Abstract
The popularity and availability of herbal extracts has increased dramatically over the last decade, providing an inexpensive manner of self-medication. Although the efficacy of individual extracts is currently being intensively studied, research regarding complex mixtures is limited. Therefore, we evaluated the effects of three complex formulations including BRC-301, a polyherbal extract; BRC-304, a mixture of vitamins, minerals, antioxidant enzymes, botanical extracts and carotenoids; and BRC-306, a proprietary blend of Uncaria tomentosa (cat’s claw) and Phytolens® on murine dendritic cells (DCs). We hypothesized that these formulations would decrease the inflammatory responsiveness and innate function of DCs. To address this hypothesis, we evaluated the effects of BRC-301, 304, and 306 on DC2.4 cells and further assessed the effects of BRC-301 on bone marrow-derived DCs (bmDCs). LPS stimulation of DC2.4 cells and bmDCs induced production of NO, TNF-α, and IL-6, a response that was modulated by concomitant treatment with non-cytotoxic concentrations of BRC-301. In contrast, only the production of NO or IL-6 by LPS-activated DC2.4 cells was affected by BRC-304 or BRC-306, respectively. Flow cytometric evaluation following concurrent BRC-301 and LPS treatment revealed an increased relative expression of CD11c, CD86, and CD54 on bmDCs and an increased frequency of bmDCs expressing MHC II. Finally, BRC-301 enhanced the uptake of FITC-conjugated ovalbumin by bmDCs. Taken together, these results suggest that these commercially available formulations modulate the innate responsiveness of murine DCs and may enhance their ability to initiate T cell-mediated immunity.
Keywords: Dendritic cells, antigen presenting cells, inflammation, natural products, dietary supplements
INTRODUCTION
The uses of Western therapies to treat inflammatory diseases are often suboptimal in terms of their associated adverse side effects and cost. Thus, many people who suffer from these disorders are turning to the use of complementary and alternative medicine (CAM) for treatment. The use of CAM in the United States has risen from 34% to 42% in the past 20 years (Mainardi, Kapoor, & Bielory 2009). The practice of traditional Chinese and Ayurvedic medicines trace back 5000 and over 2000 years, respectively (Clarke & Mullan, 2008). The increased use of CAM has been paralleled by the increased use of dietary supplements, including herbal products. However, these products are not required to pass the same safety, efficacy, and biochemical characterization requirements as seen with over-the-counter and prescription medications as exempted in the Dietary Supplemental Health and Education Act of 1994 (Berman & Staus, 2004).
Dendritic cells (DCs) are the professional antigen presenting cells (APCs) of the immune system and play an important role in both immunity and tolerance. When microorganisms invade the body, DCs are one of the first cell populations to access the site of infection, take up microbial antigens and load peptides onto their cell surfaces. DCs then migrate to the draining immune tissue where they then present antigens to T lymphocytes. In order to generate an effective T cell mediated immune response, DCs must express high levels of cell-surface major histocompatibility complex (MHC) proteins, antigen complexes, and costimulatory molecules (Figdor, de Vries, Lesterhuis, & Melief, 2004). Immune activation of DCs occurs when microbial components known as pattern-associated molecular patterns (PAMPs) bind to Toll-like receptors (TLRs) on DCs leading to upregulation of pro-inflammatory cytokines (Janeway & Medzhitov, 2002).
In the present study, three complex dietary supplements were evaluated for their potential to modulate the inflammatory responsiveness of murine DC2.4 cells and bone marrow derived DCs (bmDCs). The products BRC-301, 304, and 306 were provided by the Biotics Research Corporation and are available for purchase by the public through licensed healthcare professionals. BRC-301, KappArest®, is composed of 12 different herbs all which have a rich medicinal history in the treatment of ailments ranging from the common cold to hypertension. BRC-304, commercially available as Optic-Plus®, is a mixture of vitamins and enzymes, which is marketed for support of healthy ocular function. BRC-306, POA-Phytolens®, is a proprietary blend of Uncaria tomentosa and Phytolens®, which is sold as an anti-oxidant. Although many of the individual constituents have been investigated for their ability to alter immune cell function, little is known of the capacity of the complex mixtures as immunomodulators. Therefore, we hypothesized that these complex formulations would decrease the inflammatory responsiveness of LPS-stimulated murine DCs.
In order to evaluate the potential for these complex herbal mixtures to alter inflammatory responsiveness of DC2.4 cells, levels of nitric oxide (NO), tumor necrosis factor-α (TNF-α), and interleukin-6 (IL-6) were analyzed after a 48-hour simultaneous treatment with the TLR agonist lipopolysaccharide (LPS) and three varying concentrations of BRC-301, 304, and 306. Due to BRC-301’s documented anti-inflammatory potential, further analysis was performed using bmDCs. In addition to measuring NO and inflammatory mediators, phagocytic capacity and cell surface marker expression were evaluated as well.
METHODOLOGY
Extracts and chemicals
The Biotics Research Corporation of Rosenberg, Texas generously provided our lab with three formulations of dietary supplements (Table 1). All three tested negative for endotoxin (analyses performed by BRC). All solvents used for extraction (water, methanol, and ethanol) were HPLC grade and obtained from JT Baker. Crude extracts were prepared by extracting powders with appropriate solvents, filtering the liquid extractives, and evaporating the solvents under reduced pressure using a rotary evaporator (Yamamoto RE 400, Tokyo, Japan). BRC-301 was prepared using methanol; BRC-304 and BRC-306 were prepared using water. The extracts were dissolved in culture grade DMSO (Sigma-Aldrich, St. Louis, MO) and sterile-filtered through 0.22 μM Millipore membranes. Lipopolysaccharide (LPS) from Escherichia coli (055:B5) was obtained from Sigma-Aldrich.
