Abstract
Bats are natural reservoirs for the majority of lyssaviruses globally, and are unique among mammals in having exceptional sociality and longevity. Given these facets, and the recognized status of bats as reservoirs for rabies viruses (RABVs) in the Americas, individual bats may experience repeated exposure to RABV during their lifetime. Nevertheless, little information exists with regard to within-host infection dynamics and the role of immunological memory that may result from abortive RABV infection in bats. In this study, a cohort of big brown bats (Eptesicus fuscus) was infected intramuscularly in the left and right masseter muscles with varying doses [10−0.1–104.9 median mouse intracerebral lethal doses (MICLD50)] of an E. fuscus RABV variant isolated from a naturally infected big brown bat. Surviving bats were infected a second time at 175 days post-(primary) infection with a dose (103.9–104.9 MICLD50) of the same RABV variant. Surviving bats were infected a third time at either 175 or 305 days post-(secondary) infection with a dose (104.9 MICLD50) of the same RABV variant. When correcting for dose, similar mortality was observed following primary and secondary infection, but reduced mortality was observed following the third and last RABV challenge, despite infection with a high viral dose. Inducible RABV-neutralizing antibody titres post-infection were ephemeral among infected individuals, and dropped below levels of detection in several bats between subsequent infections. These results suggest that long-term repeated infection of bats may confer significant immunological memory and reduced susceptibility to RABV infection.
INTRODUCTION
Rabies is an acute, progressive encephalitis caused by single-stranded, negative-sense RNA viruses in the genus Lyssavirus. Rabies virus (RABV) has the broadest host range among lyssaviruses. Rabies has a case fatality ratio approaching 100 %, one of the highest of any infectious disease, although it is preventable by appropriate pre- and post-exposure prophylaxis. Bats are the primary reservoirs for 10 of 11 recognized lyssaviruses. Among bats, the distribution of RABV is limited to New World fauna, although sylvatic or domestic cycles occur in carnivores globally. In North America (NA), RABV variants tend to be species-associated among various bat and mesocarnivore taxa, indicating that intraspecific contact is the predominant mode of transmission and maintenance between animals (Smith, 1996). However, spillover infections from wildlife occur to humans, domestic animals and other sylvatic hosts (McQuiston et al., 2001; Messenger et al., 2002, 2003).
Big brown bats (Eptesicus fuscus) are one of the most widely distributed species of bat in NA, with a geographical range that extends from the southern provinces of Canada into northern South America (Hall, 1981; Koopman, 1982). Big brown bats utilize a variety of different roost structures, including natural and man-made roosts. This species is gregarious, typically roosting in colonies of 25–75 individuals, and may undergo short-distance migrations between summer maternity colonies and winter hibernacula (Kurta & Baker, 1990). Consistent with their synanthropic roosting ecology, big brown bats rank highest annually in the number of bats submitted for rabies diagnosis in the USA (Mondul et al., 2003). Although RABV from E. fuscus has been implicated only in a single indigenous human rabies case (Blanton et al., 2009; Messenger et al., 2002), and spillover into domestic animals implicated only rarely (McQuiston et al., 2001), spillover infections in wildlife are more common (Messenger et al., 2003). Furthermore, recent surveillance suggests repeated spillover and a host shift of RABV from E. fuscus into striped skunks (Mephitis mephitis) and grey foxes (Urocyon cinereoargenteus), which has led to an epizootic of rabies in Flagstaff, AZ, USA (Blanton et al., 2009; Leslie et al., 2006).
Several studies have conducted enhanced surveillance for RABV among natural colonies of big brown bats. Such investigations demonstrate collectively that exposure is temporally and geographically enzootic in natural colonies. Early studies found RABV infection prevalence ranging from 0 to 3 % among natural colonies of big brown bats in New England (Daniels et al., 1960; Girard et al., 1965). In a later study from New York, seven colonies were surveyed and 3 % (eight of 278) of big brown bats were diagnosed with RABV infection, whereas RABV-neutralizing antibodies (rabies VNA) were detected in 10 % of bats (Trimarchi & Debbie, 1977). Concurrent studies in Canada reported RABV infection prevalence of 1–6 % among natural colonies of big brown bats in Alberta (Pybus, 1986; Schowalter, 1980). Additionally, a 3 year study of over 20 colonies of big brown bats around Fort Collins, CO, USA, found rabies VNA seroprevalence ranging from 3 to 35 % among colonies, and was one of the first studies to demonstrate multi-year maintenance of rabies VNA in individual bats (O'Shea et al., 2003). Most recently, a 3 year study of 10 colonies of big brown bats in New Hampshire and Massachusetts detected rabies VNA seroprevalence in 2–17 % of bats across years (Moore et al., 2008). Although evidence of RABV infection is widespread, RABV variants circulating in big brown bat populations in NA appear to be structured geographically (Messenger et al., 2003; Smith, 1996), suggesting a complex epizootiology that is consistent with the population genetic structure of this host (Turmelle, 2002).
