Dear Editor,
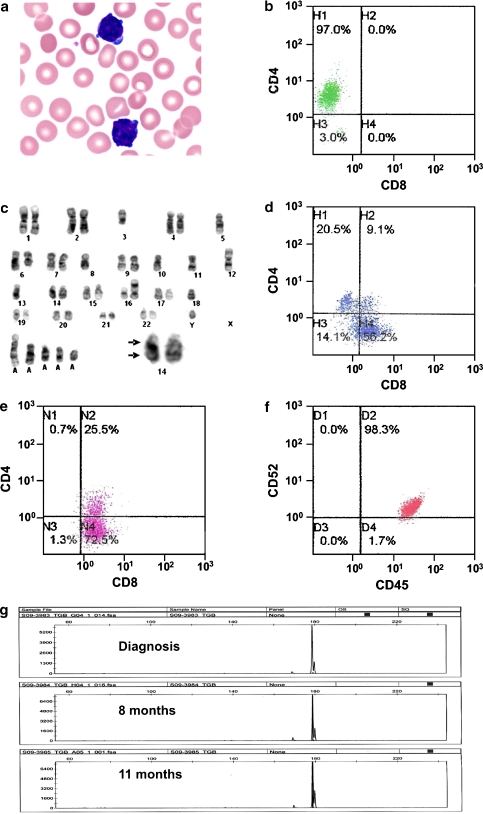
A 48-year-old man was incidentally found to have a lymphocytosis, with haemoglobin: 14.1 g/dL, white cell count: 16.6 × 109/L (77% abnormal lymphocytes), and platelet count: 160 × 109/L. The abnormal lymphocytes were small, with scanty basophilic cytoplasm showing frequent blebs (Fig. 1a). The nuclei contained condensed chromatin and inconspicuous nucleoli. They were predominantly positive for CD4 (Fig. 1b), and expressed CD2, CD5, CD7 and T cell receptor (TCR) αβ. Karyotyping showed inv(14)(q11q32) with multiple karyotypic aberrations (Fig. 1c). Serologic test for human T-lymphotropic virus 1 was negative. The diagnosis was consistent with T-prolymphocytic leukaemia (T-PLL) [1]. The patient elected not to receive treatment. Eight months afterwards, he presented with erythematous rashes, generalised lymphadenopathy and splenomegaly. A full blood count showed haemoglobin: 10.9 g/dL, white cell count: 132 × 109/L (95% abnormal lymphocytes), and platelet count: 139 × 109/L. Interestingly, the lymphocytes showed a diminution in CD4, but an increase in CD8 expression (CD4+CD8–:23.9%, CD4–CD8+: 65.5%, CD4+CD8+: 10.6%; Fig. 1d). Skin biopsy confirmed leukaemic infiltration. The patient was treated with alemtuzumab (30 mg/day thrice weekly) for 4 weeks without response. Two courses of fludarabine, mitoxantrone, dexamethasone [2] and resumption of alemtuzumab were ineffective. Immunophenotyping 11 months after diagnosis showed that the PLL cells were predominantly CD8 expressing (CD4–CD8+:73.5%, and CD4+CD8+:25.8%; Fig. 1e). Owing to the poor response to alemtuzumab, CD52 expression was investigated. The PLL cells had retained CD52 expression (Fig. 1f). To investigate if a new clone might have emerged, polymerase chain reaction for TCR gamma gene was performed. Identical peaks were demonstrated despite a change from CD4 to CD8 expression (Fig. 1g). Splenectomy was performed, followed by combination chemotherapy (dexamethasone, methotrexate, ifosphamide, etoposide, L-asparaginase). However, the patient died of sepsis 2 weeks afterwards.
Fig. 1.
Pathological features of a case of T-cell prolymphocytic leukemia (PLL). a Abnormal lymphoid cells at diagnosis, showing condensed nuclei with inconspicuous nucleoli, and scanty basophilic cytoplasm with frequent blebs (Wright–Giemsa stain, original magnification ×100). b Flow cytometric analysis at diagnosis, showing predominance of CD4+ PLL cells. c Karotyping analysis, showing inv(14)(q11q32) (arrow, insert) and other aberrations. d At 8 months, there was an increase in CD8+ PLL cells. e At 11 months, there was predominance of CD8+ PLL cells. f CD52 expression had been consistent through the clinical course. g T cell receptor gene gamma rearrangement studies, showing identical peaks at various time points in the clinical course (GeneMapper v3.7, Applied Biosystems)
This case showed a number of interesting features pertinent to the diagnosis of T-PLL. The usual morphological features of prolymphocytes, viz., small to medium cells with a single distinct nucleolus and abundant cytoplasm, were not observed. The neoplastic cells were instead mature looking. It has been proposed that a diagnosis of “mature T cell leukaemia, unclassified” might be offered to such cases [3]. However, had the case presented later when CD8 expression predominated, a diagnosis of T cell large granular lymphocyte leukaemia might have been entertained. These diagnostic difficulties were resolved with demonstration of inv(14), implying that this case should be classified as the small cell variant of T-PLL [1]. The immunophenotypical switch from CD4 to CD8 was also intriguing. Molecular analysis confirmed that the CD4 to CD8 switch occurred in the same clone, implying that the immunophenotypical switch was an intrinsic property of the T-PLL cells. This phenomenon appeared to be exceptionally rare; and to our knowledge, only one similar case had been reported, in which a change from CD4 to CD8 expression was associated with large cell transformation [4]. Interestingly, the CD8+ phenotype had also been reported to be associated with a poor response to conventional chemotherapy [5]. Such observations were recapitulated in our case, which showed a relentless deterioration with increasing CD8 expression.
Acknowledgments
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
References
- 1.Catovsky D, Muller-Hermelink HK, Ralfkiaer E. T-cell prolymphocytic leukemia. In: Swerdlow SH, Campo E, Harris NL, Jaffe ES, Pileri SA, Stein H, Thiele J, Vardiman JW, editors. WHO classification of tumours of haematopoietic and lymphoid tissues. Lyon: International Agency for Research on Cancer; 2008. pp. 270–271. [Google Scholar]
- 2.Ma SY, Au WY, Chim CS, et al. Fludarabine, mitoxantrone and dexamethasone in the treatment of indolent B- and T-cell lymphoid malignancies in Chinese patients. Br J Haematol. 2004;124:754–761. doi: 10.1111/j.1365-2141.2004.04852.x. [DOI] [PubMed] [Google Scholar]
- 3.Foucar K. Mature T-cell leukemias including T-prolymphocytic leukemia, adult T-cell leukemia/lymphoma, and Sezary syndrome. Am J Clin Pathol. 2007;127:496–510. doi: 10.1309/KWJYBCCGTB90B6AE. [DOI] [PubMed] [Google Scholar]
- 4.Kubota K, Tanaka H, Suda K, et al. Morphological and immunological change in the predominant type of leukaemic cells in a patient with T-cell chronic lymphocytic leukaemia. Scand J Haematol. 1986;36:439–443. doi: 10.1111/j.1600-0609.1986.tb02278.x. [DOI] [PubMed] [Google Scholar]
- 5.Matutes E, Brito-Babapulle V, Swansbury J, et al. Clinical and laboratory features of 78 cases of T-prolymphocytic leukemia. Blood. 1991;78:3269–3274. [PubMed] [Google Scholar]