TABLE 1.
Individual components of BRC-301, BRC-304, and BRC-306 formulations
| Test Product | Commercial Name | Active Ingredients |
|---|---|---|
| BRC-301 | KappArest® | Curcuminiods (turmeric extract) (rhizome), Boswellia serrata extract (gum), green tea extract (Camellia sinensis) (leaves), ginger extract (rhizome), rosemary extract, celery seed extract, resveratrol (Polygonum cuspidatum extract) (root), alpha-lipoic acid, Phytolens® extract (Lens esculenta extract) (husks), bioperine (from Piper nigrum). |
| BRC-304 | Optic-Plus® | Vitamins A, C, and E, thiamin riboflavin, zinc, selenium, copper, citrus bioflavonoids, bilberry extract, lutein, zeaxanthin, superoxide dismutase, catalase. |
| BRC-306 | POA- Phytolens® | Proprietary blend of Cat’s claw (Uncaria tomentosa), Phytolens® (Lens Esculenta extract). |
Cyclooxygenase (COX) assays
COX-1 and COX-2 activity was detected using the COX inhibitor screening assay per the manufacturer’s instructions (Cayman Chemical, Ann Harbor, MI). This is a non-cell based assay that utilizes recombinant enzyme in an enzyme immunoassay.
Cell culture
DC2.4 cells, a murine dendritic cell line (Zhenhai, Reznikoff, Dranoff, & Rock, 1997), were kindly provided by Dr. Kenneth Rock (University of Massachusetts Medical Center, Worcester, MA). Cells were grown in complete media (cDMEM) consisting of DMEM (GibcoBRL, Grand Island, NY), supplemented with 10% FBS (Hyclone, Logan, UT), 10 mM HEPES, 2 mM L-glutamine, and 50 μg/ml gentamicin (GibcoBRL). DC2.4 cells were maintained at 37°C in a humidified incubator with 5% CO2. Cells were maintained via weekly passage and utilized for experimentation at 60-80% confluency as previously described (Rhule, Navarro, Smith, & Shepherd, 2006). Viability of cells was determined via trypan blue exclusion.
Bone marrow-derived dendritic cells (bmDCs) were prepared using methods from Bankoti, Rase, Simones, & Shepherd (2010). Briefly, bone marrow cells were flushed from the bone shafts of C57Bl/6 mice with cDMEM and subsequently subjected to gradient centrifugation using Lympholyte®-M (Cedarlane Laboratories Limited, Hornby, Canada). Bone marrow cells were washed twice and plated at a density of 1×106 cells/ml in tissue culture flasks. Cells were cultured in media with 30 ng/ml GM-CSF for 3 days at 37°C in 5% CO2. On days 3 and 5 non-adherent cells were collected, washed, and reseeded. Fresh media and GM-CSF (30 ng/ml) was added back to the culture flask. Non-adherent, immature DCs were harvested on day 7 and purified (≥90%) using CD11c antibody and anti-APC beads (Miltenyi Biotec, Auburn, CA).
Cell activation and treatment
DC2.4 cells or purified bmDCs were cultured in 6-well plates at 1.0×106 per 1 ml of complete media. Cells were stimulated with LPS (1 μg/ml) and concomitantly treated with DMSO (0.1%) or BRC-301, BRC-304, or BRC-306 for 48 hours. Due to cytotoxicity observed at concentrations over 10 μg/ml (viability was significantly reduced to ≤70% of control), BRC-304 was tested at non-cytotoxic concentrations of 0.5, 5 and 10 μg/ml when compared to BRC-301 and 306, which were both tested at 0.5, 5, 25 μg/ml. DMSO (0.1%) was used as the vehicle control. Supernatants were collected after 48 hours based on the effects of each formulation on COX activity and then were frozen at −20°C for ensuing evaluation of cytokine production.
Cytokine assays
IL-6 and TNF-α levels in supernatants collected from cultured cells were evaluated using enzyme-linked immunosorbent assay (ELISA). Samples were analyzed per the manufacturer’s recommendations using mouse cytokine-specific BD OptEIA ELISA kits (BD PharMingen, San Diego, CA). Nitric oxide (NO) levels were measured using the Griess Reagent System (Promega, Madison, WI).
Flow cytometry
The expression of costimulatory molecules on bmDC cells was determined by flow cytometric analysis as previously described (Shepherd, Steppan, Hedstrom, & Kerkvliet, 2001). Briefly, bmDCs were harvested and washed with PAB (1% bovine serum albumin and 0.1% sodium azide in PBS). Non-specific staining of cells was blocked with 600 μg purified rat and/or hamster IgG (Jackson ImmunoResearch, West Grove, PA). Fluorochrome-conjugated antibodies CD11c-APC, MHC class II-FITC, CD86-PB, CD54-PB, CD40-PE, CD14-FITC, and their corresponding isotype controls (BD Biosciences, San Jose, CA except for CD86-PB and CD54-PB Biolegend, San Diego, CA) were used to stain cells. Sixty thousand viable cells per treatment group were collected using BD FACS Aria flow cytometer and analyzed with FACS Diva software (BD Biosciences, San Jose, CA).
