Abstract
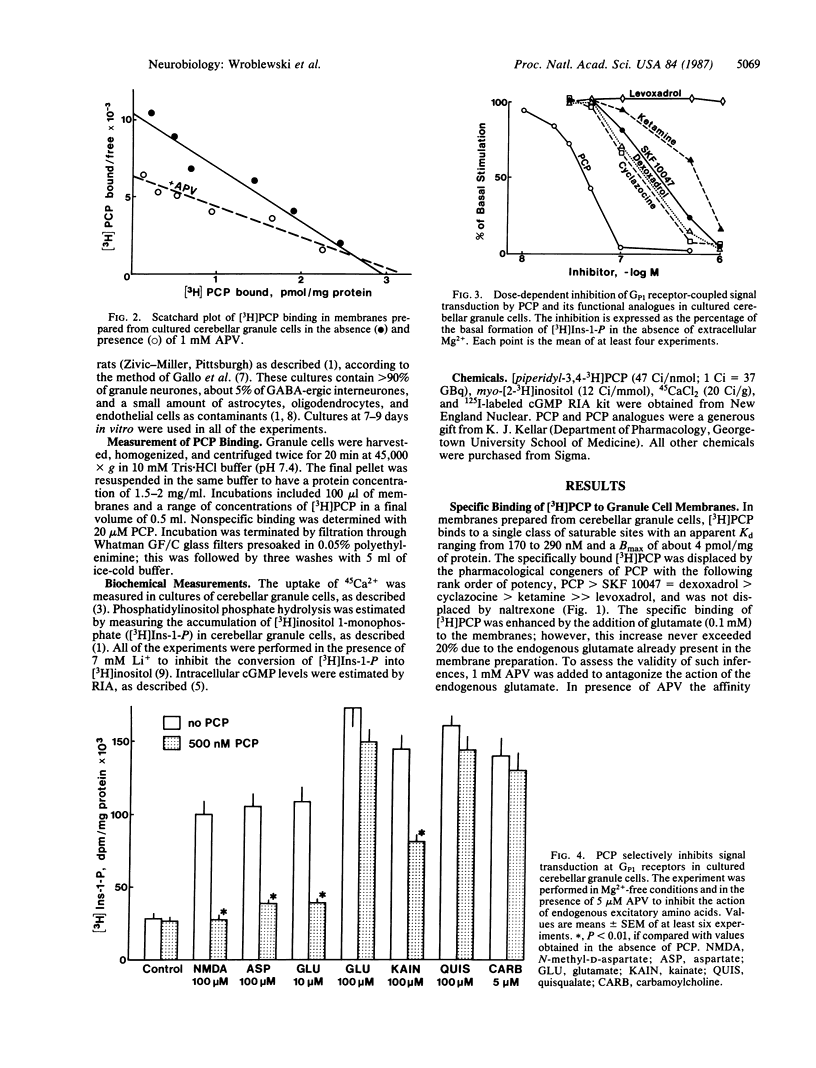
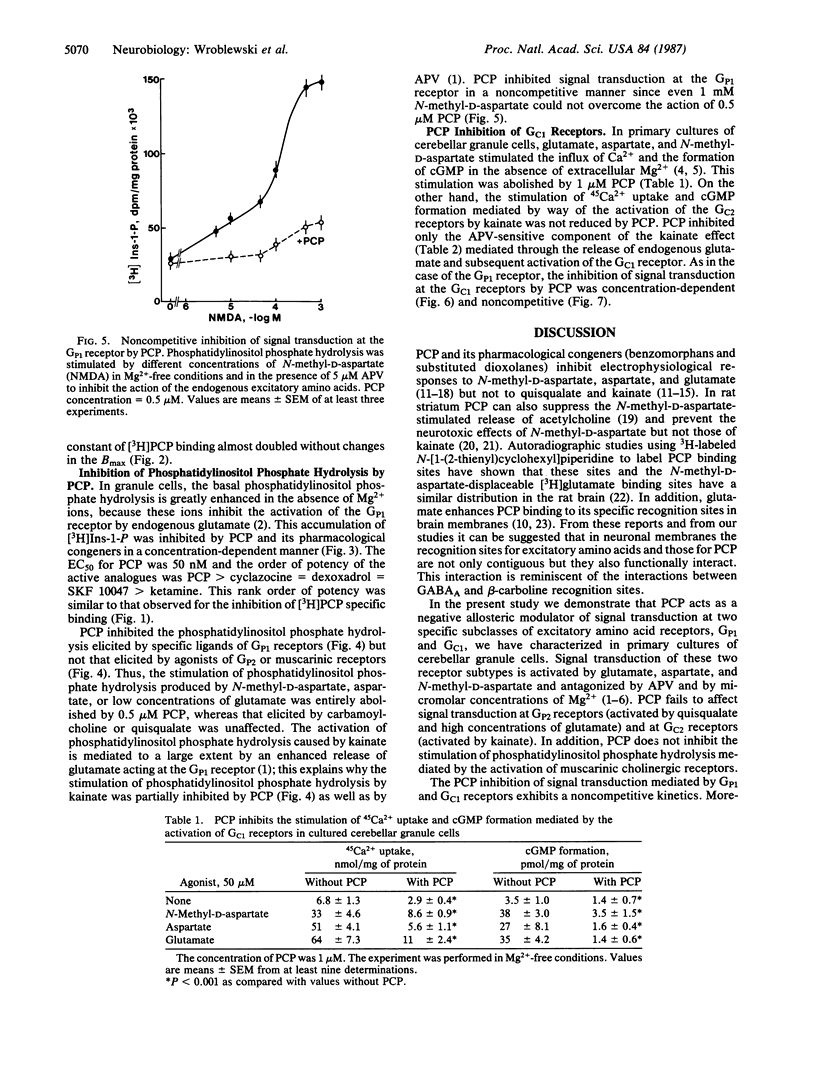
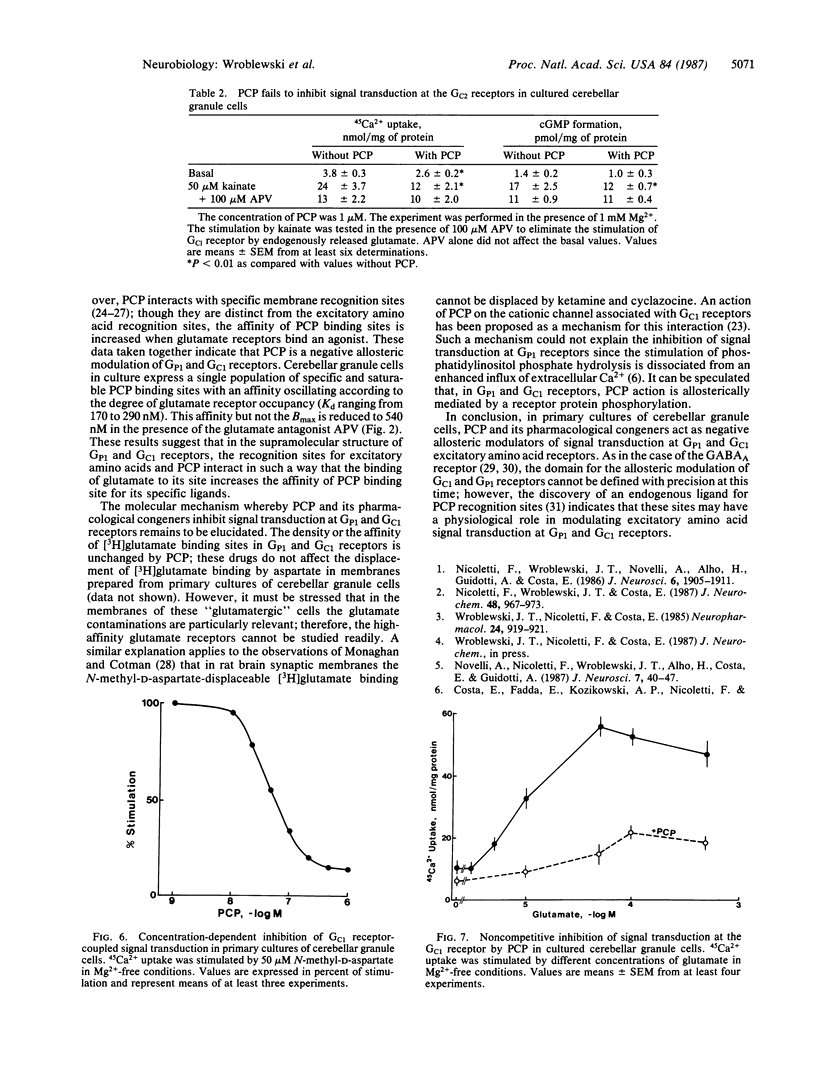
Phencyclidine (PCP) and some of its pharmacological congeners inhibit the signal transduction at specific excitatory amino acid receptors of cerebellar granule cells in primary cultures. These drugs do not bind to the transmitter recognition sites, and affinity of this specific binding site is increased by the presence of the transmitter bound to its recognition sites. PCP inhibits phosphatidylinositol phosphate hydrolysis mediated by Mg2+-sensitive glutamate receptors (GP1) but not that mediated by Mg2+-insensitive glutamate receptors (GP2). In addition, PCP inhibits Ca2+ influx and cGMP formation mediated by the activation of Mg2+-sensitive glutamate receptors (GC1) but not that mediated by Mg2+-insensitive glutamate receptors (GC2). In this cell culture the activation of phosphatidylinositol phosphate hydrolysis by muscarinic receptor agonists is not affected by PCP. Since PCP inhibits noncompetitively GP1 and GC1 signal transduction it may act as a negative allosteric modulator of signal transduction at both receptors. The pharmacological profile of PCP and its congeners delimits a class of drugs modulating allosterically the action of the primary transmitter at GP1 and GC1 receptors. These drugs need the presence of the transmitter to act and they cannot be termed inverse agonists because they are devoid of activity in the absence of the transmitter; moreover, they do not bind to the transmitter recognition site nor do they prevent the transmitter binding to its recognition sites.
