Abstract
Background
There are no treatments currently available for peanut allergy. Sublingual immunotherapy is a novel approach to the treatment of peanut allergy.
Objective
To investigate the safety, clinical effectiveness and immunologic changes with sublingual immunotherapy in peanut-allergic children.
Methods
In this double-blind, placebo-controlled study, subjects underwent 6 months of dose escalation and 6 months of maintenance dosing followed by a double-blind, placebo-controlled food challenge.
Results
Eighteen children ages 1 to 11 years completed 12 months of dosing and the food challenge. Dosing side effects were primarily oropharyngeal and uncommonly required treatment. During the double-blind, placebo-controlled food challenge, the treatment group safely ingested 20 times more peanut protein than the placebo group (median 1710 mg vs. 85 mg, p=0.011). Mechanistic studies demonstrated a decrease in prick skin test wheal size (p=0.020) and decreased basophil responsiveness after stimulation with 10−2 mcg/ml (p=0.009) and 10−3 mcg/ml (p=0.009) of peanut. Peanut-specific IgE increased over the initial 4 months (p=0.002) then steadily decreased over the remaining 8 months (p=0.003) while peanut-specific IgG4 increased during the 12 months (p=0.014). Lastly, IL-5 levels decreased after 12 months (p=0.015). No statistically significant changes were found in IL-13 levels, the percent of T regulatory cells, or IL-10 and IFN-gamma production.
Conclusion
Peanut sublingual immunotherapy is able to safely induce clinical desensitization in peanut allergic children with evidence of immunologic changes suggesting a significant change in the allergic response. Further study is required to determine if continued peanut sublingual immunotherapy is able to induce long-term immune tolerance.
Keywords: peanut allergy, sublingual immunotherapy, desensitization, food allergy
INTRODUCTION
Food allergy continues to be a significant public health problem in industrialized countries. The National Center for Health Statistics estimated that approximately 3.9% of US children in 2007 reported a food allergy in the past 12 months. This included an 18% increase in prevalence from 1997-2007.1 Of all the foods implicated, peanut remains one of the most common and is considered one of the most severe with the majority of food related life threatening and fatal allergic reactions due to peanut ingestion.2, 3 Of the more than 3 million Americans with a peanut or tree nut allergy4, fewer than 20% will “outgrow” the allergy naturally.5, 6 The current standard of care remains strict avoidance and the treatment of accidental ingestions with intramuscular epinephrine and/or antihistamines.
A significant amount of research has focused on the use of immunotherapy for the treatment of food allergy. Although subcutaneous immunotherapy (SCIT) has been successfully used in the treatment of allergic rhinitis and asthma for many years, early attempts with SCIT for food allergy resulted in an unacceptably high rate of systemic reactions.7
In the past few years, several different types of therapy for food allergy have been studied including oral immunotherapy (OIT), which involves ingesting milligrams to grams of allergen in the form of flour combined in a food vehicle. Ongoing research with OIT has shown interesting results but this type of therapy needs much more study.8-11 In contrast, sublingual immunotherapy (SLIT) involves the administration of small amounts (micrograms to milligrams) of allergen extract under the tongue. Although its use has been limited in the United States, SLIT has been used commonly in Europe as an alternative to SCIT for allergic rhinitis. It offers a novel means of treatment for food allergy and seems well suited for several reasons. First, oral Langerhans cells that take up antigen within the mouth have been shown to have tolerogenic properties, potentially accounting for the efficacy of aeroallergen SLIT.12 Second, SLIT is easily administered especially when compared to receiving injections, such as with SCIT, or ingesting large amounts of food, as with OIT. Finally, systemic reactions have been uncommon13, which may be secondary to the relatively small doses used to achieve clinical efficacy.
We present the first study on the use of SLIT in the treatment of peanut allergy in children. The goal of our double-blinded, placebo-controlled study was to evaluate the safety and efficacy of peanut SLIT after 12 months of therapy. In addition, we investigated whether any increase in reaction threshold would be accompanied by immunologic changes indicative of a significant change in the allergic response.
METHODS
Study Design
The primary outcome of the study was to evaluate the reaction threshold to peanut ingestion after 12 months of peanut SLIT therapy compared to placebo. Food challenges to assess the primary endpoint were scheduled after 12 and 18 months of SLIT therapy. A planned interim analysis was performed after 18 months of enrollment to evaluate the primary endpoint and whether food challenges at later time points would be necessary to further assess the primary endpoint.
Secondary endpoints included the frequency and severity of side effects to dosing as well as changes in several immunological parameters, including peanut-specific IgE and IgG4, basophil activation, skin test reactivity, the cytokines IL-5, IL-13, IL-10 and IFN-gamma, and T regulatory cells (TRegs).
Subject Recruitment
Subjects age 1 to 11 years were recruited from the Duke University Medical Center Allergy and Immunology clinics and local referring physician offices. Subjects with a physician documented clinical history of reaction to peanut within 60 minutes of ingestion and a CAP-FEIA peanut-specific IgE ≥ 7 kU/L (Phadia AB; Uppsala, Sweden) were enrolled. Skin prick testing to peanut was not required for enrollment. Exclusion criteria included a history of severe anaphylaxis to peanut involving respiratory failure, hypotension, or other condition necessitating care in the intensive care unit. The study was approved by Duke University’s institutional review board.
Randomization
Subjects were randomized 1:1 to receive either peanut SLIT or placebo. The primary investigators, clinical staff, subjects and families remained blinded to the treatment as well as all laboratory studies during the 12 month study period. Laboratory staff were unblinded throughout the study. Subjects were unblinded after the DBPCFC performed at 12 months.