Antigen uptake
To measure phagocytic potential, bmDCs were incubated with Alexa Fluor 488-conjugated acetylated low-density lipoprotein (LDL) (Molecular probes, Oregon, USA) for 1.5 hours or Alexa Fluor 488-ovalbumin (Molecular probes, Oregon, USA) for 12 hours. In pre-treated samples, BRC-301 was added 24 hours prior to the direct addition of Alexa Fluor 488-OVA or Alexa Fluor-LDL to the cell cultures. BRC-301 was left in the cultures during the incubation period with Alexa Fluor 488-OVA or Alexa Fluor-LDL. In concurrently treated samples, BRC-301 and Alexa Fluor-OVA were added simultaneously and incubated overnight. Antigen uptake was assessed by flow cytometry.
Statistics
All statistical analyses were performed using GraphPad Prism 4.0a for Macintosh (GraphPad Software, San Diego, CA). Data sets with multiple comparisons were evaluated by one-way analysis of variance (ANOVA) with Tukey’s multiple comparison post-hoc test and data sets with two comparisons were evaluated by t-test. Values of p < .05 were considered significant.
RESULTS
BRC extracts differentially modulate COX-1 and COX-2 activity
An initial screening was performed to determine the effects of the BRC extracts on COX enzyme activity. COX-1 is the constitutive isoform involved in homeostatic processes while the COX-2 isoform is induced during inflammatory responses. In order to assess COX inhibitory effect, the half maximal inhibitory concentration (IC50) values were determined for each natural product formulation (Table 2). BRC-306 had the greatest inhibitory effect on both COX-1 and COX-2 activity with IC50 values of 0.07 μg/ml and 0.78 μg/ml, respectively. BRC-301 and BRC- 304 also inhibited cycloxygenase activity, although to a lesser degree than BRC-306. The IC50 values were then used to calculate the COX-2:COX-1 activity ratios in order to provide a relative potential for the natural products to selectively affect COX-2 activity relative to COX-1 activity. The product formulations BRC-301 and BRC-304 were found to be selective for COX-2 with inhibition ratios of 0.75 and 0.11, respectively.
TABLE 2.
The effects of BRC extracts on COX-1 and COX-2 activity
| PRODUCT | IC50 COX-2 (μg/ml) | IC50 COX-1 (μg/ml) | Activity Ratio (COX-2:COX-1) |
|---|---|---|---|
| BRC-301 | 1.8 ± 0.1 | 2.4 ± 0.2 | 0.75 |
| BRC-304 | 3.6 ± 0.8 | 33.5 ± 4.5 | 0.11 |
| BRC-306 | 0.07 ± 0.1 | 0.78 ± 0.1 | 0.09 |
The natural product formulations were evaluated via a COX enzyme activity assay and IC50 values are shown along with the corresponding activity ratio.
BRC extracts modulate the production of inflammatory mediators by DC2.4 cells
To evaluate the innate responsiveness of DCs, we first screened the potential for all three BRC extracts to modify inflammatory mediator production by DC2.4 cells. As expected, LPS activation (1 μg/ml) of DC2.4 cells increased the production of NO, TNF-α, and IL-6 when compared to the unstimulated controls (Figures 1-3).
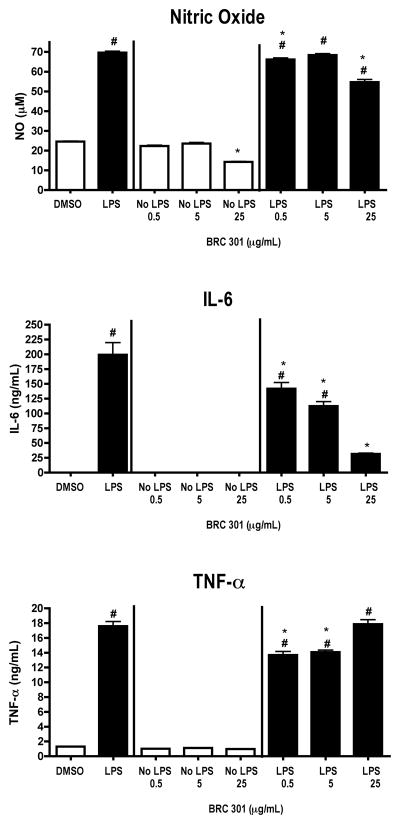
FIGURE 1.
LPS-activated DC2.4 cells produce decreased levels of NO, TNF-α, and IL-6 following exposure to BRC-301. DC2.4 cells were stimulated with LPS (1 μg/ml) and concomitantly treated with DMSO (0.1%) or BRC-301 (0.5, 5.0, 25 μg/ml) for 48 hours. Supernatants were collected and analyzed by ELISA (TNF-α and IL-6) or Griess Reagent System (NO). Results are mean ± SEM (n=3) and are representative of two separate experiments. # indicates p < .05 compared to the unstimulated control; * indicates p < .05 compared to the vehicle control.
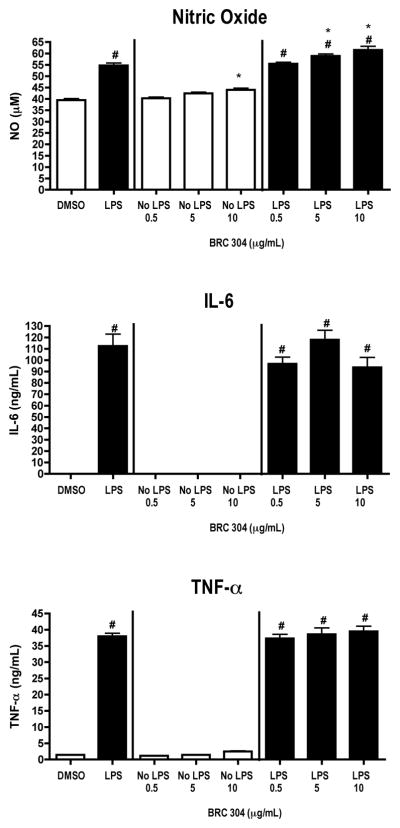
FIGURE 3.
