Abstract
Integration of sensory and motor inputs has been shown to be impaired in appendicular muscles and joints of Parkinson’s disease (PD) patients. As PD advances, axial symptoms such as gait and balance impairments appear, which often progresses to complete inability stand or walk unaided. The current study evaluates kinesthesia in the axial musculature of PD patients during active postural control to determine whether impairments similar to those found in the appendages are also present in the hip and trunk. Using axial twisting, we quantified the detection threshold and directional accuracy of the hip relative to the feet (i.e. Hip Kinesthesia) and the hip relative to the shoulders (i.e. Trunk Kinesthesia). The relation of kinesthetic threshold to disease progression as measured by UPDRS and the effect of levodopa treatment on kinesthesia were assessed in 12 PD compared to age-matched controls. Subjects stood unaided while passively twisted at a very low constant rotational velocity (1°/s). The results showed that accuracy in determining the direction of axial twisting was reduced in PD relative to healthy control subjects in the hip (PD-ON: 81%; PD-OFF: 91%; CTL=96%) and trunk (PD-ON: 81%; PD-OFF: 88%; CTL=95%). Thresholds for perception of axial twisting were increased when PD subjects were ON levodopa versus OFF in both the hip (p<0.01) and the trunk (p<0.05). The magnitude of decrease in sensitivity due to being ON levodopa was significantly correlated with the increase in UPDRS motor scores (Hip: r=0.90, p<0.01 and Trunk: r=0.60, p<0.05). This effect was not significantly correlated with equivalent levodopa dosage. PD subjects with disease onset on the left side of their body showed significantly higher axial thresholds than subjects with right PD onset (p<0.05). In conclusion, deficits in axial kinesthesia seem to contribute to the functional impairments of posture and locomotion in PD. Although levodopa has been shown to improve appendicular kinesthesia, we observed the opposite in the body axis. These findings underscore the dissociable neurophysiological circuits and dopaminergic pathways that are known to innervate these functionally distinct muscle groups.
Keywords: kinesthesia, muscle tone, hypertonicity, rigidity, levodopa, axial musculature, perceptual asymmetry
Introduction
Motor symptoms in Parkinson’s disease (PD) have been found to be associated with impairment in the integration of somatosensory inputs (Konczak, et al., 2009). Much of this evidence has focused on the kinesthetic impairments of appendicular muscles and joints (Klockgether, et al., 1995, Konczak, et al., 2007, Maschke, et al., 2003, Schneider, et al., 1987, Zia, et al., 2000). As PD progresses symptoms in gait and posture become more and more apparent (Hoehn and Yahr, 1967), which may be due to progressive impairment of proximal and axial musculature. Axial muscles also play an important role in anticipatory postural adjustments to stabilize posture during limb movements (Hesse, 1943, Massion, 1994). A recent cross-sectional study identified axial impairment as the strongest covariate of disability and reduced quality of life (Muslimovic, et al., 2008), thus, the consequences of impaired axial musculature to posture, gait, and motor control appear to be far reaching.
Measuring kinesthesia along the body axis presents a number of complications. One is that measurement techniques that are easily applied to limbs and digits because they are easy to manipulate independently and bilaterally are not always applicable to the trunk and hip. Few studies of axial kinesthesia can be found in the literature and none specifically addressing PD, while numerous assessments of the appendicular kinesthesia can be found (Klockgether, et al., 1995, Konczak, et al., 2007, Maschke, et al., 2003, Maschke, et al., 2006, Schneider, et al., 1987, Zia, et al., 2000, Zia, et al., 2002). Among them active and passive slow arm movements without visual feedback (Klockgether, et al., 1995) and elbow joint position sense during bilateral passive arm movements (Zia, et al., 2000) have shown impairments in PD limb kinesthesia. Other tests on distal muscles and joints have shown that detection thresholds to skin pressure (Schneider, et al., 1987) and weight perception on the fingers are impaired even in early stages of PD (Maschke, et al., 2006). Evidence of limb and distal muscle kinesthetic deficits in PD also comes from studies which examined vibration-induced errors during active movement of the wrist (Rickards and Cody, 1997) and ankle (Khudados, et al., 1999). However, due to the en bloc nature of the body axis many of these above techniques for limb assessment would be difficult to apply to the trunk or hips.
A second complication faced when measuring axial kinesthesia is that the threshold for detecting trunk position is known to decrease when a subject is supine compared to when standing in an active postural state (Jakobs, et al., 1985). This suggests that in order to determine what effect axial kinesthesia has on PD functional impairment, measurements should be taken while the subject is standing unsupported. To accomplish this, a unique device that we have employed in a number of postural and motor tasks recently (Franzen, et al., 2009, Gurfinkel, et al., 2006, Wright, et al., 2007a, Wright, et al., 2007b) was used in this study. This device allows axial segments to be rotated about the horizontal plane while the subject actively balances unsupported. This type of rotation involves no imposed shifts in body mass or changes in orientation of the body relative to gravity. There are a couple of advantages to these design characteristics. First, axial bending may confound threshold measures of axial musculature when reorientation of the head or a shift in center of mass occurs, since vestibular or foot proprioceptive thresholds may be reached before axial muscle and vertebral somatosensory thresholds. Second, many of the trunk’s anterior (e.g. abdominal external and internal obliques and abdominal transverses) and posterior muscles (e.g. erector spinae and multifidus) have a pinnate orientation. Therefore, these muscles will be changed in length more effectively by rotation than by hip and trunk flexion/extension. The functional importance of axial rotation and segmental coordination about the horizontal plane is evident during locomotion, and perhaps more importantly, changes in the coordination between thoracic and pelvic segments can be seen even in the earliest stages of PD gait impairment (Huxham, et al., 2008, Murray, et al., 1978, Vaugoyeau, et al., 2006, Vaugoyeau, et al., 2003). Thus, keeping the axial muscles active during measurement of kinesthesia and assessing them during functionally relevant actions is important to our understanding of the role of axial kinesthesia in postural control.
