Abstract
Multiple proteins interacting with DNA polymerases orchestrate DNA replication. Human cytomegalovirus (HCMV) encodes a DNA polymerase that includes the presumptive processivity factor UL44. UL44 is structurally homologous to the eukaryotic DNA polymerase processivity factor proliferating cell nuclear antigen (PCNA), which interacts with numerous proteins. Previous proteomic analysis has identified the HCMV protein IRS1 as a candidate protein interacting with UL44. Nuclease-resistant reciprocal co-immunoprecipitation of UL44 with IRS1 and with TRS1, which has an amino terminus identical to that of IRS1, was observed from lysate of cells infected with viruses expressing epitope-tagged UL44, epitope-tagged IRS1 or epitope-tagged TRS1. Western blotting of protein immunoprecipitated from infected cell lysate indicated that epitope-tagged IRS1 and TRS1 do not associate simultaneously with UL44. Glutathione S-transferase pull-down experiments indicated that IRS1 and TRS1 interact with UL44 via a region that is identical in both proteins. Taken together, these data suggest that IRS1 and TRS1 may compete for association with UL44 and may affect UL44 function differentially.
INTRODUCTION
Protein–protein interactions are essential for genome replication in the eukaryotic cell. Of particular importance is the interaction of proteins with the DNA polymerase processivity factor, proliferating cell nuclear antigen (PCNA). PCNA holds the catalytic subunit of the DNA polymerase, for example DNA polymerases δ and ε, on DNA to permit continuous DNA synthesis while simultaneously interacting with a range of proteins that participate in DNA synthesis and repair (Maga & Hubscher, 2003; Moldovan et al., 2007).
The presumptive processivity factor of the human cytomegalovirus (HCMV) DNA polymerase, UL44, has structural homology to PCNA, and the interaction of the HCMV DNA polymerase catalytic subunit UL54 with UL44 (Ertl & Powell, 1992) is similar to the interaction of proteins with PCNA (Appleton et al., 2004, 2006). It was hypothesized that, like PCNA, multiple proteins interact with UL44 during viral DNA replication (Appleton et al., 2004). Indeed, aside from UL54, we and others have found interactions of the viral protein UL84 and the cellular protein nucleolin with UL44 (Gao et al., 2008; Strang et al., 2009, 2010). Interaction of UL44 with the viral uracil DNA glycosylase UL114 has also been reported (Prichard et al., 2005; Ranneberg-Nilsen et al., 2008), although this interaction has been called into question (Strang et al., 2009).
We have previously conducted proteomic analysis utilizing mass spectrometry (MS) to identify proteins associated with UL44 in infected cell lysate (Strang et al., 2009, 2010). Peptides from a large number of viral and cellular proteins were identified in protein immunoprecipitating with UL44 from infected cell lysate. Among the peptides identified in protein immunoprecipitating with UL44 were peptides derived from the viral protein IRS1 (Strang et al., 2010).
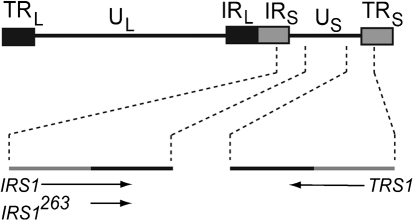
IRS1 is encoded by a reading frame extending from the internal short repeated region (IRS) into the unique short (US) domain of the viral genome (Fig. 1). Also encoded in the viral genome is the protein TRS1, produced from a reading frame extending from the terminal short repeated region (TRS) into the US domain (Fig. 1). IRS1 and TRS1, therefore, are identical at their amino termini and differ at their carboxyl termini. An alternative IRS1 transcript termed IRS1263, which encodes the 263 aa of the IRS1 carboxyl terminus, is also present in the infected cell (Romanowski & Shenk, 1997) (Fig. 1).
Fig. 1.
Positions of IRS1, IRS1263 and TRS1 open reading frames in the HCMV genome. The diagram at the top of the figure represents the entire HCMV genome. The terminal and internal repeat regions of the long unique (UL) segment of the genome (TRL and IRL, respectively) are represented as black boxes. The terminal and internal repeat regions of the short unique (US) segment of the genome (TRS and IRS, respectively) are represented as grey boxes. Dashed lines from the TRS and IRS regions of US lead to expanded areas showing the relative positions of the IRS1, IRS1263 and TRS1 coding sequences in the HCMV genome. Grey lines represent genome sequences in repeat regions; black lines represent genome sequences in the US segment. Arrows show the position and direction of transcription of the coding sequences. IRS1263 is the product of an alternative IRS1 transcript encoding the carboxyl terminus of IRS1 (aa 583–846) (Romanowski & Shenk, 1997).
Insertion of a marker cassette into the open reading frame encoding IRS1 and IRS1263 (Blankenship & Shenk, 2002) or deletion of this region (Marshall et al., 2009) does not affect virus replication notably. Insertion of a marker cassette into the open reading frame encoding TRS1 inhibits virus replication by approximately 5-fold at high m.o.i. and approximately 200-fold at low m.o.i. (Blankenship & Shenk, 2002). The defect in virus replication in virus containing the marker-cassette insert appears to be during virus assembly (Blankenship & Shenk, 2002). Simultaneous deletion of IRS1 and TRS1 from the viral genome causes a severe replication defect (Marshall et al., 2009). Several functions have been attributed to IRS1 and TRS1. In transient assays, IRS1 or TRS1 stimulates UL54 expression (Kerry et al., 1996) and TRS1 stimulates UL44 expression (Stasiak & Mocarski, 1992). Conversely, IRS1263 acts as a repressor of gene expression in transient assays, antagonizing several viral transcriptional transactivators (Romanowski & Shenk, 1997). Experiments where cloned fragments of the HCMV genome are transfected into cells to determine what viral proteins are required for replication of a plasmid containing the HCMV origin of replication (oriLyt) have shown that the presence of either IRS1 or TRS1 is necessary for plasmid replication (Pari & Anders, 1993; Pari et al., 1993). When these experiments were repeated using HCMV DNA replication proteins expressed by a strong heterologous promoter, IRS1 was dispensable (Sarisky & Hayward, 1996), indicating that, in this context, IRS1 is probably required for expression of viral DNA replication proteins, not DNA replication per se. IRS1 and TRS1 are also double-stranded RNA-binding proteins (Hakki & Geballe, 2005) required for viral evasion of protein kinase R (PKR)-mediated inhibition of protein synthesis (Child et al., 2004; Hakki et al., 2006; Marshall et al., 2009).
