Abstract
As sexual transmission of human immunodeficiency virus-1 (HIV-1) occurs via the mucosa, an ideal HIV-1 vaccine should induce both mucosal and systemic immunity. We therefore sought to evaluate the induction of mucosal responses using a DNA env prime–gp120 protein boost approach in which sequential nasal and parenteral protein administration was performed with two novel carbohydrate-based adjuvants. These adjuvants, Advax-M and Advax-P, were specifically designed for mucosal and systemic immune enhancement, respectively. Murine intranasal immunization with gp120/Advax-M adjuvant elicited gp120-specific IgA in serum and mucosal secretions that was markedly enhanced by DNA priming. Boosting of DNA-primed mice with gp120/Advax-M and gp120/Advax-P by sequential intranasal and intramuscular immunization, or vice versa, elicited persistent mucosal gp120-specific IgA, systemic IgG and memory T- and B-cell responses. Induction of homologous, but not heterologous, neutralizing activity was noted in the sera of all immunized groups. While confirmation of efficacy is required in challenge studies using non-human primates, these results suggest that the combination of DNA priming with sequential nasal and parenteral protein boosting, with appropriate mucosal and systemic adjuvants, could generate strong mucosal and systemic immunity and may block HIV-1 mucosal transmission and infection.
INTRODUCTION
Mucosal transmission of human immunodeficiency virus (HIV)-1 often leads to rapid depletion of activated CD4+CCR5+ T-cells in mucosal tissues and establishes a major reservoir for virus persistence in gut-associated lymphoid tissues (Brenchley et al., 2004; Chase et al., 2007; Pandrea et al., 2007). This indicates that an HIV vaccine should induce strong and long-lasting mucosal immunity at both the B- and T-cell level. Induction of HIV-specific IgA and CTL at critical mucosal sites should provide a first line of defence to block mucosal penetration, with systemic HIV-specific IgG and CTLs defending against parenteral HIV-1 transmission (Belyakov et al., 2006; Shattock et al., 2008; Srivastava et al., 2008; Vajdy, 2006).
Limited HIV-1 vaccine studies have shown that mucosal immunization can elicit secretory IgA, CTL and memory B- and T-cell responses in mucosal compartments that are dependent on the vaccine regimen, route of immunization and adjuvants used (Alving & Rao, 2008; Lai et al., 2007; Manrique et al., 2009; Vajdy & Singh, 2006). Mucosal adjuvants that have been studied extensively include the secreted enterotoxins of Vibrio cholerae and Escherichia coli, and mutated forms thereof [e.g. cholera toxin, Escherichia coli heat-labile toxin (LT), LTK63 (non-toxic LT mutant) and LTR72 (non-toxic LT mutant)] (Connell, 2007; Glenn et al., 2007; Stevceva & Ferrari, 2005). However, the use of these toxins as mucosal adjuvants has been impeded by safety issues, most notably cases of facial palsy in human trials of an LT-adjuvanted nasal influenza vaccine (Couch, 2004; van Ginkel et al., 2000, 2005). Many parenteral adjuvants such as CpG oligodeoxynucleotides, polymerized liposomes, microparticles and interleukins [such as IL-12 and granulocyte macrophage colony-stimulating factor (GM-CSF)], are currently being evaluated as mucosal adjuvants. However, none of these appear to be as successful as CT or LT in induction of mucosal immunity (Ahmed et al., 2005; Bradney et al., 2002; Manrique et al., 2008; Matyas et al., 2009; Staats et al., 2001). Until recently, relatively little attention has been given to the potential use of carbohydrate compounds as mucosal adjuvants. Two highly promising carbohydrate-based adjuvant systems currently in advanced-clinical and preclinical testing are Advax-P and Advax-M, respectively. These adjuvants are derived from natural sugar-containing compounds extracted from plants (Advax-P) and marine sponges (Advax-M) that have potent immune-enhancing activities (Fujii et al., 2006; Kobayashi et al., 1995; Petrovsky, 2006), and have favourable safety profiles in animal models and humans (Cooper, 1995; Cooper et al., 1991; Veldt et al., 2007). Advax-P is a microparticulate adjuvant formulation based on δ-inulin and is specifically designed for parenteral administration. Advax-P has previously proved successful for enhancing neutralizing immune responses against Japanese encephalitis virus (Lobigs et al., 2010) and seasonal and pandemic H1N1/2009 influenza virus (N. Petrovsky, personal communication), while exhibiting good tolerability. In contrast, Advax-M is a glycolipid adjuvant formulation based on α-galactosyl ceramide and is specifically designed for mucosal administration. Galactosyl ceramide and its analogues are potent natural killer T-cell agonists, enhance mucosal IgA production via a mechanism dependent on interleukin (IL)-4 and have been shown to enhance protection against heterologous influenza-virus challenge when nasally administered with an inactivated influenza antigen (Kamijuku et al., 2008).
