Abstract
The pharyngeal constriction ratio (PCR), derived directly from videofluoroscopy without the need for manometry, requires validation as a surrogate for pharyngeal strength. A correlation of −0.70 was previously identified between PCR and pharyngeal clearing pressures (PP) on separate fluoroscopic and manometric studies. As PP increases, PCR decreases. The objective of the current study was to evaluate the correlation between PCR and PP in 25 patients undergoing simultaneous fluoroscopy and pharyngeal manometry. The effect of the manometric catheter on PCR was also investigated. The correlation between the PCR and averaged pharyngeal clearing pressures was −0.72 (p < 0.001). All patients with a PCR > 0.25 had a PP < 60 mmHg. PCR did not differ significantly as a consequence of the manometric catheter. Results suggest the utility of an objective fluoroscopic measure in assessing pharyngeal strength when manometry may not be available or possible.
Keywords: Pharyngeal constriction, Pharyngeal pressure, Deglutition, Deglutition disorders
During deglutition, the oropharynx and hypopharynx must first expand to accommodate bolus material, then compress and shorten to direct bolus material through the hypopharynx and into the esophagus. If pharyngeal constriction is incomplete or untimely, resultant poor bolus transit and residue may threaten safe and effective swallowing.
Pharyngeal manometry is the current criterion standard for assessing pharyngeal contractility [1, 2]. Solid-state sensors placed in the pharynx, hypopharynx, and at the level of the upper esophageal sphincter (UES) provide important information regarding pharyngeal pressure, UES resting pressure and relaxation, and pharyngeal-UES coordination during swallowing. These measures are frequently considered in predicting the success of a cricopharyngeal myotomy. For example, if pharyngeal pressures are low, indicating poor pharyngeal strength, increasing UES opening size is not likely to significantly improve swallow function. Conversely, if UES opening is reduced and pharyngeal pressures are good, myotomy, Botox, or dilation may be beneficial [3, 4]. Unfortunately, manometry is invasive and available only at certain referral centers.
Another means of assessing pharyngeal constriction is with fluoroscopic imaging studies, which are routinely used in the evaluation of dysphagic patients [5, 6]. From the lateral view, it is possible to observe the pharyngeal air space prior to swallowing and to note changes in this space during the swallow. Filming of the patient in the anterior–posterior view provides additional information regarding the symmetry of pharyngeal constriction. One limitation of fluoroscopy is the inability to quantify the three-dimensional pharyngeal volume as it changes from a relaxed position to its point of maximum constriction during a swallow.
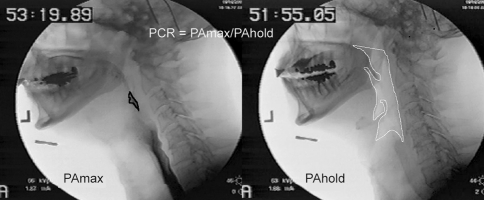
We have developed an objective surrogate measure of pharyngeal constriction that can be readily calculated from a routine fluoroscopic swallow evaluation (modified barium swallow). The pharyngeal constriction ratio (PCR) is the pharyngeal area (including residual bolus material) visible in the lateral radiographic view at the point of maximum pharyngeal constriction during swallow (PAmax) divided by the area with a 1-cc bolus held in the oral cavity (PAhold) [7]. PAmax for any size or consistency swallow is referenced to PAhold for a 1-cc bolus. Normative data for this measure have been previously defined for a large group of subjects less than 65 years of age and for a group of elderly normal subjects [8]. In younger normal subjects, PCR approximates “0” (0.03-0.04) for a 20-cc liquid barium bolus; in elderly subjects, the PCR is higher, on the order of 0.13–0.14 for a 20-cc bolus (Table 1). Across gender and age, the mean PCR is 0.07 (±0.09). Clinically, a PCR of 0.25 in any adult would be considered abnormally high (greater than 2 standard deviations from the mean).
Table 1.