The longevity, philopatry and gregarity of certain bats are exceptional among mammals (Austad & Fischer, 1991; Kunz & Pierson, 1994). Two long-term banding studies demonstrated that big brown bats can survive in the wild for up to 20 years (Goehring, 1972; Hitchcock, 1965). Given such high contact rates, exceptional longevity and evidence of regular exposure to RABV in natural colonies, big brown bats may be subject to repeated infection with RABV in the wild. However, no experimental studies have examined the effects of multiple exposures and the potential role of immunological memory to RABV infection. The objectives of this study were to determine whether abortive RABV infections confer immunity against subsequent challenge, and to evaluate the induction of rabies VNA in the anamnestic response to RABV infection in big brown bats.
RESULTS
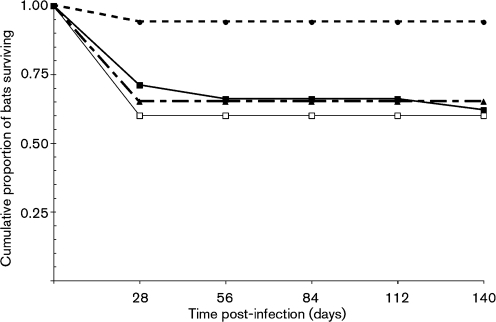
A cohort of bats was used in three RABV infection studies (Table 1). RABV sequences from brain-tissue samples for a subset of bats that were diagnosed with rabies during the study (n=14; incubation period range, 13–140 days) demonstrated 100 % sequence identity with the experimental inoculum. Cumulative survivorship of bats in the primary, secondary and tertiary infections is shown in Fig. 1.
Table 1.
Details of experimental RABV infection of bats
| Days of blood sampling for VNA determination | |
|---|---|
| Primary infections | |
| 24 January 2007 | 0, 10, 17, 31, 42, 57, 93, 104, 118, 133, 147, 174 (n=25; one bat censored) |
| 26 April 2007 | 0, 7, 13, 22, 29, 41, 55, 68, 83, 98, 145 (n=20; one bat censored) |
| Secondary infections | |
| 18 July 2007 (day 175 p.i.) | 0, 5, 14, 27, 43, 98, 147 (n=17; none censored) |
| 18 October 2007 (day 175 p.i.) | 0, 15, 27, 41, 54, 89, 133, 160 (n=9; one bat censored) |
| Tertiary infections | |
| 8 January 2008 (day 174 p.i.) | 0, 20, 48, 120 (n=10; none censored) |
| 18 August 2008 (day 305 p.i.) | 0, 29, 86, 149 (n=6; none censored) |
Fig. 1.
Cumulative survivorship of bats in the primary infection (solid line, ▪), secondary infection (dashed line; ▴) and tertiary infection (dotted line; •). Survivorship of bats in the primary infection from higher doses only (103.9–104.9 MICLD50) is also shown (□).
Primary infection
Two bats were censored due to non-specific mortality during the primary RABV infection [days 12 and 113 post-infection (p.i.)]. There was 40 % (17 of 43) mortality among the remaining bats, with demonstrable RABV antigen in the central nervous system (CNS), summarized across all RABV doses (Table 2). Dose effects on mortality to infection were not statistically significant (n=43; df=5, 37; χ2=5.9; P=0.32). Among infected bats, 35 % (15 of 43) seroconverted. Among the 17 bats that succumbed to RABV infection, only 6 % (one of 17) seroconverted, and rabies VNA in the single bat was only observed upon the day of terminal infection (day 22 p.i.). A statistically significant difference was found in the probability of seroconversion when comparing survivors with bats that succumbed to RABV infection (54 versus 6 %, respectively; n=43; df=1, 41; χ2=12.1; P<0.001). With few exceptions, maximum rabies VNA titres were observed between 31 and 42 days p.i. Substantial variation was recorded in the incubation periods of bats that succumbed to RABV infection (n=17; median, 19 days; range, 13–140 days). Higher RABV inoculation doses were correlated with shorter incubation periods in animals that succumbed (n=17; Spearman's ρ, −0.52; P=0.03).
Table 2.