An additional consideration for assessing axial rigidity is that the pathways and neurotransmitter activity connected with proximal versus axial musculature are dissociable. This was highlighted in our recent study on axial rigidity in PD, which provided evidence that, unlike appendicular rigidity, axial muscle tone is not reduced by levodopa (Wright, et al., 2007b). Therefore, it is difficult to know whether the effects of levodopa and dopamine agonist drug therapy on kinesthesia involving distal muscles can be assumed to apply to axial kinesthetic thresholds either. This is further complicated by the fact that drug effects on distal muscles are still poorly understood. At least one study has shown levodopa worsens elbow joint proprioception (O’Suilleabhain, et al., 2001) while another study suggests improvement (Klockgether, et al., 1995). Therefore, to understand the effects of drug therapy on axial kinesthesia, it should be evaluated independent of distal musculature.
A final consideration was undertaken in this study due to the well-known asymmetry of motor symptoms at PD onset, as well as a growing body of research that suggests a perceptual asymmetry exists as well (Ebersbach, et al., 1996, Harris, et al., 2003, Hovestadt, et al., 1987, Starkstein, et al., 1987, Wright, et al., 2007a). We recently found a leftward perceptuomotor asymmetry in PD during reaching tasks, which occurred independent of the side of disease onset (Wright, et al., 2007a), however, many others have found evidence for perceptual left-neglect that occurs predominantly in patients with left-sided PD onset (LPD) (Davidsdottir, et al., 2005, Ebersbach, et al., 1996, Harris, et al., 2003, Lee, et al., 2001). Though these perceptual asymmetries have been established in visuospatial and perceptuomotor tasks, whether they extend to body-centered kinesthetic asymmetries in axial musculature has not yet been investigated.
To summarize the primary goals of this study which are aimed at furthering our understanding of kinesthetic deficits in PD: 1) we evaluated axial kinesthesia and determined if it is correlated with disease progression and functional impairments, 2) we evaluated what effect levodopa drug therapy has on axial kinesthetic thresholds, and 3) we determined if there is a lateral asymmetry in axial kinesthesia that correlates with the PD affected side. As in all studies of kinesthesia, changes in sensitivity are used as a marker for how well the calibration between ascending sensory input and conscious processing is tuned and if a threshold change subsequently translates to real functional impairment.
Methods
Subjects
A total of 26 subjects participated in this study. Twelve subjects (66±11 yr old) had Parkinson’s disease (PD) and were undergoing levodopa treatment. Fourteen subjects were healthy age-matched controls (65±10 yr old). The patients were selected based on being H&Y≥1.5, which is characterized as PD with axial involvement. All subjects provided informed consent in accordance with local ethics committee regulations for human subjects’ studies and the Helsinki Declaration.
PD subjects were tested in the morning after abstaining from anti-Parkinsonian medication overnight; the wash-out period was at least 12 hrs in the OFF-medication state. Subjects OFF-medication were assessed with the motor part of the United Parkinson’s Disease Rating Scale (UPDRS) prior to participating in the experimental protocol. All subjects were able to stand for periods of greater than 15 min without rest. After the subjects completed the OFF-medication portion of the protocol, they took their normal morning dosage of medications. Once PD subjects reported that they felt “ON”, at least 45 minutes after taking their medication, the UPDRS motor score was obtained. The UPDRS scores in the ON (20.7±11.6 SD) versus OFF antiparkinson medication (33.4±13.4 SD) showed a highly significant decrease (p<0.0001). The protocol was then repeated in the ON state.
Protocol
A torsional rotation device, which consists of a steel frame surrounding a rotating platform was used to twist axial segments of the body about the horizontal plane of a freely standing subject (Gurfinkel, et al., 2006, Wright, et al., 2007b). A unique feature of the torsional rotation device is that the platform rotates the lower body relative to a fixed upper body. The frame and the rotating platform each have a fixation point for a rigid aluminum rod that attaches to the subject with a harness at the opposite end. This attachment ensures that the lower part of the body axis remains fixed relative to earth, while the upper part is rotated about a vertical axis passing through the trunk roughly collinear with the spine (Fig. 1). The platform rotated ±10° clockwise (CW=Right) and counter-clockwise (CC=Left) in a trapezoidal pattern at a very slow velocity (1°/s) with reversals in rotational direction not exceeding 12°/s2 angular acceleration (Fig. 2). Four directions of platform rotation were used in each condition: CW from 10° left to 0° (CW10-0), CW from 0° to 10° right (CW0-10), CC from 0° to 10° left (CC0-10), CC from 10° right to 0° (CC10-0), with variable length pauses between each, as well as occasional sham trials in which no rotation occurred. The subject was asked to hold a bi-directional manual switch in their hands at approximately waist-height, which they were instructed to press as soon as rotation was perceived and the direction of rotation could be identified. Because the lower body is rotated relative to the upper body, axial rotation is not veridically perceived as platform rotation. Instead, the platform is perceived as stationary and the upper body is perceived as rotating in the direction opposite of platform rotation (Wright, et al., 2007a). Therefore, a “correct” response (i.e. a HIT) is CW indication of upper body rotation, when the platform is actually rotating the lower body CC. Each direction was tested at least 3 times per subject per condition and the order of rotations was counter-balanced. The button-push induced a ±5V change, which was recorded in synchrony with the platform position at 50 samples/sec using Spike2 software (Cambridge Electronic Devices, Cambridge, UK) and analyzed offline using Matlab software (Mathworks, Natick, MA).
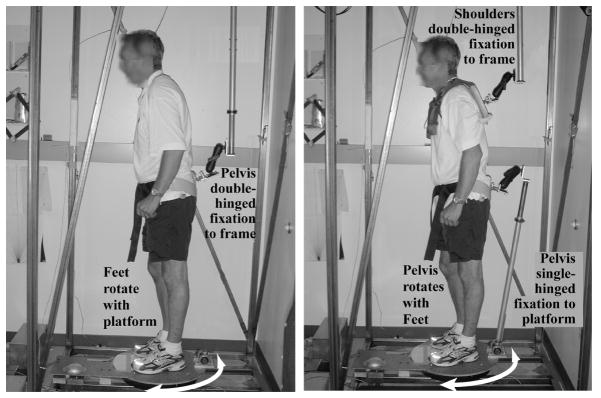
Fig. 1.