Full text
PDF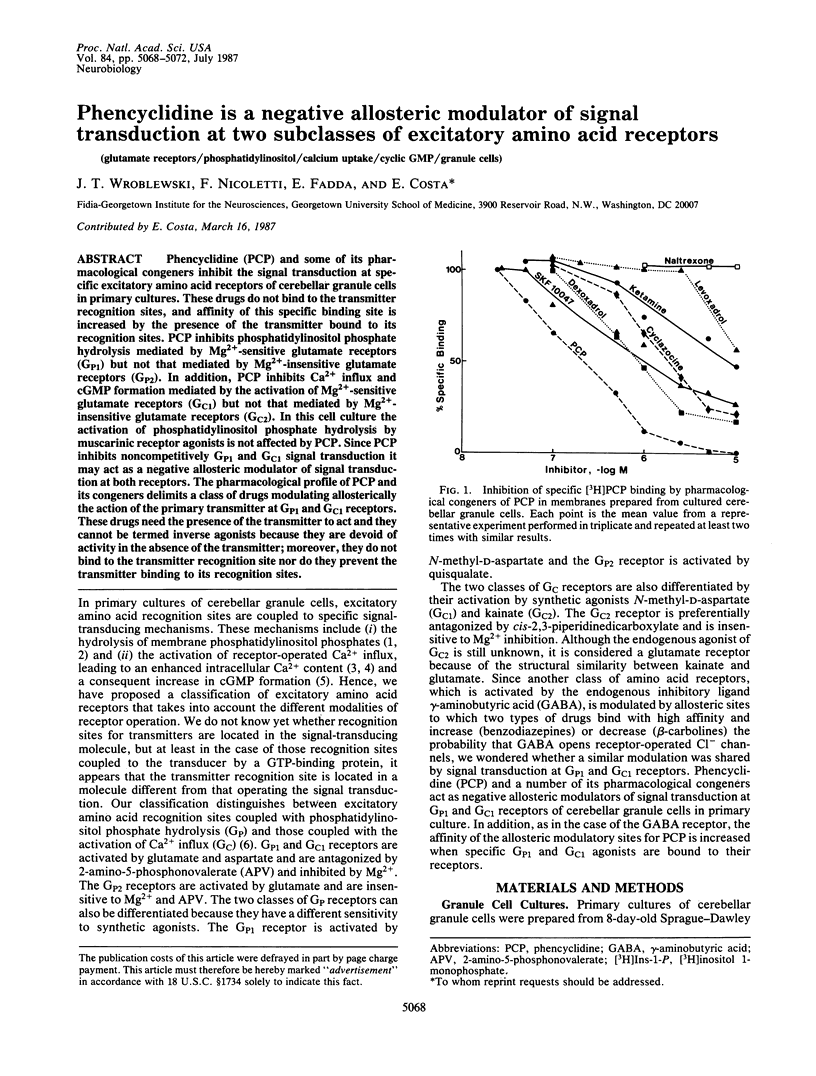

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aanonsen L. M., Wilcox G. L. Phencyclidine selectively blocks a spinal action of N-methyl-D-aspartate in mice. Neurosci Lett. 1986 Jun 18;67(2):191–197. doi: 10.1016/0304-3940(86)90396-4. [DOI] [PubMed] [Google Scholar]
- Anis N. A., Berry S. C., Burton N. R., Lodge D. The dissociative anaesthetics, ketamine and phencyclidine, selectively reduce excitation of central mammalian neurones by N-methyl-aspartate. Br J Pharmacol. 1983 Jun;79(2):565–575. doi: 10.1111/j.1476-5381.1983.tb11031.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge M. J., Downes C. P., Hanley M. R. Lithium amplifies agonist-dependent phosphatidylinositol responses in brain and salivary glands. Biochem J. 1982 Sep 15;206(3):587–595. doi: 10.1042/bj2060587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry S. C., Dawkins S. L., Lodge D. Comparison of sigma- and kappa-opiate receptor ligands as excitatory amino acid antagonists. Br J Pharmacol. 1984 Sep;83(1):179–185. doi: 10.1111/j.1476-5381.1984.tb10133.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa E., Guidotti A., Mao C. C., Suria A. New concepts on the mechanism of action of benzodiazepines. Life Sci. 1975 Jul 15;17(2):167–185. doi: 10.1016/0024-3205(75)90501-9. [DOI] [PubMed] [Google Scholar]
- Costa E., Guidotti A. Molecular mechanisms in the receptor action of benzodiazepines. Annu Rev Pharmacol Toxicol. 1979;19:531–545. doi: 10.1146/annurev.pa.19.040179.002531. [DOI] [PubMed] [Google Scholar]
