Abstract
Triple-reassortant swine influenza viruses circulating in North American pigs contain the internal genes derived from swine (matrix, non-structural and nucleoprotein), human [polymerase basic 1 (PB1)] and avian (polymerase acidic and PB2) influenza viruses forming a constellation of genes that is well conserved and is called the triple-reassortant internal gene (TRIG) cassette. In contrast, the external genes [haemagglutinin (HA) and neuraminidase (NA)] are less conserved, reflecting multiple reassortant events that have produced viruses with different combinations of HA and NA genes. This study hypothesized that maintenance of the TRIG cassette confers a selective advantage to the virus. To test this hypothesis, pigs were co-infected with the triple-reassortant H3N2 A/Swine/Texas/4199-2/98 (Tx/98) and the classical H1N1 A/Swine/Iowa/15/1930 viruses and co-housed with a group of sentinel animals. This direct contact group was subsequently moved into contact with a second group of naïve animals. Four different subtypes (H1N1, H1N2, H3N1 and H3N2) of influenza virus were identified in bronchoalveolar lavage fluid collected from the lungs of the experimentally infected pigs, with most of the viruses containing TRIG from the Tx/98 virus. Interestingly, only the intact H3N2 Tx/98 virus was transmitted from the infected pigs to the direct-contact animals and from them to the second contact group of pigs. These results demonstrated that multiple reassortments can occur within a host; however, only specific gene constellations are readily transmissible. It was concluded that certain HA and NA gene pairs, in conjunction with the TRIG cassette, may have a competitive advantage over other combinations for transmission and maintenance in swine.
INTRODUCTION
Influenza A viruses infect a wide variety of animal species including humans, horses, pigs, dogs, sea mammals and birds. All 16 haemagglutinin (HA) and nine neuraminidase (NA) subtypes of influenza A virus have been isolated from aquatic birds (Alexander, 2000; Fouchier et al., 2005; Webster et al., 1992), which are thought to be the primary reservoirs for all subtypes of influenza A viruses from which novel viruses can emerge and infect other animal species (Webster et al., 1992). Influenza A virus, a negative-strand RNA virus in the family Orthomyxoviridae, contains eight RNA segments encoding ten or 11 proteins. The segmented nature of the influenza viral genome provides opportunity for reassortment when two (or more) different influenza viruses infect the same cell or host. Although only three subtypes (H1N1, H3N2 and H1N2) of influenza A viruses are consistently isolated from pigs worldwide, pigs are known to be susceptible to infection with many subtypes of influenza A viruses (Kida et al., 1994). Because naturally occurring reassortant viruses derived from different host species have been recovered from pigs, they have been considered to be a ‘mixing vessel’, supporting potential influenza virus reassortment (Scholtissek, 1994). Involvement of pigs in interspecies transmission of influenza A viruses between birds and humans is at least partially due to their respiratory tract lining epithelial cells having receptors for both avian and human influenza viruses (Ito et al., 1998). The recent isolation of a unique H2N3 virus from pigs provided direct evidence that swine are able to act as a natural host from which novel influenza viruses may emerge (Ma et al., 2007).
The first swine influenza virus (SIV) isolate belonging to the H1N1 subtype in the USA was identified in 1930 (Shope, 1931); subsequently, a similar virus was isolated from humans (Smith et al., 1933). This H1N1 swine virus and closely related viruses are designated classical H1N1 (cH1N1) viruses and currently still circulate in swine populations worldwide. With the exception of one isolation of human H3N2 virus from pigs in Colorado in 1977 (Karasin et al., 2000b), only the cH1N1 virus was isolated from US swine prior to 1998 (Olsen, 2002). In August 1998, a novel subtype H3N2 virus (double-reassortant H3N2 virus) was isolated from pigs in North Carolina that contained HA, NA and polymerase basic 1 (PB1) genes from human influenza virus origin and the remaining five genes from the cH1N1 SIV origin (Zhou et al., 