Abstract
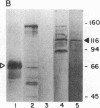
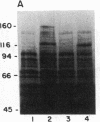
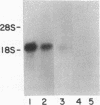
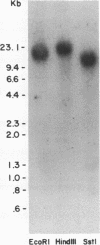
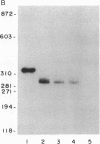
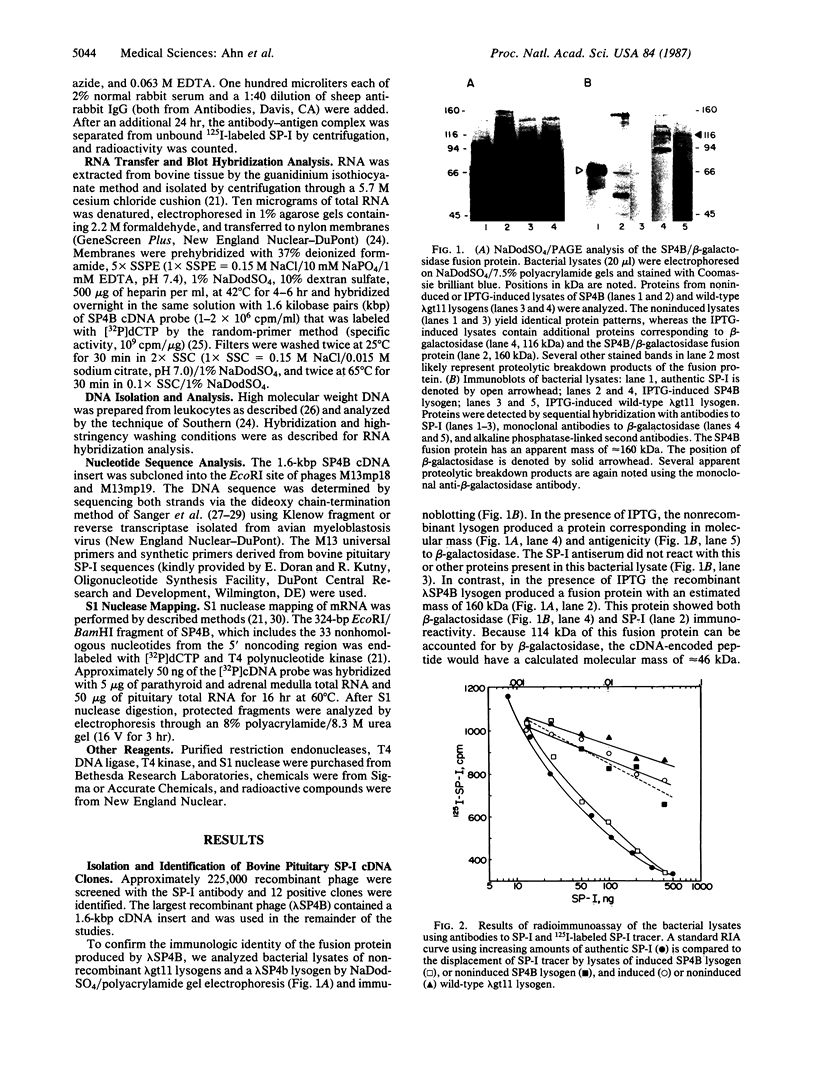
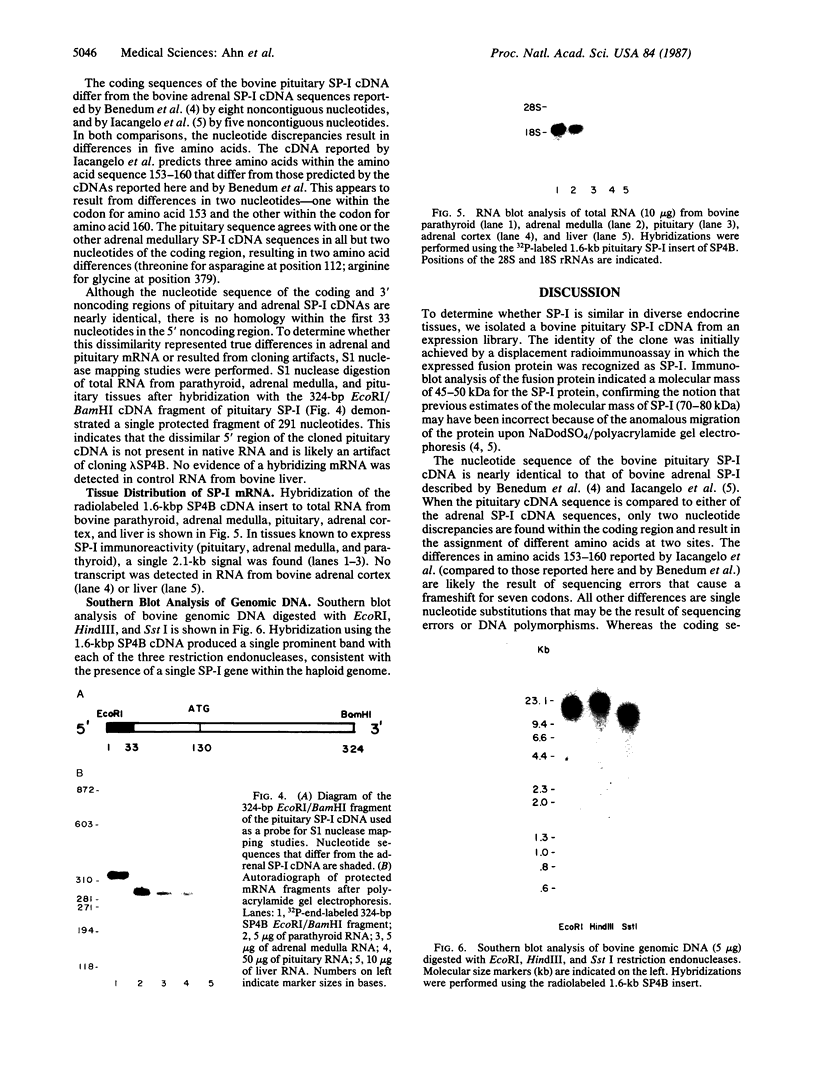
Secretory protein I (SP-I), also referred to as chromogranin A, is an acidic glycoprotein that has been found in every tissue of endocrine and neuroendocrine origin examined but never in exocrine or epithelial cells. Its co-storage and co-secretion with peptide hormones and neurotransmitters suggest that it has an important endocrine or secretory function. We have isolated cDNA clones from a bovine pituitary lambda gt11 expression library using an antiserum to parathyroid SP-I. The largest clone (SP4B) (approximately equal to 1.6 kilobases) hybridized to a transcript of 2.1 kilobases in RNA from parathyroid, pituitary, and adrenal medulla. Immunoblots of bacterial lysates derived from SP4B lysogens demonstrated specific antibody binding to an SP4B/beta-galactosidase fusion protein (160 kDa) with a cDNA-derived component of 46 kDa. Radioimmunoassay of the bacterial lysates with SP-I antiserum yielded parallel displacement curves of 125I-labeled SP-I by the SP4B lysate and authentic SP-I. SP4B contains a cDNA of 1614 nucleotides that encodes a 449-amino acid protein (calculated mass, 50 kDa). The nucleotide sequences of the pituitary SP-I cDNA and adrenal medullary SP-I cDNAs are nearly identical. Analysis of genomic DNA suggests that pituitary, adrenal, and parathyroid SP-I are products of the same gene.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams J. M., Gough N. M., Webb E. A., Tyler B. M., Jackson J., Cory S. Molecular cloning of mouse immunoglobulin heavy chain messenger ribonucleic acids coding for mu, alpha, gamma 1, gamma 2a, and gamma 3 chains. Biochemistry. 1980 Jun 10;19(12):2711–2719. doi: 10.1021/bi00553a027. [DOI] [PubMed] [Google Scholar]
- Ahn T. G., Antonarakis S. E., Kronenberg H. M., Igarashi T., Levine M. A. Familial isolated hypoparathyroidism: a molecular genetic analysis of 8 families with 23 affected persons. Medicine (Baltimore) 1986 Mar;65(2):73–81. [PubMed] [Google Scholar]