Here, we investigate the association of IRS1 and TRS1 with UL44 in infected cell lysate and in vitro. These experiments indicate that IRS1 and TRS1 associate with UL44 via a region that is identical in both proteins. Furthermore, analysis of these proteins in infected cell lysate indicates that IRS1 and TRS1 do not associate simultaneously with UL44, suggesting that IRS1 and TRS1 potentially compete for association with UL44.
RESULTS
Association of IRS1 and TRS1 with FLAG-tagged UL44 in infected cell lysate
Previously, recombinant HCMV expressing FLAG-tagged UL44 (FLAG–44) was generated and used to immunoprecipitate UL44 and associated proteins from infected cell lysate (Strang et al., 2010). Peptides derived from HCMV protein IRS1 could be detected by MS in protein immunoprecipitated with FLAG-tagged UL44, suggesting an association of FLAG-tagged UL44 and IRS1 in infected cell lysate (Strang et al., 2010). To confirm the presence of IRS1 in these experiments, protein was immunoprecipitated from lysate of cells infected with FLAG–44 or wild-type virus AD169rv and examined by Western blotting using a monoclonal antibody (mAb) recognizing IRS1 (Fig. 2). IRS1 was detected in both AD169rv- and FLAG–44-infected cell lysate (lanes 1 and 2) and protein immunoprecipitated from FLAG–44-infected cell lysate (lane 4). IRS1 was not detected in protein immunoprecipitated from AD169rv-infected cell lysate (lane 3). IRS1263 [approx. 30 kDa (Romanowski & Shenk, 1997)] could be detected in neither lysate nor immunoprecipitated protein; however, it is possible that this protein was present below the level of detection in the assay.
Fig. 2.
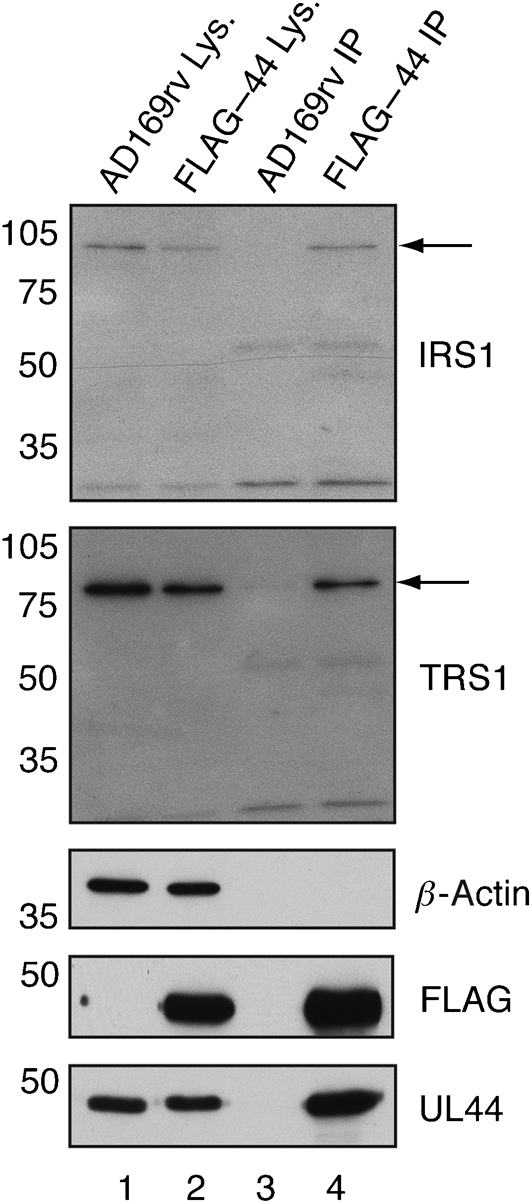
Detection of protein immunoprecipitated from FLAG–44-infected cell lysate by Western blotting. Lysate (Lys.) from AD169rv-infected (lane 1) and FLAG–44-infected (lane 2) cells and protein immunoprecipitated (IP) using an anti-FLAG antibody from those lysates (lanes 3 and 4, respectively) were separated on a 10 % polyacrylamide gel. Proteins in each lane were examined by Western blotting for the presence of UL44, FLAG, IRS1, TRS1 and β-actin using antibodies recognizing these proteins, as indicated to the right of the figure. The positions of IRS1 and TRS1 are indicated by arrows. The positions of molecular mass markers (in kDa) are indicated to the left of the figure.
As the amino acid sequence of the amino terminus of IRS1 is identical to that of the amino terminus of TRS1 (Fig. 1), it is possible that peptides found by MS in our original analysis of proteins immunoprecipitating with FLAG-tagged UL44 (Strang et al., 2010) may have been from either IRS1 or TRS1. The presence of TRS1 in immunoprecipitated protein was, therefore, also examined by Western blotting (Fig. 2). Like IRS1, TRS1 was detected in protein immunoprecipitated from FLAG–44-infected cell lysate (lane 4), but not in protein immunoprecipitated from AD169rv-infected cell lysate (lane 3), indicating an association of FLAG-tagged UL44 and TRS1 in infected cell lysate.
As a control, immunoprecipitated protein was also probed by Western blotting for the presence of β-actin. This abundant protein could not be detected in protein immunoprecipitated from either FLAG–44- or AD169rv-infected cell lysate (Fig. 2, lanes 3 and 4), indicating that the immunoprecipitation (IP) conditions used in these experiments detected specific interactions. Therefore, both IRS1 and TRS1 associate with FLAG-tagged UL44 in infected cell lysate.
Generation of recombinant HCMV expressing either FLAG-tagged IRS1 or FLAG-tagged TRS1
To perform reciprocal co-IP of UL44 and IRS1 or TRS1, recombinant HCMV expressing either FLAG-tagged IRS1 or FLAG-tagged TRS1 was generated. Using Red two-step recombination (Tischer et al., 2006), a single FLAG epitope (DYKDDDDK) was inserted immediately before the termination codon of either the IRS1 or TRS1 coding sequence in the bacterial artificial chromosome (BAC) AD169-BAC (Hobom et al., 2000). These new BACs were termed AD169-BACIRSF and AD169-BACTRSF, respectively. AD169-BAC, AD169-BACIRSF and AD169-BACTRSF were then transfected into human foreskin fibroblast (HFF) cells to generate the viruses AD169rv, IRSF and TRSF, respectively.
To determine whether insertion of the FLAG epitope into the IRS1 or TRS1 coding sequence affects virus replication, the replication kinetics of AD169rv, IRSF and TRSF infection were analysed following infection (m.o.i. of 1; Fig. 3a). At 3 days post-infection (p.i.), all viruses replicated to similar levels. By 5 days p.i., however, compared with replication of AD169rv, IRSF exhibited no more than a 2-fold decrease in virus replication. However, TRSF exhibited up to a 10-fold decrease in virus replication at the same time point. Virus replication was, therefore, only affected notably by the addition of FLAG to TRS1 and only at late time points (after 3 days p.i.).