1999). Subsequent H3N2 isolates from other states were triple-reassortant viruses containing HA, NA and PB1 genes from human influenza viruses, matrix (M), non-structural (NS) and nucleoprotein (NP) genes from classical swine viruses, and polymerase acidic (PA) and PB2 genes from avian viruses (Webby et al., 2000; Zhou et al., 1999). Subsequent reassortments occurred between the H3N2 and the cH1N1 subtypes producing H1N2 (Karasin et al., 2000a), reassortant H1N1 (rH1N1) (Webby et al., 2004) and H3N1 viruses (Lekcharoensuk et al., 2006; Ma et al., 2006). At present, the H3N2, rH1N1 and H1N2 viruses have become endemic, and co-circulate in most major swine-producing regions of both the USA and Canada (Choi et al., 2002; Richt et al., 2003; Webby et al., 2004). More recently, the introduction of human-like H1 viruses that are genetically and antigenically distinct from the classical swine H1 lineage was identified in pigs in both Canada and the USA (Karasin et al., 2006), and these viruses have spread among US swine herds (Vincent et al., 2009). All the successful reassortant SIVs that have become endemic in US swine herds to date contain similar internal genes representing the PA and PB2 genes of avian origin, NS, NP and M genes of classical swine origin, and the PB1 gene of human origin; this has been referred to as the triple-reassortant internal gene (TRIG) cassette (Ma et al., 2009; Vincent et al., 2008). This consistent finding suggests that the TRIG cassette can accept multiple HA and NA types and may contribute to a selective advantage over other influenza viruses infecting swine. The 2009 human pandemic H1N1 virus is a reassortant between North American triple-reassortant and Eurasian SIVs and has also been found in pigs in over 20 countries including the USA. Interestingly, this human virus has a modified TRIG, which contains a new M gene of the Eurasian swine lineage (Garten et al., 2009; Smith et al., 2009). To date, no studies have been conducted on the role of the TRIG cassette in the production of current endemic SIVs in North American swine herds and how this TRIG cassette affects virus replication and adaptation in pigs. In this study, we used the triple-reassortant prototype, A/Swine/Texas/4199-2/98 (H3N2) (Tx/98), and A/Swine/Iowa/15/1930 (cH1N1) (IA/30) SIVs to co-infect pigs that were then exposed to a contact group of pigs, which was subsequently exposed to a second group of pigs, to investigate reassortment between these viruses and to characterize which constellation of viral genes is critical for virus replication and efficient maintenance and transmission in pigs.
RESULTS
Generation and identification of a reverse genetics-generated IA/30 (rgIA/30) virus with novel restriction enzyme sites in each segment
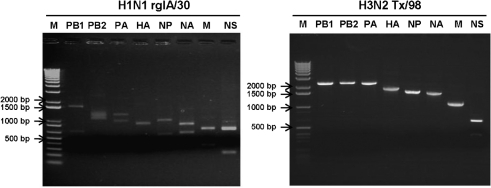
An rgIA/30 virus was rescued by an eight-plasmid reverse genetics system and the sequence was confirmed by sequencing. In order to show that the eight individual gene segments contained novel restriction sites, the RNA was amplified by RT-PCR and enzyme digestion analysis was conducted. The results showed that each gene of rgIA/30 contained a novel restriction enzyme site when compared with that of the wild-type IA/30 virus. Thus, rgIA/30 could easily be differentiated from Tx/98 virus by restriction enzyme digestion, as demonstrated in Fig. 1 and Table 1.
Fig. 1.
Introduction of novel restriction enzyme sites into each segment of rgIA/30 SIV to differentiate it from Tx/98. The introduced novel restriction enzyme sites in each gene segment of the rgIA/30 SIV were used to differentiate it from the Tx/98 virus as shown in Table 1. RT-PCR was performed for RNA isolated from rgIA/30 and Tx/98, and the PCR products were incubated with different restriction enzymes as indicated and separated on agarose gels.
Table 1.
Differentiation of rgIA/30 and Tx/98 by restriction enzyme digestion of each gene segment
Fragment sizes are given in bp.
| Virus | Gene/enzyme | |||||||
|---|---|---|---|---|---|---|---|---|
| PB1/XmaI | PB2/BspEI | PA/BamHI | HA/BspEI | NP/BpsEI | NA/BpsEI | M/SacI | NS/XhoI | |
| H3N2 Tx/98 | 2341 | 2341 | 2233 | 1778 | 1565 | 1413 | 1027 | 581 |
| 309 | ||||||||
| H1N1 rglA/30 | 1676 | 1240 | 1244 | 901 | 987 | 804 | 701 | 688 |
| 665 | 1101 | 989 | 878 | 578 | 609 | 326 | 202 | |