- Benedum U. M., Baeuerle P. A., Konecki D. S., Frank R., Powell J., Mallet J., Huttner W. B. The primary structure of bovine chromogranin A: a representative of a class of acidic secretory proteins common to a variety of peptidergic cells. EMBO J. 1986 Jul;5(7):1495–1502. doi: 10.1002/j.1460-2075.1986.tb04388.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berk A. J., Sharp P. A. Sizing and mapping of early adenovirus mRNAs by gel electrophoresis of S1 endonuclease-digested hybrids. Cell. 1977 Nov;12(3):721–732. doi: 10.1016/0092-8674(77)90272-0. [DOI] [PubMed] [Google Scholar]
- Biggin M. D., Gibson T. J., Hong G. F. Buffer gradient gels and 35S label as an aid to rapid DNA sequence determination. Proc Natl Acad Sci U S A. 1983 Jul;80(13):3963–3965. doi: 10.1073/pnas.80.13.3963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohn D. V., Elting J. J., Frick M., Elde R. Selective localization of the parathyroid secretory protein-I/adrenal medulla chromogranin A protein family in a wide variety of endocrine cells of the rat. Endocrinology. 1984 Jun;114(6):1963–1974. doi: 10.1210/endo-114-6-1963. [DOI] [PubMed] [Google Scholar]
- Cohn D. V., Kumarasamy R., Ramp W. K. Intracellular processing and secretion of parathyroid gland proteins. Vitam Horm. 1986;43:283–316. doi: 10.1016/s0083-6729(08)60423-9. [DOI] [PubMed] [Google Scholar]
- Cohn D. V., MacGregor R. R. The biosynthesis, intracellular processing, and secretion of parathormone. Endocr Rev. 1981 Winter;2(1):1–26. doi: 10.1210/edrv-2-1-1. [DOI] [PubMed] [Google Scholar]
- Cohn D. V., Morrissey J. J., Hamilton J. W., Shofstall R. E., Smardo F. L., Chu L. L. Isolation and partial characterization of secretory protein I from bovine parathyroid glands. Biochemistry. 1981 Jul 7;20(14):4135–4140. doi: 10.1021/bi00517a029. [DOI] [PubMed] [Google Scholar]
- Cohn D. V., Zangerle R., Fischer-Colbrie R., Chu L. L., Elting J. J., Hamilton J. W., Winkler H. Similarity of secretory protein I from parathyroid gland to chromogranin A from adrenal medulla. Proc Natl Acad Sci U S A. 1982 Oct;79(19):6056–6059. doi: 10.1073/pnas.79.19.6056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deftos L. J., Murray S. S., Burton D. W., Parmer R. J., O'Connor D. T., Delegeane A. M., Mellon P. L. A cloned chromogranin A (CgA) cDNA detects a 2.3Kb mRNA in diverse neuroendocrine tissues. Biochem Biophys Res Commun. 1986 May 29;137(1):418–423. doi: 10.1016/0006-291x(86)91226-x. [DOI] [PubMed] [Google Scholar]
