Abstract
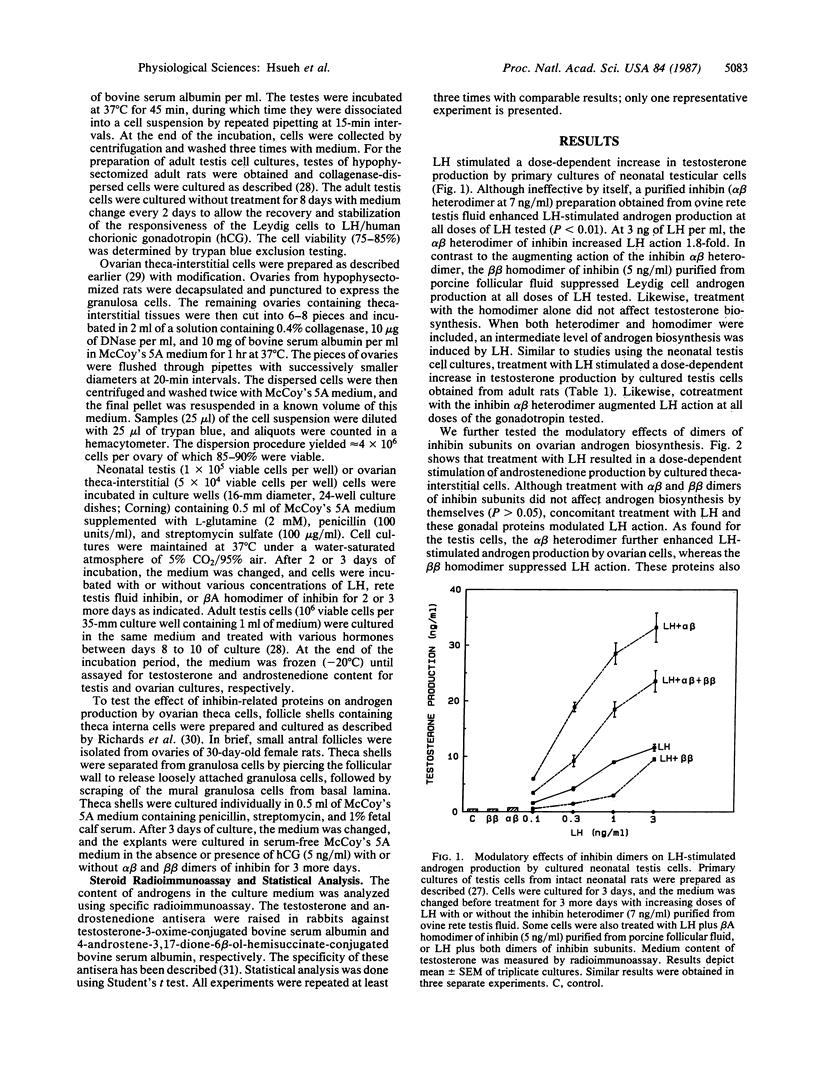
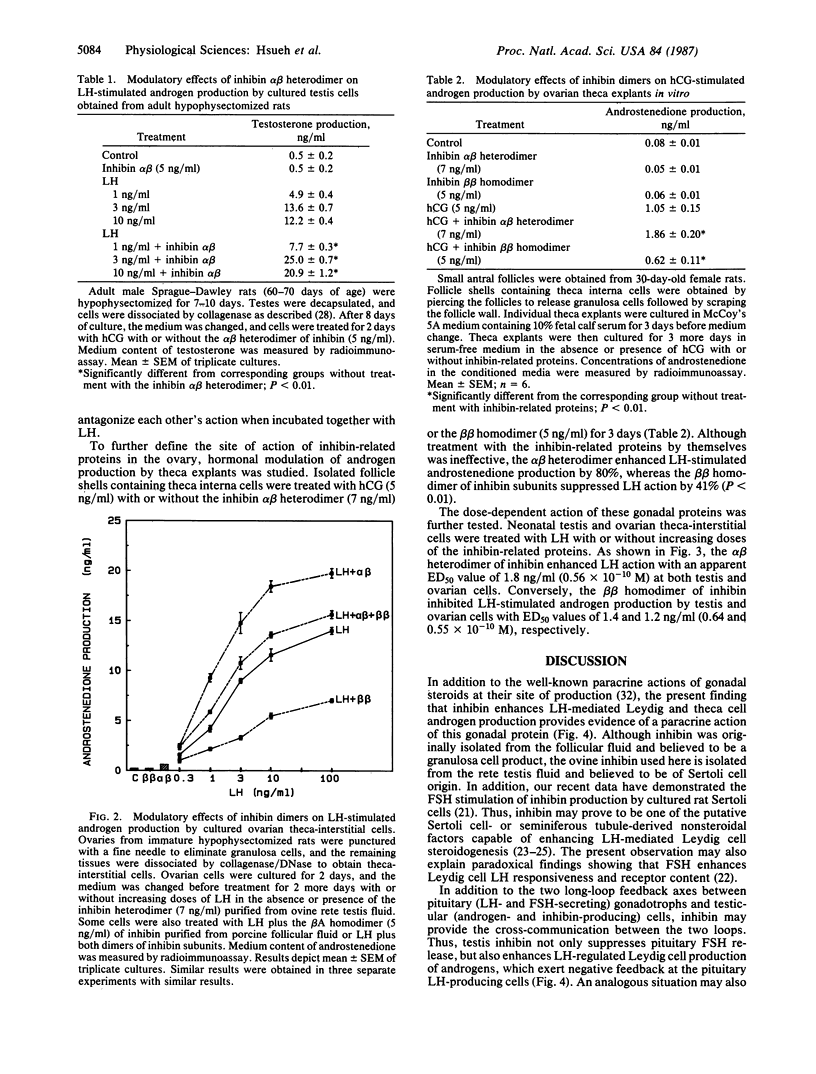
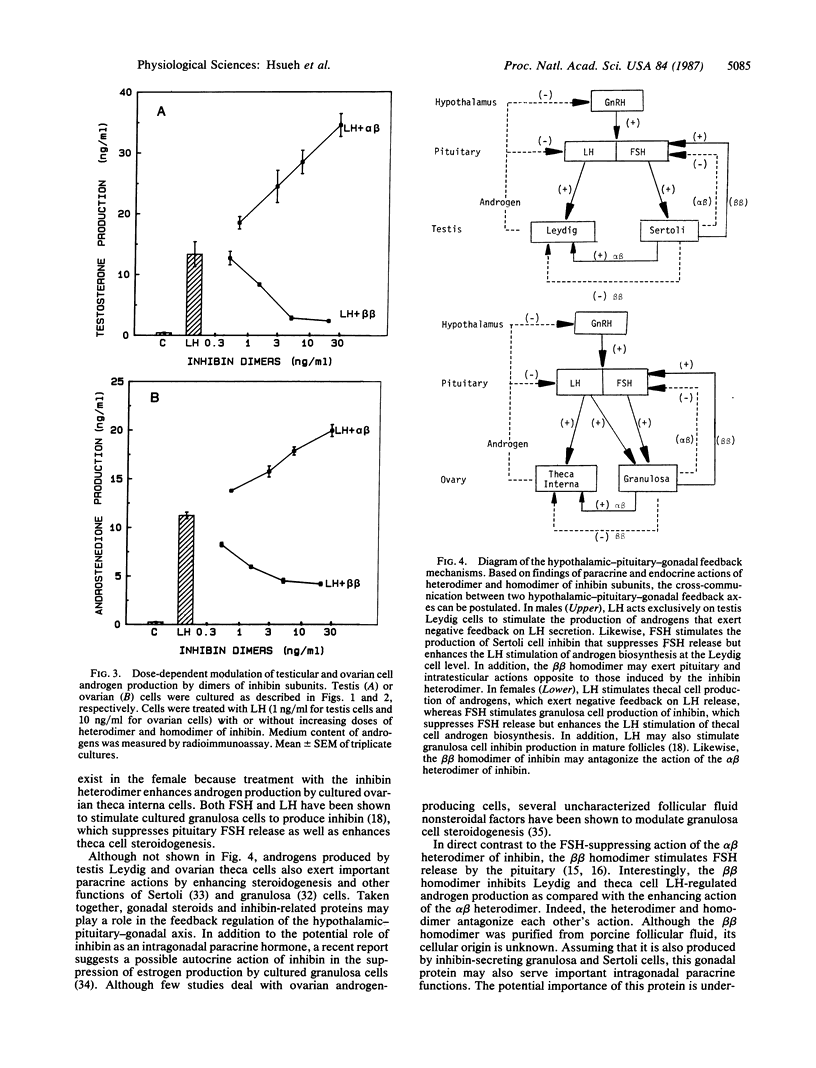
Inhibin, a gonadal hormone capable of preferential suppression of pituitary follicle-stimulating hormone (FSH) secretion, has recently been purified. The major form of this protein is an alpha beta heterodimer encoded by two separate genes. In contrast to the FSH-suppressing action of the alpha beta heterodimer, the beta beta homodimer stimulates FSH secretion. Luteinizing hormone (LH)-secreting pituitary cells and gonadal androgen-producing cells have long been shown to form a closed-loop feedback axis. Based on recent studies demonstrating the FSH stimulation of inhibin biosynthesis by ovarian granulosa and testis Sertoli cells, an additional closed-loop feedback axis exists between pituitary FSH- and gonadal inhibin-producing cells. Because uncharacterized Sertoli cell factors have been suggested to either stimulate or inhibit androgen production by testicular Leydig cells, we have tested the intragonadal paracrine actions of heterodimers and homodimers of inhibin subunits. In primary cultures of testis cells, the alpha beta heterodimer of inhibin enhances Leydig cell androgen biosynthesis stimulated by LH, whereas the beta beta homodimer suppresses androgen production. Furthermore, similar modulatory actions of inhibin-related proteins were found in cultured ovarian theca-interstitial cells and theca explants treated with LH. In contrast, treatment with the inhibin-related proteins alone did not affect gonadal steroidogenesis. Our data indicate that the inhibin-related gene products synthesized by Sertoli and granulosa cells may form heterodimers or homodimers to serve as intragonadal paracrine signals in the modulation of LH-stimulated androgen biosynthesis and allow cross-communication between the two feedback loops.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bambino T. H., Schreiber J. R., Hsueh A. J. Gonadotropin-releasing hormone and its agonist inhibit testicular luteinizing hormone receptor and steroidogenesis in immature and adult hypophysectomized rats. Endocrinology. 1980 Oct;107(4):908–917. doi: 10.1210/endo-107-4-908. [DOI] [PubMed] [Google Scholar]