- Duchen M. R., Burton N. R., Biscoe T. J. An intracellular study of the interactions of N-methyl-DL-aspartate with ketamine in the mouse hippocampal slice. Brain Res. 1985 Sep 2;342(1):149–153. doi: 10.1016/0006-8993(85)91364-2. [DOI] [PubMed] [Google Scholar]
- Gallo V., Ciotti M. T., Coletti A., Aloisi F., Levi G. Selective release of glutamate from cerebellar granule cells differentiating in culture. Proc Natl Acad Sci U S A. 1982 Dec;79(24):7919–7923. doi: 10.1073/pnas.79.24.7919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honey C. R., Miljkovic Z., MacDonald J. F. Ketamine and phencyclidine cause a voltage-dependent block of responses to L-aspartic acid. Neurosci Lett. 1985 Oct 24;61(1-2):135–139. doi: 10.1016/0304-3940(85)90414-8. [DOI] [PubMed] [Google Scholar]
- Loo P., Braunwalder A., Lehmann J., Williams M. Radioligand binding to central phencyclidine recognition sites is dependent on excitatory amino acid receptor agonists. Eur J Pharmacol. 1986 Apr 29;123(3):467–468. doi: 10.1016/0014-2999(86)90726-0. [DOI] [PubMed] [Google Scholar]
- Maragos W. F., Chu D. C., Greenamyre J. T., Penney J. B., Young A. B. High correlation between the localization of [3H]TCP binding and NMDA receptors. Eur J Pharmacol. 1986 Apr 9;123(1):173–174. doi: 10.1016/0014-2999(86)90703-x. [DOI] [PubMed] [Google Scholar]
- Maughan R. J., Watson J. S., Weir J. Strength and cross-sectional area of human skeletal muscle. J Physiol. 