- Derynck R., Content J., DeClercq E., Volckaert G., Tavernier J., Devos R., Fiers W. Isolation and structure of a human fibroblast interferon gene. Nature. 1980 Jun 19;285(5766):542–547. doi: 10.1038/285542a0. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. "A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity". Addendum. Anal Biochem. 1984 Feb;137(1):266–267. doi: 10.1016/0003-2697(84)90381-6. [DOI] [PubMed] [Google Scholar]
- Hamilton J. W., Chu L. L., Rouse J. B., Reddig K., MacGregor R. R. Structural characterization of adrenal chromogranin A and parathyroid secretory protein-I as homologs. Arch Biochem Biophys. 1986 Jan;244(1):16–26. doi: 10.1016/0003-9861(86)90089-5. [DOI] [PubMed] [Google Scholar]
- Hogue-Angeletti R. A. Nonidentity of chromogranin A and dopamine beta-monooxygenase. Arch Biochem Biophys. 1977 Dec;184(2):364–372. doi: 10.1016/0003-9861(77)90363-0. [DOI] [PubMed] [Google Scholar]
- Iacangelo A., Affolter H. U., Eiden L. E., Herbert E., Grimes M. Bovine chromogranin A sequence and distribution of its messenger RNA in endocrine tissues. Nature. 1986 Sep 4;323(6083):82–86. doi: 10.1038/323082a0. [DOI] [PubMed] [Google Scholar]
- Kruggel W., O'Connor D. T., Lewis R. V. The amino terminal sequences of bovine and human chromogranin A and secretory protein I are identical. Biochem Biophys Res Commun. 1985 Feb 28;127(1):380–383. doi: 10.1016/s0006-291x(85)80170-4. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Majzoub J. A., Dee P. C., Habener J. F. Cellular and cell-free processing of parathyroid secretory proteins. J Biol Chem. 1982 Apr 10;257(7):3581–3588. [PubMed] [Google Scholar]
- O'Connor D. T., Burton D., Deftos L. J. Chromogranin A: immunohistology reveals its universal occurrence in normal polypeptide hormone producing endocrine glands. Life Sci. 1983 Oct 24;33(17):1657–1663. doi: 10.1016/0024-3205(83)90721-x. [DOI] [PubMed] [Google Scholar]
- O'Connor D. T. Chromogranin: widespread immunoreactivity in polypeptide hormone producing tissues and in serum. Regul Pept. 1983 Jul;6(3):263–280. doi: 10.1016/0167-0115(83)90145-3. [DOI] [PubMed] [Google Scholar]
- O'Connor D. T., Frigon R. P. Chromogranin A, the major catecholamine storage vesicle soluble protein. Multiple size forms, subcellular storage, and regional distribution in chromaffin and nervous tissue elucidated by radioimmunoassay. J Biol Chem. 1984 Mar 10;259(5):3237–3247. [PubMed] [Google Scholar]