Mean PCR measured on a 20-cc liquid bolus
| Mean (±SD) PCR | p value | ||
|---|---|---|---|
| Males | Females | ||
| Age < 65 years (n = 63) | 0.04 (±.03) | 0.03 (±.03) | Ns |
| Age > 65 years (n = 88) | 0.12 (±.11) | 0.10 (±.11) | Ns |
| Combined normal subjects (N = 151) | 0.07 (±.09) | ||
| 95% CI = 0.059–0.089 | |||
ns not significant
An elevated PCR reflects reduced pharyngeal constriction and poorer pharyngeal clearing during the swallow, i.e., as pharyngeal constriction diminishes, the PCR increases. The PCR has been useful in assessing pharyngeal function in patients of differing age and gender with dysphagia secondary to a diverse assortment of disorders [8–10]. The PCR has also been utilized to monitor changes in pharyngeal function over time and after treatment, and has been found to be a strong predictor of aspiration in selected patient populations [11]. We previously reported a strong negative correlation (−0.70) between PCR and pharyngeal clearing pressures (PP) in patients undergoing manometric and fluoroscopic studies at different time points [12]. The objective of the current study was to assess the relationship between PCR and PP in patients undergoing simultaneous fluoroscopy and pharyngeal manometry, which has not been previously investigated. A further goal was to assess possible differences in PCR as a consequence of the manometry catheter.
Materials and Methods
Twenty-five consecutive individuals undergoing simultaneous fluoroscopic swallow evaluation and pharyngeal manometry at the Center for Voice and Swallowing of UC Davis were included in this study. This sample size was determined to provide appropriate power (0.80) based on a previous report of a correlation of −0.70 for nonsimultaneous PCR and PP measures. The mean age of the cohort was 60 (±17) years. Sixty-four percent (16/25) was male. The most common diagnoses were reflux (based on 24-h pH probe testing) and esophageal motility disorder (based on esophagram) (Table 2). Permission to conduct this study was obtained from the Institutional Review Board at UC Davis. Information regarding patient demographics, diagnoses, PCR, and manometric pharyngeal peak pressures was abstracted. These data are presented in Table 2.
Table 2.
Details of study patients, PCRs, and PPs
| S# | Medical history | Gender | Age | Complaint | Pharyngeal pressure (mmHg) | Pharyngeal constriction ratio (PCR) |
|---|---|---|---|---|---|---|
| 1 | Inhalation injury (burn) | M | 54 | Dysphagia for liquids and solids | 217.75 (wnl) | 0.03 (wnl) |
| 2 | GERD, vocal fold paralysis | M | 71 | Solid food sticking | 76.95 (wnl) | 0.04 (wnl) |
| 3 | Intubation injury, CVA | M | 71 | Solid food dysphagia, aspiration | 131.50 (wnl) | 0.22 (wnl) |
| 4 | GERD | F | 49 | Difficulty with pills | 77.50 (wnl) | 0.15 (wnl) |
| 5 | EMD, Barrett’s | M | 76 | J-tube dependent | 20.05 (low) | 0.82 (high) |
| 6 | GERD/FBS | F | 80 | Food sticking | 30 (low) | 0.89 (high) |
| 7 | GERD, FBS | F | 68 | Solid food dysphagia | 111.65 (wnl) | 0.03 (wnl) |
| 8 | Traumatic brain injury | M | 37 | FBS | 100.70 (wnl) | 0.02 (wnl) |
| 9 | HNCa | M | 82 | G-tube dependent | 48.80 (low) | 0.63 (high) |
| 10 | GERD | M | 51 | Solid food dysphagia | 190.80 (wnl) | 0.02 (wnl) |
| 11 | EMD | M | 76 | G-tube dependent | 35 (low) | 0.46 (high) |
| 12 | GERD | M | 29 | Solid food dysphagia | 74 (wnl) | 0.02 (wnl) |
| 13 | Vagal Schwannoma | F | 60 | Dysphagia for liquid and solids | 67 (wnl) | 0.12 (wnl) |
| 14 | CVA | F | 44 | Food sticking | 110.80 (wnl) | 0.03 (wnl) |
| 15 | GERD | M | 71 | Food sticking, coughing with meals | 29.90 (low) | 0.42 (high) |