Left – In the Hip Kinesthesia condition, the torsional platform rotates (white arrow arc) the feet relative to the pelvis that is fixed against rotation by a double-hinge to allow for normal sway excursions and provide no postural support. Right – In the Trunk Kinesthesia condition, the platform rotates (white arrow arc) the feet and pelvis together relative to the upper trunk. The pelvis is fixed to the rotating support surface by a single-hinge allowing anterior-posterior sway, while the shoulder girdle is fixed against rotation by a double-hinge to a fixation frame. Both the Trunk and Hip configurations require active postural control and provide no support. The subject held a bi-directional button with both hands at waist-height (not pictured).
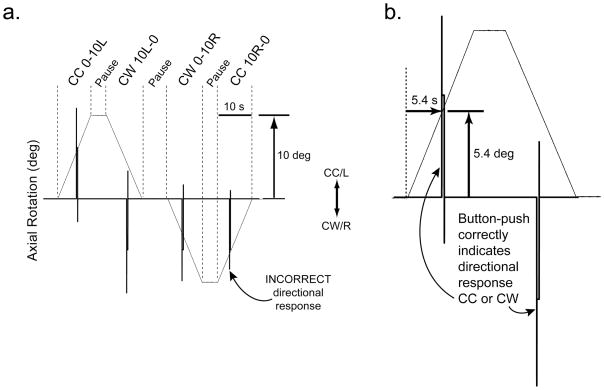
Fig. 2.
The proprioceptive stimulus and directional button-push response show how threshold and direction are measured. Axial rotation (thin line) is 10° CC/Left (up) or CW/Right (down) at 1°/s. a) The four directions with pauses in between (separated by vertical dotted lines) are CC from 0° to 10°L, CW from 10°L to 0°, CW from 0° to 10°R, or CC from 10°R to 0°, where 0° is considered a neutral, untwisted bodily configuration. Voltage signals (thick vertical spikes) show directional button-push response (CC=up, CW=down). The CW directional spike in the bottom left corner depicts a MISS, whereas all other spikes depict HITS. b) An expanded view of CC 0-10L, pause, CW 10L-0 test phases. If the response (thick line) occurs 5.4 seconds after platform rotation onset (dotted line), then the amount of axial rotation equals 5.4°, which is the kinesthetic threshold for that trial. An upward voltage spike indicates the subject perceived CC axial rotation and a downward voltage spike indicates the perception of CW axial rotation. To avoid more than 10° of axial abduction, CW 0-10L was always followed by CC 10L-0 and CC 0-10R was followed by CW 10R-0, however adduction from 10° to 0° could be followed by either CW 0-10R or CC 0-10L.
In both experimental conditions, Trunk and Hip Kinesthesia, we collected two dependent measures: threshold of detection of axial rotation and direction of perceived axial rotation. In one condition (PD: n=12; CTL: n=14), we quantified the thresholds to axial rotation of the trunk relative to the fixed hips (Trunk Kinesthesia). A harness, snugly attached to the subject’s shoulders on one end was attached on the other end to the steel frame by a double-hinge that allowed the subject to sway in the horizontal plane, but not rotate in yaw. In other words, the shoulders were rigidly fixed to the external rigid frame to prevent torsional rotation while the platform under the subject rotated the feet and pelvis. The feet and pelvis were rotated together by the platform, thus isolating twisting to the thoracolumbar region (Fig. 1, left). To rotate the hips with the feet, a rod connected to the platform was fixated to the hips. The subject wore a padded, rigid-framed, butterfly-shaped pelvic band taken from a thoracolumbar orthoses. The pelvic band was modified with an attachment for linking to the platform rod. The link between the pelvic band and the platform was single-hinged to allow free anterior-posterior translation, while allowing no rotation of the hips relative to the feet.
In the other condition (PD: n=9; CTL: n=12), the kinesthetic threshold of hips relative to lower body axial rotation (Hip Kinesthesia) was tested using the same rotation parameters as during the Trunk condition. The fixations of the hip allowed the legs to be passively rotated relative to a fixed pelvis. The pelvic band was attached via a double-hinged bar to the rigid frame, while the feet were rotated by the support platform. The shoulders were completely free in this condition. As the platform rotated the feet, the pelvis remained fixed relative to earth, thus isolating the hip and lower extremities (Fig. 1, right). Again, subjects held a bi-directional button in both hands at waist-height to indicate the onset and direction of perceived direction.
In both conditions, the double-hinged connections to the platform and frame allowed for unrestrained postural sway translations in the horizontal plane without rotation, which created a relatively natural mechanical environment for quiet stance. Subjects were required to maintain standing equilibrium, but the unconstrained motions in the horizontal plane precluded using the fixations as a spatial reference. The fixation linkages provided the degrees of freedom necessary to spontaneously sway within the normal base-of-support, therefore kinesthetic thresholds were measured with the subject in an active postural state engaged in maintaining upright posture, rather than in an less active state such as during supported stance or while lying supine. The attachments to the body and fixation linkages were counterweighted with springs so no unnatural loading could affect postural behavior. Because the Hip and Trunk attachments took time to remove and reattach, the trials were grouped such that all Hip condition trials were run together and all Trunk condition trials together. Also, the attachments for the Hip condition were not built until a few subjects had already been tested in the Trunk condition, however, all subsequent subjects were run in counterbalanced order. This order was also counterbalanced in ON and OFF conditions for PD subjects.
Data Analysis
For both Hip and Trunk conditions, the threshold for detecting axial rotation was determined for each subject in each direction. At least three trials per direction were collected for each subject and a mean was calculated for the thresholds. Either repeated-measures ANOVA (PD-ON versus PD-OFF medication) or one-way ANOVA (PD versus controls) were used to compare threshold values. Subjects’ button-push response also indicated direction of rotation, so the accuracy of each response was tabulated and an overall percent “HIT” rate was assessed for each subject in each condition. If a subject indicated the wrong direction or failed to respond before the 10° limit of rotation was reached, it was counted as a “MISS”. Non-parametric Fischer Exact tests for small samples were used to assess any differences in HIT/MISS rates between PD-ON, PD-OFF, and controls. The Pearson’s product moment correlation was used to quantify the interrelation of variables in all regressions. Paired t-tests were used to compare PD subjects ON versus OFF medication. Unpaired t-tests were used to compare PD subjects to controls. Significance was set at α ≤ 0.05.