Fig. 3.
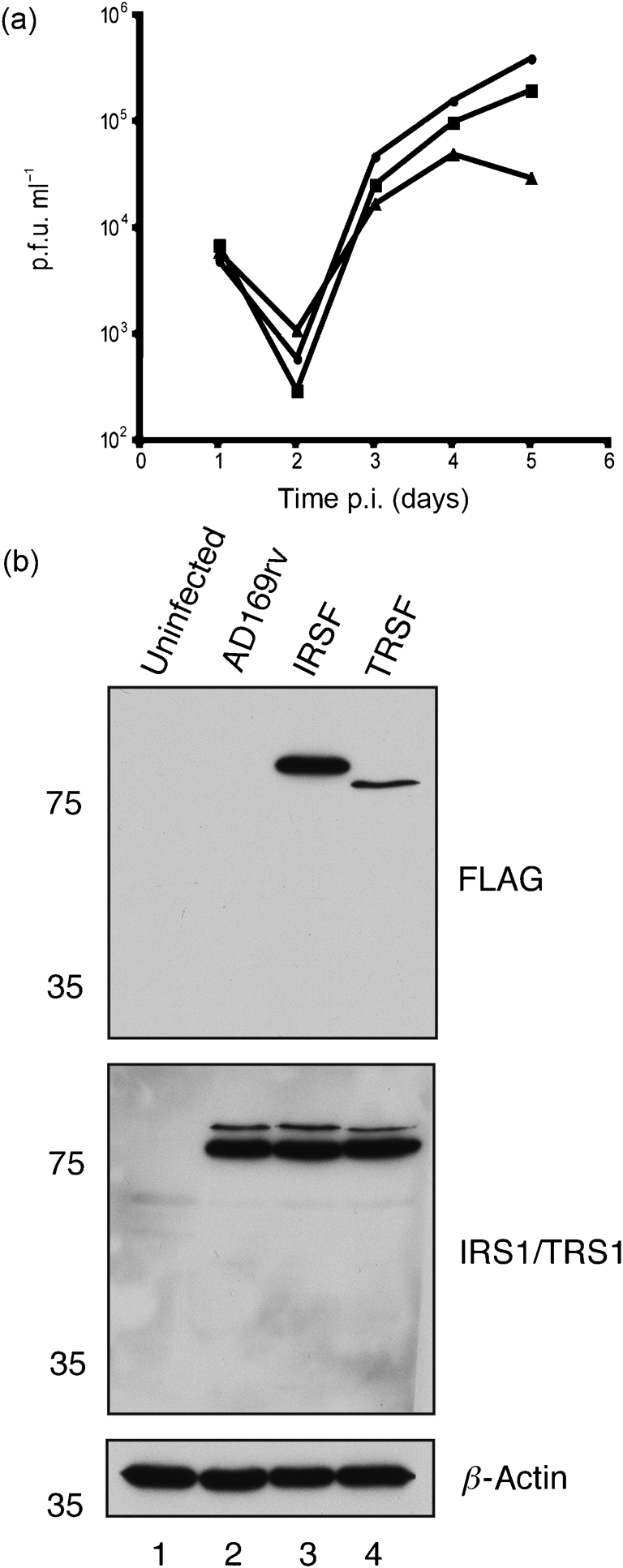
Characterization of recombinant virus. (a) Replication of AD169rv (•), IRSF (▪) and TRSF (▴) viruses. HFF cells were infected at an m.o.i. of 1 and virus supernatant was harvested at the indicated time points. Virus titre is represented as p.f.u. ml−1 on HFF cells. Data are representative of two experiments. (b) Western blotting of infected cells. Cell lysates of uninfected HFF cells (lane 1) or HFF cells infected at an m.o.i. of 1 with AD169rv (lane 2), IRSF (lane 3) or TRSF (lane 4) viruses were prepared 72 h p.i. Proteins in each lane were examined by Western blotting for the presence of FLAG-tagged IRS1 and FLAG-tagged TRS1 (top panel), IRS1 and TRS1 (middle panel) and β-actin (bottom panel) using antibodies recognizing these proteins or FLAG, as indicated to the right of the figure. Note that, in the middle panel, a combination of antibodies recognizing IRS1 or TRS1 was utilized and that FLAG-tagged versions of each protein migrate more slowly than wild-type proteins. The positions of molecular mass markers (in kDa) are indicated to the left of the figure.
Levels of FLAG-tagged and untagged IRS1 and TRS1 proteins in AD169rv-, IRSF- and TRSF-infected cell lysate were also assayed by Western blotting 72 h p.i. (Fig. 3b). The amount of β-actin in each sample was also assayed, which demonstrated equivalent loading of samples in each lane. Using a mAb recognizing FLAG, a single band corresponding to FLAG-tagged IRS1 was observed in IRSF-infected cell lysate (Fig. 3b, lane 3), but in no other sample. Similarly, a single band corresponding to FLAG-tagged TRS1 was observed in TRSF-infected cell lysate (Fig. 3b, lane 4), but in no other sample. Using a mixture of mAbs recognizing either IRS1 or TRS1, the level of FLAG-tagged IRS1 in IRSF-infected cell lysate (Fig. 3b, lane 3) was comparable to the level of IRS1 in AD169rv- and TRSF-infected cell lysate (Fig. 3b, lanes 2 and 4). Likewise, the level of FLAG-tagged TRS1 in TRSF-infected cell lysate (Fig. 3b, lane 4) was comparable to the level of TRS1 in AD169rv- and IRSF-infected cell lysate (Fig. 3b, lanes 2 and 3). Addition of the FLAG tag to IRS1 or TRS1, therefore, has no obvious effect on the accumulation of IRS1 or TRS1 in infected cells up to 72 h p.i.
As addition of the FLAG tag to either IRS1 or TRS1 had no obvious effect on protein or virus production up to 72 h p.i., it was decided to conduct all subsequent assays at this time point.