The hypothesis of the current study is that induction of optimal mucosal and systemic immunity to HIV-1 may require a multimodal vaccine approach. Initial DNA immunization would maximize helper T-cell and Bmem priming. Subsequent intranasal (IN) and parenteral immunization with protein, using two novel adjuvants, would boost systemic and mucosal responses. Mucosal and systemic immune responses were evaluated in mice following priming with DNA encoding HIV-1 envelope (env) and boosting with gp120 protein combined with either Advax-M or Advax-P adjuvants delivered via mucosal, parenteral or mucosal/parenteral combination routes. Results presented here demonstrate that this DNA prime–protein boost vaccine-regimen strategy, incorporating Advax-M and/or Advax-P adjuvants, elicits robust and durable immune effector and memory responses in both mucosal and systemic compartments and may therefore contribute to enhanced protection against HIV-1.
RESULTS
Generation of systemic and mucosal immune responses following DNA priming and protein boosting by mucosal or parenteral routes
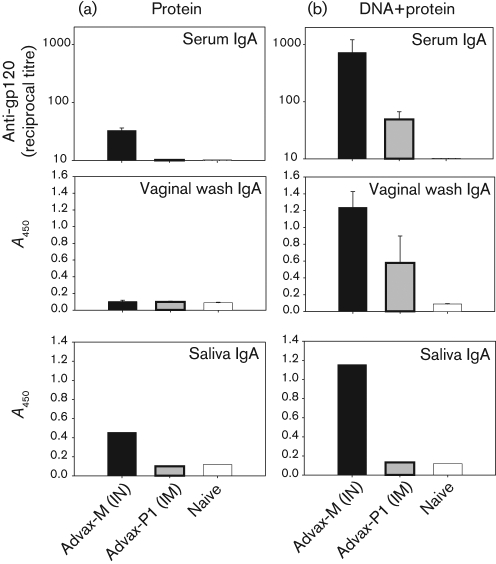
To provide a baseline adjuvant comparison, BALB/c mice were immunized with recombinant-HIV-1Ba-L gp120 formulated in two novel adjuvants, Advax-M and Advax-P1, delivered by IN and intramuscular (IM) routes, respectively (Fig. 1a). Anti-gp120 IgG was measured in serum and anti-gp120 IgA was measured in serum, saliva and vaginal wash samples. Serum anti-gp120 IgA titres were detected in mice immunized with Advax-M-formulated gp120 but not with Advax-P1-adjuvanted gp120 (P<0.01; Fig. 2a, upper panel). A similar trend was noted for pooled saliva from test groups, in which IgA was only detected in mice immunized with gp120/Advax-M (Fig. 2a, lower panel). For vaginal wash samples, anti-gp120 IgA was not detectable in any groups that had been immunized with adjuvanted protein alone (Fig. 2a, middle panel). Anti-gp120 IgG responses were noted in both test groups and were found to be greater in the Advax-P1 group compared with the Advax-M group (data not shown).
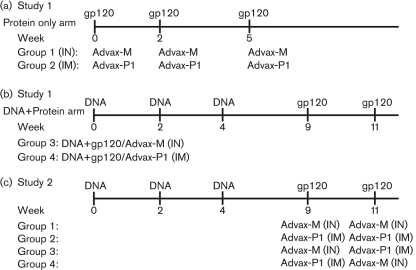
Fig. 1.