Bat response to RABV infection
| Bat ID* | Primary infection | Secondary infection | Tertiary infection | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dose† | Incubation period (days) | Base VNA‡ | Max. VNA‡ | Dose† | Incubation period (days) | Base VNA‡ | Max. VNA‡ | Dose† | Incubation period (days) | Base VNA‡ | Max. VNA‡ | |
| 487 | −0.1 | 135 | <0.02 | <0.02 | ||||||||
| 486 | −0.1 | <0.02 | 0.02 | 4.9 | 19 | <0.02 | 5.22 | |||||
| 488 | −0.1 | 0.02 | 0.02 | 4.9 | <0.02 | 0.08 | 4.9 | 0.03 | 0.02 | |||
| 491 | −0.1 | <0.02 | <0.02 | 4.9 | 21 | <0.02 | <0.02 | |||||
| 492 | −0.1 | <0.02 | 0.02 | 4.9 | <0.02 | 0.02 | 4.9 | <0.02 | 0.02 | |||
| 464 | 0.9 | <0.02 | 0.10 | 4.9 | 19 | <0.02 | <0.02 | |||||
| 484 | 0.9 | <0.02 | 0.15 | 4.9 | <0.02 | 0.52 | 4.9 | 0.17 | <0.02 | |||
| 485 | 0.9 | 0.05 | 0.05 | 4.9 | <0.02 | 0.17 | 4.9 | 0.16 | 0.17 | |||
| 1892 | 0.9 | <0.02 | 0.83 | 4.9 | 0.17 | 0.49 | 4.9 | 0.27 | 0.39 | |||
| 462 | 1.9 | 32 | <0.02 | <0.02 | ||||||||
| 1862 | 1.9 | 38 | <0.02 | <0.02 | ||||||||
| 460 | 1.9 | <0.02 | 0.05 | 4.9 | 27 | <0.02 | <0.02 | |||||
| 461 | 1.9 | 0.02 | 0.83 | 4.9 | <0.02 | 0.03 | 4.9 | <0.02 | 0.02 | |||
| 494 | 1.9 | <0.02 | 0.07 | 4.9 | 19 | <0.02 | <0.02 | |||||
| 84 | 1.9 | 13 | 0.04 | 0.13 | ||||||||
| 82 | 1.9 | 19 | <0.02 | <0.02 | ||||||||
| 95 | 1.9 | 140 | <0.02 | <0.02 | ||||||||
| 83 | 1.9 | <0.02 | <0.02 | 3.9 | 0.02 | 0.10 | 4.9 | <0.02 | <0.02 | |||
| 79 | 1.9 | <0.02 | 0.02 | 3.9 | 21 | <0.02 | 0.19 | |||||
| 1883 | 2.9 | 15 | <0.02 | <0.02 | ||||||||
| 454 | 2.9 | 26 | <0.02 | 0.02 | ||||||||
| 455 | 2.9 | 26 | <0.02 | 0.02 | ||||||||
| 456 | 2.9 | <0.02 | 1.66 | 4.9 | 14 | <0.02 | 0.02 | |||||
| 457 | 2.9 | <0.02 | 0.41 | 4.9 | 23 | <0.02 | <0.02 | |||||
| 87 | 2.9 | 15 | <0.02 | <0.02 | ||||||||
| 96 | 2.9 | 22 | <0.02 | 0.19 | ||||||||
| 88 | 2.9 | <0.02 | 0.09 | 3.9 | <0.02 | 0.16 | 4.9 | 0.03 | 0.03 | |||
| 89 | 2.9 | <0.02 | 0.21 | 3.9 | <0.02 | 0.29 | 4.9 | <0.02 | <0.02 | |||
| 90 | 2.9 | <0.02 | 0.02 | 3.9 | 19 | <0.02 | 1.26 | |||||
| 497 | 3.9 | 27 | 0.02 | 0.02 | ||||||||
| 440 | 3.9 | <0.02 | 0.16 | 4.9 | 0.09 | 1.33 | 4.9 | 0.30 | 0.29 | |||
| 442 | 3.9 | <0.02 | 1.66 | 4.9 | 0.07 | >9.39 | 4.9 | 0.21 | >9.39 | |||
| 498 | 3.9 | <0.02 | 0.05 | 4.9 | <0.02 | 0.24 | 4.9 | 0.07 | 0.06 | |||
| 1881 | 3.9 | <0.02 | 0.10 | 4.9 | <0.02 | 0.06 | 4.9 | 0.06 | <0.02 | |||
| 74 | 3.9 | 17 | <0.02 | <0.02 | ||||||||
| 76 | 3.9 | 18 | <0.02 | 0.02 | ||||||||
| 77 | 3.9 | 19 | <0.02 | <0.02 | ||||||||
| 78 | 3.9 | <0.02 | 0.02 | 3.9 | <0.02 | 0.11 | 4.9 | <0.02 | 0.02 | |||
| 71 | 4.9 | 13 | <0.02 | <0.02 | ||||||||
| 490 | 4.9 | 16 | <0.02 | <0.02 | ||||||||
| 467 | 4.9 | <0.02 | 0.08 | 3.9 | 0.02 | 0.29 | 4.9 | 0.03 | 0.29 | |||
| 63 | 4.9 | 0.02 | 0.03 | 3.9 | <0.02 | 0.21 | 4.9 | 22 | <0.02 | |||
| 68§ | 4.9 | <0.02 | 0.16 | 3.9 | <0.02 | 0.09 | ||||||
*Includes all non-censored bats during primary infection.