The UPDRS Part III was used to measure disease progression and severity in the PD subjects. The UPDRS Part III includes items to clinically quantify the level of various motor abilities in PD subjects. These items are grouped according to their body location and their functional relevance. Our study analyzed the functional measure “postural instability and gait difficulty” (PIGD), which includes UPDRS items 27–30. Also, a measure of axial symptoms included UPDRS item 20 resting tremor of legs, item 22 (neck and leg rigidity), item 26 (leg agility), and items 27–30 (PIGD). We used the variable of kinesthetic thresholds when ON – OFF medication to examine the change in kinesthetic threshold across these states. Correlating this variable with overall clinical measures such as disease severity, PIGD, and axial symptoms informs us of any relation between the patient’s functional state and improvement or decline in the experimental dependent measure.
Results
The accuracy in determining the direction of axial rotation was impaired in PD subjects relative to healthy age-matched controls. Kinesthetic deficits were also evident by the higher thresholds for detecting the onset of axial rotation. Sensitivity to axial rotation was lower when ON levodopa medication versus OFF. The change in threshold when ON versus OFF medication was also significantly correlated with disease severity. Side of disease onset also affected PD sensitivity.
Directional sensitivity
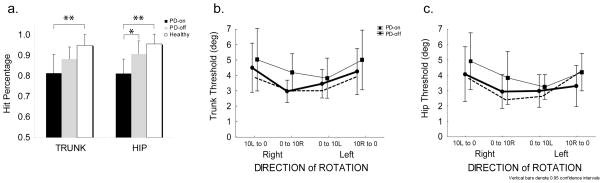
Although all groups performed above 50% chance in both Hip and Trunk Kinesthesia conditions, significant differences in accuracy of directional indication were observed between subject groups (Fig 3a). In the Hip condition, PD-ON performed at 81%, PD-OFF at 91% and controls at 96% HIT-rates. The difference between PD-ON versus OFF was significant (p<0.03), and PD-ON versus controls (p<0.001). In the Trunk condition, PD-ON accuracy was 81%, PD-OFF was 88%, and controls were 95%. PD subjects ON medication performed significantly worse than controls (p<0.002), and had a tendency to perform worse than controls when OFF medication (p=0.06, n.s) (Fig. 3b).
Fig. 3.
Accuracy of perceiving the direction of axial rotation and threshold of detecting the onset of axial rotation. a) The average percentage of accurate responses for PD subjects ON (black bar) or OFF (gray bar) medication, and age-matched control (white bar) show a significant difference (*, p<0.05; **, p<0.01) between groups during TRUNK (left) and HIP (right) rotation, (bars indicate standard error), b) thresholds for detection of TRUNK rotation in PD subjects is greater when ON (thin line, squares) versus OFF (circles) medication (p<0.05), c) thresholds for detection of HIP rotation in PD subjects is greater when ON versus OFF medication (p<0.01). In b. and c., control subjects are indicated by dotted line and error bars indicate 95% confidence intervals.
Threshold of sensitivity
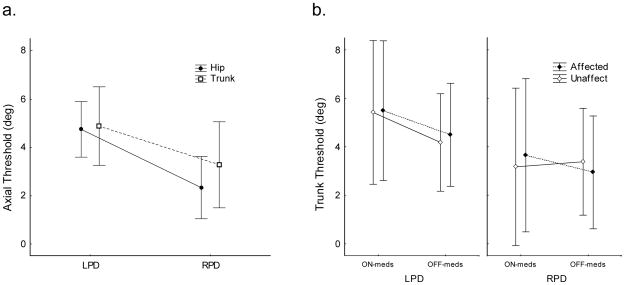
The sensitivity threshold to rotation of the hip was significantly higher in PD subjects (F1,8=11.7, p=0.009) when ON medication compared to when OFF (Fig 3c). The trunk kinesthetic threshold was less affected when ON versus OFF, showing only a non-significant trend (F1,10=4.60, p=0.058, n.s.) that was dependent on whether rotating from a symmetrical or asymmetrical axial state (Fig 3b). The effect of starting rotation from an asymmetrical state (“10L to 0” and “10R to 0”, in Fig 3) compared to starting rotation from a symmetrical straight-ahead facing state (“0 to10L” and “0 to 10R”) showed a higher threshold in all subjects, controls and PD alike. Moreover, in PD subjects this directional effect was significant both when ON (p<0.05) and OFF (p<0.05) medication. Both hip and trunk kinesthetic sensitivity in PD subjects whose disease onset was on the left (LPD) was significantly higher than for those whose disease onset was on the right side (RPD) (F1,7=6.78, p<0.05) (Fig. 4a). A number of significant statistical interactions were also found when we analyzed the effects of rotation direction and medication. We found a significant statistical interaction when rotating the trunk toward the affected side due to medication (F1,9=5.15, p<0.05). RPD subjects had higher axial sensitivity than LPD subjects and only showed a reduction in sensitivity when rotated towards their affected side. Whereas LPD, in addition to having lower sensitivity, their sensitivity decreased even more when ON-meds, regardless of whether they were rotated to the affected or unaffected side (Fig. 4b).
Fig. 4.
Asymmetries in PD with left (LPD) versus right (RPD) disease onset. a) LPD have higher axial kinesthetic thresholds than RPD in both the trunk and hip (p<0.05), b) trunk kinesthetic threshold shows an interaction between LPD and RPD when ON versus OFF medication, depending on whether rotating the Affected or Unaffected Side. The sensitivity of the unaffected side of RPD did not decrease when ON-medication (p<0.05). Error bars indicate 95% confidence intervals.
Correlations in kinesthetic sensitivity relative to dopamine effects
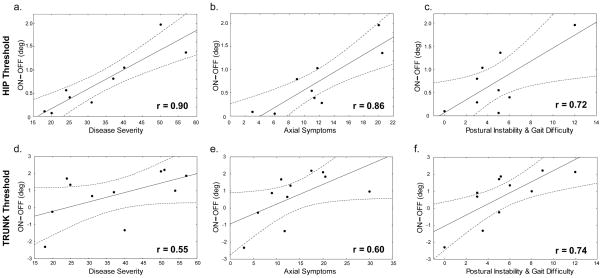
The relationship between clinical UPDRS measures and the change in kinesthetic sensitivity due to levodopa usage revealed that the differences in threshold when ON and OFF medication (i.e. “ON – OFF”) were positively correlated with clinical measures (Fig. 5). This correlation was most robust for the hip thresholds (r=0.90, p<0.01, Fig. 5a). When only considering the axial symptoms as measured by the axial part of the UPDRS, a significant positive correlation with the levodopa-induced change in hip threshold was also found (r=0.86, p<0.01, Fig. 5b). The change in trunk kinesthetic threshold “ON – OFF” had slightly weaker correlations with the full UPDRS scale (r=0.55, p=0.08, Fig. 5d) and axial symptoms (r=0.60, p<0.05, Fig. 5e). The “ON – OFF” effect of levodopa on axial kinesthetic thresholds also showed significant positive correlations with postural instability and gait difficulties (PIGD) in the hip (r=0.72, p<0.05, Fig. 5c) and trunk (r=0.74, p<0.01, Fig. 5f).