Murine immunization schedule. (a) Mice (n=5) were immunized with recombinant gp120 (25 μg) via the IM route for Advax-P1 (1 mg) or via the IN route for Advax-M (2 μg) at weeks 0, 2 and 5. (b) Alternatively, mice (n=5) were immunized with DNA (100 μg) at weeks 0, 2 and 4, and with adjuvanted gp120 protein at weeks 9 and 11. (c) Mice (n=10) were immunized intramuscularly with DNA (100 μg) at weeks 0, 2 and 4 and boosted with adjuvanted gp120 (25 μg) for either Advax-M or Advax-P1 via the IN or IM routes, respectively, at weeks 9 and 11. Combination delivery strategies were tested in which gp120 was administered by the IM route for Advax-P1 at week 9 and by the IN route for Advax-M at week 11 or vice versa.
Fig. 2.
Generation of anti-gp120 IgA antibodies in serum and mucosal compartments of mice immunized with DNA and adjuvanted protein. At 2 weeks post-protein immunization, anti-gp120 IgA responses, assayed by ELISA, were determined for serum (upper panels), vaginal wash samples (middle panels) and saliva (lower panels) following protein (a), and DNA prime–protein boost (b), immunization. Mean serum titres±sem values are shown. For vaginal-wash samples (1 : 2 dilution) and saliva (1 : 5 dilution), mean A450 measurements±sem are shown for the diluted samples.
Earlier studies in mice and macaques demonstrated that, in DNA-primed animals boosted parenterally with QS-21-adjuvanted protein, systemic IgG and T-cell responses were induced (Cristillo et al., 2006; Pal et al., 2005, 2006). A similar DNA prime–protein boost vaccine regimen was used here to compare mucosal and systemic immune responses elicited by Advax-M and Advax-P1 given via IN and IM routes, respectively. Mice primed with three administrations of DNA were boosted twice with adjuvanted gp120 by IN or IM routes (Fig. 1b). Serum anti-gp120 IgA levels increased in all test groups relative to adjuvanted protein-only immunization (P<0.01) and were greatest in the Advax-M group (Fig. 2b, upper panel). Whereas vaginal wash samples from mice immunized with adjuvanted protein showed no detectable anti-gp120 IgA, antibody levels were markedly augmented in Advax-M and Advax-P1 groups following prime–boost immunization (P<0.05) (Fig. 2b, middle panel). When evaluated further in pooled saliva, anti-gp120 IgA responses were only detected in the Advax-M-boosted groups (Fig. 2b, lower panel).
Increased serum-anti-gp120 IgG titres were noted in all adjuvant groups following DNA prime–protein boost compared with protein-only immunization (data not shown). Serum antibody responses, characterized by IgG subclasses (Table 1), revealed that immunization with DNA or protein alone resulted in a predominantly IgG1 response. DNA prime–protein boost immunization incorporating Advax-M or Advax-P1 resulted in a broader response including IgG1, IgG2a and IgG2b, with minimal IgG3 responses detected in the Advax-P1 group. The relative proportions of specific IgG isotypes elicited following prime–boost immunization varied depending upon the adjuvant used. Advax-M yielded profiles in which IgG1>IgG2b>IgG2a and Advax-P1 generated responses in which IgG1>IgG2a>IgG2b.
Table 1.
Anti-gp120-specific IgG titres
Median values±sem of anti-gp120 IgG-isotype reciprocal titres.
| Vaccine/route | IgG1 | IgG2a | IgG2b | IgG3 |
|---|---|---|---|---|
| DNA (IM) only | 23 040±6271 | 980±387 | 715±415 | 25±8 |
| gp120/Advax-M (IN) | 10 880±1920 | 10±10 | 440±147 | <25 |
| gp120/Advax-P1 (IM) | 28 160±9406 | 60±37 | 920±582 | <25 |
| DNA+gp120/Advax-M (IN) | 204 800±153 600 | 21 920±11 962 | 56 960±37 868 | <25 |
| DNA+gp120/Advax-P1 (IM) | 81 920±30 720 | 24 320±11 113 | 8480±2673 | 170±158 |
| DNA+gp120/Advax-P1 (IM)+gp120/Advax-M (IN) | 104 960±41 358 | 5960±2837 | 2720±480 | <25 |
| DNA+gp120/Advax-M (IN)+gp120/Advax-P1 (IM) | 327 680±122 880 | 62 120±37 351 | 14 720±9308 | 180±155 |
Mucosal and systemic responses observed in the Advax test groups were compared with previous results obtained with QS-21. No IgA responses were detected in mucosal washes of mice immunized with QS-21-adjuvanted gp120 or following DNA prime–protein boost immunization. In sera, minimal IgA responses were only detected after DNA prime–protein boost immunization (data not shown).