- Benahmed M., Grenot C., Tabone E., Sanchez P., Morera A. M. FSH regulates cultured Leydig cell function via Sertoli cell proteins: an in vitro study. Biochem Biophys Res Commun. 1985 Oct 30;132(2):729–734. doi: 10.1016/0006-291x(85)91193-3. [DOI] [PubMed] [Google Scholar]
- Bicsak T. A., Tucker E. M., Cappel S., Vaughan J., Rivier J., Vale W., Hsueh A. J. Hormonal regulation of granulosa cell inhibin biosynthesis. Endocrinology. 1986 Dec;119(6):2711–2719. doi: 10.1210/endo-119-6-2711. [DOI] [PubMed] [Google Scholar]
- Bicsak T. A., Vale W., Vaughan J., Tucker E. M., Cappel S., Hsueh A. J. Hormonal regulation of inhibin production by cultured Sertoli cells. Mol Cell Endocrinol. 1987 Feb;49(2-3):211–217. doi: 10.1016/0303-7207(87)90215-2. [DOI] [PubMed] [Google Scholar]
- Cate R. L., Mattaliano R. J., Hession C., Tizard R., Farber N. M., Cheung A., Ninfa E. G., Frey A. Z., Gash D. J., Chow E. P. Isolation of the bovine and human genes for Müllerian inhibiting substance and expression of the human gene in animal cells. Cell. 1986 Jun 6;45(5):685–698. doi: 10.1016/0092-8674(86)90783-x. [DOI] [PubMed] [Google Scholar]
- Chen Y. I., Payne A. H., Kelch R. P. FSH stimulation of Leydig cell function in the hypophysectomized immature rat. Proc Soc Exp Biol Med. 1976 Dec;153(3):473–475. doi: 10.3181/00379727-153-39571. [DOI] [PubMed] [Google Scholar]
- De Jong F. H., Sharpe R. M. Evidence for inhibin-like activity in bovine follicular fluid. Nature. 1976 Sep 2;263(5572):71–72. doi: 10.1038/263071a0. [DOI] [PubMed] [Google Scholar]
- Erickson G. F., Hsueh A. J. Secretion of "inhibin" by rat granulosa cells in vitro. Endocrinology. 1978 Nov;103(5):1960–1963. doi: 10.1210/endo-103-5-1960. [DOI] [PubMed] [Google Scholar]
- Eto Y., Tsuji T., Takezawa M., Takano S., Yokogawa Y., Shibai H. Purification and characterization of erythroid differentiation factor (EDF) isolated from human leukemia cell line THP-1. Biochem Biophys Res Commun. 1987 Feb 13;142(3):1095–1103. doi: 10.1016/0006-291x(87)91528-2. [DOI] [PubMed] [Google Scholar]