Fig. 5.
Levodopa induced changes in threshold when ON versus OFF are positively correlated with disease severity (UPDRS scores when OFF-meds), axial symptoms (Axial-UPDRS scores OFF meds), and postural instability and gait disturbance (PIGD-UPDRS scores OFF meds). A positive value for “ON minus OFF” (y-axis) indicates that the threshold increased when ON-meds, i.e. sensitivity decreased when ON-meds. (Top row) a) Disease severity (r=0.90, p<0.01), b) axial symptoms (r=0.86, p<0.01), and c) PIGD (r=0.72, p<0.05) positively correlated with levodopa induced change in HIP threshold. (Bottom row) d) Disease severity (r=0.55, p=0.08, n.s.), and e) axial symptoms (r=0.60, p<0.05) showed weaker correlations with the levodopa induced change in TRUNK threshold than in the hip, but f) PIGD had a similar correlation in the trunk (r=0.74, p<0.01) as in the hip. Dotted lines indicate 95% CI.
Discussion
Our study showed that PD subjects were significantly less accurate in determining the direction of axial rotation than age-matched healthy control subjects. We found that levodopa drug therapy had an unexpected negative effect on axial kinesthesia by increasing the thresholds in both the hip and trunk. Clinical measures of disease progression and functional impairment were significantly correlated with the change in axial kinesthetic thresholds when going ON and OFF their antiparkinson medication. Finally, the side of PD onset affected the axial kinesthetic thresholds, with LPD showing greater symptoms.
Effects of levodopa on axial kinesthesia
A very clear relation between axial kinesthetic sensitivity and levodopa usage in PD was found in this study. However, levodopa has a negative impact on axial kinesthesia. Although we also saw increases in threshold and worse accuracy when OFF medication relative to healthy controls, the differences were significantly larger when ON. The size of the kinesthetic deficit was not correlated with levodopa dosage amount, however, it was related to overall disease severity. This relationship was not a direct correlation with UPDRS scores though. That is, UPDRS scores increased neither with the kinesthetic thresholds when OFF medication nor with the kinesthetic thresholds when ON medication. Rather, PD subjects whose disease symptoms were rated worst showed the greatest difference in their axial kinesthetic threshold when ON compared to OFF medication (“ON – OFF”). In other words, increases in disease severity correlated with the greatest increase in kinesthesia due to medication (see Fig 5a and 5d). By looking at the change when ON versus OFF medication, we found that the most severe PD subjects may not necessarily have the lowest axial sensitivity, but rather it was those PD subjects with the largest “ON – OFF” threshold difference. That no correlation with levodopa dosage size was found may be explained by the fact that individuals with the most advanced disease symptoms do not necessarily take the most medication. To summarize the antiparkinson drug effects, the fluctuation from ON to OFF appears to have a more debilitating effect on axial kinesthetic sensitivity than dosage size, but being ON medication does result in a significant decrease in sensitivity.
Hypertonicity and Kinesthesia
In a previous study with many of the same PD patients used in this study, we measured axial rigidity (i.e. the resistance to axial rotation) (Wright, et al., 2007b). Despite finding a relation between disease severity and kinesthetic thresholds (Fig 5), no significant correlation was found between the level of hypertonicity and kinesthetic thresholds. This finding seems to run counter to the hypothesis that increasing rigidity underlies increasing kinesthetic axial thresholds (Klockgether, et al., 1995). However, we cannot discount the fact that there may not be a one-to-one relation between the two variables, but instead an all-or-none relationship. In other words, an upper limit for tonic level of muscle activity may exist. Above this limit improper integration of sensory information can alter the kinesthetic threshold, but by how much it alters the threshold depends on other factors not identified by our current methods.
Altered axial kinesthesia affects functional measures
Individual parkinsonian symptoms have been shown to have dissociable effects on behavioral outcomes (Zetusky, et al., 1985). For example, PD “fallers” tend to have postural instability, hypertonicity, and bradykinesia, but not tremor, and falling is not found to be reversible by dopamine therapy (Koller, et al., 1989). A look at axial symptoms in PD reveals that these directly impact posture and gait (Franzen, et al., 2009, Van Emmerik, et al., 1999, Wright, et al., 2007b). Although some studies have suggested that PD patients can be classified within subgroups, such as those with tremor-dominance and those with dominant symptoms of postural instability and gait difficulty (Jankovic, et al., 1990, Zetusky, et al., 1985), we did not set our inclusion/exclusion criteria based on these classifications. However, regression analysis using subsets of the UPDRS scores were used to determine how specific sets of related symptoms may correlate with kinesthetic deficits. Two of these subsets included axial symptoms and a more specific subset for postural impairment and gait difficulties. The “ON – OFF” threshold difference was strongly correlated with axial symptoms (Fig. 5b,e), while even stronger correlations were found with PIGD (Fig. 5c,f). Since there was no correlation between kinesthetic threshold when ON relative to PIGD, we can surmise that increasing kinesthetic thresholds do not directly correlate with worsening PIGD. So by itself, being ON levodopa does not predict a worsening in PIGD or axial symptoms in our sample, rather the negative impact seems to derive from the size of the difference in sensitivity when ON versus OFF medication.