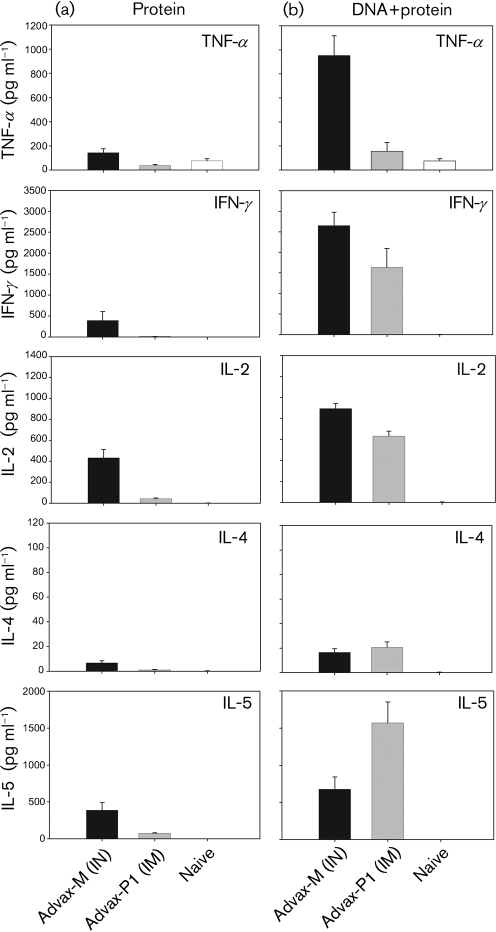
Th1 cytokine responses were noted in mice immunized with gp120/Advax-M, with lower cytokine levels (P<0.01 vs Advax-M) observed in mice immunized with gp120/Advax-P1 (Fig. 3a). For Th2 cytokines, IL-5 was elicited in mice immunized with protein/Advax-M and to a lesser extent with protein/Advax-P1 (P<0.01) (Fig. 3a). IL-4 levels were either minimal or not detected in both groups. Following prime–boost immunization (Fig. 3b), Th1 cytokine levels increased in both groups relative to adjuvanted protein-only immunization. For the Advax-M group, tumour necrosis factor α (TNF-α), gamma interferon (IFN-γ) and IL-2 were significantly (P<0.01) augmented relative to protein-only immunization. For the Advax-P1 group, increased cytokine levels were only statistically significant for IFN-γ and IL-2 (P<0.01). Consistent with the Th1 cytokines, a trend of greater Th2 cytokine levels was noted following prime–boost immunization compared with protein-only immunization (Fig. 3b). This increase in Th2 cytokines was statistically significant in the Advax-P1 but not Advax-M group. Mice immunized with DNA followed by QS-21-adjuvanted protein induced comparable Th1 and Th2 cytokines as noted with Advax-P1 (data not shown).
Fig. 3.
T-cell responses in splenocytes of mice immunized with DNA and adjuvanted protein. Secreted Th1 (TNF-α, IFN-γ and IL-2) and Th2 (IL-5 and IL-4) cytokines were quantified, by CBA, following a 24 h ex vivo stimulation of splenocytes with 1 μg Env peptide pool ml−1, from protein-immunized (a) and DNA prime–protein boosted (b) mice. Mean cytokine responses for each group±sem values are shown.