Association of UL44 with FLAG-tagged IRS1 and FLAG-tagged TRS1
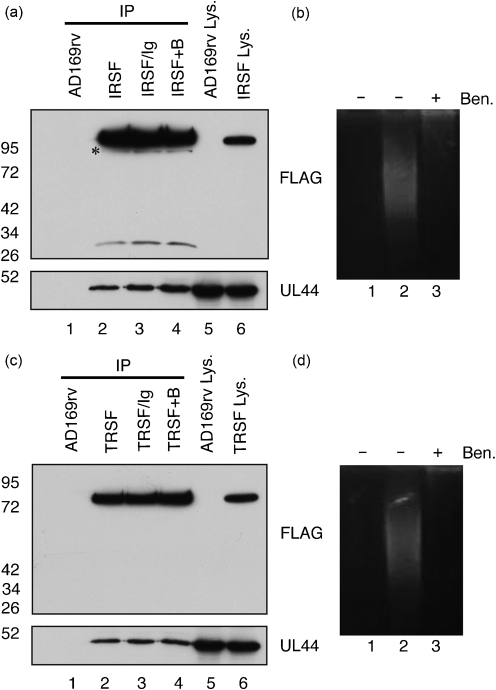
Lysate was prepared 72 h p.i. from cells infected with AD169rv or IRSF and protein was immunoprecipitated from those lysates using beads bearing anti-FLAG antibody. Immunoprecipitated protein was examined by Western blotting (Fig. 4a). Using a mAb recognizing FLAG (Fig. 4a, top panel), a band corresponding to full-length FLAG-tagged IRS1 was observed in IRSF-infected cell lysate (lane 6) and protein immunoprecipitated from IRSF-infected cell lysate (lane 2). A band of approximately 30 kDa could be observed in protein immunoprecipitated from IRSF-infected cell lysate (lane 2). It is likely that this band corresponds to the protein IRS1263 (Romanowski & Shenk, 1997). Another band with a molecular mass lower than that of the full-length FLAG-tagged IRS1 was also observed in protein immunoprecipitated from IRSF-infected cell lysate (lane 2, marked with an asterisk). The identity of this protein is unclear, but it may correspond to one of the less than full-length IRS1 proteins observed by Romanowski & Shenk (1997). No proteins could be observed in AD169rv-infected cell lysate (lane 5) or protein immunoprecipitated from AD169rv-infected cell lysate (lane 1). Using antibody recognizing UL44 (Fig. 3a, bottom panel), UL44 could be observed in both cell lysates (lanes 5 and 6) and in protein immunoprecipitated from IRSF-infected cell lysate (lane 2). It could not be detected in protein immunoprecipitated from AD169rv-infected cell lysate (lane 1).
Fig. 4.
Detection of protein immunoprecipitated from IRSF- and TRSF-infected cell lysate by Western blotting. (a, c) Proteins immunoprecipitated (IP) from lysate of AD169rv- and IRSF-infected cells (a) or AD169rv- and TRSF-infected cells (c) prepared 72 h p.i. were separated on a 10 % polyacrylamide gel and examined by Western blotting using antibodies recognizing FLAG (top panels) or UL44 (bottom panels). Immunoprecipitated proteins are examined in lanes 1–4. Lysates precleared with control antibody before IP (IRSF/Ig or TRSF/Ig) are shown in lane 3 of both figures. Lysates treated with Benzonase (IRS+B or TRS+B) are shown in lane 4 of both figures. Lysates (Lys.) used in the IP are examined in lanes 5 and 6. The positions of molecular mass markers (in kDa) are indicated to the left of the figure. The novel IRS1 band discussed in the text is marked with an asterisk. (b, d) Cell lysate used in the IP in lanes 2 and 4 of (a) and (c) was run out on an ethidium bromide-stained 0.8 % agarose gel [(b) and (d), respectively]. Lanes: 1, no sample; 2, IP in the absence of Benzonase (Ben.); 3, IP in the presence of Ben.
Lysate was also prepared from cells infected with AD169rv or TRSF and protein immunoprecipitated using beads bearing anti-FLAG antibody. Immunoprecipitated protein was also examined by Western blotting (Fig. 4c). Using a mAb recognizing FLAG (Fig. 4c, top panel), a band corresponding to full-length FLAG-tagged TRS1 was observed in TRSF-infected cell lysate (lane 6) and protein immunoprecipitated from TRSF-infected cell lysate (lane 2). No proteins could be observed in AD169rv-infected cell lysate (lane 5) or protein immunoprecipitated from AD169rv-infected cell lysate (lane 1). Using antibody recognizing UL44 (Fig. 4c, bottom panel), UL44 could be observed in both cell lysates (lanes 5 and 6) and in protein immunoprecipitated from TRSF-infected cell lysate (lane 2). It could not be detected in protein immunoprecipitated from AD169rv-infected cell lysate (lane 1). UL44, therefore, associates with IRS1 and TRS1 in infected cell lysate. Taken together, the data shown in Fig. 4(a, c) confirm by reciprocal co-IP the association of UL44 with IRS1 and TRS1 in infected cell lysate observed in Fig. 2.
UL44 could also be detected in protein immunoprecipitating with FLAG-tagged IRS1 and FLAG-tagged TRS1 when protein was eluted from beads in the presence of FLAG peptide (data not shown), indicating that the association of UL44 with beads is not due to non-specific interaction of the proteins with beads. Additionally, lysates were also precleared with antibody of the same isotype as the anti-FLAG antibody used for IP. The immunoprecipitated protein from these experiments is shown in Fig. 4(a, c), lane 3. Preclearing lysate with control antibody did not appear to affect the levels of IRS1 or TRS1 proteins immunoprecipitated from lysate or the level of UL44 associated with them. It is unlikely, therefore, that the presence of these proteins in immunoprecipitated protein is due to non-specific interaction of the proteins with antibody during IP.
Nucleic acid-binding proteins can associate during IP due to their adjacent binding on nucleic acid, rather than due to protein–protein interaction (Lai & Herr, 1992; Taylor & Knipe, 2004). To determine whether nucleic acid is required for association of UL44 and IRS1 or UL44 and TRS1 in cell lysate during IP, IP was also carried out in the presence of the non-specific nuclease Benzonase (Novagen) (Fig. 4a, c, lane 4). The presence of Benzonase during IP did not appear to affect the levels of IRS1 or TRS1 proteins immunoprecipitated from lysate or the level of UL44 associated with them. To confirm the action of Benzonase on nucleic acid, the cell lysates from which protein in lanes 2 and 4 of Fig. 4(a, c) was immunoprecipitated were examined on an ethidium bromide-stained agarose gel (Fig. 4b, d). In untreated lysate (Fig. 4b, d, lane 2), robust staining of nucleic acid was observed. In lysate treated with Benzonase (Fig. 4b, d, lane 3), little or no nucleic acid could be observed, confirming the action of Benzonase in the infected cell lysate during IP. It is, therefore, unlikely that the association of UL44 with IRS1 or UL44 with TRS1 during IP is due to their adjacent binding to nucleic acid during IP.