| 16 | GERD | F | 80 | Food sticking | 31.5 (low) | 0.83 (high) |
| 17 | Zenker’s diverticulum | F | 85 | Food sticking, solid food dysphagia | 78 (wnl) | 0.24 (wnl) |
| 18 | EMD, neuromuscular | M | 71 | Food sticking, coughing with meals | 18 (low) | 0.49 (high)) |
| 19 | Head/neck penetrating injury | M | 53 | Food sticking, throat clearing with liquids | 40 (low) | 0.35 (high) |
| 20 | Thyroid ca | F | 29 | Coughing with meals/sticking food | 119.9 (wnl) | 0.01 (wnl) |
| 21 | Esophageal | M | 66 | Solid food dysphagia | 107.6 (wnl) | 0.05 (wnl) |
| 22 | EMD, neuromuscular | M | 78 | Solid food dysphagia | 48.70 (low) | 0.67 (high) |
| 23 | CVA | M | 41 | G-tube dependent | 58 (low) | 0.58 (high) |
| 24 | CVA | M | 42 | G-tube dependent | 43 (low) | 0.45 (high) |
| 25 | TBI | F | 53 | Recurrent pneumonia | 83 (wnl) | 0.06 (wnl) |
CVA cerebrovascular accident, HnCa head and neck cancer, FBS foreign body sensation, GERD gastroesophageal reflux disease, Lung Ca lung cancer, TBI traumatic brain injury, Thyroid Ca thyroid cancer, EMD esophageal motility disorder; PP and PCR are described as normal (wnl within normal limits) or abnormal (PP low, PCR high)
All radiographic studies were conducted at UC Davis in accordance with the routine radiographic protocols approved by the institution. Equipment used included a properly collimated OEC Medical Systems 9800 Radiographic/Fluoroscopic unit that provided a 63-kV, 1.2-mA type output for the full field-of-view mode (12-in. input phosphor diameter). In accordance with our standard protocol, subjects swallowed 1- and 3-cc paste boluses and 20-cc liquid bolus (EZ-PAQUE Barium Sulfate Suspension, 60% w/v; 41% w/w, E-Z-EM, Inc., Westbury, NY) from a spoon or cup. Bolus size was carefully measured with a syringe or graduated medicine cup. A radiopaque disk of known diameter was placed on each subject’s midchin so that displacements of structures can be calculated in the lateral view. When the protocol and other tasks in lateral view were complete, the disk was moved to a position over the anterior edge of the posterior pharyngeal wall at the level of the UES for filming in the anterior–posterior view. In the current study, patients swallowed 1-, 3-, and 15-20-cc liquid boluses (largest bolus possible) with the manometry catheter in place. The catheter was then removed and the three swallows were repeated. Fluoroscopy studies were recorded digitally for later playback and analysis using a Sony MD-1000 DVD recorder (Sony Corp. of America, New York, NY). PCR and PP were calculated for the largest liquid bolus the patient could tolerate, typically 15-20 cc.
As noted, for all measures that require comparison of a structure’s position at two different points, our convention is to reference maximum displacement of the structure to its position with a 1-cc bolus held in the oral cavity. This baseline position, representing the denominator of PCR, is referred to as “hold.” In the current study, frames representing pharyngeal area with a 1-cc bolus held in the oral cavity and the point of maximum pharyngeal constriction during swallowing of the 20-cc liquid bolus, respectively, were captured using WinDVD 7 (Corel, Ottawa, ON, Canada), a software program that permits playback of digitally recorded materials. Using software designed for measurement purposes (Image J, National Institute of Mental Health, Bethesda, MD), the unobliterated pharyngeal space, as evidenced by residual air space and/or bolus material present in the pharynx, was traced for both images and the area of each was calculated. The measures are illustrated in Fig. 1.