Induction of persistent mucosal- and systemic- anti-gp120 antibodies following DNA prime–protein boost immunization
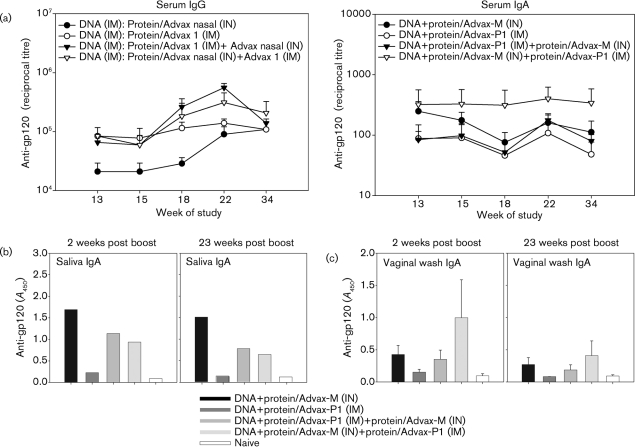
Given that both systemic and mucosal anti-gp120 IgA responses were seen in mice immunized with Advax-M or Advax-P1, an additional study was conducted to evaluate sequential IN/IM protein boost strategies using these Advax adjuvants in DNA-primed animals. BALB/c mice were immunized with DNA at 0, 2 and 4 weeks and adjuvanted gp120 at 9 and 11 weeks (Fig. 1c). Protein was formulated either in Advax-M or Advax-P1 adjuvant and delivered by IN (IN/IN) or IM (IM/IM) routes, respectively, or in combination (IM/IN, IN/IM). At 2 weeks post-final protein immunization, serum anti-gp120 IgG was found to be comparable in the IM/IM, IM/IN and IN/IM test groups (Fig. 4a, left panel). A trend of lower IgG responses was noted in the IN/IN group but the difference between these levels and those of the other test groups was not found to be statistically significant (Fig. 4a, left panel). To evaluate the decline in titres over time, as previously noted for DNA prime–protein boost immunizations (Pal et al., 2006), anti-gp120 IgG levels were monitored up to week 23 post-boost immunization. Interestingly, anti-gp120 IgG titres increased in all test groups between 2 and 23 weeks post-boost immunization and were comparable in all groups at week 23. Serum antibody responses, characterized by IgG subclass, found broad subclass responses for IM/IN and IN/IM groups, in which IgG1>IgG2a>IgG2b (Table 1). Higher titres for each IgG subclass were seen in the IN/IM group compared with the IM/IN group. IgG3 responses were minimally detected in the IN/IM group but not the IM/IN group, consistent with the asymmetry of the immune response according to the specific order in which IN and IM booster immunizations were administered.
Fig. 4.
Durability of anti-gp120 IgG and serum and mucosal IgA responses following immunization of mice via parenteral, mucosal and combination routes. Anti-gp120 IgG (a, left panel) and IgA (a, right panel) responses, assayed by ELISA, were determined for serum samples of DNA prime–protein boosted mice at weeks 13, 15, 18, 22 and 34 of the study. IgA was also measured at weeks 13 and 34 of the study for saliva (b, 1 : 5 dilution) and vaginal-wash samples (c, 1 : 2 dilution). ELISA titres and A450 are reported.
At 2 weeks post-final protein boost immunization (week 13 of the study), anti-gp120 IgA titres were detected in the sera of immunized mice from all test groups and the levels persisted up to 23 weeks post-immunization (week 34 of the study) (Fig. 4a, right panel). A trend for increased serum IgA titres was observed in mice primed with DNA and boosted with adjuvanted protein delivered via the IN/IM route compared with the other test groups; however, this difference was not statistically significant. Anti-gp120 IgA was seen in mucosal samples of immunized mice including pooled saliva (Fig. 4b) and vaginal wash samples (Fig. 4c). Anti-gp120 IgA in the saliva persisted up to 23 weeks post-boost immunization. In vaginal-wash samples, IgA titres were relatively unchanged at 23 weeks post-boost immunization compared to 2 weeks post-boost in all groups.
In order to determine the functional properties of the antibodies elicited by these vaccine regimens, the neutralizing activity of sera collected at 2 weeks following the final immunization was assayed against homologous (SHIVBa-L and pseudovirus HIV-1BaL.26) and heterologous (AC10.0 and QH0692) HIV-1 isolates. Since the volume of serum collected for each mouse was limited, neutralization assays were performed on pooled sera for each immunized group. For these assays, two types of homologous viruses encoding HIV-1Ba-L envelope were selected. HIV-1BaL.26 is a pseudovirus, whereas SHIVBa-L is a live virus capable of replicating in non-human primates and has been used in previous vaccine-efficacy studies (Pal et al., 2003, 2006). As shown in Table 2, homologous neutralizing activity was detected in all immunized groups against HIV-1BaL.26 and SHIVBa-L, with higher titres against SHIVBa-L. Amongst the combination delivery strategies, mice immunized by the IN/IM route elicited higher neutralizing activity compared with the IM/IN route. Heterologous neutralization against AC10 and QH0692 was not detected in any of the immunized groups.
Table 2.