†Dose expressed in units of log10(MICLD50).
‡Rabies VNA expressed in units of IU ml−1.
§Bat that was censored during the secondary infection.
Secondary infection
Despite routine seroconversion during the primary infection, 88 % (23 of 26) of bats were seronegative prior to the secondary infection (Table 2). One animal was censored during the secondary infection (bat 68, day 89 p.i.). Mortality to the secondary infection (36 %, nine of 25 bats) was not statistically significantly lower than that observed in the primary infection (P=0.49). During the secondary infection, 60 % (15 of 25) of bats seroconverted. Among the nine bats that succumbed to RABV infection, 33 % seroconverted (three of nine). The titres for these three bats were respectively observed 5 days prior to death (21 days incubation), 6 days prior to death (19 days incubation) and upon the day of death (19 days incubation). A statistically significant difference was found in the probability of seroconversion when comparing survivors with bats that succumbed (75 versus 33 %, respectively; n=25; df=1, 23; χ2=4.2; P=0.04). The incubation periods of animals that succumbed ranged from 14 to 27 days (median, 19 days).
Tertiary infection
For the cohort of bats observed for 174 days post-secondary infection (i.e. 349 days post-primary infection), 60 % (six of 10) had a positive rabies VNA titre immediately prior to the start of the tertiary infection (Table 1). For the cohort of bats observed for 305 days post-secondary infection (i.e. 480 days post-primary infection), none (of six) was seropositive immediately prior to the start of the tertiary infection. One animal succumbed during the tertiary infection with an incubation period of 22 days, but the maximum rabies VNA titre and seroconversion status were not determined. Mortality following tertiary exposure was significantly lower (6 %, one of 16 bats) compared with mortality following primary (Fisher's exact test, P=0.01) and secondary (Fisher's exact test, P=0.03) RABV infections. During the tertiary infection period, 27 % (four of 15) of surviving bats demonstrated apparent seroconversion.
DISCUSSION
Despite ample evidence that bats are reservoirs of several emerging viral zoonoses and that several taxa have exceptional sociality and longevity, there have been no studies that have examined the response to repeated virus infection in bats. The immunological response to peripheral RABV infection includes both innate and adaptive immune components and signalling molecules (Lafon, 2007). Helper and cytotoxic T cells are activated to recognize and clear virus particles respectively outside and inside infected cells, which presumably primes both cell types for any subsequent response to infection (i.e. anamnestic response). However, once the virus gains access to the CNS, the host adaptive immune response may be severely limited (Lafon, 2007). Pathogenic variants of RABV tend to invade the CNS and cause an acute, progressive infection, whereas less virulent viruses may have limited invasion of the CNS and cause abortive infection (Dietzschold et al., 2008; Wang et al., 2005). However, studies have demonstrated that RABV pathogenicity correlates inversely with the rate of viral RNA synthesis, production of viral glycoprotein and assembly of infectious particles, which may result in limited host immune detection and response (Dietzschold et al., 2008). Regardless of RABV pathogenicity, similar host adaptive immune responses are triggered in the periphery during initial time periods p.i. More pathogenic viruses may impair or evade the host immune response during later periods p.i. (Lafon, 2007). Furthermore, the effect of inoculum dose on pathogenicity is complex, considering that low doses of RABV may not establish a productive peripheral infection, whereas high doses of RABV may evade or overwhelm the host peripheral immune response and rapidly invade the CNS.