Viruses isolated from the primary co-infected pigs
Six 6-week-old pigs were co-infected with the cH1N1 rgIA/30 and triple-reasssortant H3N2 Tx/98 SIV viruses. No acute respiratory clinical signs were observed in challenged pigs. Necropsy revealed extensive macroscopic lung lesions (plum-coloured, consolidated areas) in all inoculated pigs at days 3, 5 and 7 post-infection (p.i.). Virus was isolated from each bronchoalveolar lavage fluid (BALF) sample collected from the four pigs necropsied on days 3 and 5 p.i.; no virus was isolated from the two pigs necropsied on day 7 p.i. After performing plaque purification three times, the whole genome of 71 single viruses (about 18 viruses per pig) was characterized by RT-PCR, enzyme digestion and sequencing. Four different subtypes of influenza virus (H1N1, H1N2, H3N1 and H3N2) were identified from the BALF samples of primary co-infected pigs (Table 2). Two isolates from the 71 single viruses were double-reassortant (human/swine) viruses without the PA and PB2 polymerase genes of the Tx/98 virus that were originally derived from avian influenza viruses. Surprisingly, the major subtype of isolated viruses was rH1N1 (54.9 %, 39/71) and none of the parental H1N1 IA/30 virus was identified from BALF samples of co-infected pigs (Table 2). H3N1 (11/71) virus comprised 15.5 % of the isolated 71 viruses; H1N2 and the original parental H3N2 viruses each comprised 14.1 % (10/71) and the novel H3N2 (nH3N2) viruses containing internal gene segments from cH1N1 rgIA/30 comprised only 1.4 % (1/71) (Table 2). Interestingly, 42.3 % (30/71) of the total isolated viruses (ten rH1N1, six H3N1, four H1N2 and ten H3N2 viruses) contained the TRIG cassette, whilst 18.3 % (13/71) of viruses (eight rH1N1, one H3N1 and four H1N2 viruses) picked up a modified TRIG cassette with the M gene from IA/30 and the other five genes from Tx/98 (Table 3). The polymerase complex (avian PA and PB2, human PB1) was found in 70.4 % (50/71) of the total viruses isolated from co-infected pigs' lungs and in all isolated H1N2 and H3N2 viruses except for one H1N2 virus containing swine PA and PB1 genes from the classical IA/30 virus. All isolated H3N2 viruses were identical to the parental Tx/98 virus except for one (nH3N2) containing the NS segment from IA/30. Notably, the majority of the PB2 (97.2 %, 69/71), NP (93.0 %, 66/71), PB1 (81.7 %, 58/71), PA (77.5 %, 55/71) and NS (77.5 %, 55/71) segments of isolated SIVs from BALF samples from co-infected pigs were derived from the contemporary triple-reassortant Tx/98 SIV, whereas the majority of the HA (69.0 %, 49/71) and NA (70.4 %, 50/71) segments were derived from IA/30. The M segment was derived almost equally from both the Tx/98 and IA/30 viruses (Table 4).
Table 2.
Influenza viruses isolated from BALF and nasal swab samples from primary co-infected and contact pigs
Results are shown as percentages, with the number of viruses/total number of viruses shown in parentheses.
| Virus | Primary co-infected | Contact group 1 | Contact group 2 | |||
|---|---|---|---|---|---|---|
| BALF | Nasal swab | BALF | Nasal swab | BALF | Nasal swab | |
| cH1N1 | 0 (0/71) | 0 (0/40) | 0 (0/40) | 0 (0/40) | 0 (0/40) | 0 (0/40) |
| rH1N1* | 54.9 (39/71) | 0 (0/40) | 0 (0/40) | 0 (0/40) | 0 (0/40) | 0 (0/40) |
| H1N2 | 14.1 (10/71) | 0 (0/40) | 0 (0/40) | 0 (0/40) | 0 (0/40) | 0 (0/40) |
| H3N1 | 15.5 (11/71) | 0 (0/40) | 0 (0/40) | 0 (0/40) | 0 (0/40) | 0 (0/40) |
| H3N2 | 14.1 (10/71) | 100 (40/40) | 100 (40/40) | 100 (40/40) | 100 (40/40) | 100 (40/40) |
| nH3N2† | 1.4 (1/71) | 0 (0/40) | 0 (0/40) | 0 (0/40) | 0 (0/40) | 0 (0/40) |
*Reassortant H1N1.
†Novel H3N2.
Table 3.
Combinations of internal gene segments of viruses isolated from the lungs of co-infected pigs
| Virus subtype | Combination of viral internal gene segments | ||
|---|---|---|---|
| TRIG cassette* | Modified TRIG cassette† | Other combination | |
| rH1N1 (39) | 10 | 8 | 21 |
| H1N2 (10) | 4 | 4 | 2 |
| H3N1 (11) | 6 | 1 | 4 |
| H3N2 (11) | 10 | 0 | 1 |
*TRIG cassette: six internal genes (PB1, PB2, PA, NP, M and NS) derived from the H3N2 Tx/98 virus in this study.
†Modified TRIG cassette: five internal genes (PB1, PB2, PA, NP and NS) derived from the H3N2 Tx/98 virus and the M gene from the IA/30 virus in this study.
Table 4.