Neutralizing activity of sera following immunizations of gp120 with Advax adjuvants
A neutralization assay was conducted with pooled sera from each group of animals. Neutralization titres represent the dilution of serum inhibiting 50 % of infection compared with an untreated control infection. The lowest dilution of serum tested was 1 : 10.
| Vaccine/route | BaL.26 | SHIVBa-L | AC10.0 | QH0692 |
|---|---|---|---|---|
| gp120/Advax-M (IN) | 16 | 70 | <10 | <10 |
| gp120/Advax-P1 (IM) | 27 | 160 | <10 | <10 |
| DNA+gp120/Advax-M (IN) | 26 | 100 | <10 | <10 |
| DNA+gp120/Advax-P1 (IM) | 21 | 92 | <10 | <10 |
| DNA+gp120/Advax-P1 (IM)+gp120/Advax-M (IN) | 10 | 24 | <10 | <10 |
| DNA+gp120/Advax-M (IN)+gp120/Advax-P1 (IM) | 32 | 160 | <10 | <10 |
Generation of robust and persistent Th1/Th2 cytokine responses following DNA prime–protein boost immunization
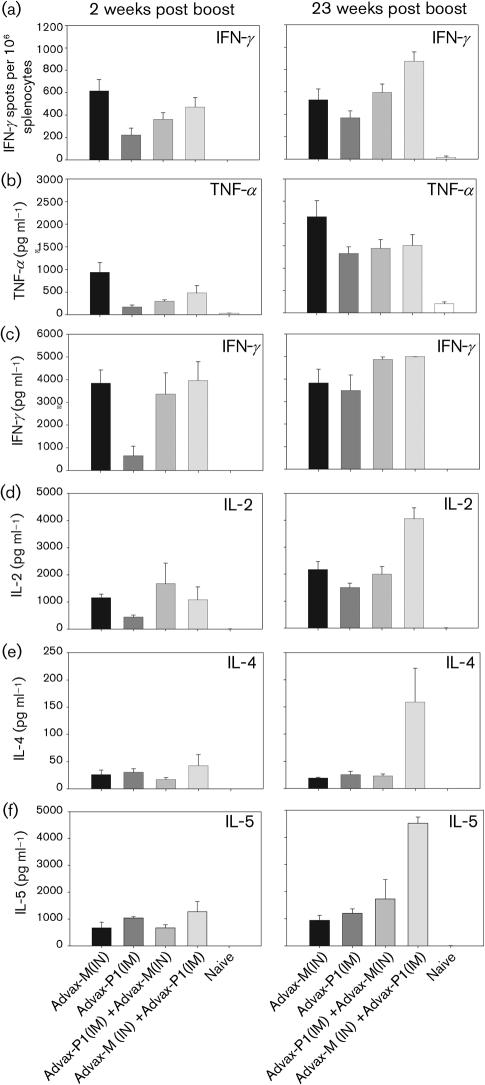
At 2 weeks post-final immunization, gp120-specific IFN-γ-secreting splenocytes were detected in all immunized mice (Fig. 5a, left panel). A trend towards increased IFN-γ was noted in mice primed with DNA and boosted with Advax-M-adjuvanted protein administered via the IN/IN route compared with the other groups. However, this trend was only statistically significant when comparing Advax-M (IN/IN) and Advax-P1 (IM/IM) test groups (P<0.05). Th1 (TNF-α and IFN-γ) cytokines, measured by cytometric bead array (CBA) at 2 weeks post-final immunization (Fig. 5b–f, left panel), were comparable in the IN/IN, IM/IN and IN/IM test groups but lower in the IM/IM group (P<0.05). In contrast, IL-2 and Th2 (IL-4 and IL-5) cytokine levels were not significantly different between groups. Sustained high levels of gp120-specific IFN-γ-secreting splenocytes were observed at 23 weeks post-final immunization by ELISPOT (Fig. 5a, right panel) and CBA assays (Fig. 5c, right panel). For other Th1 cytokines evaluated, TNFα levels observed at 23 weeks post-immunization were augmented in all test groups relative to the levels at 2 weeks post-immunization (Fig. 5b, right panel). For IL-2, IL-4 and IL-5, levels were markedly enhanced only in mice immunized via IN/IM routes (Fig. 5d–f, right panels).
Fig. 5.