LPS-activated DC2.4 cells produce decreased levels of IL-6 following exposure to BRC-306. DC2.4 cells were stimulated with LPS (1 μg/ml) and concomitantly treated with DMSO (0.1%) or BRC-306 (0.5, 5.0, 25 μg/ml) for 48 hours. Supernatants were collected and analyzed by ELISA (TNF-α and IL-6) or Griess Reagent System (NO). Results are mean ± SEM (n=3) and are representative of two separate experiments. # indicates p < .05 compared to unstimulated control; * indicates p < .05 compared to the vehicle control.
NO production by LPS-activated DC2.4 cells decreased at both 0.5 μg/ml and 25 μg/ml BRC-301 when compared to respective controls. Decreased production of NO in unstimulated DC2.4 cells was also observed at 25 μg/ml. In addition, BRC-301 inhibited IL-6 production at all three concentrations tested. TNF-α levels were decreased at both 0.5 and 5.0 μg/ml (Figure 1). BRC-301 had no effect on the cellular viability of DC2.4 cells (Table 3).
Table 3.
Dendritic cell viability following treatment with BRC extracts.
| A. Relative percent viability compared to the DMSO control | ||||
|---|---|---|---|---|
| Relative Extract Concentrationa | BRC-301 DC2.4 | BRC-304 DC2.4 | BRC-306 DC2.4 | BRC-301 bmDCs |
| Low | 100 ±2 | 92 ±10 | 98 ±1 | 107 ±3 |
| Medium | 99 ±2 | 85 ±2* | 94 ±1 | 116 ±5* |
| High | 101 ±3 | 96 ±2 | 100 ±1 | 107 ±8 |
|
B. Relative percent viability compared to the LPS/DMSO control | ||||
| Relative Extract Concentrationa | BRC-301 DC2.4 | BRC-304 DC2.4 | BRC-306 DC2.4 | BRC-301 bmDCs |
| Low | 94 ±4 | 100 ±4 | 99 ±4 | 91 ±4 |
| Medium | 97 ±5 | 102 ±3 | 92 ±2 | 97 ±1 |
| High | 101 ±7 | 95 ±3 | 95 ±5 | 92 ±5 |
DC2.4 and bmDCs were cultured (n=3) in 6-well plates (1×106 per well) with DMSO, LPS (+DMSO), and/or varying concentrations of extracts for 48 hours. Viability of extract-treated cells was calculated as percent of either the DMSO-treated (A) or LPS/DMSO-treated (B) controls plus SEM. Data are representative of three experiments (n=4-6).
Extract concentrations varied among extracts as follows: for all extracts tested, the low and medium concentrations tested were 0.5 and 5.0 μg/ml, respectively. For BRC-301 and BRC-306, the high concentration tested was 25 μg/ml while 10 μg/ml was the highest concentration tested for BRC-304 due to decreased viability at higher concentrations.
indicates p < .05
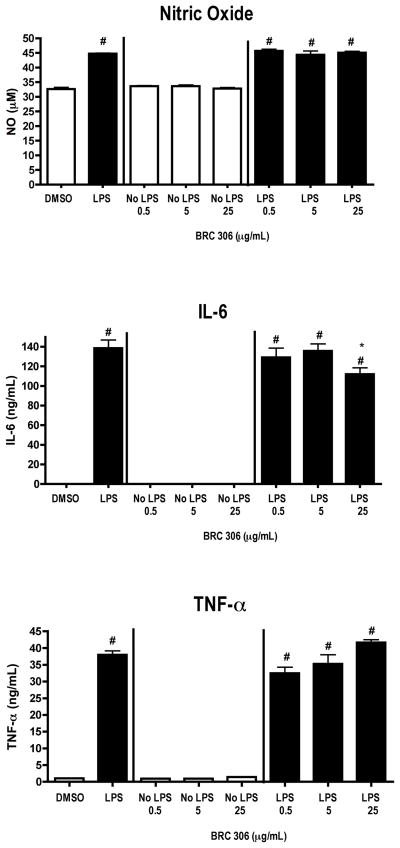
In contrast to the inhibitory effects of BRC-301, BRC-304 increased NO levels at both 5 and 10 μg/ml concentrations of BRC-304. BRC-304 also increased the basal release of NO by unstimulated DC2.4 cells at the 10 μg/ml concentration. No effects of BRC-304 were observed on the basal or LPS-induced production of IL-6 and TNF-α (Figure 2). BRC-304 had only minimal effects on the cellular viability of the cultured DCs, decreasing the viability of unstimulated DC2.4 cells at the 5.0 μg/ml concentration tested (Table 3).
FIGURE 2.
LPS-activated DC2.4 cells produce increased levels of NO following exposure to BRC-304. DC2.4 cells were stimulated with LPS (1 μg/ml) and concomitantly treated with DMSO (0.1%) or BRC-304 (0.5, 5.0, 10 μg/ml) for 48 hours. Supernatants were collected and analyzed by ELISA (TNF-α and IL-6) or Griess Reagent System (NO). Results are mean ± SEM (n=3) and are representative of two separate experiments. # indicates p < .05 compared to the unstimulated control; * indicates p < .05 compared to the vehicle control.