Peanut and Placebo Sublingual Drops
Peanut and placebo sublingual drops were obtained from Greer Laboratories, Inc (Lenoir, NC). The treatment arm received crude peanut extract (1:20 w/v) fully dissolved in 0.2% phenol and 50% - 55% glycerinated saline to a maximum peanut protein concentration of 5000 mcg/ml. Ara h 2 content was conservatively estimated to be 6% of peanut protein concentration. The placebo group received a glycerinated saline solution plus phenol with caramel coloring. Dilutions were made using glycerinated saline and doses were prescribed as 1 to 8 pumps (50 microliters/pump) of a 1/1000, 1/100, 1/10, or stock dilution of peanut SLIT or placebo (Table E1 in the Online Repository).
SLIT Protocol
Subjects were instructed to continue a strict peanut-free diet for the duration of the study. Subjects were required to have an epinephrine auto-injector with them at all times. All observed dosing was performed on the Duke Clinical Research Unit (DCRU) with ready access to epinephrine, diphenhydramine and albuterol. Subjects were restricted from eating 15 minutes before and 30 minutes after dosing. Study drug was administered sublingually, held for 2 minutes and then swallowed.
Dose escalation phase
During the initial visit, a single starting dose of 0.25 mcg of peanut protein (1 pump of 1/1000 dilution) or placebo was administered with a 2 hour observation period. Subjects then returned for 13 biweekly observed dose escalation visits. Doses were increased by 25-100% until the maintenance dose of 2000 mcg of peanut protein (8 pumps of 1/1 stock dilution) was reached. After each observed dose escalation, subjects continued the same dose daily at home for 2 weeks. Diaries were provided for families to document dosing and side effects.
Maintenance phase
The 2000 mcg maintenance dose was chosen based on the results of our pilot study of peanut SLIT (unpublished data) and on the limitations of the peanut SLIT concentration. With no immunologic changes detected in our pilot study using 45 mcg of peanut SLIT twice daily, we looked to significantly increase the dose for this study. With our peanut SLIT solution already maximally concentrated, we focused on the maximum amount of liquid a young child could be reasonably expected to hold sublingually. We decided on 8 pumps (400 microliters) of our fully concentrated peanut SLIT solution taken once daily which resulted in our maintenance dose of 2000 mcg and was an approximately 22 times increase from the pilot study dose. After reaching the 2000 mcg maintenance dose, subjects continued daily maintenance dosing at home for approximately 6 months before returning for the double-blind, placebo-controlled food challenge.
Double-blind, Placebo-controlled Food Challenge (DBPCFC)
Subjects underwent a double-blind, placebo-controlled food challenge (DBPCFC) after completing the dose escalation and maintenance phases. Prior to the food challenge, subjects were restricted from using antihistamines (short acting, 72 hours; long acting, 7 days) and beta-agonists (12 hours). A study nurse or physician on the DCRU administered all challenges. The active portion of the DBPCFC consisted of 9 increasing doses of peanut protein in the form of peanut flour mixed in a vehicle food. Doses were given every 20 minutes up to a cumulative dose of 2500 mg peanut protein. The placebo portion consisted of an equal amount of oat flour mixed in a vehicle food and given in the same increments. A coin flip determined the order of the peanut and placebo portions during the challenge day and there was a minimum 2 hour observation period between portions. DBPCFC outcomes were reported as the cumulative dose ingested prior to the symptom eliciting dose requiring treatment and discontinuation of the challenge. Symptoms alone or in combination that were observed in our study which resulted in challenge discontinuation included diffuse hives, severe nasal congestion, lip and tongue swelling, throat pain, coughing, moderate to severe abdominal pain, and vomiting.
Safety Monitoring
All dose escalations and DBPCFCs were monitored by a study nurse or physician. Parents were instructed to monitor subjects for 2 hours after home dosing and document all dosing and side effects in home diaries. Side effects were grouped into skin, upper respiratory, chest, and abdominal symptoms. Timing relative to dosing and all treatments were also recorded. A food allergy action plan was provided to all families. Study personnel were available by phone and pager at all times during the study.
Titrated Skin Prick Testing
Titrated skin prick testing (SPT) with peanut extract (Greer Laboratories, Inc, Lenoir, NC) and controls were performed at baseline, 4 months, 8 months, and the day of the DBPCFC. The most dilute concentration of peanut extract (1:20, 1:200, 1:2000, 1:20000, 1:200000) resulting in a wheal ≥ 5 mm at baseline was followed for the duration of the study. Wheal size was calculated as the average of the largest diameter and the perpendicular midpoint diameter. Reaction to peanut was reported as the peanut wheal size minus the wheal size of the saline negative control.
Mechanistic Studies
Basophil activation
Whole blood was obtained at baseline and the day of the DBPCFC and stimulated in the presence of IL-3 with several dilutions of crude peanut extract (100 mcg/ml, 10−1 mcg/ml, 10−2 mcg/ml, 10−3 mcg/ml), anti-IgE (1 mcg/ml), and media alone.14 The crude peanut extract (CPE) was prepared as previously described.15 After a 30 minute incubation, the reaction was stopped with EDTA and antibodies were added. Following another 30 minute incubation at 4°C, RBCs were lysed and leukocytes were fixed for flow cytometric analysis of activation markers. Basophils were identified as CD123+ CD203c+ lin− (CD3, CD14, CD19, CD41) events. Activation was assessed by CD63 (LAMP-3) as the primary marker of activation.
Immunoglobulins
Serum was obtained at baseline, 4 months, 8 months, and the day of the DBPCFC. Peanut-specific IgE and IgG4 levels were measured using the Phadia AB ImmunoCAP 100 instrument (Uppsala, Sweden) per the manufacturer’s instructions.
