission directly by affecting the physical and social environments of communities.76 These interventions may significantly affect TB transmission by improving housing quality in terms of indoor pollution, air ventilation, and available space—all factors associated with increased household TB transmission.69,77–79 Urban regeneration programs might also affect the web of social relationships and social organization within communities and in turn influence individuals’ behavior.80 So, for example, communities engaged in such programs may promote attitudes and health-seeking behaviors that reduce TB transmission. The social links among individuals are also the social circuits along which information may flow about how to recognize and respond to TB risks and stigma.
Second, urban regeneration programs may have indirect effects by shaping the standard of living of community residents by increasing access to health services, transport, markets, schooling, and occupation opportunities for community members. For example, participatory slum upgrading engages communities in both the identification of environmental problems and the execution of projects to address these. In the Community Managed Settlement Upgrading Project in one of the major informal settlements in the city of Dar es Salaam, Tanzania, community members developed skills and education, gained income from water-vending and solid-waste management, and accessed loans for home improvement.81 Another example comes from an evaluation of 25 small-scale community-based projects implemented in Bangladesh, Senegal, Thailand, and Zambia that involved water sanitation, electrification, irrigation, bridge construction, and health care infrastructure delivery.82 These initiatives had the potential to reduce poverty by raising productivity of the local economy. Further, they could significantly affect human health indicators, particularly malaria, pneumonia, and diarrhea in children.
Although experience is growing in implementing interventions aimed at housing policy and urban regeneration, recent systematic reviews of the health effect of urban regeneration and housing improvements conclude that an independent or additive effect of these interventions for health promotion purposes is still to be demonstrated.83,84 The TB community can make important contributions in this field.
PUTTING IDEAS INTO ACTION
The ideas we have outlined for social protection and urban regeneration interventions that contribute to TB control remain just that: ideas. To our knowledge, these interventions have not yet been evaluated regarding their relevance for TB control. Although decisions about whether to deliver social protection or slum-upgrading programs will be influenced by a range of factors, we believe that evidence of the effectiveness of these approaches in contributing to TB control is vital. Without such evidence, action will remain slow and uncoordinated.
Planning interdisciplinary evaluations of the effect of such programs on TB epidemiology will be complex. As a first step toward these aims, one of us (C. A. E.) has had the opportunity to develop these ideas in practice with his involvement in the Fighting Poverty to Control TB project, based in 16 contiguous shantytown communities with a population of approximately 750 000 people in Northern Lima, Peru. The project aims to develop and rigorously evaluate socioeconomic interventions for strengthening TB control. Interventions, which are targeted to TB patients and their families, include improving access to training for work, providing microcredit loans, and supporting the development of microenterprises. A further intervention component promotes health rights for enhancing equitable access to health care, thereby uniting and empowering TB-affected households for advocacy in regular community meetings.
By early 2010, the project had recruited more than 1000 TB patients and 5500 household contacts in 2 years. These families reported high rates of poverty, experience of stigmatization, and depression. More than 95% of households given the opportunity chose to participate in the interventions. Early results suggested increased poverty-reduction activities, including use of microcredit loans that have been more effective than the village-banking system, with moderate loan repayment and training completion rates to date. These poverty-reduction interventions are effective incentives for participation in the health rights activities. Learning opportunities continue to arise in the project.
CONCLUSIONS
Few may doubt that in an ideal world, the provision of these interventions would be desirable and would contribute to lower TB incidence in the long term. However, we expect considerable debate on their feasibility and short- and medium-term impact. Currently, however, little evidence is available to move this debate forward. We believe now is the time for a rapid scaleup of innovation, action, interdisciplinary planning, and evaluation in this area.
Our focus on tackling the social determinants of TB should not undermine other ongoing efforts. Millions of people have been successfully treated worldwide through DOTS. Increased investment in diagnostics and treatment of TB remains a priority. We also do not seek to burden already stretched TB control programs with the sole responsibility for delivering the policies and programs we have discussed. By their nature, these initiatives may require leadership from other sectors. However, we do suggest that TB control might be strengthened if national TB control programs were more actively involved in designing, developing, and motivating initiatives to improve living conditions in places where TB is a major public health problem.