To extend our understanding of the association of UL44, IRS1 and TRS1 in infected cell lysate, IP of proteins from IRSF- and TRSF-infected cell lysate using anti-FLAG antibody was repeated, and immunoprecipitated protein was examined by Western blotting using antibodies recognizing FLAG, IRS1, TRS1 and UL44 (Fig. 5). As shown in Fig. 4, the IRS1 proteins and TRS1 could be detected in immunoprecipitated protein using an antibody recognizing FLAG (Fig. 5, top panel), as could UL44 using an antibody recognizing UL44 (Fig. 5, bottom panel). The blot was also probed with a combination of antibodies recognizing either IRS1 or TRS1 (Fig. 5, middle panel). Both IRS1 and TRS1 were observed in all cell lysates (lanes 4–6). FLAG-tagged IRS1, but not TRS1, could be detected in protein immunoprecipitated from IRSF-infected cell lysate (lane 2). FLAG-tagged TRS1, but not IRS1, could be detected in protein immunoprecipitated from TRSF-infected cell lysate (lane 3). The minor IRS1 proteins detected by antibody recognizing FLAG in protein immunoprecipitated from IRSF-infected cell lysate (Figs 4a and 5a, top panels) could not be detected with the antibody recognizing IRS1 (not shown). It is likely that these proteins were present at levels below the level of detection with antibody recognizing IRS1. Taken together, these data indicate that FLAG-tagged IRS1 and TRS1 do not interact simultaneously with UL44, nor do they interact with each other in infected cell lysate.
Fig. 5.
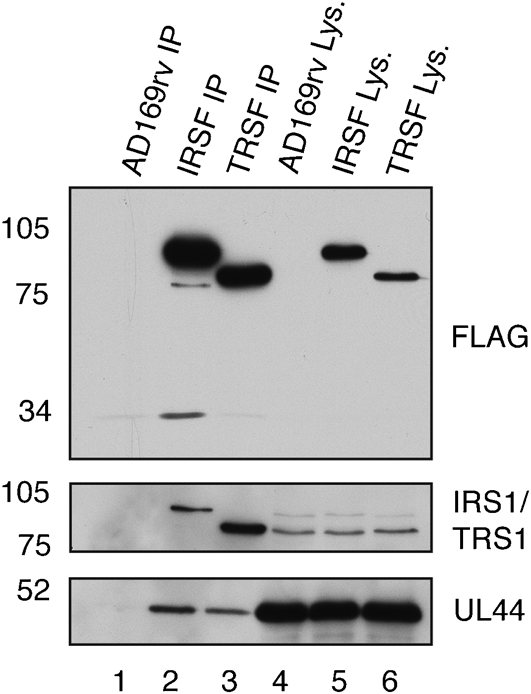
Detection by Western blotting of IRS1 and TRS1 in protein immunoprecipitated from IRSF- and TRSF-infected cell lysate. Lysate from AD169rv-infected (lane 4), IRSF-infected (lane 5) and TRSF-infected (lane 6) cells and protein immunoprecipitated using an anti-FLAG antibody from those lysates (lanes 1–3, respectively) were separated on a 10 % polyacrylamide gel. Proteins in each lane were examined by Western blotting for the presence of FLAG-tagged protein (top panel), IRS1 or TRS1 (middle panel) or UL44 (bottom panel) using antibodies recognizing these proteins, as indicated to the right of the figure. Note that, in the middle panel, a combination of antibodies recognizing IRS1 or TRS1 was utilized. The positions of molecular mass markers (in kDa) are indicated to the left of the figure.
Association of IRS1 and TRS1 with UL44 in glutathione S-transferase (GST) pull-down experiments
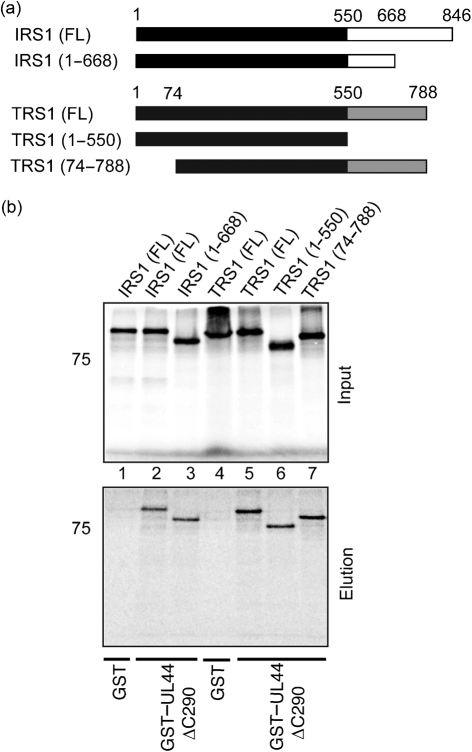
To determine what regions of IRS1 and TRS1 are required for association with UL44, the ability of IRS1 and TRS1 mutants to bind UL44 in GST pull-down experiments was examined. A range of IRS1 and TRS1 mutants (Fig. 6a) were tested for their ability to bind GST or a fusion protein of GST joined to a truncated form of UL44 lacking the protein's carboxyl-terminal 143 residues, UL44ΔC290 (GST–UL44ΔC290) (Fig. 6b). Full-length UL44 was not used, as the carboxyl terminus of this protein is readily cleaved in bacteria (Appleton et al., 2004), so that generating substantial quantities of full-length protein is problematic. Radiolabelled IRS1 and TRS1 proteins bound to GST–UL44ΔC290 (lanes 2 and 5, bottom panel, respectively), but not GST (lanes 1 and 4, bottom panel, respectively), indicating an association of IRS1 and TRS1 with the UL44 portion of the GST–UL44ΔC290 fusion protein. None of a partial truncation of the IRS1 carboxyl terminus [IRS1(1–668), lane 3, bottom panel], a complete truncation of the TRS1 carboxyl terminus [TRS1(1–550), lane 6, bottom panel] or a partial truncation of the amino terminus shared by IRS1 and TRS1 [TRS1(74–788), lane 7, bottom panel] appeared to have any effect on binding of protein to GST–UL44ΔC290, indicating that the deleted regions are not required for association with UL44. Residues within the amino terminus that is identical in both proteins are, therefore, sufficient for association of IRS1 or TRS1 with UL44. Interaction of TRS1(74–788) with GST–UL44ΔC290 in our assays indicates that the extreme amino terminus of IRS1 or TRS1 (residues 1–73) is, however, not required for UL44 binding. As the carboxyl terminus of IRS1 is not required for interaction with UL44, these data may help to explain the inability to observe IRS1263, produced from a transcript encoding the carboxyl terminus of IRS1 (Fig. 1), in protein immunoprecipitating with UL44 (Fig. 2).
Fig. 6.