It has been proposed before that a primary disturbance to PD function may lie in the changes in the gating and integration of sensory inputs which then affect motor outputs (Abbruzzese and Berardelli, 2003, Konczak, et al., 2009). Because the mapping between sensory inputs and motor outputs is adaptable and in need of up-to-date precise calibration, motor behavior may be very susceptible to the fluctuations in kinesthetic sensitivity that occur when going back and forth between ON and OFF. Furthermore, even if the PD patient is very consistent and compliant in adhering to his dosage routine, spontaneous fluctuation between subjectively feeling “ON” and “OFF” their medication still can occur and is a well-known phenomenon in PD (see UPDRS Part IV, Complications in Therapy, Items 36–39). It has been reported that levodopa medication may have no effect or even negative effect on postural stability and gait problems (Horak, et al., 1996, Koller, et al., 1989). This negative effect on postural and motor impairments may be due to the frequent and unpredictable ON/OFF fluctuations, insofar as the fluctuations affect the fine tuning of sensorimotor control necessary for these tasks.
Asymmetry in kinesthesia depends on disease onset side
The current findings revealed an asymmetry in axial sensitivity, which depends on the side affected at Parkinson’s disease onset. Subjects with left disease onset (LPD) showed significantly higher kinesthetic thresholds in both the hip and trunk. Functional asymmetries in PD have been linked to the side of nigrostriatal neuron loss in humans (Kempster, et al., 1989, Kumar, et al., 2003) and a similar explanation may underlie these axial asymmetries. Bilateral basal ganglia pathways project to areas in the brainstem involved in locomotor and postural control, such as the midbrain locomotor region and pedunculopontine tegmental nucleus (Takakusaki, et al., 2004, Takakusaki, et al., 2008). Lateral axial asymmetries in PD may be directly caused by contralateral basal ganglia–cortical–spinal loops. Conversely, as the Hoehn and Yahr scale indicates, unilateral symptoms start distally with axial symptoms only beginning to appear at level 2 and above. Therefore, axial deficits may be compensations for early appendicular deficits (Wright, et al., 2007a, Wright, et al., 2007b). Postural stability and controlled mobility require constant activity of axial muscles and any change to the center of mass via movement of the distal parts of the body must be compensated by proximal segmental stabilization. Thus, a chronic lateralized change starting with the appendages may induce a chronic axial compensation.
Our findings that LPD have greater kinesthetic deficits than RPD, suggest a causal link with the perceptual deficits that many others have described in LPD. However, most of the prior evidence comes from visual and visuospatial tasks (Davidsdottir, et al., 2005, Ebersbach, et al., 1996, Harris, et al., 2003, Lee, et al., 2001, Starkstein, et al., 1987). In our study, we are focused on a purely proprioceptive input that does not need to be mapped to exocentric coordinates, i.e. deciding between clockwise or counterclockwise rotation could be made entirely within an egocentric mapping. Using such body-centered coordinates, we surmise that this process does not overlap with that driving visual spatial motor imagery (Amick, et al., 2006). Furthermore, our kinesthetic measure excluded visual feedback, which ensures us that the dopamine-affected receptors in the retina which may underlie visual bias in PD (Bodis-Wollner, 2003, Harris, et al., 2003) is not the same mechanism underlying this axial kinesthetic asymmetry. Although our current findings do not determine if kinesthetic asymmetry precedes the motor asymmetry and visual biases or vice versa, the fact that both visual and kinesthetic asymmetries seem to predominantly affect LPD may mean that both originate from early asymmetrical nigro-striatal neuron loss.
Conclusions
The kinesthetic sensitivity of axial musculature is impaired in PD, especially when using levodopa medication. The kinesthetic deficit may be specific to a patient’s subjective state of feeling ON or OFF caused by their medication. As the disease progresses, medication tends to become less efficacious resulting in ON/OFF fluctuations. Increasing the dosage does not usually help. The current evidence suggests that it is not simply a matter of PD axial thresholds being outside the population norms, rather it is the size of within-patient threshold change that occurs when going back and forth between ON and OFF. It would be interesting to investigate whether the calibration of other sensorimotor, perceptual, or cognitive processes is also sensitive to the size of the threshold changes in PD. Notwithstanding side-effects like subjective ON/OFF fluctuations, increased axial rigidity, decreased kinesthetic sensitivity, and secondary symptoms like dyskinesia, PD patients still highly prefer to take their medication. However, a growing body of evidence appears to show that despite the “freeing” effects of levodopa therapy, there are downsides of which both patients and clinicians should be aware.
Table 1.
PD Subject Information
| PD Sub | Side | Duration of PD | Age | Ht | Wt | Sex | UPDRS Motor Part III | H&Y OFF | Medication | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total | Rigid OFF | Axis OFF | PIGD OFF | L-dopa | Other | |||||||||