Genetic make-up of viruses isolated from the lungs of co-infected pigs
Results are shown as percentages, with the number of gene segments from the virus/total number of viruses shown in parentheses.
| Virus | Gene | |||||||
|---|---|---|---|---|---|---|---|---|
| PB1 | PB2 | PA | HA | NP | NA | M | NS | |
| IA/30 | 18.3 (13/71) | 2.8 (2/71) | 22.5 (16/71) | 69.0 (49/71) | 7.0 (5/71) | 70.4 (50/71) | 43.7 (31/71) | 22.5 (16/71) |
| Tx/98 | 81.7 (58/71) | 97.2 (69/71) | 77.5 (55/71) | 31.0 (22/71) | 93.0 (66/71) | 29.6 (21/71) | 56.3 (40/71) | 77.5 (55/71) |
Transmissible viruses detected from primary co-infected and contact pigs
Neither group of contact pigs showed any clinical signs. Necropsy at days 4, 6 and 8 post-contact (p.c.) revealed minimal macroscopic lung lesions in all contact pigs. In the primary co-infected pigs, virus was isolated from nasal swab samples on days 3 and 5 p.i., but not on day 7 p.i. Virus was isolated from nasal swabs and BALF samples of pigs in both contact groups on days 4 and 6 p.c., but no virus was isolated from pigs necropsied on days 8 and 14 p.c. To identify further the transmitted viruses shed from the primary co-infected pigs and among the contact pigs, general (whole-sample) and individual (single-plaque) analysis of the viral gene components was conducted for the nasal swab and BALF samples. Only the virus containing all eight segments of the Tx/98 virus was found by general analysis in the nasal swab samples from the primary co-infected pigs and both groups of contact pigs as well as in the BALF samples from both groups of contact pigs. This was confirmed by individual analysis of 40 viruses (approx. ten viruses per pig) picked from nasal swab samples from primary co-infected pigs and from nasal swabs and BALF samples from each group of contact pigs, respectively (Table 2).
DISCUSSION
Since the first triple-reassortant H3N2 SIV was isolated from US swine populations in 1998, all triple-reassortant SIVs including H1N1, H1N2 and H3N2 subtypes circulating in North American swine herds have contained a similar TRIG cassette (Vincent et al., 2008). In this reassortment and transmission study, four different subtypes of influenza virus (H3N2, H1N2, H3N1 and H1N1) with different genetic combinations were found in the lungs of pigs co-infected with the triple-reassortant H3N2 Tx/98 and cH1N1 rgIA/30 viruses, providing further evidence that pigs can be a ‘mixing vessel’ for different subtypes of influenza viruses. In the reassortant viruses from lungs of co-infected pigs, most of the internal genes (PB1, PB2, PA, NP and NS) were from the triple-reassortant Tx/98 virus whilst the surface genes (HA and NA) were from the cH1N1 rgIA/30 virus; the M genes were derived almost equally from both initial viruses. Most viruses isolated from the lungs of co-infected pigs had the cH1N1 surface proteins with the original TRIG cassette, confirming that this original TRIG cassette can accept and support different combinations of HA and NA genes, as in the field. Noticeably, some viruses from the lungs of co-infected pigs also contained the modified TRIG cassette with the IA/30 M gene, suggesting that the modified TRIG cassette is also able to accept different HA and NA types. The appearance of the human 2009 pandemic swine-origin H1N1 virus further confirmed this point, as this virus also contains a modified TRIG cassette with the M gene from the Eurasian SIV (Garten et al., 2009). The significance of the TRIG cassette for virus replication and transmission as well as adaptation between human and swine hosts needs to be investigated in future studies.
The polymerase subunits PB2, PB1 and PA are central to the replication cycle of influenza virus and are required for viral RNA replication and transcription. Viral-like reporter replication (the functional ribonucleoproteins constituted by co-transfecting an expressing viral-like reporter RNA plasmid with four plasmids expressing PB1, PB2, PA and NP into COS-1 cells) was more efficient when PB2 and NP were both derived from the same avian or human virus or when PB1 was derived from an avian virus (Naffakh et al., 2000). However, current circulating SIVs in North America contain the avian PA and PB2, the human PB1 and the swine NP genes, similar to the pandemic H1N1 virus, and the role of the novel polymerase complex in current SIVs is still not completely understood. In this study, two double-reassortant (human/swine) H1N1 and H3N1 viruses without the avian PA and PB2 polymerase genes were isolated from the lungs of co-infected pigs. However, these viruses were not successfully transmitted from co-infected pigs to contact animals. This result coincides with the finding that the double-reassortant H3N2 virus was initially isolated in one US herd, carrying an HA gene with identical residues in critical receptor-binding regions similar to subsequently isolated triple-reassortant H3N2 viruses, and was subsequently displaced by the triple-reassortant H3N2 SIVs (Vincent et al., 2008; Webby et al., 2004). Currently, the triple-reassortant H3N2 viruses are endemic in US swine herds. This suggests that the introduction of avian PA and PB2 genes is one of the critical factors for the triple-reassortant SIVs becoming well established in pigs (Webby et al., 2000). In this study, more than 70 % of isolates from the lungs of co-infected pigs contained this novel polymerase complex (avian PA and PB2 and human PB1 genes). This novel polymerase complex is also found in currently circulating triple-reassortant SIVs, indicating its importance in virus replication and adaptation; this might explain why the triple-reassortant virus containing the novel polymerase complex successfully spread in the pig population. Previous studies have shown that the current swine viruses in North America appear to have an increased rate of antigenic drift and reassortment and have the ability to evade established herd immunity (Richt et al., 2003; Vincent et al., 2006) due to acquisition of the avian PA and PB2 genes and the human PB1 gene. The exact role of each novel polymerase gene remains unknown and needs to be investigated.