1983 May;338:37–49. doi: 10.1113/jphysiol.1983.sp014658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monaghan D. T., Cotman C. W. Identification and properties of N-methyl-D-aspartate receptors in rat brain synaptic plasma membranes. Proc Natl Acad Sci U S A. 1986 Oct;83(19):7532–7536. doi: 10.1073/pnas.83.19.7532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicoletti F., Wroblewski J. T., Costa E. Magnesium ions inhibit the stimulation of inositol phospholipid hydrolysis by endogenous excitatory amino acids in primary cultures of cerebellar granule cells. J Neurochem. 1987 Mar;48(3):967–973. doi: 10.1111/j.1471-4159.1987.tb05611.x. [DOI] [PubMed] [Google Scholar]
- Nicoletti F., Wroblewski J. T., Novelli A., Alho H., Guidotti A., Costa E. The activation of inositol phospholipid metabolism as a signal-transducing system for excitatory amino acids in primary cultures of cerebellar granule cells. J Neurosci. 1986 Jul;6(7):1905–1911. doi: 10.1523/JNEUROSCI.06-07-01905.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novelli A., Nicoletti F., Wroblewski J. T., Alho H., Costa E., Guidotti A. Excitatory amino acid receptors coupled with guanylate cyclase in primary cultures of cerebellar granule cells. J Neurosci. 1987 Jan;7(1):40–47. doi: 10.1523/JNEUROSCI.07-01-00040.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quirion R., DiMaggio D. A., French E. D., Contreras P. C., Shiloach J., Pert C. B., Everist H., Pert A., O'Donohue T. L. Evidence for an endogenous peptide ligand for the phencyclidine receptor. Peptides. 1984 Sep-Oct;5(5):967–973. doi: 10.1016/0196-9781(84)90124-4. [DOI] [PubMed] [Google Scholar]