Cytokines
Heparinized blood was obtained at baseline, 4 months, 8 months, and the day of the DBPCFC. Peripheral blood mononuclear cells (PBMCs) were isolated using Fico/Lite LymphoH density gradient sedimentation (Atlanta Biologicals, Lawrenceville, GA) and suspended in 10% autologous plasma and culture media (RPMI-1640 with 2 mmol/L L-glutamine, 25 mmol/L HEPES buffer with 100 IU/mL penicillin and 100 mcg/mL streptomycin; Mediatech, Manassas, VA). The PBMCs were plated in triplicate at a concentration of 4 × 105 cells/well with CPE (200 mcg/ml), tetanus toxoid (5 mcg/ml; EMD Biosciences, Darmstadt, Germany), concanavalin A, or media alone. Next, the PBMCs were incubated at 37°C in a 5% CO2 humidified atmosphere for 72 hours. Finally, supernatants were collected and the levels of four relevant cytokines, IL-5, IL-13, IL-10, and IFN-gamma, were measured by ELISA (R&D Systems, Minneapolis, MN). Cytokine levels in culture media controls were subtracted from CPE levels prior to reporting.
T regulatory cells (TRegs)
Isolated PBMCs from baseline and the day of the DBPCFC were plated with CPE (200 mcg/ml) or media alone and incubated for 7 days. Cells were surface stained for CD4 and CD25, then intracellularly stained for FoxP3. Flow cytometry was performed and CD4+ CD25+ lymphocytes were gated for FoxP3 status using FlowJo software (TreeStar, Ashland, OR). TRegs were reported as the percentage of CD4+ cells that were also CD25+ and FoxP3+.
Statistical Analysis
To evaluate the primary endpoint, food challenge outcomes between the groups were compared using the Wilcoxon Rank-Sum test (SAS 9.2, Chapel Hill, NC) to control for outliers in our relatively small cohort. Fisher’s exact test (SAS 9.2, Chapel Hill, NC) was used to compare baseline characteristics of subjects on active treatment and placebo. With regards to secondary outcomes, to address the expected rise then fall in peanut-specific IgE over the length of the study, interval changes (0-4 mo, 0-8 mo, 0-12 mo, 4-8 mo, 4-12 mo, and 8-12 mo) were compared between the 2 groups using a Bonferroni corrected p-value < 0.008 for significance. For SPT, basophil, IgG4, cytokine, and TReg analysis, the groups were compared at baseline and at 12 months using a p-value <0.05 for significance. The Wilcoxon Rank-Sum test was used for all secondary outcome group comparisons.
RESULTS
Study Population
At the time of the interim analysis, eighteen subjects completed 12 months of dosing and the DBPCFC with no drop outs. Eleven of these subjects were randomized to peanut SLIT while only 7 subjects were randomized to placebo. This skewing from the 1:1 randomization scheme occurred because of 2 reasons. First, the timing of our interim analysis was based on the first subject reaching the 18 month time point and not on the number of subjects in each cohort. Second, subjects were not challenged in the same order that they were enrolled and randomized. There were no statistical differences between the groups at baseline (Table I). The median age at enrollment was 5.2 years (range, 1.6 – 10.5 years). Five subjects (28%) had additional food allergies most commonly egg allergy. Most subjects had a history of atopic dermatitis (67%), asthma (50%), or allergic rhinitis (61%).
Table I.
Baseline Subject Characteristics
| Active | Placebo | |
|---|---|---|
| Number | 11 | 7 |
| Median age, yrs (range) | 5.8 (2.8--10.5) | 4.7 (1.6--7.4) |
| Sex | 7 male, 4 female | 5 male, 2 female |
| Race | 10 white, 1 asian | 7 white |
| Asthma | 5 (45%) | 4 (57%) |
| Atopic dermatitis | 9 (82%) | 3 (43%) |
| Allergic rhinitis | 8 (73%) | 3 (43%) |
| Other food allergy | 5 (45%) | 1 (14%) |
| Median peanut IgE, kU/L (range) | 33.5 (8.5--1260) | 31.1 (15--639) |
Dosing Safety
Compliance was similar in both groups with subjects on peanut SLIT taking 95.4% of doses and those on placebo taking 93.8% of doses. Symptoms were reported with 11.5% of peanut doses and 8.6% of placebo doses (Table II). The majority of reactions for those on peanut were transient oropharyngeal itching (9.3%) whereas skin itching was most common for those on placebo (6.5%). Of the 4182 active peanut doses, 11 (0.26%) home doses required treatment with an antihistamine. One home dose (0.02%) also required albuterol for mild wheezing. No placebo doses required antihistamine or albuterol treatment and no epinephrine was required for any doses during the study. The symptoms that necessitated treatment included lip swelling, throat itching, finger swelling, itchy skin, and one episode of wheezing.
Table II.
SLIT Dosing Safety
| Active (N = 11) | Placebo (N = 7) | ||
|---|---|---|---|
| Total Doses | 4182 | 2875 | |
| Reactions | 480 (11.5%) | 248 (8.6%) | |
| Symptoms | Oropharyngeal | 391 (9.3%) | 43 (1.5%) |
| Skin | 25 (0.6%) | 188 (6.5%) | |
| Upper Respiratory | 59 (1.4%) | 54 (1.9%) | |
| Chest | 2 (0.05%) | 0 | |
| Abdominal | 50 (1.2%) | 53 (1.8%) | |
| Treatment | Antihistamine | 11 (0.3%) | 0 |
| Epinephrine | 0 | 0 | |
| Albuterol | 1 (0.02%) | 0 |
Double-Blind, Placebo-Controlled Food Challenge
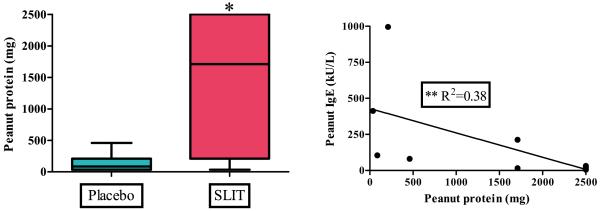
At the time of the interim analysis, eighteen enrolled subjects completed the 2500 mg peanut protein DBPCFC after 12 months of SLIT (Figure 1A). The 11 subjects randomized to active peanut SLIT treatment safely ingested a median cumulative dose of 1710 mg of peanut protein compared to the 7 subjects on placebo who ingested a median cumulative dose of 85 mg [p=0.011]. A modest but significant association (r2=0.38, p=0.043) was found between the outcome of the DBPCFC and the level of peanut-specific IgE obtained on the day of challenge (Figure 1B). No association was found between DBPCFC outcome and the peanut SPT, peanut-specific IgG4, or basophil responsiveness from the day of challenge.