The cH1N1 IA/30 virus generated by reverse genetics (Weingartl et al., 2009) has been shown to have a pathogenicity in pigs similar to the wild-type IA/30 virus (Lekcharoensuk et al., 2005; Vincent et al., 2008). However, no parental IA/30 virus generated by reverse genetics was found in BALF samples and nasal swabs from co-infected pigs, indicating that the parental triple-reassortant H3N2 viruses found in the lungs of the co-infected animals replicated more efficiently than the parental IA/30 virus. These results confirmed our previous findings that the IA/30 virus does not shed efficiently via the noses of pigs when compared with other H1N1 SIVs (Vincent et al., 2008). The rH1N1 virus levels were fourfold higher in the lungs of co-infected pigs than the other three subtypes (H3N2, H1N2 and H3N1). Eighteen H1N1 isolates contained the TRIG or modified TRIG cassette; however, these triple-reassortant H1N1 viruses were not isolated from the nasal swab samples of primary co-infected pigs and from contact animals, and were not successfully transmitted to contact pigs, indicating that the triple-reassortant H1N1 virus did not transmit efficiently among pigs in the presence of other viruses. Although other subtypes of virus containing the same TRIG cassette as the triple-reassortant Tx/98 virus were present in the lungs, only the parental Tx/98 H3N2 virus was transmitted from primary co-infected pigs to two groups of sentinel animals and became established in the contact pigs. The seroconversion data of contact pigs on day 14 p.c. to the Tx/98 virus [haemagglutination titre (HI) titre of 640] vs the IA/30 virus (HI titre of <20) support the assumption that the Tx/98 virus had a tremendous advantage when replicating in pigs (data not shown). These results indicated that the H3N2 Tx/98 virus has an optimal genetic constellation that contributes to efficient replication and successful transmission among pigs when compared with the IA/30 and other reassortant viruses. If currently circulating cH1N1 virus other than IA/30 had been used in this study, the results may have been different, and not only the parental Tx/98 H3N2 virus but also other viruses may have been transmitted to the sentinel animals. Although the triple-reassortant H1N1, H1N2 and H3N1 subtypes have been isolated from swine herds in the field, in this particular study, the similar subtype viruses containing the TRIG cassette of the Tx/98 virus were not able to transmit efficiently and be maintained in the small groups of pigs in the presence of the H3N2 Tx/98 virus. This finding suggests that a prerequisite for an influenza virus to establish and maintain itself in pigs is the right combination of HA and NA genes as well as the TRIG cassette, which may be reflected in the field situation with North American SIVs. The current pandemic H1N1 virus may be another example – a virus that contains the modified TRIG cassette, the NA from Eurasian swine virus and the HA from the North American triple-reassortant swine virus (Garten et al., 2009). This virus has infected humans and has been transmitted effectively from humans to pigs and to other species (WHO, 2009), and has caused over 15 000 human deaths worldwide as of February 2010 (WHO, 2010). The virulence and transmission capacity of this virus suggest that the genetic constellation of the pandemic H1N1 virus is optimal for its effective replication, transmission and adaptation to different hosts. In this study, only the Tx/98 virus was successfully transmitted and established in pigs, even though other subtypes of virus containing a similar TRIG cassette were produced simultaneously. These results indicate that reassortment will occur when pigs are infected with different viruses, but only the virus with an optimal gene constellation will have the opportunity to establish itself in herds under similar selective pressure. Therefore, the old dogma of the pig as a ‘mixing vessel’ needs to be readdressed. In a scenario where efficient reassortment occurs in the lung, only the fittest virus will be maintained in a pig population.
METHODS
Viruses and cells.
Influenza viruses H1N1 IA/30 and H3N2 Tx/98 were propagated in 10-day-old embryonated chicken eggs. Human embryonic kidney 293T cells (constitutively expressing the simian virus 40 large T antigen) were maintained in Opti-MEM supplemented with 10 % fetal bovine serum (FBS; HyClone) and 1 % antibiotics (Invitrogen), and Madin–Darby canine kidney (MDCK) cells were maintained in Eagle's minimum essential medium (MEM) with 5 % FBS and antibiotics. Cells were infected with the corresponding viruses and incubated with infecting MEM containing 0.3 % BSA, 1 μg l-1-tosylamido-2-phenylethyl chloromethyl ketone-treated trypsin ml−1, 1× l-glutamine (Invitrogen), 1× MEM vitamins (Invitrogen) and 1 % antibiotics.