Binding of IRS1 and TRS1 mutants to UL44 in vitro. (a) Schematic of IRS1 and TRS1 proteins used. Full-length (FL) IRS1 and TRS1 proteins are shown, as are mutants of these proteins. Each mutation is noted to the left of the figure. The position of certain amino acids in each protein in the diagram above both IRS1 and TRS1 proteins is noted. Black regions represent the amino terminus common to IRS1 and TRS1; white and grey regions represent the unique carboxyl termini of IRS1 and TRS1, respectively. (b) GST or GST–UL44ΔC290 was incubated with radiolabelled IRS1 or TRS1 proteins and passed over a glutathione column. The GST protein used in each reaction is noted below the figure; the radiolabelled protein used in each reaction is noted above the figure. The top panel of the figure shows the proteins used in each reaction (Input) and the bottom panel shows the proteins eluted from the glutathione column (Elution). The positions of molecular mass markers (in kDa) are indicated to the left of each figure.
DISCUSSION
Previous studies suggested that the viral protein IRS1, like UL54, UL84 and nucleolin (Appleton et al., 2004; Ertl & Powell, 1992; Gao et al., 2008; Loregian et al., 2004a, b; Strang et al., 2009, 2010), associates with UL44 in infected cell lysate (Strang et al., 2010). In this study, association of UL44 with IRS1 or with TRS1, which shares a common amino terminus with IRS1, was demonstrated by reciprocal co-IP from lysate of cells infected with virus expressing FLAG-tagged UL44, IRS1 or TRS1. Analysis of protein immunoprecipitating with FLAG-tagged IRS1 and TRS1 indicates that these proteins do not interact simultaneously with UL44. Interaction of UL44 with either IRS1 or TRS1 was also observed in vitro using GST pull-down assays, and residues within the amino terminus that are identical in both proteins were shown to be sufficient for association of IRS1 or TRS1 with UL44. Taken together, these data suggest that FLAG-tagged IRS1 and TRS1 associate independently with UL44, potentially competing for association with UL44.
Our experiments utilized viruses expressing either FLAG-tagged IRS1 (IRSF) or FLAG-tagged TRS1 (TRSF). Addition of the FLAG tag to either IRS1 or TRS1 did not affect accumulation of these proteins at 3 days p.i. Virus replication was not affected by addition of the tag to IRS1. However, TRSF exhibited reduced virus replication at late time points (after 3 days p.i.). Presently, we cannot exclude the possibility that TRSF possesses a mutation outside TRS1 that is the cause of the defect in virus replication. Nevertheless, this replication defect requires us to interpret our results with additional caution. Mitigating this concern, we conducted co-IP experiments with these viruses using samples prepared 3 days p.i., a time point at which TRSF replication was not reduced. Additionally, we not only detected the association of FLAG-tagged IRS1 or TRS1 with UL44 in IRSF- and TRSF-infected cell lysate, but we also detected the association of untagged IRS1 and TRS1 with FLAG-tagged UL44 in FLAG–44-infected cell lysate. Finally, the observations made using IRSF and TRSF that IRS1 or TRS1 do not associate simultaneously with UL44 in infected cell lysate tally with our observation that identical regions of untagged IRS1 and TRS1 interact with UL44.
A mutant similar to TRSF in which a haemagglutinin tag was added to the carboxyl terminus of TRS1 exhibited an approximately 2-fold defect in virus replication in the context of an IRS1 deletion (Marshall et al., 2009). A rather different TRS1 mutant, in which a segment of the unique carboxyl terminus of TRS1 was substituted by a marker cassette, exhibited a more profound defect on virus replication, with a 5-fold reduction at high m.o.i. and 20-fold reduction at low m.o.i. (Blankenship & Shenk, 2002). Replication of this mutant was reduced as early as 72 h p.i. at both high and low m.o.i. (Blankenship & Shenk, 2002). In cells infected with this virus, small decreases in expression of certain viral mRNAs and proteins were observed and several viral tegument proteins were found to be mislocalized (Blankenship & Shenk, 2002). TRS1 is, therefore, important for efficient virus replication in the presence and absence of IRS1. Further analysis of the extreme carboxyl terminus of TRS1 is required to determine what functions that region provides and how the presence of tags at the carboxyl terminus affects those functions.
Our experiments indicate that both IRS1 and TRS1 associate with UL44 via their amino termini, a region that is identical in both proteins. It is likely, therefore, that the same residues required for IRS1 association with UL44 are required for association of TRS1 with UL44. The residues of UL44 that are required for interaction are presently unknown, although our data indicate that the carboxyl terminus of UL44 is not required. UL44 possesses a hydrophobic crevice similar in structure to that used by proteins to bind to PCNA (Appleton et al., 2004, 2006). It is possible that IRS1 or TRS1 interacts with UL44 by binding within this hydrophobic crevice. If so, then, given that UL44 is a dimer (Appleton et al., 2004), our finding that FLAG-tagged IRS1 and TRS1 do not interact simultaneously with UL44 in infected cells suggests that each of these proteins would complex with UL44 in a crevice on one monomer, whilst the crevice on the other monomer would already be occupied. It should be noted, however, that not all UL44-interacting proteins interact with UL44 at its hydrophobic crevice, as we have shown that UL84 does not bind UL44 within this region (Strang et al., 2009). Another possibility is that IRS1 or TRS1 interacts with UL44 via its dimer interface.
The observation that the same region in IRS1 and TRS1 mediates association with UL44, but that these proteins do not associate simultaneously with UL44, suggests that these proteins may compete for association with UL44. Moreover, these data also suggest that UL44 is a component of different protein complexes in infected cell lysate, including those that contain, among other proteins, UL44 and IRS1 or UL44 and TRS1. It will be interesting to determine whether other proteins known to interact with UL44, such as UL54, UL84 and nucleolin (Ertl & Powell, 1992; Gao et al., 2008; Strang et al., 2009, 2010), are also present in complexes that contain UL44 and IRS1 or UL44 and TRS1, and whether IRS1 and TRS1 provide the same function in these complexes.
Several reports indicate that IRS1 and TRS1 are important for gene expression (Kerry et al., 1996; Sarisky & Hayward, 1996; Stasiak & Mocarski, 1992). UL44 has also been implicated in viral gene expression (Isomura et al., 2007, 2008). In this context, IRS1 or TRS1 could, therefore, conceivably act as a transcriptional co-factor with UL44 in the infected cell. Alternatively, UL44 may act with IRS1 and TRS1 to affect gene expression by interacting with these proteins while they interact with and relocate PKR (Hakki et al., 2006). We currently have no evidence that IRS1 or TRS1 interaction with PKR is relevant to their function in association with UL44. PKR was not found to associate with UL44 and IRS1 or TRS1 in our previous studies (Strang et al., 2009, 2010). However, it is possible that PKR was present below the level of detection in our assays.