Fig. 1.
PCR is calculated by dividing pharyngeal area at point of maximum constriction during a swallow by the area with a 1-cc bolus held in the oral cavity
Pharyngeal manometry and UES manometry were performed using a Koenigsberg 9-channel probe (Sandhill EFT catheter; Sandhill Scientific Inc., Highlands Ranch, CO). The 4.5-mm-diameter catheter has two circumferential solid-state pressure sensors at 5 and 10 cm from the tip and three unidirectional pressure sensors at 15, 20, and 25 cm. The catheter was inserted transnasally into the esophagus just below the UES. Baseline esophageal and pharyngeal pressures were then established. The UES pressure was determined by a 0.5-cm station pull-through technique. The distal circumferential sensor was placed with fluoroscopic guidance just proximal to the high-pressure zone of the UES. This positioned the pharyngeal sensor approximately 5 cm above the UES. As noted, pharyngeal–UES pressures were recorded during successive swallows of 1-, 3-, and 20-cc (or largest bolus tolerated) liquid boluses. An average pharyngeal peak pressure was calculated for all study participants for the largest bolus swallowed. The normal range of peak pressures reported for the instrumentation used (Sandhill EFT catheter) is 60–192 mmHg. For purposes of this study, pressures below 60 mmHg were considered below the normal range.
All data were abstracted and coded into SPSS 11.0 for Macintosh (SPSS, Inc., Chicago, IL). The Pearson product–moment correlation coefficient was used to evaluate the correlation between the PCR and the average pharyngeal pressure on manometry. Differences in PCR and PP with and without the manometry catheter in place were assessed with a paired-samples t test for dependent means (p < 0.05).
Results
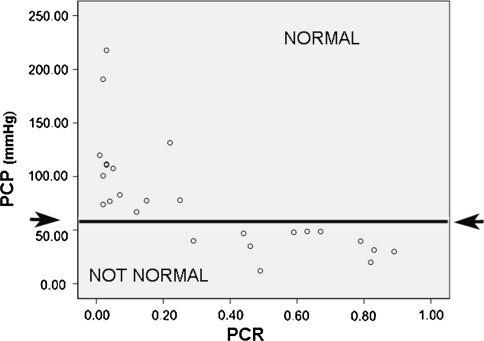
The mean PCR for the 25 subjects was 0.32 ± 0.31. The mean pharyngeal pressure (PP) was 78 ± 50 mmHg. The correlation between the PCR and PP was −0.716 (p < 0.000) (Table 3). Data for PCR and PP are presented for each subject in Table 2 and Fig. 2.
Table 3.
Summary of Pearson product-moment correlation
| Pharyngeal pressure | PCR | |
|---|---|---|
| Pharyngeal pressure | 1.000 | −0.716a |
| Pearson correlation significance (2-tailed) N | 0.000 | |
| 25 | 25 | |
| PCR | −0.716a | 1.000 |
| Pearson correlation significance (2-tailed) N | 0.000 | |
| 25 | 2 |
aCorrelation is significant at the 0.000 level (2-tailed)
Fig. 2.
Pharyngeal clearing pressure (PP) is represented on the vertical axis and pharyngeal constriction values (PCR) are displayed on the horizontal axis. All patients with low PP (<60 mmHg) had elevated PCRs (>0.25). All patients with normal pressures also had normal PCRs
Each patient’s PCR was also inspected with respect to the normative data previously reported and presented here in Table 1. In this analysis, PCRs were considered for patients with PP > 60 mmHg (normal) and for patients with PP < 60 mmHg (abnormal). No patient with PP < 60 mmHg had a PCR that fell within the normal range (mean ± 1 SD) for his/her age and gender. Of the 14 patients with PP > 60 mmHg, only two had a PCR that was outside the normal range (mean ± 1 SD) for age and gender. These patients had a PP of 77 mmHg and a PCR of 0.15 and a PP of 67 mmHg and a PCR of 0.12, respectively. Combined across age and gender, normal PCR for a 20-cc bolus is 0.07 ± 0.09 (Table 1). A PCR of 0.25 (mean + 2 SD) is considered likely to interfere with pharyngeal clearing in any individual. It is of interest that in the current study no patient with PCR > 0.25 had normal PPs, and no patient with PCR < 0.25 had abnormal PPs.