| ON | OFF | |||||||||||||
| 1 | L | 14 | 64 | 160 | 59 | F | 13 | 22 | 3 | 13 | 6 | 2.5 | 300 | Am, DA |
| 2 | L | 10 | 81 | 157 | 52.2 | F | 5 | 24 | 1 | 11 | 5 | 2 | 500 | DA, Sel |
| 3 | L | 7 | 69 | 178 | 104.3 | M | 20 | 35 | 9 | 12 | 4 | 3 | 1000 | |
| 4 | L | 10 | 71 | 178 | 77.1 | M | 39 | 55 | 12 | 21 | 5 | 3.5 | 1050 | |
| 5 | L | 4 | 53 | 165 | 70.3 | F | 17 | 37 | 5 | 9 | 3 | 3 | 300 | DA |
| 6 | L | 8 | 79 | 178 | 77.1 | M | 29 | 33 | 8 | 18 | 9 | 3 | 600 | DA |
| 7 | R | 18 | 59 | 173 | 74.8 | M | 36 | 50 | 8 | 20 | 12 | 3 | 500 | Am, DA |
| 8 | R | 17 | 74 | 175 | 66.7 | M | 33 | 54 | 16 | 30 | 8 | 3 | 1200 | Am, DA, Sel |
| 9 | R | 7 | 51 | 178 | 68 | M | 5 | 21 | 4 | 8 | 2 | 2 | 500 | DA |
| 10 | R | 7 | 50 | 170 | 70.3 | M | 19 | 20 | 3 | 6 | 5 | 1.5 | 1250 | Am, DA |
| 11 | R | 7 | 64 | 180 | 90.7 | M | 9 | 18 | 2 | 3 | 0 | 2 | 300 | DA, AntiCh |
| 12 | R | 9 | 73 | 173 | 77.1 | M | 23 | 32 | 5 | 12 | 3 | 2 | 300 | DA |
| Avg | - | 9.8 | 65.7 | 172 | 74.0 | - | 20.7 | 33.4 | 6.3 | 13.6 | 5.1 | 2.5 | - | - |
| Avg | Controls (n=14) | 64.5 | 170 | 71.0 | - | - | - | - | - | - | - | - | ||
Duration of PD in years, Ht – height in cm, Wt – weight in kg, Side – side of disease onset, Rigid – Rigidity (UPDRS Item 22), Axis – Axial Symptoms in UPDRS (Items 20, 22, 26–30), PIGD – Postural Instability and Gait Difficulty (UPDRS Items 27–30), L-dopa – daily dose in mg/day, AntiCh – anti-cholinergic, Am – amantadine, DA – dopamine agonist, Sel – Selegeline
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Abbruzzese G, Berardelli A. Sensorimotor integration in movement disorders. Mov Disord. 2003;18:231–240. doi: 10.1002/mds.10327. [DOI] [PubMed] [Google Scholar]
- 2.Amick MM, Schendan HE, Ganis G, Cronin-Golomb A. Frontostriatal circuits are necessary for visuomotor transformation: mental rotation in Parkinson’s disease. Neuropsychologia. 2006;44:339–349. doi: 10.1016/j.neuropsychologia.2005.06.002. [DOI] [PubMed] [Google Scholar]
- 3.Bodis-Wollner I. Neuropsychological and perceptual defects in Parkinson’s disease. Parkinsonism Relat Disord. 2003;9(Suppl 2):S83–89. doi: 10.1016/s1353-8020(03)00022-1. [DOI] [PubMed] [Google Scholar]
- 4.Davidsdottir S, Cronin-Golomb A, Lee A. Visual and spatial symptoms in Parkinson’s disease. Vision Res. 2005;45:1285–1296. doi: 10.1016/j.visres.2004.11.006. [DOI] [PubMed] [Google Scholar]
- 5.Ebersbach G, Trottenberg T, Hattig H, Schelosky L, Schrag A, Poewe W. Directional bias of initial visual exploration. A symptom of neglect in Parkinson’s disease. Brain. 1996;119 (Pt 1):79–87. doi: 10.1093/brain/119.1.79. [DOI] [PubMed] [Google Scholar]
- 6.Franzen E, Paquette C, Gurfinkel VS, Cordo PJ, Nutt JG, Horak FB. Reduced performance in balance, walking and turning tasks is associated with increased neck tone in Parkinson’s disease. Exp Neurol. 2009;219:430–438. doi: 10.1016/j.expneurol.2009.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gurfinkel V, Cacciatore TW, Cordo P, Horak F, Nutt J, Skoss R. Postural muscle tone in the body axis of healthy humans. J Neurophysiol. 2006;96:2678–2687. doi: 10.1152/jn.00406.2006. [DOI] [PubMed] [Google Scholar]
- 8.Harris JP, Atkinson EA, Lee AC, Nithi K, Fowler MS. Hemispace differences in the visual perception of size in left hemiParkinson’s disease. Neuropsychologia. 2003;41:795–807. doi: 10.1016/s0028-3932(02)00285-3. [DOI] [PubMed] [Google Scholar]
- 9.Hesse WR. Teleokinetische und ereismaticsch Kräftesyseme in der Biomotorik. Helvetica Physiol Acta. 1943;1:C62–C63. [Google Scholar]
- 10.Hoehn MM, Yahr MD. Parkinsonism: onset, progression and mortality. Neurology. 1967;17:427–442. doi: 10.1212/wnl.17.5.427. [DOI] [PubMed] [Google Scholar]
- 11.Horak FB, Frank J, Nutt J. Effects of dopamine on postural control in parkinsonian subjects: scaling, set, and tone. J Neurophysiol. 1996;75:2380–2396. doi: 10.1152/jn.1996.75.6.2380. [DOI] [PubMed] [Google Scholar]
- 12.Hovestadt A, de Jong GJ, Meerwaldt JD. Spatial disorientation as an early symptom of Parkinson’s disease. Neurology. 1987;37:485–487. doi: 10.1212/wnl.37.3.485. [DOI] [PubMed] [Google Scholar]
- 13.Huxham F, Baker R, Morris ME, Iansek R. Head and trunk rotation during walking turns in Parkinson’s disease. Mov Disord. 2008;23:1391–1397. doi: 10.1002/mds.21943. [DOI] [PubMed] [Google Scholar]
- 14.Jakobs T, Miller JA, Schultz AB. Trunk position sense in the frontal plane. Exp Neurol. 1985;90:129–138. doi: 10.1016/0014-4886(85)90046-9. [DOI] [PubMed] [Google Scholar]
- 15.Jankovic J, McDermott M, Carter J, Gauthier S, Goetz C, Golbe L, Huber S, Koller W, Olanow C, Shoulson I, et al. Variable expression of Parkinson’s disease: a base-line analysis of the DATATOP cohort. The Parkinson Study Group. Neurology. 1990;40:1529–1534. doi: 10.1212/wnl.40.10.1529. [DOI] [PubMed] [Google Scholar]
- 16.Kempster PA, Gibb WR, Stern GM, Lees AJ. Asymmetry of substantia nigra neuronal loss in Parkinson’s disease and its relevance to the mechanism of levodopa related motor fluctuations. J Neurol Neurosurg Psychiatry. 1989;52:72–76. doi: 10.1136/jnnp.52.1.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Khudados E, Cody FW, O’Boyle DJ. Proprioceptive regulation of voluntary ankle movements, demonstrated using muscle vibration, is impaired by Parkinson’s disease. J Neurol Neurosurg Psychiatry. 1999;67:504–510. doi: 10.1136/jnnp.67.4.504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Klockgether T, Borutta M, Rapp H, Spieker S, Dichgans J. A defect of kinesthesia in Parkinson’s disease. Mov Disord. 1995;10:460–465. doi: 10.1002/mds.870100410. [DOI] [PubMed] [Google Scholar]