Figure 1.
DBPCFC after 12 months of SLIT or placebo.
[A] When challenged to 2500 mg peanut protein, peanut SLIT subjects demonstrate an increased reaction threshold. Boxes represent interquartile range with a line at the median. Bars represent min and max values. * indicates p=0.011.
[B] Association of DBPCFC outcomes with peanut-IgE from the day of challenge for active treatment. ** indicates p=0.043.
Titrated Skin Prick Testing
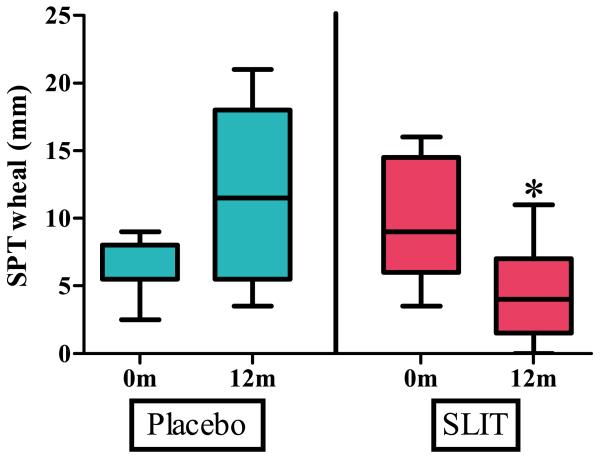
Titrated skin prick testing (SPT) to peanut was not statistically different between the groups at baseline with a median wheal size of 9 mm (range 4.5 – 16 mm) in the active group and 5.5 mm (range 2.5 – 9 mm) in the placebo group. After 12 months, SPT in the active treatment group was significantly smaller than in the placebo group [p=0.020] with a median wheal size of 4 mm (range 0 – 11 mm) in the active treatment group and 11.5 mm (range 3.5 – 21 mm) in the placebo group (Figure 2). The increase in median wheal size for the placebo group from baseline to 12 months was not significant.
Figure 2.
Titrated skin prick testing at baseline and after 12 months of peanut SLIT or placebo. After 12 months, peanut SLIT results in a decreased wheal size to peanut skin prick test. Boxes represent interquartile range with a line at the median. Bars represent min and max values. * indicates p=0.020.
Basophil Activation
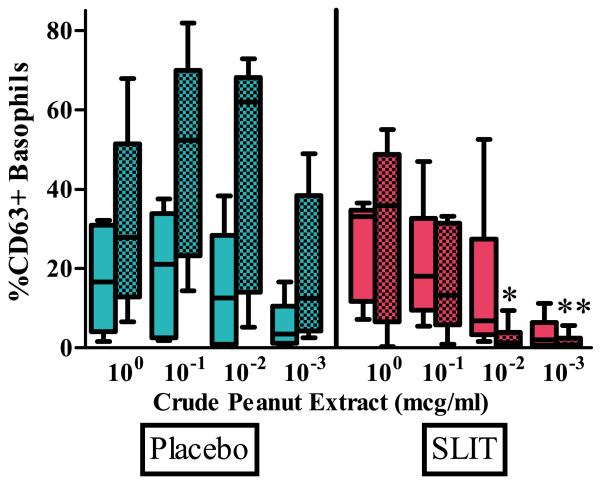
6 treatment and 5 placebo subjects had both baseline and 12 month measurements available for analysis of basophil activation (Figure 3). There was no statistical difference in the percentage of CD63+ basophils at baseline between active treatment and placebo when stimulated with 100 mcg/ml, 10−1 mcg/ml, 10−2 mcg/ml, and 10−3 mcg/ml of crude peanut extract. After 12 months, a significantly lower percentage of CD63+ basophils was found in the active treatment group compared to placebo when stimulated with 10−2 mcg/ml and 10−3 mcg/ml of crude peanut extract [p=0.009, p=0.009]. No difference was seen between the groups after stimulation with 100 and 10−1 mcg/ml of crude peanut extract.
Figure 3.
Percent of CD63+ activated basophils at baseline and after 12 months of peanut SLIT or placebo.
After 12 months, peanut SLIT results in a decreased percent of activated basophils when stimulated with 10−2 and 10−3 mcg/ml of crude peanut extract. Clear boxes - baseline; shaded boxes - 12 month. Boxes represent interquartile range with a line at the median. Bars represent min and max values. * indicates p=0.009, **indicates p=0.009.