Interaction between Epstein–Barr virus (EBV) DNA polymerase processivity factor BMRF1 and the viral transcriptional transactivator BZLF1 has been reported (Zhang et al., 1996). The interaction of these proteins appears to inhibit BZLF1-mediated transcription of BMRF1. Also, the presence of both BZLF1 and BRMF1 enhances transcription of BHLF1, a gene found within the EBV oriLyt. Direct interaction between BZLF1 and BMRF1 is, however, not required for this function (Zhang et al., 1996). The action of BZLF1 and BMRF1 at oriLyt is thought to be necessary for virus replication (Zhang et al., 1997). Further study will clarify whether, as in EBV replication, interaction of the HCMV DNA polymerase processivity factor (UL44) and proteins involved in gene expression (IRS1 or TRS1) facilitate multiple functions in HCMV replication, such as viral DNA replication and transcription.
METHODS
IP of proteins with FLAG antibody.
HFF cells (ATCC no. CRL-1684; 2.5×106 cells) were infected in 100 mm dishes with the viruses indicated in the text at an m.o.i. of 3. Seventy-two hours p.i., plates were washed and IP from clarified cell lysate was carried out as described previously (Kamil & Coen, 2007). Where indicated, clarified lysate was precleared before IP by adding 20 μl protein A–Sepharose beads and 5 μg isotype-control antibody (Bethyl Laboratories) and incubating at 4 °C with rotation for 3 h. Beads were removed by centrifugation. Where indicated, 800 U Benzonase (Novagen) was added to clarified lysate before addition of FLAG antibody.
Western blotting.
Western blotting of proteins separated on 10 % polyacrylamide gels was carried out as described elsewhere (Strang & Stow, 2005), using as primary antibodies mAbs recognizing UL44 (Virusys; 1 : 1000 dilution) or β-actin (Sigma; 1 : 5000 dilution) or antibodies recognizing the unique carboxyl termini of IRS1 (mAb 8B3, 1 : 100 dilution) or TRS1 (mAb 9A1, 1 : 100 dilution) (Romanowski & Shenk, 1997) (generously provided by Tom Shenk, Princeton University, Princeton, NJ, USA). In IP experiments, anti-mouse TruBlot antibody conjugated to horseradish peroxidase (HRP) (eBioscience), which recognizes the native but not the denatured form of antibody, was used to detect primary antibodies. Goat anti-mouse HRP-conjugated antibody (Southern Biotech) was used to detect primary antibodies in all other experiments. In all blots to detect FLAG-tagged protein, anti-FLAG antibody M2 conjugated to HRP (Sigma; 1 : 1000 dilution) was used. Chemiluminescence solution (Pierce) was used in all experiments to detect HRP.
Mutation of the BACs and reconstitution of virus.
A single FLAG epitope (DYKDDDDK) was inserted immediately before the termination codon of the IRS1 or TRS1 coding sequence in the BAC AD169-BAC (Hobom et al., 2000) using two-step Red recombination (Tischer et al., 2006) in Escherichia coli strain GS1783 (a kind gift from Gregory Smith, Northwestern University, Evanston, IL, USA). Primer sequences are available at https://coen.med.harvard.edu. BACs AD169-BAC, AD169-IRSF and AD169-TRSF were electroporated into HFF cells as described by Tischer et al. (2006) with plasmids pCGN71 (Baldick et al., 1997) expressing the viral transcriptional transactivator pp71 and pBRep-Cre (Hobom et al., 2000) to generate viruses AD169rv, IRSF and TRSF, respectively.
GST pull-down assays.
Recombinant GST and GST–UL44ΔC290 proteins were purified from E. coli BL21(DE3)pLysS (Novagen) harbouring plasmids pD15–GST or pD15–UL44ΔC290 as described elsewhere (Loregian et al., 2004a). In vitro transcription–translation of plasmids expressing IRS1, TRS1 or luciferase proteins was performed using the TNT T7 coupled transcription–translation system (Promega) in the presence of [35S]methionine (Amersham Pharmacia Biotech) according to the manufacturer's instructions. Plasmids expressing full-length and mutant IRS1 or TRS1 have been described elsewhere (Hakki & Geballe, 2005; Hakki et al., 2006). A plasmid expressing luciferase was obtained from Promega as part of the TNT T7 kit. GST pull-down reactions were performed as described elsewhere (Strang et al., 2009).
Acknowledgments
We gratefully acknowledge the advice and encouragement received from all members of the Coen laboratory and thank Tom Shenk (Princeton University, Princeton, NJ, USA) and Gregory Smith (Northwestern University, Evanston, IL, USA) for their kind provision of reagents. This work was supported in part by NIH grants AI19838 and AI26077 to D. M. C., NIH grant AI26672 to A. P. G. and an award from the William Randolph Hearst Fund to B. L. S.