The issues we seek to address are complex. For example, the emergence of HIV and multidrug-resistant TB raises questions about whether socioeconomic development in modern developing countries would indeed reproduce the same effect in TB trends observed in North America and Europe during the 19th century.42 But these questions are unanswerable without better evidence. Intervention-based research that explores the complex interaction between biological and structural phenomena driving the current TB epidemic is needed.
Gaps still exist in our understanding of the extent to which socioeconomic determinants drive the current TB epidemic, the underlying processes linking socioeconomic determinants to TB, and how to best address these determinants. However, we believe that taking TB control forward is both desirable and possible and that current recognition of the importance of addressing the social determinants of health provides a real opportunity to expand the current paradigm for TB control. Key to success will be the capacity to design research in which different disciplines can develop a shared approach and common conceptual framework.85 A great deal will be learned as partnerships involving actors from within and beyond the health sector conduct rigorous evaluations of the impact of economic and development aid programs on TB control.
Acknowledgments
While work on this article was undertaken without specific funding, we acknowledge funding support for tuberculosis research that underpinned the development of these ideas from the Wellcome Trust, the UK Government Department for International Development, the World Health Organization, Innovation for Health and Development, and the Foundation for Innovative New Diagnostics.
References
- 1.Raviglione MC. TB prevention, care and control, 2010–2015: framing global and WHO strategic priorities. Paper presented at: 40th Union World Conference on Lung Health; December 3, 2009; Cancun, Mexico [Google Scholar]
- 2.Lonnroth K, Jaramillo E, Williams BG, Dye C, Raviglione M. Drivers of tuberculosis epidemics: the role of risk factors and social determinants. Soc Sci Med. 2009;68(12):2240–2246 [DOI] [PubMed] [Google Scholar]
- 3.Commission on Social Determinants of Health Commission on Social Determinants of Health—final report. Available at: http://www.who.int/social_determinants/thecommission/finalreport/en/index.html. Accessed February 16, 2010
- 4.Jackson S, Sleigh AC, Wang GJ, Liu XL. Poverty and the economic effects of TB in rural China. Int J Tuberc Lung Dis. 2006;10(10):1104–1110 [PubMed] [Google Scholar]
- 5.Muniyandi M, Ramachandran R, Gopi PG, et al. The prevalence of tuberculosis in different economic strata: a community survey from South India. Int J Tuberc Lung Dis. 2007;11(9):1042–1045 [PubMed] [Google Scholar]
- 6.Lonnroth K, Williams BG, Cegielski P, Dye C. A consistent log-linear relationship between tuberculosis incidence and body mass index. Int J Epidemiol. 2010;39(1):149–155 [DOI] [PubMed] [Google Scholar]
- 7.Cegielski JP, McMurray DN. The relationship between malnutrition and tuberculosis: evidence from studies in humans and experimental animals. Int J Tuberc Lung Dis. 2004;8(3):286–298 [PubMed] [Google Scholar]
- 8.Pakasi TA, Karyadi E, Dolmans WM, van der Meer JW, van der Velden K. Malnutrition and socio-demographic factors associated with pulmonary tuberculosis in Timor and Rote Islands, Indonesia. Int J Tuberc Lung Dis. 2009;13(6):755–759 [PubMed] [Google Scholar]
- 9.Doherty MJ, Davies PD, Bellis MA, Tocque K. Tuberculosis in England and Wales. Ethnic origin is more important than social deprivation. BMJ. 1995;311(6998):187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stout JE, Saharia KK, Nageswaran S, Ahmed A, Hamilton CD. Racial and ethnic disparities in pediatric tuberculosis in North Carolina. Arch Pediatr Adolesc Med. 2006;160(6):631–637 [DOI] [PubMed] [Google Scholar]