BRC-306 had little effect on the inflammatory responsiveness of DC2.4 cells. However, at the highest concentration tested (25 μg/ml), there was a significant decrease in IL-6 production. No effects were observed on the production of NO and TNF-α byDC2.4 cells when treated with BRC-306 (Figure 3). BRC-306 had no effect on the cellular viability of DC2.4 cells (Table 3).
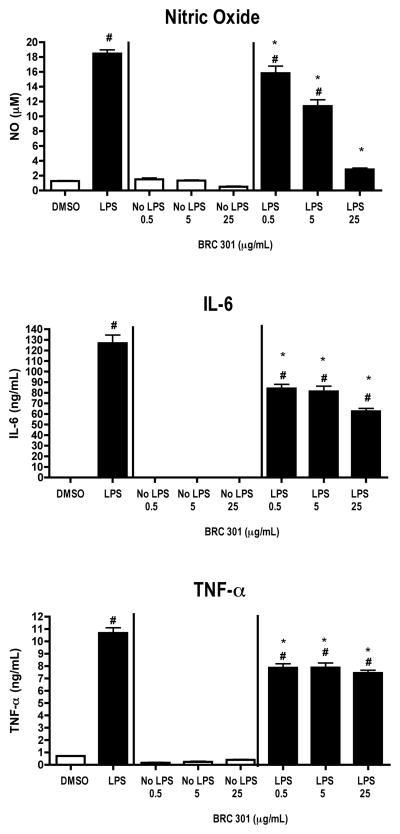
BRC-301 decreases inflammatory mediator production by bmDCs
To further evaluate the potential of BRC-301 to modulate the responsiveness of DCs to LPS, we characterized the effects of this complex herbal extract on primary, murine bmDCs. Consistent with the effects observed on DC2.4 cells, BRC-301 inhibited the production of NO, IL-6, and TNF-α by LPS-stimulated bmDCs (Figure 4). BRC-301 had no effect on the cellular viability of the bmDCs, except for an increased viability of unstimulated bmDCs at the 5.0 μg/ml concentration (Table 3).
FIGURE 4.
LPS-activated bmDCs produce decreased levels of inflammatory mediators following exposure to BRC-301. GM-CSF-derived bmDC cells were stimulated with LPS (1 μg/ml) and concomitantly treated with DMSO (0.1%) or BRC-301 (0.5, 5.0, 25 μg/ml) for 48 hours. Supernatants were collected and analyzed by ELISA (TNF-α and IL-6) or Griess Reagent System (NO). Results are mean ± SEM (n=3) and are representative of two separate experiments. # indicates p < .05 compared to unstimulated control; * indicates p < .05 compared to the vehicle control.
BRC-301 modulates the uptake of soluble antigens by bmDCs
Phagocytosis of Alexa Fluor-ovalbumin by bmDCs increased when samples were pre-treated for 24 hours with 25 μg/ml of BRC-301 prior to antigen exposure (Figure 5). Similarly, uptake of Alexa Fluor-ovalbumin was increased when added simultaneously with BRC-301 for 24 hours. In contrast, no effect of BRC-301 was observed on the uptake of LDL by bmDCs (data not shown).
FIGURE 5.
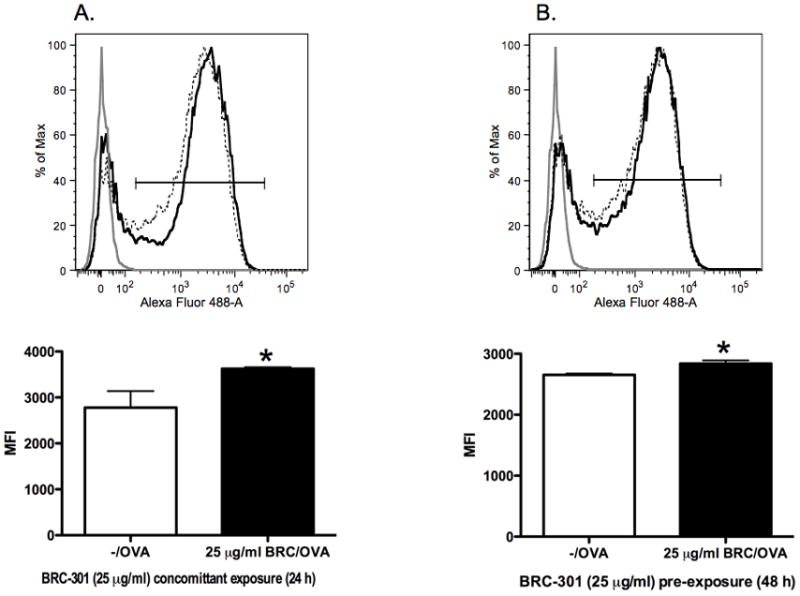
Phagocytosis of soluble antigen by bmDCs increases following exposure to BRC-301. BmDCs were treated with DMSO (0.1%) or BRC-301 (25 μg/ml) under the two following conditions. BRC-301 and Alexa Fluor 488-ovalbumin were added simultaneously and incubated overnight (A). BRC-301 was added 24 hours prior to exposure to Alexa Fluor 488-ovalbumin in pre-treated samples and cultured for an additional 24 hour incubation period (B). Cells were then harvested and analyzed by flow cytometry. Light gray lines represent control cells that received no antigen; dotted lines represent vehicle-treated bmDCs exposed to antigen; solid lines represent BRC-301-treated bmDCs exposed to antigen. Results are reported as Mean Fluorescence Intensity (MFI) ± SEM (n=3) and are representative of two separate experiments. * indicates p < .05 compared to the vehicle control.