- Rosa P., Hille A., Lee R. W., Zanini A., De Camilli P., Huttner W. B. Secretogranins I and II: two tyrosine-sulfated secretory proteins common to a variety of cells secreting peptides by the regulated pathway. J Cell Biol. 1985 Nov;101(5 Pt 1):1999–2011. doi: 10.1083/jcb.101.5.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sage H. J., Smith W. J., Kirshner N. Mechanism of secretion from the adrenal medulla. I. A microquantitative immunologic assay for bovine adrenal catecholamine storage vesicle protein and its application to studies of the secretory process. Mol Pharmacol. 1967 Jan;3(1):81–89. [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serck-Hanssen G., O'Connor D. T. Immunological identification and characterization of chromogranins coded by poly(A) mRNA from bovine adrenal medulla and pituitary gland and human phaeochromocytoma. J Biol Chem. 1984 Sep 25;259(18):11597–11600. [PubMed] [Google Scholar]
- Somogyi P., Hodgson A. J., DePotter R. W., Fischer-Colbrie R., Schober M., Winkler H., Chubb I. W. Chromogranin immunoreactivity in the central nervous system. Immunochemical characterisation, distribution and relationship to catecholamine and enkephalin pathways. Brain Res. 1984 Dec;320(2-3):193–230. doi: 10.1016/0165-0173(84)90007-9. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Weaver C. A., Gordon D. F., Kemper B. Introduction by molecular cloning of artifactual inverted sequences at the 5' terminus of the sense strand of bovine parathyroid hormone cDNA. Proc Natl Acad Sci U S A. 1981 Jul;78(7):4073–4077. doi: 10.1073/pnas.78.7.4073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson B. S., Lloyd R. V. Detection of chromogranin in neuroendocrine cells with a monoclonal antibody. Am J Pathol. 1984 Jun;115(3):458–468. [PMC free article] [PubMed] [Google Scholar]
- Winkler H. The biogenesis of adrenal chromaffin granules. Neuroscience. 1977;2(5):657–683. doi: 10.1016/0306-4522(77)90022-7. [DOI] [PubMed] [Google Scholar]
- Young R. A., Davis R. W. Efficient isolation of genes by using antibody probes. Proc Natl Acad Sci U S A. 1983 Mar;80(5):1194–1198. doi: 10.1073/pnas.80.5.1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young R. A., Davis R. W. Yeast RNA polymerase II genes: isolation with antibody probes. Science. 1983 Nov 18;222(4625):778–782. doi: 10.1126/science.6356359. [DOI] [PubMed] [Google Scholar]