- 11.Volmink J, Garner P. Directly observed therapy for treating tuberculosis. Cochrane Database Syst Rev. 2007;(4):CD003343. [DOI] [PubMed] [Google Scholar]
- 12.Raviglione MC, Pio A. Evolution of WHO policies for tuberculosis control, 1948–2001. Lancet. 2002;359(9308):775–780 [DOI] [PubMed] [Google Scholar]
- 13.Dye C, Lonnroth K, Jaramillo E, Williams BG, Raviglione M. Trends in tuberculosis incidence and their determinants in 134 countries. Bull World Health Organ. 2009;87(9):683–691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14a.Rusen ID, Ait-Khaled N, Alarcon E, et al. Cochrane systematic review of directly observed therapy for treating tuberculosis: good analysis of the wrong outcome. Int J Tuberc Lung Dis. 2007;11(2):120–121 [PubMed] [Google Scholar]
- 14b.World Health Organization The global plan to stop TB 2011-2015: transforming the fight towards elimination of tuberculosis. Available at: http://www.stoptb.org/assets/documents/global/plan/TB_GlobalPlanToStopTB2011-2015.pdf. Accessed February 11, 2011
- 15.Global Tuberculosis Control: Surveillance, Planning, Financing. Geneva, Switzerland: World Health Organization; 2009 [Google Scholar]
- 16.World Development Report 1993: Investing in Health. Washington, DC: World Bank; 1993 [Google Scholar]
- 17.Laxminarayan R, Klein E, Dye C, Floyd K, Darley S, Adeji O. Economic Benefit of Tuberculosis Control. Washington DC: World Bank; 2007. Policy Research Working Paper 4295 [Google Scholar]
- 18.Obermeyer Z, Abbott-Klafter J, Murray CJ. Has the DOTS strategy improved case finding or treatment success? An empirical assessment. PLoS ONE. 2008;3(3):e1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Declaration of Commitment on HIV/AIDS Paper presented at: United Nations General Assembly Special Session on HIV/AIDS; June 25–27, 2001; New York, NY [Google Scholar]
- 20.Campbell C, MacPhail C. Peer education, gender and the development of critical consciousness: participatory HIV prevention by South African youth. Soc Sci Med. 2002;55(2):331–345 [DOI] [PubMed] [Google Scholar]
- 21.Hargreaves JR, Bonell CP, Boler T, et al. Systematic review exploring time-trends in the association between educational attainment and risk of HIV infection in sub-Saharan Africa. AIDS. 2008;22(2):403–414 [DOI] [PubMed] [Google Scholar]
- 22.Jewkes RK, Levin JB, Penn-Kekana LA. Gender inequalities, intimate partner violence and HIV preventive practices: findings of a South African cross-sectional study. Soc Sci Med. 2003;56(1):125–134 [DOI] [PubMed] [Google Scholar]
- 23.Sumartojo E. Structural factors in HIV prevention: concepts, examples, and implications for research. AIDS. 2000;14(suppl 1):S3–S10 [DOI] [PubMed] [Google Scholar]
- 24.Wojcicki JM. Socioeconomic status as a risk factor for HIV infection in women in East, Central and Southern Africa: a systematic review. J Biosoc Sci. 2005;37(1):1–36 [DOI] [PubMed] [Google Scholar]
- 25.Blankenship KM, Bray SJ, Merson MH. Structural interventions in public health. AIDS. 2000;14(suppl 1):S11–S21 [DOI] [PubMed] [Google Scholar]
- 26.Campbell C, Nair Y, Maimane S. Building contexts that support effective community responses to HIV/AIDS: a South African case study. Am J Community Psychol. 2007;39(3–4):347–363 [DOI] [PubMed] [Google Scholar]
- 27.Pronyk PM, Hargreaves JR, Kim JC, et al. Effect of a structural intervention for the prevention of intimate-partner violence and HIV in rural South Africa: a cluster randomised trial. Lancet. 2006;368(9551):1973–1983 [DOI] [PubMed] [Google Scholar]
- 28.Sweat MD, Denison J. Reducing HIV incidence in developing countries with social and structural interventions. AIDS. 1995;9(suppl A):s251–s257 [PubMed] [Google Scholar]
- 29.Human Rights, Health and Poverty Reduction Strategies. Geneva, Switzerland: World Health Organization; 2008 [Google Scholar]