Immunoglobulins
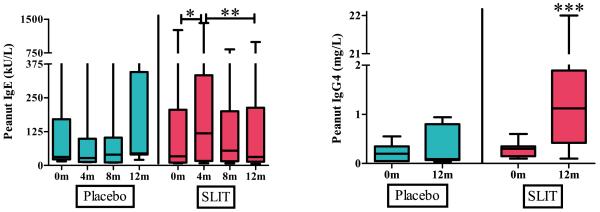
Baseline peanut-specific IgE was not statistically different between the groups with a median level of 33.5 kU/L (range 8.5 – 1260 kU/L) in the active treatment group and 31.1 kU/L (range 15 – 639 kU/L) in the placebo group. During the initial 4 months of SLIT, peanut-specific IgE in the active treatment group increased significantly compared to placebo [p=0.002] up to a median level of 118.5 kU/L. Over the subsequent 8 months of the study, peanut-specific IgE in the active treatment group decreased significantly compared to placebo [p=0.003] to a median level of 31.4 kU/L (Figure 4A). There was no difference in peanut-specific IgG4 between the groups at baseline with levels of 0.3 mg/L (range 0.1 – 0.6 mg/L) and 0.2 mg/L (range 0.05 – 0.55 mg/L) in the active treatment and placebo groups respectively. After 12 months, peanut-specific IgG4 was significantly higher in the active treatment group with a level of 1.12 mg/L (range 0.1 – 22 mg/L) compared to placebo [p=0.014] which remained virtually unchanged at 0.09 mg/L (range 0.04 – 0.94 mg/L) (Figure 4B).
Figure 4.
Levels of peanut-specific immunoglobulins at baseline and after 12 months of peanut SLIT or placebo.
[A] After 12 months, peanut SLIT results in an increase in peanut IgE over 4 months, followed by a decrease over the subsequent 8 months. * indicates p=0.002, ** indicates p=0.003.
[B] After 12 months, peanut results in an increase in peanut IgG4. *** indicates p=0.014. Boxes represent interquartile range with a line at the median. Bars represent min and max values.
Cytokine Analysis
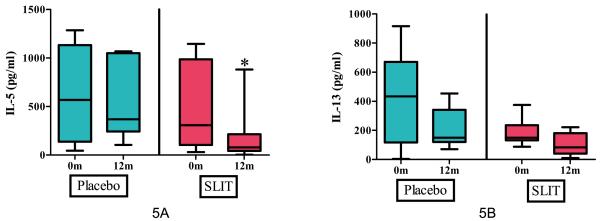
Baseline IL-5 levels in the active treatment group (308.6 pg/ml, range 32.4 – 1145.9 pg/ml) and placebo group (569.9 pg/ml, range 45.9 – 1286.1 pg/ml) were not statistically different. After 12 months, IL-5 was significantly lower in the active treatment group (79 pg/ml, range 4 – 881.3 pg/ml) compared to placebo (368.9 pg/ml, range 104.9 – 1068.5 pg/ml) [p=0.015] (Figure 5A). IL-13 in the active treatment group decreased from a median of 147.1 pg/ml (range 0 – 374.8 pg/ml) at baseline to 62.1 pg/ml (range 0 – 221.7 pg/ml) after 12 months. However, this level was not significant compared to placebo [p=0.06] which also decreased from a median baseline of 369.6 pg/ml (range 0 – 915.3 pg/ml) to 149.55 pg/ml (range 71.1 – 453.3 pg/ml) after 12 months (Figure 5B). No differences between the two groups were found in IL-10 or IFN-gamma at baseline or at 12 months (data not shown).
Figure 5.
Cytokine secretion after stimulation with crude peanut extract at baseline and after 12 months of peanut SLIT or placebo.
[A] After 12 months, peanut SLIT results in a decrease in IL-5 secretion. * indicates p=0.015.
[B] After 12 months, a decrease in IL-13 secretion was found in both peanut SLIT and placebo. Boxes represent interquartile range with a line at the median. Bars represent min and max values.
TReg Analysis
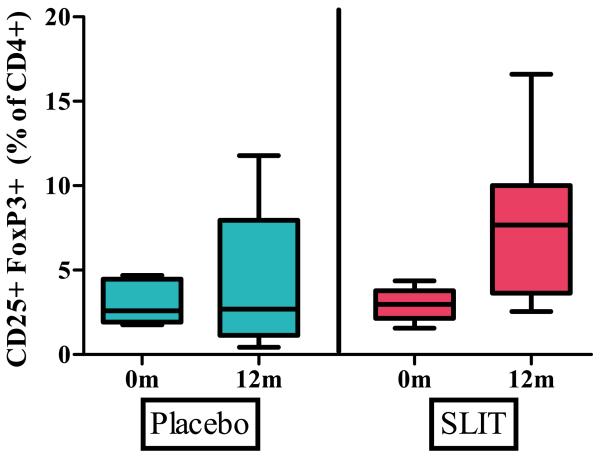
Quantitative analysis of TRegs demonstrated no statistical difference at baseline between active treatment (median 2.98%, range 1.56% - 4.36 %) and placebo (median 2.59%, range 1.76% - 4.7%). After 12 months, an increased percent of TRegs was seen in active treatment (7.68%, range 2.55% - 16.61) compared to placebo (2.69%, range 0.43% - 11.78%) but this result did not reach significance [p=0.145] (Figure 6).
Figure 6.
Percent of T regulatory cells at baseline and after 12 months of peanut SLIT or placebo.
Although an increase in TRegs was seen after 12 months of peanut SLIT, this did not reach significance. Boxes represent interquartile range with a line at the median. Bars represent the min and max values.