HI assays.
Before infection, sera from all experimental pigs were tested by HI assay (Palmer, 1975). For HI assays, sera were heat inactivated at 56 °C, treated with a 20 % suspension of kaolin (Sigma-Aldrich) to eliminate non-specific inhibitors, and adsorbed with 0.5 % chicken or turkey red blood cells. A HI assay was performed to test antibodies against a panel of reference SIV strains including H1N1 A/Swine/Iowa/1973, H3N2 Tx/98 and A/Swine/North Carolina/2001, a variant H1N1 (representing a β-cluster triple-reassortant H1N1 virus).
Cloning and generation of the IA/30 virus by reverse genetics.
To identify each segment of the rgIA/30 virus from the wild-type IA/30 and Tx/98 viruses, each gene of the IA/30 virus was engineered to contain an internal unique restriction site as a genetic tag (silent mutation); therefore, universal primers were used in combination with specific internal primers carrying the silent mutations to generate two PCR products for each gene. The full length of each gene segment was amplified utilizing two PCR products as templates with the universal primers (primers are available upon request). The full-length PCR products were cloned into pGEM-T Easy vector (Promega) and confirmed by sequencing. Subsequently, the full-length PCR products were subcloned into pDZ vector, leading to plasmids pDZ-PB1, -PB2, -PA, -NP, -HA, -NA, -M and -NS (Lekcharoensuk et al., 2005). pDZ is an ambisense expression plasmid with a human RNA polymerase I promoter transcribing negative-sense genomic RNA and a chicken β-actin promoter for expression of the recombinant gene products (Quinlivan et al., 2005). Viruses were recovered using a mixture of 293T and MDCK cells as described previously (Hoffmann et al., 2000). Rescued viruses were confirmed by restriction digestion analysis and viral titres were determined by plaque assay on MDCK cells.
Reassortment and transmission experiments in pigs.
Pigs were obtained from a healthy herd free of SIVs and porcine reproductive and respiratory syndrome virus. All animal experiments were conducted in compliance with the Institutional Animal Care and Use Committee of the National Animal Disease Center (IA, USA). The inoculation protocol has been described elsewhere (Richt et al., 2003). Six 6-week-old crossbred pigs were co-inoculated intratracheally with 106 p.f.u. per pig of the egg-derived H1N1 rgIA/30 and 106 p.f.u. per pig of the egg-derived triple-reassortant H3N2 Tx/98 SIVs. Two pigs were euthanized on each of days 3, 5 and 7 p.i. On day 3 p.i., eight pigs (contact group 1) were co-housed with the infected pigs. The last two pigs from the co-infected group and two pigs from contact group 1 were euthanized at day 7 p.i. (4 days p.c. for contact group 1). The remaining six pigs from contact group 1 were moved to a new biocontainment room, which housed another eight pigs (contact group 2). Two pigs from contact group 1 were euthanized on days 6, 8 and 14 p.c. Likewise, two pigs from contact group 2 were euthanized on days 4, 6, 8 and 14 p.c. All infected and contact pigs were bled, and nasal swab and BALF samples were collected for further experiments.
Identification of the genetic component of viruses isolated from BALF and nasal swabs.
Each BALF sample from co-infected pigs was serially diluted tenfold and inoculated onto a monolayer of MDCK cells grown in six-well plates. Single plaques with different sizes were randomly selected (approx. 18 plaques per BALF sample) and plaque purification was performed three times in order to purify the virus. The purified viruses were amplified either in cell culture or in 10-day-old chicken embryonated eggs and RNA was extracted from the supernatant or allantoic fluid for RT-PCR. All eight viral segments of each virus were characterized by restriction enzyme digestion, or by sequencing if necessary. To detect the viruses transmitted from the primary co-infected pigs to contact pigs and among the contact pigs, general (whole-sample) and individual (single-plaque) analysis of the viral gene component was conducted for the nasal swabs from all experimental pigs and the BALF samples from contact animals. For general analysis, all nasal swab samples were amplified directly and RNA was extracted from the amplified samples and BALF. An RT-PCR was conducted to amplify each segment and PCR products were characterized by restriction enzyme digestion and sequencing. For individual analysis, a single virion was plaque purified three times from the amplified nasal swabs (approx. ten plaques per nasal swab) and BALFs (approx. ten plaques per BALF sample), and then each segment of the viral genome was amplified and characterized by restriction enzyme digestion and sequencing as the general analysis. All gene segments of transmissible viruses were compared with those of the parental viruses and the viruses isolated from co-infected pigs. This allowed us to determine the genetic components of successfully transmissible viruses.