- 30.Commission on Social Determinants of Health A conceptual framework for action on the social determinants of health. Available at: http://www.who.int/social_determinants/resources/csdh_framework_action_05_07.pdf. Accessed February 16, 2010
- 31.Lienhardt C. From exposure to disease: the role of environmental factors in susceptibility to and development of tuberculosis. Epidemiol Rev. 2001;23(2):288–301 [DOI] [PubMed] [Google Scholar]
- 32.Hill PC, Jackson-Sillah D, Donkor SA, Otu J, Adegbola RA, Lienhardt C. Risk factors for pulmonary tuberculosis: a clinic-based case control study in The Gambia. BMC Public Health. 2006;6:156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Boccia D, Hargreaves J, Ayles H, Fielding K, Simwinga M, Godfrey-Faussett P. Tuberculosis infection in Zambia: the association with relative wealth. Am J Trop Med Hyg. 2009;80(6):1004–1011 [PMC free article] [PubMed] [Google Scholar]
- 34.Baker M, Das D, Venugopal K, Howden-Chapman P. Tuberculosis associated with household crowding in a developed country. J Epidemiol Community Health. 2008;62(8):715–721 [DOI] [PubMed] [Google Scholar]
- 35.Van Lettow M, Kumwenda JJ, Harries AD, et al. Malnutrition and the severity of lung disease in adults with pulmonary tuberculosis in Malawi. Int J Tuberc Lung Dis. 2004;8(2):211–217 [PubMed] [Google Scholar]
- 36.Kanara N, Cain KP, Chhum V, et al. Association between distance to HIV testing site and uptake of HIV testing for tuberculosis patients in Cambodia. Int J Tuberc Lung Dis. 2009;13(2):226–231 [PubMed] [Google Scholar]
- 37.Somma D, Thomas BE, Karim F, et al. Gender and socio-cultural determinants of TB-related stigma in Bangladesh, India, Malawi and Colombia. Int J Tuberc Lung Dis. 2008;12(7):856–866 [PubMed] [Google Scholar]
- 38.Kim J, Pronyk P, Barnett T, Watts C. Exploring the role of economic empowerment in HIV prevention. AIDS. 2008;22(suppl 4):S57–S71 [DOI] [PubMed] [Google Scholar]
- 39.Bernays S, Rhodes T, Barnett T. Hope: a new way to look at the HIV epidemic. AIDS. 2007;21(suppl 5):S5–S11 [DOI] [PubMed] [Google Scholar]
- 40.Gilbert L, Walker L. Treading the path of least resistance: HIV/AIDS and social inequalities a South African case study. Soc Sci Med. 2002;54(7):1093–1110 [DOI] [PubMed] [Google Scholar]
- 41.Blankenship KM, Friedman SR, Dworkin S, Mantell JE. Structural interventions: concepts, challenges and opportunities for research. J Urban Health. 2006;83(1):59–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Grange JM, Gandy M, Farmer P, Zumla A. Historical declines in tuberculosis: nature, nurture and the biosocial model. Int J Tuberc Lung Dis. 2001;5(3):208–212 [PubMed] [Google Scholar]
- 43.Rothman KJ, Adami HO, Trichopoulos D. Should the mission of epidemiology include the eradication of poverty? Lancet. 1998;352(9130):810–813 [DOI] [PubMed] [Google Scholar]
- 44.Dubos RJ. The White Plague: Tuberculosis, Man, and Society. New Brunswick, NJ: Rutgers University Press; 1987 [Google Scholar]
- 45.Adato M, Bassett L. What Is the Potential of Cash Transfers to Strengthen Families Affected by HIV and AIDS? A Review of the Evidence on Impacts and Key Policy Debates. Boston, MA: Joint Learning Initiative on Children and AIDS; 2008 [Google Scholar]
- 46.Grantham-McGregor S, Cheung YB, Cueto S, Glewwe P, Richter L, Strupp B. Developmental potential in the first 5 years for children in developing countries. Lancet. 2007;369(9555):60–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Adato M, Hoddinott J. Social Protection: Opportunities for Africa. (IFPRI) Policy Brief 5. Washington, DC: International Food Policy Research Institute; 2008 [Google Scholar]
- 48.Adato M, Bassett L. Social protection to support vulnerable children and families: the potential of cash transfers to protect education, health and nutrition. AIDS Care. 2009;21(S1):60–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fiszbein A, Schady NR. Conditional cash transfers: reducing current and future poverty. World Bank Policy Research Report. Washington, DC: The World Bank; 2009 [Google Scholar]