BRC-301 increases the expression of accessory molecules on LPS-activated bmDCs
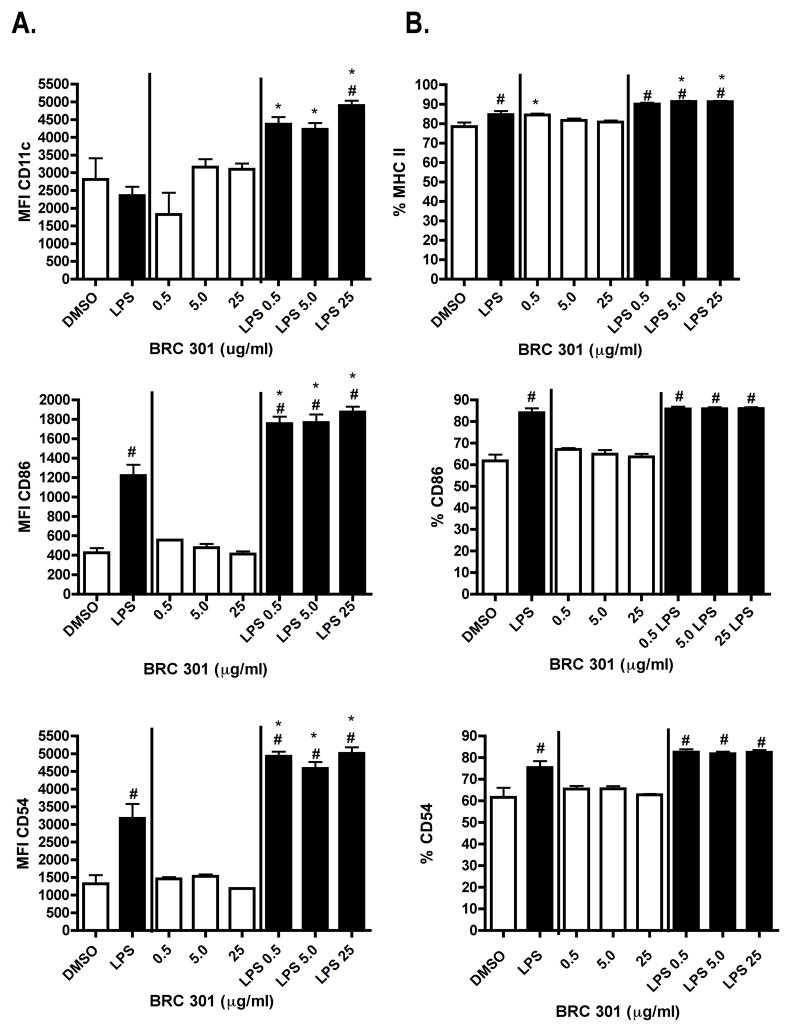
As DCs become activated, they increase their surface expression of costimulatory molecules, which play an important role in the generation of T cell-mediated immune responses. BRC-301 enhanced the expression of several important cell surface proteins on bmDCs (Figure 6). In unstimulated bmDCs, BRC-301 minimally affected the expression of accessory molecules except for the increased frequency of bmDCs expressing MHC class II at the lowest concentration tested (0.5 μg/ml) compared to vehicle control. As expected, LPS activation increased expression of costimulatory molecules on the bmDCs. Relative expression of CD11c, a murine DC lineage marker, was also increased on bmDCs following LPS activation and treatment with BRC-301. Furthermore, the frequency of LPS-stimulated bmDCs expressing MHC class II was increased at the higher concentrations (10 and 25 μg/ml) tested. The relative expression of the costimulatory molecules, CD86 and CD54, was significantly increased on LPS-activated bmDCs. In contrast, BRC-301 did not affect the LPS- induced expression of CD14 and CD40 (data not shown).
FIGURE 6.
BRC-301 affects the expression of accessory molecules on bmDCs. BmDCs were treated with BRC-301 (0.5, 5.0, 25 μg/ml) or DMSO (0.1%) and stimulated with LPS (1 μg/ml) or unstimulated for 48 hours. The Mean Fluorescence Intensity (A) and percent positive (B) profiles were determined by FACS analysis. Results are reported as MFI ± SEM (n=3) and are representative of two independent experiments. # indicates p < .05 compared to the unstimulated control; * indicates p < .05 compared to the vehicle control.
DISCUSSION AND CONCLUSIONS
The use of natural products to maintain or improve health has steadily increased in the United States over the last two decades. Moreover, formulating new products that have the ability to improve health by reducing inflammation is desirable because of the potential for long-term effectiveness, decreased toxicities, and lower costs. These products, however, are not regulated to the same extent as are over-the-counter and prescription drugs. Therefore, the safety and efficacy of most of these products are not well known.
BRC-301, BRC-304, and BRC-306 were evaluated for their immunomodulatory effects on DCs due to their preferential inhibition of COX-2 in an initial screening assay (Benson et al., 2010). BRC-306 was the most selective COX-2 inhibitor of these formulations, and it was also more selective than some pharmaceuticals including ibuprofen, naproxen, and indomethacin (Seaver & Smith, 2004). This formulation, however, was less selective than rofecoxib. Since highly selective COX-2 inhibitors have been pulled from the market in recent years due to their adverse cardiovascular effects, the goal is to find a compound that is selective for COX-2 to reduce inflammation but not too highly selective.