The results of this study support previous suggestions that high doses of pathogenic RABV lead to more rapid CNS invasion (Baer & Bales, 1967). For example, a significant negative correlation was observed between inoculum dose and incubation period in the primary infection of bats. Baer & Bales (1967) suggested that longer incubation periods lead to the development of higher viral titres in the CNS and salivary glands upon clinical presentation in Brazilian free-tailed bats (Tadarida brasiliensis). Although titration of virus in brains and salivary glands from clinically rabid bats was not conducted in the present study, a possible negative-feedback link between dose, incubation period and virus shedding of clinically infected bats warrants additional investigation.
Significant differences were observed in the seroconversion probabilities of survivors versus bats that developed clinical illness among cohorts of naïve (i.e. primary infection) and previously infected (i.e. secondary or tertiary infection) bats. These results do support a role of rabies VNA in the successful clearance of a peripheral RABV infection, as higher seroconversion probabilities were consistently observed among surviving bats. Nevertheless, many bats that did not seroconvert also survived RABV infection, indicating that other innate or adaptive immune factors may contribute to RABV clearance. Furthermore, bats may have had prior natural exposure to RABV before captivity, despite being rabies VNA-seronegative at the start of the study, which would obscure the interpretation of response to primary RABV infection. Complex interactions occur between natural individual variation in the immune response among wild bats that allows RABV to replicate differentially in the periphery and invade the CNS, and obvious variation in RABV pathogenicity that leads to subversion of the host immune response (Dietzschold et al., 2008; Smith, 1981; Smith et al., 1982; Wang et al., 2005).
Immunological memory does appear to be conferred to bats following abortive infection. Rather, the results of this study suggest that a single exposure may not protect bats significantly against subsequent infections. If primary infection doses had been uniformly higher [e.g. >103.9 median mouse intracerebral lethal doses (MICLD50)], then immune-system priming and anamnestic response may have been more apparent in the secondary infections. Uneven sampling precluded more robust testing for a relationship between primary virus inoculum dose and the degree of immune-system priming between the primary and secondary infections. The relationship between dose and strength of priming using street RABVs is not well-characterized in bats or other wildlife. Mortality was not reduced significantly until the third infection, despite a low proportion of bats that seroconverted. Thus, these results suggest a reduced role of rabies VNA in the clearance of RABV infection in bats that have experienced multiple exposures. Development of specific markers to measure other factors associated with immune responses, particularly interferon and cytotoxic T-cell activity, is needed for a more thorough characterization of immune response and memory to virus infection in bats.
Data from field studies suggest that rabies VNA titres in recaptured big brown bats fluctuate through time, indicating repeated RABV exposure in natural colonies (O'Shea et al., 2003). In the current study, a proportion of bats responded to primary experimental RABV infection by development of rabies VNA, although levels dropped below the limit of detection (i.e. <0.02 IU ml−1) by 6 months p.i., which is consistent with previous studies (Jackson et al., 2008). Following secondary infection, rabies VNA persisted longer in infected bats that seroconverted, lasting beyond 6 months but less than 1 year p.i., before levels dropped below the limit of detection. However, repeated observations of bats that did not develop a rabies VNA response to experimental infection in this study provide evidence that field serology may be limited for inference to past exposure history of seronegative bats. Although studies tend to report only seroprevalence (i.e. proportions of seropositive individuals), rabies VNA titres of bats in natural colonies are generally low, although a few bats may have high titres (e.g. >2 IU ml−1) (Turmelle et al., 2010). The results of the current study suggest that bats with high rabies VNA titres may have experienced repeated RABV exposure, as few bats responded with high rabies VNA titres to experimental RABV infection.
The observation of limited rabies VNA titres or the absence of apparent seroconversion in response to experimental infection with high doses of RABV is consistent with the concept that pathogenic RABV may limit replication rate and produce few infectious particles, thereby evading or minimally activating host responses in the periphery (Dietzschold et al., 2008). In the primary infection, bats 487 and 95 were infected with low doses of RABV and exhibited incubation periods of 135 and 140 days, respectively, but neither bat demonstrated a rabies VNA response at any time p.i. Thus, RABV is clearly able to evade successful immune recognition or response, perhaps through periods of quiescence or via extremely low levels of virus replication. Additional studies targeting variation in virus pathogenicity and peripheral immune recognition and activation may improve our understanding of street RABV persistence in wildlife reservoirs, particularly in long-lived social hosts such as bats, as several species are capable of maintaining long-term enzootic transmission despite fluctuating rabies VNA seroprevalence in natural colonies. Such approaches to elucidation of pathogen maintenance and within-host dynamics offer a fresh perspective to emerging infectious diseases of bats and other species (Dimitrov & Hallam, 2009).
METHODS
Animal collection and care.