- Quirion R., Hammer R. P., Jr, Herkenham M., Pert C. B. Phencyclidine (angel dust)/sigma "opiate" receptor: visualization by tritium-sensitive film. Proc Natl Acad Sci U S A. 1981 Sep;78(9):5881–5885. doi: 10.1073/pnas.78.9.5881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snell L. D., Johnson K. M. Antagonism of N-methyl-D-aspartate-induced transmitter release in the rat striatum by phencyclidine-like drugs and its relationship to turning behavior. J Pharmacol Exp Ther. 1985 Oct;235(1):50–57. [PubMed] [Google Scholar]
- Thomson A. M., West D. C., Lodge D. An N-methylaspartate receptor-mediated synapse in rat cerebral cortex: a site of action of ketamine? Nature. 1985 Feb 7;313(6002):479–481. doi: 10.1038/313479a0. [DOI] [PubMed] [Google Scholar]
- Vignon J., Vincent J. P., Bidard J. N., Kamenka J. M., Geneste P., Monier S., Lazdunski M. Biochemical properties of the brain phencyclidine receptor. Eur J Pharmacol. 1982 Jul 30;81(4):531–542. doi: 10.1016/0014-2999(82)90342-9. [DOI] [PubMed] [Google Scholar]
- Vincent J. P., Kartalovski B., Geneste P., Kamenka J. M., Lazdunski M. Interaction of phencyclidine ("angel dust") with a specific receptor in rat brain membranes. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4678–4682. doi: 10.1073/pnas.76.9.4678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wroblewski J. T., Nicoletti F., Costa E. Different coupling of excitatory amino acid receptors with Ca2+ channels in primary cultures of cerebellar granule cells. Neuropharmacology. 1985 Sep;24(9):919–921. doi: 10.1016/0028-3908(85)90046-2. [DOI] [PubMed] [Google Scholar]
- Zukin S. R., Zukin R. S. Specific [3H]phencyclidine binding in rat central nervous system. Proc Natl Acad Sci U S A. 1979 Oct;76(10):5372–5376. doi: 10.1073/pnas.76.10.5372. [DOI] [PMC free article] [PubMed] [Google Scholar]