Interestingly, of the three products tested in our study, BRC-301 demonstrated the greatest potential for modulating the inflammatory response of DC2.4 cells. There was a reduction in NO, IL-6 and TNF-α levels in the supernatants of all three concentrations tested. Importantly, these effects were not attributable to decreases in cellular viability indicating that this extract was not adversely affecting these cultured murine DCs. Therefore, these results suggest that BRC-301 may inhibit the LPS-induced inflammatory responsiveness of DCs by modulating signal transduction pathways involved in the generation of NO, IL-6 and TNF-α. This effect may be the result of specific inhibition of nuclear factor-kappa B (NF-κB), a transcription factor involved in the activation of many inflammatory mediator genes, although this possibility remains to be evaluated. It is worthwhile to note that the anti-inflammatory effects of BRC-301 are not limited to DCs, as we recently found that BRC-301 also elicited suppressive effects on pro-inflammatory cytokine production by LPS-stimulated RAW264.7 macrophages, another important population of innate immune cells that function as antigen presenting cells (Benson et al., 2010). Thus, it is possible that this extract may alter the responsiveness of additional cell populations (immune and non-immune) that act together to reduce inflammation, effects that warrant in vivo studies to test this possibility.
Similarly, BRC-301 was effective in its capacity to modulate the primary bmDCs. Although BRC-301 did not affect the basal levels of the three inflammatory mediators evaluated, it did inhibit the release of NO, IL-6 and TNF-α by LPS activated bmDCs. However, the extent of inhibition varied between the inflammatory mediators. NO production at 25 μg/ml of BRC-301 showed the greatest level of inhibition, thus suggesting that it may selectively affect specific signal transduction pathways. Furthermore, BRC-301 increased the phagocytic capacity of the bmDCs to internalize ovalbumin, but had no effect on the receptor mediated phagocytosis of acetylated LDL. The effects of BRC-301 on uptake of antigen may indicate a selective enhancement of antigen clearance and antigen presentation to T cells, effects that would be expected to accelerate resolution of infection and reduce associated inflammation. However, defining the biological significance of these effects will require additional investigations. In addition to the anti-inflammatory effects that were observed, BRC-301 increased expression of several cell surface markers (CD86, and CD54), which may enhance the ability of the bmDCs to interact with T lymphocytes. In comparison, a recent study reported the effects of aspirin and ibuprofen on murine DCs (Kim, Lee, Im, Kim, & Lee, 2010). After 18h of treatment, ibuprofen, but not aspirin, increased the expression of several accessory molecules on unstimulated DC2.4 cells but did not affect their phagocytic capability. In addition, in bmDCs, both aspirin and ibuprofen blocked the presentation of exogenous ovalbumin to T cells. These results imply that DC functions are adversely sensitive to NSAIDs, effects that make these pharmaceuticals less than desirable in the treatment of chronic inflammation.
Many of the individual constituents of the natural product mixture BRC-301 have been rigorously studied individually and have been shown to modulate inflammation specifically through the inhibition of the NF-κB pathway. For example, Curcumin longa was shown to inhibit TNF-α induced NF-κB activation in human myelomonoblastic leukemia cells in a study conducted by Singh and Aggrawal (1995). Furthermore, pretreatment with curcumin in LPS stimulated murine bmDCs suppressed the NF-κB p65 nuclear translocation as well as inhibited LPS-induced NF-κB promoter activity (Kim et al., 2005). In human monocyte-derived DCs, treatment with curcumin decreased the levels of IL-12, IL-10, TNF-α and IL-6 following activation with LPS or Poly I:C (Shirley, Montpetit, Lockey, & Mohapatra, 2008). An additional component of BRC-301, Boswellia serrata, is also well known for its anti-inflammatory activity. It has been shown in numerous studies to be useful in treating ailments including Crohn’s disease, ulcerative colitis, and arthritis (Gerhardt, Seifert, Buvari, Vogelsang, & Repges, 2001; Gupta et al. 1997; Krieglstein et al. 2001; Sharma, Bani, & Singh, 1989). When administered together, these anti-inflammatory natural products decreased inflammatory mediators when tested in murine DCs.
Collectively, our data suggest that BRC-301 may act as an effective anti-inflammatory product with little-to-no toxicity. Because of its promising anti-inflammatory effects in vitro, BRC-301 should be considered for additional testing using in vivo models of chronic inflammation and infectious disease in addition to determining the specific mechanisms underlying its anti-inflammatory effects.
Acknowledgments
This project was supported by the Biotics Research Corporation (Rosenberg, TX, USA). The authors would like to acknowledge the assistance of the Fluorescence Cytometry Core Facility of the University of Montana, supported in part by NIH grant (P20RR017670).
Contributor Information
Andrea K. Miller, Center for Environmental Health Sciences, University of Montana, USA.
Jenna M. Benson, Center for Environmental Health Sciences, Department of Biomedical and Pharmaceutical Sciences, University of Montana, USA.
Dave N. Muanza, Phytochemistry Laboratory, Biotics Research Corporation, USA.
Jerry R. Smith, Department of Biomedical and Pharmaceutical Sciences, University of Montana, USA.
David M. Shepherd, Center for Environmental Health Sciences, Department of Biomedical and Pharmaceutical Sciences, University of Montana, USA.