Experimental procedures and animal care were performed in compliance with the CDC Institutional Animal Care and Use Committee guidelines. Forty-five adult big brown bats were captured from building roosts in Georgia (GA permit #29-WSF-05-14). All bats were aged on the basis of the degree of closure of the phalangeal epiphyses (Brunet-Rossinni & Wilkinson, 2009). Bats were held captive in quarantine for at least 1 month prior to use, and marked individually with metal bands on the forearm. Samples of baseline sera for rabies VNA and oral swabs for RABV RNA were negative, which was used in assigning a presumptive naïve status of all bats at the start of the experiment. After quarantine, bats were separated randomly into cage groups of four or five individuals each, allowing an even distribution of males and females when possible. Each group was housed in a separate 813×305×254 mm stainless steel cage, with all cages held collectively in a room at 24–27 °C and 30 % humidity.
Experimental infections.
The overall infection and sampling scheme is listed in Table 1. The RABV used for these experiments was collected from the salivary glands of a naturally infected big brown bat in Pennsylvania during 2006 (CDC A06-3684). This virus was passaged once through murine neuroblastoma cell culture. On day 0, all 45 bats were inoculated intramuscularly (i.m.) into both the left and right masseter muscles with varying doses of RABV (10−0.1–104.9 MICLD50; Table 2) in 0.05 ml. Bats were observed for 175 days p.i. Twenty-six bats that survived the initial infection were reinfected on day 175 p.i. with 103.9 or 104.9 MICLD50 of the same RABV variant in 0.05 ml. Bats in the second experiment were observed for either 174 or 305 days p.i. On day 174 or 305 post-secondary infection (i.e. day 349 or 480 post-primary infection), 16 surviving bats were infected a third time with 104.9 MICLD50 of the same RABV variant in 0.05 ml. Bats in the third experiment were observed for 140 days p.i., for a combined observation period of 489–620 days for all bats.
Blood samples were obtained routinely from a peripheral wing vein during each experiment (Table 1) or by the intracardiac route for terminal exsanguination at euthanasia (Voigt & Cruz-Neto, 2009), and collected in sterile heparinized microcapillary tubes. Plasma was separated by low-speed centrifugation (2200 g) and stored at −20 °C until processing. Bats were euthanized, by exsanguination under anaesthesia followed by intracardiac injection of a barbiturate solution (i.e. pentobarbital sodium and phenytoin sodium), upon presentation of two or more definitive clinical signs of RABV infection (e.g. increased aggression/reclusion, acute weight loss, ataxia, atypical vocalizations, paresis or paralysis). Brain tissue was collected and tested for RABV antigen by the direct fluorescent antibody (DFA) test, as described by Dean et al. (1996), using a fluorescein isothiocyanate-labelled monoclonal antibody conjugate (Fujirebio Diagnostics, Inc.). For a subset of DFA-positive bats, total RNA was extracted from the brain tissue of individual bats and the RABV nucleoprotein gene was amplified and sequenced as described by Trimarchi & Smith (2002).
Detection of rabies VNA.
A modified rapid fluorescent focus inhibition test (Jackson et al., 2008; Smith et al., 1996), using RABV challenge virus standard (CVS-11, V399), was used to assay rabies VNA in the blood plasma of individual bats. The rabies VNA end-point titres of individual bats were calculated by the method of Reed & Muench (1938) and were converted to IU ml−1 by comparison with the US Standard Rabies Immune Globulin (SRIG; Laboratory of Standards and Testing, Food and Drug Administration, USA) diluted to 2 IU ml−1. Any values >0.06 IU ml−1, equivalent to >50 % reduction of 50 focus-forming doses at a 1 : 13 dilution in this study, were considered as evidence of neutralization according to prior routine assay criteria (Smith et al., 1996). Maximum rabies VNA titres during the course of each infection are reported for individual bats. Titres that both were positive and exhibited a fourfold or greater increase above baseline values of the primary infection were considered evidence of seroconversion p.i.
Statistical analyses.
All statistics were performed using jmp v.7 (SAS Institute). All bats that were censored due to non-specific deaths were excluded from the statistical calculations, including two bats during the primary infection and one bat during the secondary infection. A nominal logistic regression model was used to test for effects of categorical variables on infection or seroconversion outcomes using the likelihood ratio test statistic (α=0.05). Spearman's rank order correlation tested for a non-parametric correlation between incubation period and inoculum dose. Fisher's exact one-tailed test was used to test the hypothesis that mortality in a subsequent infection was lower than in the previous infection (α=0.05).