DISCUSSION
Despite significant ongoing research, no treatments for peanut allergy are available. The standard of care remains avoidance. SLIT is a novel and possibly safer approach to desensitization and may have the potential to induce long-term oral tolerance. In this first double-blind, placebo-controlled study of peanut SLIT, subjects underwent 6 months of build-up dosing followed by 6 months of maintenance dosing. Subjects receiving peanut SLIT had a significant increase in reaction threshold after safely ingesting a median cumulative dose of 1710 mg of peanut protein, or the equivalent of 6–7 peanuts. This was significantly more than those on placebo, who only safely ingested a median cumulative dose of 85 mg, less than 1 peanut. This level of desensitization is clinically significant in that it likely represents protection from accidental ingestions of peanut that are often caused by the ingestion of less than 100 mg of peanut.16 To investigate possible predictors of food challenge outcomes, we correlated the results of DBPCFCs with immunological data obtained on the day of challenge including SPT, peanut-specific IgE and IgG4, as well as basophil stimulation assays. An association between DBPCFC and a lower peanut IgE but not SPT, IgG4, or basophils was found in the treatment group. This association is likely modest, at best, as the r2 of 0.38 indicates that factors in addition to allergen-specific IgE are involved.
Subjects on peanut SLIT displayed immunologic changes similar to those seen in patients receiving SCIT for environmental allergies. Aeroallergen SCIT has suppressive effects on mast cells and basophils.17 We found a significant decrease in SPT wheal diameter in the active treatment group after the 12 month study period. This finding is indicative of a decrease in mast cell reactivity. Basophil reactivity was also diminished after 12 months of peanut SLIT as indicated by the significantly lower percent of CD63+ activated basophils after stimulation with peanut extract.
With respect to humoral changes, peanut-specific IgE increased significantly in the active treatment group during the first 4 months to a median of almost 4 times the baseline level. No significant change in peanut-specific IgE was noted in the placebo group. Over the remaining 8 months, peanut-specific IgE in the active treatment group decreased significantly compared to the placebo group that remained stable. On the other hand, peanut-specific IgG4, which is thought to block the binding of allergen to effector cells18, was significantly increased compared to placebo, which remained unchanged.
The effects of immunotherapy on mast cells, basophils and B-cells are thought to be a result of the down-regulation of IL-4, IL-5, and IL-13 as well as the increased production of IL-10 and TGF-beta by TRegs.17, 19 TRegs are also thought to suppress the effects of TH2 cells.19 This central role of TRegs is supported by a recent study of grass pollen SLIT20 by Allam, et al, which demonstrated increased production of IL-10 and TGF-beta by oral Langerhans cells and an increased number of IL-10 and TGF-beta producing TRegs after SLIT therapy. We did not see an increase in IL-10 production with peanut SLIT; however, we were able to show an increased percentage of TRegs which may have reached significance with a larger sample size. The TRegs reported by Allam, et al, are hypothesized to be IL-10 and TGF-beta producing type 1 TRegs or TH3 cells20. A possible explanation for our discrepancy in IL-10 production is that in contrast to grass pollen SLIT, peanut SLIT may preferentially induce naturally occurring FoxP3 positive TRegs which are thought to mediate their effects less through secreted cytokines and more by direct cell contact. Although we were unable to define the role of TRegs in peanut SLIT, our results clearly show a down-regulation of the TH2 response as represented by a decrease in IL-5 production after 12 months of peanut SLIT. Production of another TH2 cytokine, IL-13, also decreased after 12 months of peanut SLIT but this was not significant as a parallel decrease was seen in the placebo group during this time period. We are unaware of any studies demonstrating decreases in IL-13 production during peanut avoidance and thus believe that this result is more likely an artifact of our small sample size rather than a true phenomenon. Lastly, we measured the production of a representative TH1 cytokine, IFN-gamma, but did not see an increase that would indicate that peanut SLIT causes a shift from a TH2 response towards a TH1 response. The decrease in IL-5 production detected in our study is in contrast with recently published results by our group on OIT for peanut allergy which showed an increase in IL-5 production after therapy 21. In another recently published study on peanut OIT22, Blumchen, et al, also demonstrated decreased levels of IL-5 after therapy. We suspect that the discrepant results found in our OIT study may have been a result of the relatively low levels of IL-5 measured. These low levels combined with a sample size of 5 may have amplified typical biologic variation and resulted in the discrepancy. Another possible explanation could be that the different methodology needed to measure the low levels may have introduced more error. We believe that the results of our study, which are in agreement with Blumchen, are more plausible as they support the hypothesis of a downregulation of the TH2 response by immunotherapy.
Few studies have examined SLIT for the treatment of food allergy. There have been case reports on its effective use for the treatment of kiwi allergy22, 23 and a trial lacking clinical benefit in peach allergy.24 In 2005, Enrique described a double-blinded study of SLIT in the treatment of hazelnut allergy.25 Subjects on active treatment tolerated a significantly larger amount of hazelnut but the results were difficult to extrapolate to peanut allergy as half of the subjects had oral allergy syndrome, a limited form of oral pruritus due to epitopes cross-reactive with pollens, rather than IgE-mediated food allergy.
Although reports on SLIT for food allergy have been limited, SLIT has been used extensively in Europe as an alternative to SCIT for the treatment of allergic rhinitis and asthma. A 2006 AAAAI/ACAAI joint task force report on aeroallergen SLIT found that two-thirds of studies reported an improvement in symptom scores.13 Although an increase in allergen-specific IgG4 was commonly reported, changes in allergen-specific IgE were rare and reported changes in cytokines were inconsistent. The report also showed that regarding the safety of aeroallergen SLIT, local oropharyngeal itching was the most common side effect reported. Similarly, oropharyngeal itching was the most commonly reported side effect in our study with subjects on active treatment reporting transient oropharyngeal itching with 9.3% of doses. Systemic reactions have been quite uncommon in aeroallergen SLIT.13 In our study, only 0.3% of active treatment doses required treatment with an antihistamine. Only 1 dose (0.02%) required treatment with a beta-agonist. Most importantly, epinephrine was not required for any SLIT dosing.