Acknowledgments
We thank Deb Clouser, Kevin Hassall, Hannah Polashek, Deb Adolphson, Brian Pottebaum and Jason Huegel for animal studies and technical assistance, and the DNA Sequence Unit at the National Animal Disease Center for sequencing assistance. This project was supported by USDA/ARS CRIS 3625-3200-088 and the National Institute of Allergy and Infectious Diseases, National Institutes of Health, Department of Health and Human Services, under contracts HHSN266200700005C and HHSN266200700010C, and by Center for Disease Control and Prevention Grant U01 CI000357-01.
References
- Alexander, D. J. (2000). A review of avian influenza in different bird species. Vet Microbiol 74, 3–13. [DOI] [PubMed] [Google Scholar]
- Choi, Y. K., Goyal, S. M. & Joo, H. S. (2002). Prevalence of swine influenza virus subtypes on swine farms in the United States. Arch Virol 147, 1209–1220. [DOI] [PubMed] [Google Scholar]
- Fouchier, R. A., Munster, V., Wallensten, A., Bestebroer, T. M., Herfst, S., Smith, D., Rimmelzwaan, G. F., Olsen, B. & Osterhaus, A. D. (2005). Characterization of a novel influenza A virus hemagglutinin subtype (H16) obtained from black-headed gulls. J Virol 79, 2814–2822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garten, R. J., Davis, C. T., Russell, C. A., Shu, B., Lindstrom, S., Balish, A., Sessions, W. M., Xu, X., Skepner, E. & other authors (2009). Antigenic and genetic characteristics of swine-origin 2009 A(H1N1) influenza viruses circulating in humans. Science 325, 197–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann, E., Neumann, G., Kawaoka, Y., Hobom, G. & Webster, R. G. (2000). A DNA transfection system for generation of influenza A virus from eight plasmids. Proc Natl Acad Sci U S A 97, 6108–6113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito, T., Couceiro, J. N., Kelm, S., Baum, L. G., Krauss, S., Castrucci, M. R., Donatelli, I., Kida, H., Paulson, J. C. & other authors (1998). Molecular basis for the generation in pigs of influenza A viruses with pandemic potential. J Virol 72, 7367–7373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karasin, A. I., Olsen, C. W. & Anderson, G. A. (2000a). Genetic characterization of an H1N2 influenza virus isolated from a pig in Indiana. J Clin Microbiol 38, 2453–2456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karasin, A. I., Schutten, M. M., Cooper, L. A., Smith, C. B., Subbarao, K., Anderson, G. A., Carman, S. & Olsen, C. W. (2000b). Genetic characterization of H3N2 influenza viruses isolated from pigs in North America, 1977–1999: evidence for wholly human and reassortant virus genotypes. Virus Res 68, 71–85. [DOI] [PubMed] [Google Scholar]
- Karasin, A. I., Carman, S. & Olsen, C. W. (2006). Identification of human H1N2 and human–swine reassortant H1N2 and H1N1 influenza A viruses among pigs in Ontario, Canada (2003 to 2005). J Clin Microbiol 44, 1123–1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kida, H., Ito, T., Yasuda, J., Shimizu, Y., Itakura, C., Shortridge, K. F., Kawaoka, Y. & Webster, R. G. (1994). Potential for transmission of avian influenza viruses to pigs. J Gen Virol 75, 2183–2188. [DOI] [PubMed] [Google Scholar]
- Lekcharoensuk, P., Vincent, A. L., Lager, K. M., Solórzano, A., García-Sastre, A. & Richt, J. A. (2005). Establishment of a pig model for the 1930 H1N1 swine influenza virus and application of reverse genetics. In XIII International Congress of Virology, 23–28 July 2005, San Francisco, CA, USA, p. 42.
- Lekcharoensuk, P., Lager, K. M., Vemulapalli, R., Woodruff, M., Vincent, A. L. & Richt, J. A. (2006). Novel swine influenza virus subtype H3N1, United States. Emerg Infect Dis 12, 787–794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma, W., Gramer, M., Rossow, K. & Yoon, K. J. (2006). Isolation and genetic characterization of new reassortant H3N1 swine influenza virus from pigs in the midwestern United States. J Virol 80, 5092–5096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma, W., Vincent, A. L., Gramer, M. R., Brockwell, C. B., Lager, K. M., Janke, B. H., Gauger, P. C., Patnayak, D. P., Webby, R. J. & Richt, J. A. (2007). Identification of H2N3 influenza A viruses from swine in the United States. Proc Natl Acad Sci U S A 104, 20949–20954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma, W., Lager, K. M., Vincent, A. L., Janke, B. H., Gramer, M. R. & Richt, J. A. (2009). The role of swine in the generation of novel influenza viruses. Zoonoses Public Health 56, 326–337. [DOI] [PubMed] [Google Scholar]
- Naffakh, N., Massin, P., Escriou, N., Crescenzo-Chaigne, B. & van der Werf, S. (2000). Genetic analysis of the compatibility between polymerase proteins from human and avian strains of influenza A viruses. J Gen Virol 81, 1283–1291. [DOI] [PubMed] [Google Scholar]
- Olsen, C. W. (2002). The emergence of novel swine influenza viruses in North America. Virus Res 85, 199–210. [DOI] [PubMed] [Google Scholar]
- Palmer, D. F., Coleman, M. T., Dowdle, W. D. & Schild, G. O. (1975). Advanced Laboratory Techniques for Influenza Diagnosis. Washington, DC: US Department of Health, Education, and Welfare.