Acknowledgments
This research was supported in part by a National Science Foundation–National Institutes of Health Ecology of Infectious Disease grant (#0430418 to G. F. M.), and also an Environmental Protection Agency Science-To-Achieve-Results (STAR) Fellowship to A. S. T. The authors thank staff on the Rabies Team at the CDC and the Animal Resources Branch in Lawrenceville, GA, USA, for their expertise. Special thanks go also to I. Kuzmin, B. Panasuk, D. Palmer and J. Ellison for technical assistance during the study. Use of trade names and commercial sources is for identification only and does not imply endorsement by the US Department of Health and Human Services. The findings and conclusions in this report are those of the authors and do not necessarily represent the views of their institutions.
References
- Austad, S. N. & Fischer, K. E. (1991). Mammalian aging, metabolism, and ecology: evidence from the bats and marsupials. J Gerontol 46, B47–B53. [DOI] [PubMed] [Google Scholar]
- Baer, G. M. & Bales, G. L. (1967). Experimental rabies infection in the Mexican freetail bat. J Infect Dis 117, 82–90. [DOI] [PubMed] [Google Scholar]
- Blanton, J. D., Robertson, K., Palmer, D. & Rupprecht, C. E. (2009). Rabies surveillance in the United States during 2008. J Am Vet Med Assoc 235, 676–689. [DOI] [PubMed] [Google Scholar]
- Brunet-Rossinni, A. K. & Wilkinson, G. S. (2009). Methods for age estimation and the study of senescence in bats. In Ecological and Behavioral Methods for the Study of Bats, pp. 315–325. Edited by T. H. Kunz & S. Parsons. Baltimore, MD: Johns Hopkins University Press.
- Daniels, J. B., Stuart, G., Wheeler, R. E., Gifford, C., Ahearn, J. P., Philbrook, R., Hayes, R. O. & MacCready, R. A. (1960). A search for encephalitis and rabies in bats of eastern Massachusetts. N Engl J Med 263, 516–520. [Google Scholar]
- Dean, D. J., Abelseth, M. K. & Atanasiu, P. (1996). The fluorescent antibody test. In Laboratory Techniques in Rabies, 4th edn, pp. 88–93. Edited by F. X. Meslin, M. M. Kaplan & H. Koprowski. Geneva: WHO.
- Dietzschold, B., Li, J., Faber, M. & Schnell, M. (2008). Concepts in the pathogenesis of rabies. Future Virol 3, 481–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimitrov, D. T. & Hallam, T. G. (2009). Effects of immune system diversity and physical variation of immunotypic mixing on the dynamics of rabies in bats. J Biol Dyn 3, 164–179. [DOI] [PubMed] [Google Scholar]
- Girard, K. F., Hitchcock, H. B., Edsall, G. & MacCready, R. A. (1965). Rabies in bats in southern New England. N Engl J Med 272, 75–80. [DOI] [PubMed] [Google Scholar]
- Goehring, H. H. (1972). Twenty-year study of Eptesicus fuscus in Minnesota. J Mammal 53, 201–207. [Google Scholar]
- Hall, E. R. (1981). The Mammals of North America. New York: Wiley.
- Hitchcock, H. B. (1965). Twenty-three years of bat banding in Ontario and Quebec. Can Field Nat 79, 4–14. [Google Scholar]
- Jackson, F. R., Turmelle, A. S., Farino, D. M., Franka, R., McCracken, G. F. & Rupprecht, C. E. (2008). Experimental rabies virus infection of big brown bats (Eptesicus fuscus). J Wildl Dis 44, 612–621. [DOI] [PubMed] [Google Scholar]
- Koopman, K. F. (1982). Biogeography of the bats of South America. In Mammalian Biology in South America, pp. 273–302. Edited by M. A. Mares & H. H. Genoways. Pittsburgh, PA: University of Pittsburgh.
- Kunz, T. H. & Pierson, E. D. (1994). Bats of the world: an introduction. In Bats of the World, pp. 1–46. Edited by R. M. Nowak. Baltimore, MD: Johns Hopkins University Press.
- Kurta, A. & Baker, R. H. (1990). Eptesicus fuscus. Mamm Species 356, 1–10. [Google Scholar]
- Lafon, M. (2007). Immunology. In Rabies, 2nd edn, pp. 489–504. Edited by A. Jackson & W. Wunner. London: Academic Press.