There are several notable limitations to our study. We did not include an entry food challenge and were therefore unable to determine individual changes in reaction threshold. Future studies will incorporate an entry challenge. Nevertheless, a 20-fold greater amount of ingested peanut protein in the active treatment group compared to placebo group indicates a high likelihood of clinical efficacy. An ongoing double-blinded, placebo-controlled randomized trial of peanut SLIT through the Consortium of Food Allergy Research (ClinicalTrials.gov # NCT00580606) involves a larger number of subjects from multiple centers and should provide further insight into the efficacy of SLIT. Regarding the induction of oral tolerance, we have not examined the long-term effects of peanut SLIT after stopping the therapy. Changes in peanut-specific IgE and IgG4, as well as the decrease in IL-5 are promising and may suggest the beginning of longer term tolerance to peanut, but continued study is needed.
In summary, our study shows that peanut-specific SLIT can be a safe and effective form of immunotherapy for peanut-allergic children. SLIT induces significant desensitization after 12 months of therapy. Immunological data demonstrate a diminished allergic response in mast cells and basophils, decreasing peanut-specific IgE and increasing peanut-specific IgG4, and a decrease in IL-5 production.
ACKNOWLEDGEMENTS
We would like to thank Xiaohong Yue, Hua-Mei Zhang, and Nikolas Kamilaris of the Burks lab for their assistance with the laboratory assays, Alison Edie, FNP, and Jane Hainline, RN, for assistance with dosing and followup visits, and Henry Beresford, Duke University School of Nursing, for development and maintenance of the patient database. We would also like to thank the Duke Clinical Research Unit for providing the clinic space and staff support necessary to conduct our study.
Funding support provided by the National Institutes of Health: NCCAM R01–AT004435–03, 5T32–AI007062–32, the American Academy of Allergy, Asthma, and Immunology/Food Allergy Initiative Howard Gittis Memorial 3rd Year/4th Year Fellowship/Instructor Award (J.A.B.) and the Wallace Research Foundation (WRF2010.01). Additional support for the project was provided by Grant Number UL1RR024128 from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH) and NIH Roadmap for Medical Research, and its contents are solely the responsibility of the authors and do not necessarily represent the official view of NCRR or NIH.
Abbreviations
- DBPCFC
double-blind, placebo-controlled food challenge
- OIT
oral immunotherapy
- PBMC
peripheral blood mononuclear cell
- SCIT
subcutaneous immunotherapy
- SLIT
sublingual immunotherapy
- SPT
skin prick testing
- TRegs
T regulatory cells
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Branum AM, Lukacs SL. Food allergy among U.S. children: trends in prevalence and hospitalizations. NCHS Data Brief. 2008 Oct 10;:1–8. [PubMed] [Google Scholar]
- 2.Bock SA, Munoz-Furlong A, Sampson HA. Fatalities due to anaphylactic reactions to foods. J Allergy Clin Immunol. 2001 Jan;107(1):191–3. doi: 10.1067/mai.2001.112031. [DOI] [PubMed] [Google Scholar]
- 3.Bock SA, Munoz-Furlong A, Sampson HA. Further fatalities caused by anaphylactic reactions to food, 2001-2006. J Allergy Clin Immunol. 2007 Apr;119(4):1016–8. doi: 10.1016/j.jaci.2006.12.622. [DOI] [PubMed] [Google Scholar]
- 4.Sicherer SH, Munoz-Furlong A, Godbold JH, Sampson HA. US prevalence of self-reported peanut, tree nut, and sesame allergy: 11-year follow-up. J Allergy Clin Immunol. 2010 Jun;125(6):1322–6. doi: 10.1016/j.jaci.2010.03.029. [DOI] [PubMed] [Google Scholar]
- 5.Hourihane JO, Roberts SA, Warner JO. Resolution of peanut allergy: case-control study. BMJ. 1998 Apr 25;316(7140):1271–5. doi: 10.1136/bmj.316.7140.1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Skolnick HS, Conover-Walker MK, Koerner CB, Sampson HA, Burks W, Wood RA. The natural history of peanut allergy. J Allergy Clin Immunol. 2001 Feb;107(2):367–74. doi: 10.1067/mai.2001.112129. [DOI] [PubMed] [Google Scholar]
- 7.Nelson HS, Lahr J, Rule R, Bock A, Leung D. Treatment of anaphylactic sensitivity to peanuts by immunotherapy with injections of aqueous peanut extract. J Allergy Clin Immunol. 1997 Jun;99(6 Pt 1):744–51. doi: 10.1016/s0091-6749(97)80006-1. [DOI] [PubMed] [Google Scholar]
- 8.Jones SM, Pons L, Roberts JL, Scurlock AM, Perry TT, Kulis M, et al. Clinical efficacy and immune regulation with peanut oral immunotherapy. J Allergy Clin Immunol. 2009 Aug;124(2):292–300. e1–97. doi: 10.1016/j.jaci.2009.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Clark AT, Islam S, King Y, Deighton J, Anagnostou K, Ewan PW. Successful oral tolerance induction in severe peanut allergy. Allergy. 2009 Aug;64(8):1218–20. doi: 10.1111/j.1398-9995.2009.01982.x. [DOI] [PubMed] [Google Scholar]