- Quinlivan, M., Zamarin, D., García-Sastre, A., Cullinane, A., Chambers, T. & Palese, P. (2005). Attenuation of equine influenza viruses through truncations of the NS1 protein. J Virol 79, 8431–8439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richt, J. A., Lager, K. M., Janke, B. H., Woods, R. D., Webster, R. G. & Webby, R. J. (2003). Pathogenic and antigenic properties of phylogenetically distinct reassortant H3N2 swine influenza viruses cocirculating in the United States. J Clin Microbiol 41, 3198–3205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholtissek, C. (1994). Source for influenza pandemics. Eur J Epidemiol 10, 455–458. [DOI] [PubMed] [Google Scholar]
- Shope, R. E. (1931). Swine influenza. III. Filtration experiments and etiology. J Exp Med 54, 373–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith, W., Andrewes, C. H. & Laidlaw, P. P. (1933). A virus obtained from influenza patients. Lancet ii, 66–68. [Google Scholar]
- Smith, G. J., Vijaykrishna, D., Bahl, J., Lycett, S. J., Worobey, M., Pybus, O. G., Ma, S. K., Cheung, C. L., Raghwani, J. & other authors (2009). Origins and evolutionary genomics of the 2009 swine-origin H1N1 influenza A epidemic. Nature 459, 1122–1125. [DOI] [PubMed] [Google Scholar]
- Vincent, A. L., Lager, K. M., Ma, W., Lekcharoensuk, P., Gramer, M. R., Loiacono, C. & Richt, J. A. (2006). Evaluation of hemagglutinin subtype 1 swine influenza viruses from the United States. Vet Microbiol 118, 212–222. [DOI] [PubMed] [Google Scholar]
- Vincent, A. L., Ma, W., Lager, K. M., Janke, B. H. & Richt, J. A. (2008). Swine influenza viruses a North American perspective. Adv Virus Res 72, 127–154. [DOI] [PubMed] [Google Scholar]
- Vincent, A. L., Ma, W., Lager, K. M., Gramer, M. R., Richt, J. A. & Janke, B. H. (2009). Characterization of a newly emerged genetic cluster of H1N1 and H1N2 swine influenza virus in the United States. Virus Genes 39, 176–185. [DOI] [PubMed] [Google Scholar]
- Webby, R. J., Swenson, S. L., Krauss, S. L., Gerrish, P. J., Goyal, S. M. & Webster, R. G. (2000). Evolution of swine H3N2 influenza viruses in the United States. J Virol 74, 8243–8251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webby, R. J., Rossow, K., Erickson, G., Sims, Y. & Webster, R. (2004). Multiple lineages of antigenically and genetically diverse influenza A virus co-circulate in the United States swine population. Virus Res 103, 67–73. [DOI] [PubMed] [Google Scholar]
- Webster, R. G., Bean, W. J., Gorman, O. T., Chambers, T. M. & Kawaoka, Y. (1992). Evolution and ecology of influenza A viruses. Microbiol Rev 56, 152–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weingartl, H. M., Albrecht, R. A., Lager, K., Babiuk, S., Marszal, P., Neufeld, J., Embury-Hyatt, C., Lekcharoensuk, P., Tumpey, T. M. & other authors (2009). Experimental infection of pigs with the human 1918 pandemic influenza virus. J Virol 83, 4287–4296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO (2009). Infection of farmed animals with the pandemic virus. In Pandemic (H1N1) 2009 Briefing Note 15. http://www.who.int/csr/disease/swineflu/notes/briefing_20091105/en/index.html.
- WHO (2010). Pandemic (H1N1) 2009 – Update 87. http://www.who.int/csr/don/2010_02_12/en/index.html.
- Zhou, N. N., Senne, D. A., Landgraf, J. S., Swenson, S. L., Erickson, G., Rossow, K., Liu, L., Yoon, K., Krauss, S. & Webster, R. G. (1999). Genetic reassortment of avian, swine, and human influenza A viruses in American pigs. J Virol 73, 8851–8856. [DOI] [PMC free article] [PubMed] [Google Scholar]