- 50.Hoddinott J, Skoufias E, Washburn R. The Impact of PROGRESA on Consumption: A Final Report. Washington, DC: International Food Policy Research Institute; 2000 [Google Scholar]
- 51.Attanasio O, Mesnard A. The impact of a conditional cash transfer programme on consumption in Colombia. Fisc Stud. 2006;27(4):421–442 [Google Scholar]
- 52.Sridar D, Duffield A. A review of the impact of cash transfers programmes on child nutritional status and some implications for Save the Children UK programmes. October 2006. Available at: http://www.savethechildren.org.uk/en/docs/cash_transfer_prog_nutrition.pdf. Accessed January 20, 2011
- 53.Hoddinott J, Bassett L. Conditional cash transfer programs and nutrition in Latin America: assessment of impacts and strategies for improvement. Working paper #9. : Iniciativa America Latina y Caribe Sin Hambre Working Papers. Rome, Italy: United Nations Food and Agricultural Organization; 2009 [Google Scholar]
- 54.Attanasio O, Gomez LC, Heredia P, Vera-Hernandez M. The Short-Term Impact of a Conditional Cash Subsidy on Child Health and Nutrition in Colombia. London, UK: Institute of Fiscal Studies; 2005 [Google Scholar]
- 55.Gertler P. The Impact of PROGRESA on Health. Washington, DC: International Food Policy Research Institute; 2000 [Google Scholar]
- 56.Lagarde M, Haines A, Palmer N. Conditional cash transfers for improving uptake of health interventions in low- and middle-income countries: a systematic review. JAMA. 2007;298(16):1900–1910 [DOI] [PubMed] [Google Scholar]
- 57.Mohindra KS, Haddad S. Evaluating the unintended health consequences of poverty alleviation strategies: or what is the relevance of Mohammed Yunus to public health? Can J Public Health. 2008;99(1):66–68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Daley-Harris S. State of the Microcredit Summit Campaign—Report 2009. Washington, DC: RESULTS Educational Fund; 2009 [Google Scholar]
- 59.Goldberg N. Measuring the Impact of Microfinance: Taking Stock of What We Know. Washington, DC: Grameen Foundation; 2005 [Google Scholar]
- 60.Edstrom J, Samuels F. HIV, nutrition, food and livelihoods in Sub-Saharan Africa. Evidence, debates and reflections for guidance. June 25, 2007. Institute for Development Studies in collaboration with Overseas Development Institute Available at: http://www.eldis.org/vfile/upload/1/document/0812/DFIDAIDSLivelihoods.pdf. Accessed January 20, 2011
- 61.Pronyk PM, Hargreaves JR, Morduch J. Microfinance programs and better health: prospects for sub-Saharan Africa. JAMA. 2007;298(16):1925–1927 [DOI] [PubMed] [Google Scholar]
- 62a.Devereux S, Mthinda C, Power F, Sakala P, Suka A. An Evaluation of Concern Worldwide's Dowa Emergency Cash Transfer Project (DECT) in Malawi, 2006/07. Lilongwe, Malawi: Concern Worldwide; 2007 [Google Scholar]
- 62b.Schubert B, Webb D, Temin M, Masabane P. The Impact of Social Cash Transfers on Children Affected by HIV and AIDS: Evidence from Zambia, Malawi, and South Africa. Lilongwe, Malawi: UNICEF ESARO; 2007 [Google Scholar]
- 63.Adato M, Hoddinott J. Conditional cash transfers in the second decade: current debates and new frontiers. : Adato M, Hoddinott J, Conditional Cash Transfers in Latin America. Baltimore, MD: Johns Hopkins University Press; 2010:351–371 [Google Scholar]
- 64.Baird S, Mcintosh C, Ozler B. Cash or Condition? Evidence From a Randomized Cash Transfer Program. Washington, DC: World Bank; 2010. Policy Research Working Paper [Google Scholar]
- 65.Hunter JM, Thomas MO. Hypothesis of leprosy, tuberculosis and urbanization in Africa. Soc Sci Med. 1984;19(1):27–57 [DOI] [PubMed] [Google Scholar]
- 66.Corbett EL, Bandason T, Cheung Y-B, et al. Prevalent infectious tuberculosis in Harare, Zimbabwe: burden, risk factors and implications for control. Int J Tuberc Lung Dis. 2009;13(10):1231–1237 [PMC free article] [PubMed] [Google Scholar]