- Forage R. G., Ring J. M., Brown R. W., McInerney B. V., Cobon G. S., Gregson R. P., Robertson D. M., Morgan F. J., Hearn M. T., Findlay J. K. Cloning and sequence analysis of cDNA species coding for the two subunits of inhibin from bovine follicular fluid. Proc Natl Acad Sci U S A. 1986 May;83(10):3091–3095. doi: 10.1073/pnas.83.10.3091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franchimont P., Verstraelen-Proyard J., Hazee-Hagelstein M. T., Renard C., Demoulin A., Bourguignon J. P., Hustin J. Inhibin: from concept to reality. Vitam Horm. 1979;37:243–302. doi: 10.1016/s0083-6729(08)61071-7. [DOI] [PubMed] [Google Scholar]
- Fukuda M., Miyamoto K., Hasegawa Y., Nomura M., Igarashi M., Kangawa K., Matsuo H. Isolation of bovine follicular fluid inhibin of about 32 kDa. Mol Cell Endocrinol. 1986 Jan;44(1):55–60. doi: 10.1016/0303-7207(86)90105-x. [DOI] [PubMed] [Google Scholar]
- Grady R. R., Charlesworth M. C., Schwartz N. B. Characterization of the FSH-suppressing activity in follicular fluid. Recent Prog Horm Res. 1982;38:409–456. doi: 10.1016/b978-0-12-571138-8.50015-9. [DOI] [PubMed] [Google Scholar]
- Hsueh A. J., Adashi E. Y., Jones P. B., Welsh T. H., Jr Hormonal regulation of the differentiation of cultured ovarian granulosa cells. Endocr Rev. 1984 Winter;5(1):76–127. doi: 10.1210/edrv-5-1-76. [DOI] [PubMed] [Google Scholar]
- Hsueh A. J. Gonadotropin stimulation of testosterone production in primary culture of adult rat testis cells. Biochem Biophys Res Commun. 1980 Nov 28;97(2):506–512. doi: 10.1016/0006-291x(80)90292-2. [DOI] [PubMed] [Google Scholar]
- Ling N., Ying S. Y., Ueno N., Esch F., Denoroy L., Guillemin R. Isolation and partial characterization of a Mr 32,000 protein with inhibin activity from porcine follicular fluid. Proc Natl Acad Sci U S A. 1985 Nov;82(21):7217–7221. doi: 10.1073/pnas.82.21.7217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling N., Ying S. Y., Ueno N., Shimasaki S., Esch F., Hotta M., Guillemin R. Pituitary FSH is released by a heterodimer of the beta-subunits from the two forms of inhibin. Nature. 1986 Jun 19;321(6072):779–782. doi: 10.1038/321779a0. [DOI] [PubMed] [Google Scholar]
- Magoffin D. A., Erickson G. F. Prolactin inhibition of luteinizing hormone-stimulated androgen synthesis in ovarian interstitial cells cultured in defined medium: mechanism of action. Endocrinology. 1982 Dec;111(6):2001–2007. doi: 10.1210/endo-111-6-2001. [DOI] [PubMed] [Google Scholar]