- Leslie, M. J., Messenger, S., Rohde, R. E., Smith, J., Cheshier, R., Hanlon, C. & Rupprecht, C. E. (2006). Bat-associated rabies virus in skunks. Emerg Infect Dis 12, 1274–1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McQuiston, J. H., Yager, P. A., Smith, J. S. & Rupprecht, C. E. (2001). Epidemiologic characteristics of rabies virus variants in dogs and cats in the United States, 1999. J Am Vet Med Assoc 218, 1939–1942. [DOI] [PubMed] [Google Scholar]
- Messenger, S. L., Smith, J. S. & Rupprecht, C. E. (2002). Emerging epidemiology of bat-associated cryptic cases of rabies in humans in the United States. Clin Infect Dis 35, 738–747. [DOI] [PubMed] [Google Scholar]
- Messenger, S. L., Smith, J. S., Orciari, L. A., Yager, P. A. & Rupprecht, C. E. (2003). Emerging pattern of rabies deaths and increased viral infectivity. Emerg Infect Dis 9, 151–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mondul, A. M., Krebs, J. W. & Childs, J. E. (2003). Trends in national surveillance for rabies among bats in the United States (1993–2000). J Am Vet Med Assoc 222, 633–639. [DOI] [PubMed] [Google Scholar]
- Moore, M. S., Jackson, F. R., Turmelle, A. S., Panasuk, B. J., Mendonca, M. T., Rupprecht, C. E., Kunz, T. H. & McCracken, G. F. (2008). Rabies exposure, relative immune function, and life-history traits in the big brown bat (Eptesicus fuscus). Bat Res News 49, 150 [Google Scholar]
- O'Shea, T. J., Shankar, V., Bowen, R. A., Rupprecht, C. E. & Wimsatt, J. H. (2003). Do bats acquire immunity to rabies? Evidence from the field. Bat Res News 44, 161 [Google Scholar]
- Pybus, M. J. (1986). Rabies in insectivorous bats of western Canada, 1979 to 1983. J Wildl Dis 22, 307–313. [DOI] [PubMed] [Google Scholar]
- Reed, L. J. & Muench, H. (1938). A simple method of estimating fifty per cent endpoints. Am J Hyg 27, 493–497. [Google Scholar]
- Schowalter, D. B. (1980). Characteristics of bat rabies in Alberta. Can J Comp Med 44, 70–76. [PMC free article] [PubMed] [Google Scholar]
- Smith, J. S. (1981). Mouse model for abortive rabies infection of the central nervous system. Infect Immun 31, 297–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith, J. S. (1996). New aspects of rabies with emphasis on epidemiology, diagnosis, and prevention of the disease in the United States. Clin Microbiol Rev 9, 166–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith, J. S., McCelland, C. L., Reid, F. L. & Baer, G. M. (1982). Dual role of the immune response in street rabiesvirus infection of mice. Infect Immun 35, 213–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith, J. S., Yager, P. & Baer, G. (1996). A rapid fluorescent focus inhibition test (RFFIT) for determining rabies virus-neutralizing antibody. In Laboratory Techniques in Rabies, pp. 181–192. Edited by F. X. Meslin, M. M. Kaplan & H. Koprowski. Geneva: WHO.
- Trimarchi, C. & Debbie, J. G. (1977). Naturally occurring rabies virus and neutralizing antibody in two species of insectivorous bats of New York state. J Wildl Dis 13, 366–369. [DOI] [PubMed] [Google Scholar]
- Trimarchi, C. V. & Smith, J. (2002). Diagnostic evaluation. In Rabies, pp. 308–344. Edited by A. C. Jackson & W. H. Wunner. San Diego, CA: Academic Press.
- Turmelle, A. S. (2002). Phylogeography and population structure of the big brown bat (Eptesicus fuscus). Honors thesis, Boston University, Boston, MA, USA.
- Turmelle, A. S., Allen, L. C., Jackson, F. R., Kunz, T. H., Rupprecht, C. & McCracken, G. F. (2010). Ecology of rabies virus exposure in colonies of Brazilian free-tailed bats (Tadarida brasiliensis) at natural and man-made roosts in Texas. Vector Borne Zoonotic Dis 10, 165–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voigt, C. & Cruz-Neto, A. (2009). Energetic analysis of bats. In Ecological and Behavioral Methods for the Study of Bats, pp. 623–645. Edited by T. H. Kunz & S. Parsons. Baltimore, MD: Johns Hopkins University Press.
- Wang, Z. W., Sarmento, L., Wang, Y., Li, X. Q., Dhingra, V., Tseggai, T., Jiang, B. & Fu, Z. F. (2005). Attenuated rabies virus activates, while pathogenic rabies virus evades, the host innate immune responses in the central nervous system. J Virol 79, 12554–12565. [DOI] [PMC free article] [PubMed] [Google Scholar]