- 10.Patriarca G, Nucera E, Roncallo C, Pollastrini E, Bartolozzi F, De Pasquale T, et al. Oral desensitizing treatment in food allergy: clinical and immunological results. Aliment Pharmacol Ther. 2003 Feb;17(3):459–65. doi: 10.1046/j.1365-2036.2003.01468.x. [DOI] [PubMed] [Google Scholar]
- 11.Bauer A, Ekanayake Mudiyanselage S, Wigger-Alberti W, Elsner P. Oral rush desensitization to milk. Allergy. 1999 Aug;54(8):894–5. doi: 10.1034/j.1398-9995.1999.00228.x. [DOI] [PubMed] [Google Scholar]
- 12.Allam JP, Peng WM, Appel T, Wenghoefer M, Niederhagen B, Bieber T, et al. Toll-like receptor 4 ligation enforces tolerogenic properties of oral mucosal Langerhans cells. J Allergy Clin Immunol. 2008 Feb;121(2):368–74 e1. doi: 10.1016/j.jaci.2007.09.045. [DOI] [PubMed] [Google Scholar]
- 13.Cox LS, Larenas Linnemann D, Nolte H, Weldon D, Finegold I, Nelson HS. Sublingual immunotherapy: a comprehensive review. J Allergy Clin Immunol. 2006 May;117(5):1021–35. doi: 10.1016/j.jaci.2006.02.040. [DOI] [PubMed] [Google Scholar]
- 14.Wanich N, Nowak-Wegrzyn A, Sampson HA, Shreffler WG. Allergen-specific basophil suppression associated with clinical tolerance in patients with milk allergy. J Allergy Clin Immunol. 2009 Apr;123(4):789–94 e20. doi: 10.1016/j.jaci.2008.12.1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pons L, Ponnappan U, Hall RA, Simpson P, Cockrell G, West CM, et al. Soy immunotherapy for peanut-allergic mice: modulation of the peanut-allergic response. J Allergy Clin Immunol. 2004 Oct;114(4):915–21. doi: 10.1016/j.jaci.2004.06.049. [DOI] [PubMed] [Google Scholar]
- 16.Taylor SL, Moneret-Vautrin DA, Crevel RW, Sheffield D, Morisset M, Dumont P, et al. Threshold dose for peanut: Risk characterization based upon diagnostic oral challenge of a series of 286 peanut-allergic individuals. Food Chem Toxicol. 2010 Mar;48(3):814–9. doi: 10.1016/j.fct.2009.12.013. [DOI] [PubMed] [Google Scholar]
- 17.Akdis M, Akdis CA. Mechanisms of allergen-specific immunotherapy. J Allergy Clin Immunol. 2007 Apr;119(4):780–91. doi: 10.1016/j.jaci.2007.01.022. [DOI] [PubMed] [Google Scholar]
- 18.Till SJ, Francis JN, Nouri-Aria K, Durham SR. Mechanisms of immunotherapy. J Allergy Clin Immunol. 2004 Jun;113(6):1025–34. doi: 10.1016/j.jaci.2004.03.024. quiz 35. [DOI] [PubMed] [Google Scholar]
- 19.Akdis M, Blaser K, Akdis CA. T regulatory cells in allergy: novel concepts in the pathogenesis, prevention, and treatment of allergic diseases. J Allergy Clin Immunol. 2005 Nov;116(5):961–8. doi: 10.1016/j.jaci.2005.09.004. quiz 9. [DOI] [PubMed] [Google Scholar]
- 20.Allam JP, Wurtzen PA, Reinartz M, Winter J, Vrtala S, Chen KW, et al. Phl p 5 resorption in human oral mucosa leads to dose-dependent and time-dependent allergen binding by oral mucosal Langerhans cells, attenuates their maturation, and enhances their migratory and TGF-beta1 and IL-10-producing properties. J Allergy Clin Immunol. 2010 Sep;126(3):638–45 e1. doi: 10.1016/j.jaci.2010.04.039. [DOI] [PubMed] [Google Scholar]
- 21.Jones SM, Pons L, Roberts JL, Scurlock AM, Perry TT, Kulis M, et al. Clinical efficacy and immune regulation with peanut oral immunotherapy. J Allergy Clin Immunol. 2009 Aug;124(2):292–300. e1–97. doi: 10.1016/j.jaci.2009.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Blumchen K, Ulbricht H, Staden U, Dobberstein K, Beschorner J, de Oliveira LC, et al. Oral peanut immunotherapy in children with peanut anaphylaxis. J Allergy Clin Immunol. 2010 Jul;126(1):83–91 e1. doi: 10.1016/j.jaci.2010.04.030. [DOI] [PubMed] [Google Scholar]
- 23.Mempel M, Rakoski J, Ring J, Ollert M. Severe anaphylaxis to kiwi fruit: Immunologic changes related to successful sublingual allergen immunotherapy. J Allergy Clin Immunol. 2003 Jun;111(6):1406–9. doi: 10.1067/mai.2003.1497. [DOI] [PubMed] [Google Scholar]
- 24.Kerzl R, Simonowa A, Ring J, Ollert M, Mempel M. Life-threatening anaphylaxis to kiwi fruit: protective sublingual allergen immunotherapy effect persists even after discontinuation. J Allergy Clin Immunol. 2007 Feb;119(2):507–8. doi: 10.1016/j.jaci.2006.09.041. [DOI] [PubMed] [Google Scholar]
- 25.Fernandez-Rivas M, Garrido Fernandez S, Nadal JA, Diaz de Durana MD, Garcia BE, Gonzalez-Mancebo E, et al. Randomized double-blind, placebo-controlled trial of sublingual immunotherapy with a Pru p 3 quantified peach extract. Allergy. 2009 Jun;64(6):876–83. doi: 10.1111/j.1398-9995.2008.01921.x. [DOI] [PubMed] [Google Scholar]
- 26.Enrique E, Pineda F, Malek T, Bartra J, Basagana M, Tella R, et al. Sublingual immunotherapy for hazelnut food allergy: a randomized, double-blind, placebo-controlled study with a standardized hazelnut extract. J Allergy Clin Immunol. 2005 Nov;116(5):1073–9. doi: 10.1016/j.jaci.2005.08.027. [DOI] [PubMed] [Google Scholar]