- Mason A. J., Hayflick J. S., Ling N., Esch F., Ueno N., Ying S. Y., Guillemin R., Niall H., Seeburg P. H. Complementary DNA sequences of ovarian follicular fluid inhibin show precursor structure and homology with transforming growth factor-beta. Nature. 1985 Dec 19;318(6047):659–663. doi: 10.1038/318659a0. [DOI] [PubMed] [Google Scholar]
- Mason A. J., Niall H. D., Seeburg P. H. Structure of two human ovarian inhibins. Biochem Biophys Res Commun. 1986 Mar 28;135(3):957–964. doi: 10.1016/0006-291x(86)91021-1. [DOI] [PubMed] [Google Scholar]
- McCullagh D. R. DUAL ENDOCRINE ACTIVITY OF THE TESTES. Science. 1932 Jul 1;76(1957):19–20. doi: 10.1126/science.76.1957.19. [DOI] [PubMed] [Google Scholar]
- Meidan R., Lim P., McAllister J. M., Hsueh A. J. Hormonal regulation of androgen biosynthesis by primary cultures of testis cells from neonatal rats. Endocrinology. 1985 Jun;116(6):2473–2482. doi: 10.1210/endo-116-6-2473. [DOI] [PubMed] [Google Scholar]
- Miyamoto K., Hasegawa Y., Fukuda M., Nomura M., Igarashi M., Kangawa K., Matsuo H. Isolation of porcine follicular fluid inhibin of 32K daltons. Biochem Biophys Res Commun. 1985 Jun 14;129(2):396–403. doi: 10.1016/0006-291x(85)90164-0. [DOI] [PubMed] [Google Scholar]
- Richards J. S., Hedin L., Caston L. Differentiation of rat ovarian thecal cells: evidence for functional luteinization. Endocrinology. 1986 Apr;118(4):1660–1668. doi: 10.1210/endo-118-4-1660. [DOI] [PubMed] [Google Scholar]
- Rivier J., Spiess J., McClintock R., Vaughan J., Vale W. Purification and partial characterization of inhibin from porcine follicular fluid. Biochem Biophys Res Commun. 1985 Nov 27;133(1):120–127. doi: 10.1016/0006-291x(85)91849-2. [DOI] [PubMed] [Google Scholar]
- Robertson D. M., Foulds L. M., Leversha L., Morgan F. J., Hearn M. T., Burger H. G., Wettenhall R. E., de Kretser D. M. Isolation of inhibin from bovine follicular fluid. Biochem Biophys Res Commun. 1985 Jan 16;126(1):220–226. doi: 10.1016/0006-291x(85)90594-7. [DOI] [PubMed] [Google Scholar]