A second objective of the study was to evaluate the effects of the manometry catheter on the PCR measure. The mean PCR was 0.32 ± 0.32 with the manometry catheter in place and 0.33 ± 0.29 when measured with no catheter. A t test comparing the two means revealed that this difference was not significant.
Discussion
This investigation revealed a strong inverse correlation between the PCR and pharyngeal clearing pressure on manometry. As PP decreases, the PCR increases. These findings support PCR as a valid surrogate measure of pharyngeal constriction. In fact, all individuals with abnormal pharyngeal pressure on manometry (<60 mmHg) demonstrated a PCR > 0.25. When considered with respect to normative data, the relationship between PCR and PP would seem to be of particular value.
Tongue-base (oropharyngeal) and hypopharyngeal pressures were averaged in this study for purposes of comparison to PCR. In two patients, discrepancies in oropharyngeal and hypopharyngeal pressures were observed, one within normal limits (>60 mmHg) and the other abnormal (<60 mmHg). Fluoroscopic observations for these patients were consistent with the pressure data. For example, when good oropharyngeal but poor hypopharyngeal constriction was observed, manometric pressures were normal and abnormal, respectively, at the tongue base and just above the esophagus. In the current study, the averaged pressure values in both patients were low and PCR values were high. It should be recognized, however, that even with an elevated PCR, pressure generation in the pharynx may differ according to site.
As noted previously, manometric studies are useful for evaluating patients who may be candidates for cricopharyngeal myotomy or other procedures designed to improve UES opening. However, manometry is not used in every clinical setting. Fluoroscopy is widely available, and PCR can be obtained using equipment that is standard in most clinics or hospitals. When the UES opening is reduced and intervention with myotomy, Botox, or dilation is being considered, calculation of PCR may help predict the likelihood that swallowing will improve with treatment. Patients with a reduced UES opening and PCR ≤ 0.25, indicating adequate pharyngeal strength, may be excellent candidates for intervention. If the UES opening is severely obstructed, the pharynx may not be able to clear well even if pharyngeal strength is normal. In such cases, calculation of PCR on a smaller bolus size may be helpful. The UES opening varies directly with bolus volume so that smaller bolus sizes are likely to be managed more easily when the UES is moderately or severely obstructed. If PCR is within normal limits on a smaller bolus size in patients with an obstructed UES opening, this is an indication of good pharyngeal strength, again predicting a good outcome with UES intervention. If poor pharyngeal constriction is related to a weak pharynx, or to both reduced UES opening and pharyngeal weakness, observation across swallows of various bolus sizes may help elaborate the factors involved. Future investigation is necessary to confirm the predictive value of PCR in UES myotomy or other intervention. The additional use of PCR related to other treatment considerations, for example, Zenker’s diverticulum, has been previously reported [13, 14].