- 19.Koller WC, Glatt S, Vetere-Overfield B, Hassanein R. Falls and Parkinson’s disease. Clin Neuropharmacol. 1989;12:98–105. doi: 10.1097/00002826-198904000-00003. [DOI] [PubMed] [Google Scholar]
- 20.Konczak J, Corcos DM, Horak F, Poizner H, Shapiro M, Tuite P, Volkmann J, Maschke M. Proprioception and motor control in Parkinson’s disease. J Mot Behav. 2009;41:543–552. doi: 10.3200/35-09-002. [DOI] [PubMed] [Google Scholar]
- 21.Konczak J, Krawczewski K, Tuite P, Maschke M. The perception of passive motion in Parkinson’s disease. J Neurol. 2007;254:655–663. doi: 10.1007/s00415-006-0426-2. [DOI] [PubMed] [Google Scholar]
- 22.Kumar A, Mann S, Sossi V, Ruth TJ, Stoessl AJ, Schulzer M, Lee CS. [11C]DTBZ-PET correlates of levodopa responses in asymmetric Parkinson’s disease. Brain. 2003;126:2648–2655. doi: 10.1093/brain/awg270. [DOI] [PubMed] [Google Scholar]
- 23.Lee AC, Harris JP, Atkinson EA, Fowler MS. Disruption of estimation of body-scaled aperture width in Hemiparkinson’s disease. Neuropsychologia. 2001;39:1097–1104. doi: 10.1016/s0028-3932(01)00032-x. [DOI] [PubMed] [Google Scholar]
- 24.Maschke M, Gomez CM, Tuite PJ, Konczak J. Dysfunction of the basal ganglia, but not the cerebellum, impairs kinaesthesia. Brain. 2003;126:2312–2322. doi: 10.1093/brain/awg230. [DOI] [PubMed] [Google Scholar]
- 25.Maschke M, Tuite PJ, Krawczewski K, Pickett K, Konczak J. Perception of heaviness in Parkinson’s disease. Mov Disord. 2006;21:1013–1018. doi: 10.1002/mds.20876. [DOI] [PubMed] [Google Scholar]
- 26.Massion J. Postural control system. Curr Opin Neurobiol. 1994;4:877–887. doi: 10.1016/0959-4388(94)90137-6. [DOI] [PubMed] [Google Scholar]
- 27.Murray MP, Sepic SB, Gardner GM, Downs WJ. Walking patterns of men with parkinsonism. Am J Phys Med. 1978;57:278–294. [PubMed] [Google Scholar]
- 28.Muslimovic D, Post B, Speelman JD, Schmand B, de Haan RJ. Determinants of disability and quality of life in mild to moderate Parkinson disease. Neurology. 2008;70:2241–2247. doi: 10.1212/01.wnl.0000313835.33830.80. [DOI] [PubMed] [Google Scholar]
- 29.O’Suilleabhain P, Bullard J, Dewey RB. Proprioception in Parkinson’s disease is acutely depressed by dopaminergic medications. J Neurol Neurosurg Psychiatry. 2001;71:607–610. doi: 10.1136/jnnp.71.5.607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rickards C, Cody FW. Proprioceptive control of wrist movements in Parkinson’s disease. Reduced muscle vibration-induced errors. Brain. 1997;120 (Pt 6):977–990. doi: 10.1093/brain/120.6.977. [DOI] [PubMed] [Google Scholar]
- 31.Schneider JS, Diamond SG, Markham CH. Parkinson’s disease: sensory and motor problems in arms and hands. Neurology. 1987;37:951–956. doi: 10.1212/wnl.37.6.951. [DOI] [PubMed] [Google Scholar]
- 32.Starkstein S, Leiguarda R, Gershanik O, Berthier M. Neuropsychological disturbances in hemiparkinson’s disease. Neurology. 1987;37:1762–1764. doi: 10.1212/wnl.37.11.1762. [DOI] [PubMed] [Google Scholar]
- 33.Takakusaki K, Oohinata-Sugimoto J, Saitoh K, Habaguchi T. Role of basal ganglia-brainstem systems in the control of postural muscle tone and locomotion. Prog Brain Res. 2004;143:231–237. doi: 10.1016/S0079-6123(03)43023-9. [DOI] [PubMed] [Google Scholar]
- 34.Takakusaki K, Tomita N, Yano M. Substrates for normal gait and pathophysiology of gait disturbances with respect to the basal ganglia dysfunction. J Neurol. 2008;255(Suppl 4):19–29. doi: 10.1007/s00415-008-4004-7. [DOI] [PubMed] [Google Scholar]
- 35.Van Emmerik RE, Wagenaar RC, Winogrodzka A, Wolters EC. Identification of axial rigidity during locomotion in Parkinson disease. Arch Phys Med Rehabil. 1999;80:186–191. doi: 10.1016/s0003-9993(99)90119-3. [DOI] [PubMed] [Google Scholar]
- 36.Vaugoyeau M, Viallet F, Aurenty R, Assaiante C, Mesure S, Massion J. Axial rotation in Parkinson’s disease. J Neurol Neurosurg Psychiatry. 2006;77:815–821. doi: 10.1136/jnnp.2004.050666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vaugoyeau M, Viallet F, Mesure S, Massion J. Coordination of axial rotation and step execution: deficits in Parkinson’s disease. Gait Posture. 2003;18:150–157. doi: 10.1016/s0966-6362(03)00034-1. [DOI] [PubMed] [Google Scholar]
- 38.Wright WG, Gurfinkel V, King L, Horak F. Parkinson’s disease shows perceptuomotor asymmetry unrelated to motor symptoms. Neurosci Lett. 2007a;417:10–15. doi: 10.1016/j.neulet.2007.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wright WG, Gurfinkel VS, Nutt J, Horak FB, Cordo PJ. Axial hypertonicity in Parkinson’s disease: direct measurements of trunk and hip torque. Exp Neurol. 2007b;208:38–46. doi: 10.1016/j.expneurol.2007.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zetusky WJ, Jankovic J, Pirozzolo FJ. The heterogeneity of Parkinson’s disease: clinical and prognostic implications. Neurology. 1985;35:522–526. doi: 10.1212/wnl.35.4.522. [DOI] [PubMed] [Google Scholar]
- 41.Zia S, Cody F, O’Boyle D. Joint position sense is impaired by Parkinson’s disease. Ann Neurol. 2000;47:218–228. [PubMed] [Google Scholar]
- 42.Zia S, Cody FW, O’Boyle DJ. Identification of unilateral elbow-joint position is impaired by Parkinson’s disease. Clin Anat. 2002;15:23–31. doi: 10.1002/ca.1087. [DOI] [PubMed] [Google Scholar]