- 67.Lonnroth K, Raviglione M. Global epidemiology of tuberculosis: prospects for control. Semin Respir Crit Care Med. 2008;29(5):481–491 [DOI] [PubMed] [Google Scholar]
- 68.Wanyeki I, Olson S, Brassard P, et al. Dwellings, crowding, and tuberculosis in Montreal. Soc Sci Med. 2006;63(2):501–511 [DOI] [PubMed] [Google Scholar]
- 69.Canadian Tuberculosis Committee Housing conditions that serve as risk factors for tuberculosis infection and disease. An Advisory Committee Statement (ACS). Can Commun Dis Rep. 2007;33(ACS-9):1–13 [PubMed] [Google Scholar]
- 70.Konteh FH. Urban sanitation and health in the developing world: reminiscing the nineteenth century industrial nations. Health Place. 2009;15(1):69–78 [DOI] [PubMed] [Google Scholar]
- 71.Verver S, Warren RM, Munch Z, et al. Transmission of tuberculosis in a high incidence urban community in South Africa. Int J Epidemiol. 2004;33(2):351–357 [DOI] [PubMed] [Google Scholar]
- 72.Garcia-Garcia M, Palacios-Martinez M, Ponce-de-Leon A, et al. The role of core groups in transmitting Mycobacterium tuberculosis in a high prevalence community in Southern Mexico. Int J Tuberc Lung Dis. 2000;4(1):12–17 [PubMed] [Google Scholar]
- 73.Klovdahl AS, Graviss EA, Yaganehdoost A, et al. Networks and tuberculosis: an undetected community outbreak involving public places. Soc Sci Med. 2001;52(5):681–694 [DOI] [PubMed] [Google Scholar]
- 74.Mangura BT, Napolitano EC, Passannante MR, McDonald RJ, Reichman LB. Mycobacterium tuberculosis miniepidemic in a church gospel choir. Chest. 1998;113(1):234–237 [DOI] [PubMed] [Google Scholar]
- 75.United Nations Millennium Development Goals Goal 7: ensure environmental sustainability. Available at: http://www.un.org/millenniumgoals/environ.shtml. Accessed January 20, 2011
- 76.Robert SA. Neighborhood socioeconomic context and adult health. The mediating role of individual health behaviors and psychosocial factors. Ann N Y Acad Sci. 1999;896:465–468 [DOI] [PubMed] [Google Scholar]
- 77.Lin HH, Ezzati M, Murray M. Tobacco smoke, indoor air pollution and tuberculosis: a systematic review and meta-analysis. PLoS Med. 2007;4(1):e20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Rehfuess E, Corvalan C, Neira M. Indoor air pollution: 4000 deaths a day must no longer be ignored. Bull World Health Organ. 2006;84(7):508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Perez-Padilla R, Perez-Guzman C, Baez-Saldana R, Torres-Cruz A. Cooking with biomass stoves and tuberculosis: a case control study. Int J Tuberc Lung Dis. 2001;5(5):441–447 [PubMed] [Google Scholar]
- 80.Sampson RJ, Morenoff JD, Gannon-Rowley T. Assessing “neighborhood effect”: social processes and new directions in research. Annu Rev Sociol. 2002;28:443–478 [Google Scholar]
- 81.Sheuya SA. Improving the health and lives of people living in slums. Ann N Y Acad Sci. 2008;1136:298–306 [DOI] [PubMed] [Google Scholar]
- 82.Jahan S, McCleery R. Making Infrastructure Work for the Poor. Synthesis Report for Four Country Studies: Bangladesh, Senegal, Thailand and Zambia. New York, NY: United Nations Development Program; 2005 [Google Scholar]
- 83.Bambra C, Gibson M, Petticrew M, Sowden A, Whitehead M, Wright K. Tackling the wider social determinants of health and health inequalities: evidence from systematic reviews. Public Health Research Consortium. Available at: http://www.york.ac.uk/phrc/D2-06%20Final%20Report.pdf. Accessed January 20, 2011 [DOI] [PMC free article] [PubMed]
- 84.Thomson H, Atkinson R, Petticrew M, Kearns A. Do urban regeneration programmes improve public health and reduce health inequalities? A synthesis of the evidence from UK policy and practice (1980–2004). J Epidemiol Community Health. 2006;60(2):108–115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Rosenfield PL. The potential of transdisciplinary research for sustaining and extending linkages between the health and social sciences. Soc Sci Med. 1992;35(11):1343–1357 [DOI] [PubMed] [Google Scholar]