References
- Appleton, B. A., Loregian, A., Filman, D. J., Coen, D. M. & Hogle, J. M. (2004). The cytomegalovirus DNA polymerase subunit UL44 forms a C clamp-shaped dimer. Mol Cell 15, 233–244. [DOI] [PubMed] [Google Scholar]
- Appleton, B. A., Brooks, J., Loregian, A., Filman, D. J., Coen, D. M. & Hogle, J. M. (2006). Crystal structure of the cytomegalovirus DNA polymerase subunit UL44 in complex with the C terminus from the catalytic subunit. Differences in structure and function relative to unliganded UL44. J Biol Chem 281, 5224–5232. [DOI] [PubMed] [Google Scholar]
- Baldick, C. J., Jr, Marchini, A., Patterson, C. E. & Shenk, T. (1997). Human cytomegalovirus tegument protein pp71 (ppUL82) enhances the infectivity of viral DNA and accelerates the infectious cycle. J Virol 71, 4400–4408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blankenship, C. A. & Shenk, T. (2002). Mutant human cytomegalovirus lacking the immediate-early TRS1 coding region exhibits a late defect. J Virol 76, 12290–12299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Child, S. J., Hakki, M., De Niro, K. L. & Geballe, A. P. (2004). Evasion of cellular antiviral responses by human cytomegalovirus TRS1 and IRS1. J Virol 78, 197–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ertl, P. F. & Powell, K. L. (1992). Physical and functional interaction of human cytomegalovirus DNA polymerase and its accessory protein (ICP36) expressed in insect cells. J Virol 66, 4126–4133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao, Y., Colletti, K. & Pari, G. S. (2008). Identification of human cytomegalovirus UL84 virus- and cell-encoded binding partners by using proteomics analysis. J Virol 82, 96–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hakki, M. & Geballe, A. P. (2005). Double-stranded RNA binding by human cytomegalovirus pTRS1. J Virol 79, 7311–7318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hakki, M., Marshall, E. E., De Niro, K. L. & Geballe, A. P. (2006). Binding and nuclear relocalization of protein kinase R by human cytomegalovirus TRS1. J Virol 80, 11817–11826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hobom, U., Brune, W., Messerle, M., Hahn, G. & Koszinowski, U. H. (2000). Fast screening procedures for random transposon libraries of cloned herpesvirus genomes: mutational analysis of human cytomegalovirus envelope glycoprotein genes. J Virol 74, 7720–7729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isomura, H., Stinski, M. F., Kudoh, A., Nakayama, S., Iwahori, S., Sato, Y. & Tsurumi, T. (2007). The late promoter of the human cytomegalovirus viral DNA polymerase processivity factor has an impact on delayed early and late viral gene products but not on viral DNA synthesis. J Virol 81, 6197–6206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isomura, H., Stinski, M. F., Kudoh, A., Murata, T., Nakayama, S., Sato, Y., Iwahori, S. & Tsurumi, T. (2008). Noncanonical TATA sequence in the UL44 late promoter of human cytomegalovirus is required for the accumulation of late viral transcripts. J Virol 82, 1638–1646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamil, J. P. & Coen, D. M. (2007). Human cytomegalovirus protein kinase UL97 forms a complex with the tegument phosphoprotein pp65. J Virol 81, 10659–10668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerry, J. A., Priddy, M. A., Jervey, T. Y., Kohler, C. P., Staley, T. L., Vanson, C. D., Jones, T. R., Iskenderian, A. C., Anders, D. G. & Stenberg, R. M. (1996). Multiple regulatory events influence human cytomegalovirus DNA polymerase (UL54) expression during viral infection. J Virol 70, 373–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai, J. S. & Herr, W. (1992). Ethidium bromide provides a simple tool for identifying genuine DNA-independent protein associations. Proc Natl Acad Sci U S A 89, 6958–6962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loregian, A., Appleton, B. A., Hogle, J. M. & Coen, D. M. (2004a). Residues of human cytomegalovirus DNA polymerase catalytic subunit UL54 that are necessary and sufficient for interaction with the accessory protein UL44. J Virol 78, 158–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loregian, A., Appleton, B. A., Hogle, J. M. & Coen, D. M. (2004b). Specific residues in the connector loop of the human cytomegalovirus DNA polymerase accessory protein UL44 are crucial for interaction with the UL54 catalytic subunit. J Virol 78, 9084–9092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maga, G. & Hubscher, U. (2003). Proliferating cell nuclear antigen (PCNA): a dancer with many partners. J Cell Sci 116, 3051–3060. [DOI] [PubMed] [Google Scholar]
- Marshall, E. E., Bierle, C. J., Brune, W. & Geballe, A. P. (2009). Essential role for either TRS1 or IRS1 in human cytomegalovirus replication. J Virol 83, 4112–4120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moldovan, G. L., Pfander, B. & Jentsch, S. (2007). PCNA, the maestro of the replication fork. Cell 129, 665–679. [DOI] [PubMed] [Google Scholar]
- Pari, G. S. & Anders, D. G. (1993). Eleven loci encoding trans-acting factors are required for transient complementation of human cytomegalovirus oriLyt-dependent DNA replication. J Virol 67, 6979–6988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pari, G. S., Kacica, M. A. & Anders, D. G. (1993). Open reading frames UL44, IRS1/TRS1, and UL36–38 are required for transient complementation of human cytomegalovirus oriLyt-dependent DNA synthesis. J Virol 67, 2575–2582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prichard, M. N., Lawlor, H., Duke, G. M., Mo, C., Wang, Z., Dixon, M., Kemble, G. & Kern, E. R. (2005). Human cytomegalovirus uracil DNA glycosylase associates with ppUL44 and accelerates the accumulation of viral DNA. Virol J 2, 55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranneberg-Nilsen, T., Dale, H. A., Luna, L., Slettebakk, R., Sundheim, O., Rollag, H. & Bjoras, M. (2008). Characterization of human cytomegalovirus uracil DNA glycosylase (UL114) and its interaction with polymerase processivity factor (UL44). J Mol Biol 381, 276–288. [DOI] [PubMed] [Google Scholar]
- Romanowski, M. J. & Shenk, T. (1997). Characterization of the human cytomegalovirus irs1 and trs1 genes: a second immediate-early transcription unit within irs1 whose product antagonizes transcriptional activation. J Virol 71, 1485–1496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarisky, R. T. & Hayward, G. S. (1996). Evidence that the UL84 gene product of human cytomegalovirus is essential for promoting oriLyt-dependent DNA replication and formation of replication compartments in cotransfection assays. J Virol 70, 7398–7413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stasiak, P. C. & Mocarski, E. S. (1992). Transactivation of the cytomegalovirus ICP36 gene promoter requires the alpha gene product TRS1 in addition to IE1 and IE2. J Virol 66, 1050–1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strang, B. L. & Stow, N. D. (2005). Circularization of the herpes simplex virus type 1 genome upon lytic infection. J Virol 79, 12487–12494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strang, B. L., Sinigalia, E., Silva, L. A., Coen, D. M. & Loregian, A. (2009). Analysis of the association of the human cytomegalovirus DNA polymerase subunit UL44 with the viral DNA replication factor UL84. J Virol 83, 7581–7589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strang, B. L., Boulant, S. & Coen, D. M. (2010). Nucleolin can associate with the human cytomegalovirus DNA polymerase accessory subunit UL44 and is necessary for viral replication. J Virol 84, 1771–1784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor, T. J. & Knipe, D. M. (2004). Proteomics of herpes simplex virus replication compartments: association of cellular DNA replication, repair, recombination, and chromatin remodeling proteins with ICP8. J Virol 78, 5856–5866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tischer, B. K., von Einem, J., Kaufer, B. & Osterrieder, N. (2006). Two-step Red-mediated recombination for versatile high-efficiency markerless DNA manipulation in Escherichia coli. Biotechniques 40, 191–197. [DOI] [PubMed] [Google Scholar]
- Zhang, Q., Hong, Y., Dorsky, D., Holley-Guthrie, E., Zalani, S., Elshiekh, N. A., Kiehl, A., Le, T. & Kenney, S. (1996). Functional and physical interactions between the Epstein–Barr virus (EBV) proteins BZLF1 and BMRF1: effects on EBV transcription and lytic replication. J Virol 70, 5131–5142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, Q., Holley-Guthrie, E., Ge, J. Q., Dorsky, D. & Kenney, S. (1997). The Epstein–Barr virus (EBV) DNA polymerase accessory protein, BMRF1, activates the essential downstream component of the EBV oriLyt. Virology 230, 22–34. [DOI] [PubMed] [Google Scholar]