- Schwartz N. B., Channing C. P. Evidence for ovarian "inhibin": suppression of the secondary rise in serum follicle stimulating hormone levels in proestrous rats by injection of porcine follicular fluid. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5721–5724. doi: 10.1073/pnas.74.12.5721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharpe R. M., Cooper I. Intratesticular secretion of a factor(s) with major stimulatory effects on Leydig cell testosterone secretion in vitro. Mol Cell Endocrinol. 1984 Sep;37(2):159–168. doi: 10.1016/0303-7207(84)90048-0. [DOI] [PubMed] [Google Scholar]
- Steinberger A., Steinberger E. Secretion of an FSH-inhibiting factor by cultured Sertoli cells. Endocrinology. 1976 Sep;99(3):918–921. doi: 10.1210/endo-99-3-918. [DOI] [PubMed] [Google Scholar]
- Syed V., Khan S. A., Ritzen E. M. Stage-specific inhibition of interstitial cell testosterone secretion by rat seminiferous tubules in vitro. Mol Cell Endocrinol. 1985 May;40(2-3):257–264. doi: 10.1016/0303-7207(85)90181-9. [DOI] [PubMed] [Google Scholar]
- Uilenbroek J. T., Tiller R., de Jong F. H., Vels F. Specific suppression of follicle-stimulating hormone secretion in gonadectomized male and female rats with intrasplenic ovarian transplants. J Endocrinol. 1978 Sep;78(3):399–406. doi: 10.1677/joe.0.0780399. [DOI] [PubMed] [Google Scholar]
- Vale W., Rivier J., Vaughan J., McClintock R., Corrigan A., Woo W., Karr D., Spiess J. Purification and characterization of an FSH releasing protein from porcine ovarian follicular fluid. Nature. 1986 Jun 19;321(6072):776–779. doi: 10.1038/321776a0. [DOI] [PubMed] [Google Scholar]
- Verhoeven G., Cailleau J. A factor in spent media from Sertoli-cell-enriched cultures that stimulates steroidogenesis in Leydig cells. Mol Cell Endocrinol. 1985 Apr;40(1):57–68. doi: 10.1016/0303-7207(85)90158-3. [DOI] [PubMed] [Google Scholar]
- Ying S. Y., Becker A., Ling N., Ueno N., Guillemin R. Inhibin and beta type transforming growth factor (TGF beta) have opposite modulating effects on the follicle stimulating hormone (FSH)-induced aromatase activity of cultured rat granulosa cells. Biochem Biophys Res Commun. 1986 May 14;136(3):969–975. doi: 10.1016/0006-291x(86)90427-4. [DOI] [PubMed] [Google Scholar]