References
- Bankoti J, Rase B, Simones T, Shepherd DM. Functional and phenotypic effects of AhR activation in inflammatory dendritic cells. Toxicol Appl Pharmacol. 2010 doi: 10.1016/j.taap.2010.03.013. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benson JM, Miller AK, Cooper N, Muanza DN, Smith JR, Shepherd DM. Anti-inflammatory effects of natural product formulations on murine macrophages. J Diet Suppl. 2010 doi: 10.3109/19390211.2010.489035. in press. [DOI] [PubMed] [Google Scholar]
- Berman JD, Straus SE. Implementing a research agenda for complementary and alternative medicine. Annu Rev Med. 2004;55:239–254. doi: 10.1146/annurev.med.55.091902.103657. [DOI] [PubMed] [Google Scholar]
- Clarke JO, Mullin GE. A review of complementary and alternative approaches to immunomodulation. Nutr Clin Pract. 2008;23:49–62. doi: 10.1177/011542650802300149. [DOI] [PubMed] [Google Scholar]
- Figdor CG, de Vries JM, Lesterhuis JW, Melief CJM. Dendritic cell immunotherapy: mapping the way. Nature Med. 2004;10:475–480. doi: 10.1038/nm1039. [DOI] [PubMed] [Google Scholar]
- Gerhardt HF, Seifert P, Buvari H, Vogelsang H, Repges R. Therapy of active Crohn disease with Boswellia serrata extract H 15. Z Gastroenterol. 2001;39(1):7–11. doi: 10.1055/s-2001-10708. [DOI] [PubMed] [Google Scholar]
- Gupta IA, Parihar P, Malhotra GB, Singh R, Ludtke H, Safayhi H, Ammon HP. Effects of Boswellia serrata gum resin in patients with ulcerative colitis. Eur J Med Res. 1997;2(1):37–43. [PubMed] [Google Scholar]
- Janeway CA, Medzhitov R. Innate immune recognition. Annu Rev Immunol. 2002;20:197–216. doi: 10.1146/annurev.immunol.20.083001.084359. [DOI] [PubMed] [Google Scholar]
- Kim GY, Kim KH, Lee SH, Yoon MS, Lee HJ, Moon DO, Lee CM, Ahn SC, Park YC, Park YM. Curcumin inhibits immunostimulatory function of dendritic cells: MAPKs and translocation of NF-κB as potential targets. J Immunol. 2005;174:8116–8124. doi: 10.4049/jimmunol.174.12.8116. [DOI] [PubMed] [Google Scholar]
- Kim HJ, Lee YH, Im SA, Kim K, Lee CK. Cyclooxygenase inhibitors, aspirin and ibuprofen, inhibit MHC-restricted antigen presentation in dendritic cells. Immune Netw. 2010;10(3):92–98. doi: 10.4110/in.2010.10.3.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krieglstein CF, Anthoni C, Rijcken EJ, Laukötter M, Spiegel HU, Boden SE, Schweizer S, Safayhi H, Senninger N, Schürmann G. Acetyl-11-keto-beta-boswellic acid, a constituent of a herbal medicine from Boswellia serrata resin, attenuates experimental ileitis. 2001;16(2):88–95. doi: 10.1007/s003840100292. [DOI] [PubMed] [Google Scholar]
- Mainardi T, Kapoor S, Bielory L. Complementary and alternative medicine: herbs, phytochemicals and vitamins and their immunologic effects. J Allergy Clin Immunol. 2009;123:283–294. doi: 10.1016/j.jaci.2008.12.023. [DOI] [PubMed] [Google Scholar]
- Rhule A, Navarro S, Smith JR, Shepherd DM. Panax notoginseng attenuates LPS-induced pro-inflammatory mediators in RAW264.7 cells. J Ethnopharmacol. 2006;106(1):121–128. doi: 10.1016/j.jep.2005.12.012. [DOI] [PubMed] [Google Scholar]
- Seaver B, Smith JR. Inhibition of COX isoforms by nutraceuticals. J Herb Pharmacother. 2004;4:11–18. [PubMed] [Google Scholar]
- Sharma ML, Bani S, Singh GB. Anti-arthritic activity of boswellic acids in bovine serum albumin (BSA)-induced arthritis. Int J Immunopharacol. 1989;11(6):647–652. doi: 10.1016/0192-0561(89)90150-1. [DOI] [PubMed] [Google Scholar]
- Shepherd DM, Steppan LB, Hedstrom OR. Anti-CD40 Treatment of 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD)-exposed C57Bl/6 mice induces activation of antigen presenting cells yet fails to overcome TCDD-induced suppression of allograft immunity. Toxicol Appl Pharmacol. 2001;170:10–22. doi: 10.1006/taap.2000.9080. [DOI] [PubMed] [Google Scholar]
- Shirley SA, Montpetit AJ, Lockey RF, Mohapatra SS. Curcumin prevents human dendritic cell response to immune stimulants. Biochem Biophys Res Commun. 2008;374:431–436. doi: 10.1016/j.bbrc.2008.07.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh S, Aggrawal BB. Activation of transcription factor NF-κB is suppressed by curcumin. J Biol Chem. 1995;270(42):24995–5000. doi: 10.1074/jbc.270.42.24995. [DOI] [PubMed] [Google Scholar]
- Zhenhai S, Reznikoff G, Dranoff G, Rock K. Cloned dendritic cells can present exogenous antigens on both MHC class I and class II molecules. J Immunol. 1997;158:2723–2730. [PubMed] [Google Scholar]