Conclusion
This study demonstrated a high inverse correlation (−0.72) between the PCR and peak pharyngeal pressure on manometry. As pharyngeal constriction diminishes, PCR increases. The PCR appears to be a valid objective surrogate measure of pharyngeal strength. PCR did not differ significantly with and without the manometry catheter in place, suggesting that the presence of the catheter does not affect this fluoroscopic measure. Limitations of the study include the small subject size, large normal range for manometric pressures, and, as yet, no outcome data based on the predictive capability of PCR. However, preliminary findings provide support for the potential utility of continued investigation of this measure.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Biographies
Rebecca Leonard
PhD
Catherine J. Rees
MD
Peter Belafsky
MD, PhD
Jacqui Allen
MD
References
- 1.Brasseur JG, Dodds WJ. Interpretation of intraluminal manometric measurements in terms of swallowing mechanics. Dysphagia. 1991;6:100–119. doi: 10.1007/BF02493487. [DOI] [PubMed] [Google Scholar]
- 2.Castell JA, Castell DO. Modern solid state computerized manometry of the pharyngoesophageal segment. Dysphagia. 1993;8:270–275. doi: 10.1007/BF01354550. [DOI] [PubMed] [Google Scholar]
- 3.Ali GN, Wallace KL, Laundl TM, Hunt DR, deCarle DJ, Cook IJ. Predictors of outcome following cricopharyngeal disruption for phayrngeal dysphagia. Dysphagia. 1997;12:133–139. doi: 10.1007/PL00009527. [DOI] [PubMed] [Google Scholar]
- 4.Kelly JH. Management of upper esophageal sphincter disorders: indications and complications of myotomy. Am J Med. 2000;108(Suppl 4a):43–46. doi: 10.1016/S0002-9343(99)00334-4. [DOI] [PubMed] [Google Scholar]
- 5.Logemann JA. Manual for the Videofluorographic Study of Swallowing. Boston, MA: College-Hill Press; 1986. [Google Scholar]
- 6.Leonard R, McKenzie S. Dynamic swallow study: instrumentation and measurement techniques. In: Leonard R, Kendall K, editors. Dysphagia assessment and treatment planning: a team approach. San Diego, CA: Singular Publishing Group, Inc; 2008. [Google Scholar]
- 7.Leonard RJ, Kendall KA, McKenzie S, Goncalves MI, Walker A. Structural displacements in normal swallowing: a videofluoroscopic study. Dysphagia. 2000;15:146–152. doi: 10.1007/s004550010017. [DOI] [PubMed] [Google Scholar]
- 8.Leonard R, Kendall KA, McKenzie S. Structural displacements affecting pharyngeal constriction in nondysphagic elderly and nonelderly adults. Dysphagia. 2004;19:133–141. doi: 10.1007/s00455-003-0508-6. [DOI] [PubMed] [Google Scholar]
- 9.Kendall KA, Leonard RJ. Pharyngeal constriction in elderly dysphagic patients compared with young and elderly nondysphagic controls. Dysphagia. 2001;16:272–278. doi: 10.1007/s00455-001-0086-4. [DOI] [PubMed] [Google Scholar]
- 10.Yip H, Leonard R, Belafsky PC. Can a fluoroscopic estimation of pharyngeal constriction predict aspiration? Otolaryngol Head Neck Surg. 2006;135:215–217. doi: 10.1016/j.otohns.2006.03.016. [DOI] [PubMed] [Google Scholar]
- 11.Leonard RJ, Kendall KA, Johnson R, McKenzie S. Swallowing in myotonic muscular dystrophy: a videofluoroscopic study. Arch Phys Med Rehabil. 2001;82:979–985. doi: 10.1053/apmr.2001.23962. [DOI] [PubMed] [Google Scholar]
- 12.Leonard RJ, Belafsky PC, Rees CJ. Relationship between fluoroscopic and manometric measures of pharyngeal constriction: the pharyngeal constriction ratio. Ann Otol Rhinol Laryngol. 2006;12:897–901. doi: 10.1177/000348940611501207. [DOI] [PubMed] [Google Scholar]
- 13.Leonard RJ, Belafsky PC, McKenzie S. Pharyngeal adaptation in Zenker’s diverticulum: the faux-pharyngoesophageal segment. Otolaryngol Head Neck Surg. 2008;139:424–428. doi: 10.1016/j.otohns.2008.06.009. [DOI] [PubMed] [Google Scholar]
- 14.Pollard R, Marks SL, Leonard R, Belafsky PC. Preliminary evaluation of the pharyngeal constriction ratio (PCR) for fluoroscopic determination of pharyngeal constriction in dysphagic dogs. Vet Radiol Ultrasound. 2007;48(3):221–226. doi: 10.1111/j.1740-8261.2007.00232.x. [DOI] [PubMed] [Google Scholar]