Durability of gp120-specific Th1 and Th2 cytokine recall responses. Cellular responses were measured, following ex vivo Env peptide stimulation, by IFN-γ ELISPOT and CBA assays at 2 (left panels) and 23 weeks (right panels) post-protein boost. IFN-γ production, measured by ELISPOT assay (a), and secreted TNF-α (b), IFN-γ (c), IL-2 (d) IL-4 (e) and IL-5 (f) levels measured by CBA, are shown.
Generation of memory B- and T-cell responses following DNA prime–protein boost with Advax adjuvants
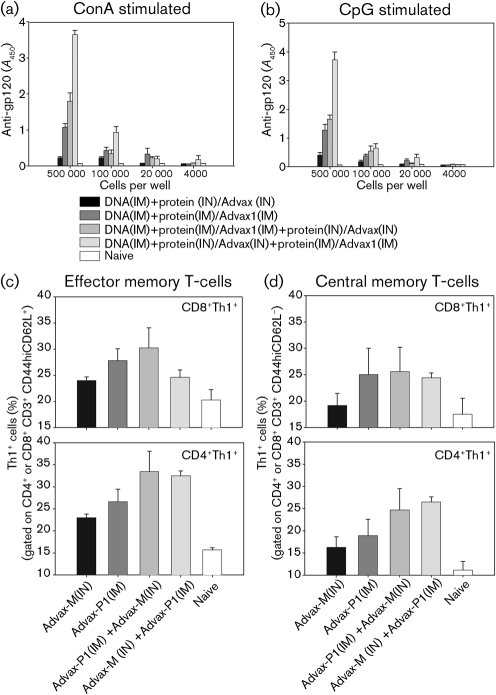
Persistent levels of anti-gp120 antibodies (Fig. 4) and sustained T-cell responses (Fig. 5) following prime–boost immunization led us to assess memory B- and T-cell responses. Splenocytes from immunized mice (23 weeks post-immunization) were stimulated with either CpG ODN2006 or concanavalin A (ConA), which have previously been shown to activate Bmem to become antibody-secreting cells (Guan et al., 2009; Slifka & Ahmed, 1996; Traggiai et al., 2004). Following a 7-day stimulation, anti-gp120 IgG responses were measured in the media (Fig. 6a, b). Under both stimulation conditions, anti-gp120 IgG responses were found to be greater in the IN/IM group relative to the IN/IN or IM/IM groups, but were not significantly different from the IM/IN group. As expected, responses were found to decline with decreasing numbers of splenocytes used. For memory T-cell responses, both CD8 and CD4 effector memory (CD44hiCD62L−; Fig. 6c) and central memory (CD44hiCD62L+; Fig. 6d) T-cells producing Th1 cytokines were observed in all test groups by using multiparameter FACS analysis. While a trend towards greater cytokine responses was found in DNA-primed mice that were boosted by IM/IM, IM/IN and IN/IM regimens, relative to the IN/IN regimen, these differences were not statistically significant.
Fig. 6.
Generation of B- and T-cell memory following immunization of mice via parenteral, mucosal and combination routes. At 23 weeks post-final immunization, splenocytes (1×105, 2×104 and 4×103) were stimulated for 7 days with a ConA supernatant mixture with mitomycin-treated splenocyte feeder cells (a). Alternatively, splenocytes (1×105, 2×104 and 4×103) were stimulated for 7 days with 4 mg CpG-ODN 2006 ml−1 with mitomycin-treated feeder cells (b). gp120-specific antibodies were measured in the media by ELISA, and A450 readings are shown. Alternatively, splenocytes (1.25×106 cells ml−1) were stimulated for 24 h with 1 μg Env peptide pool ml−1 at 37 °C, 5 % CO2 in the presence of 1 μg GolgiPlug (BD Bio Sciences) ml−1 for the final 6 h of stimulation. CD8 and CD4 effector memory (CD44hiCD62L−) (c) and central memory (CD44hiCD62L+) (d) T-cell responses, as measured by Th1 cytokine production.
DISCUSSION
Mucosal immunity is important for protection against pathogens, including HIV-1 (Asahi-Ozaki et al., 2004; Ozawa et al., 2009; Tamura et al., 1988; Watanabe et al., 2002). Early HIV-1 vaccine trials were focused on systemic immunity and were not designed specifically to elicit mucosal immune responses. Given the failures (Bradac & Dieffenbach, 2009; Robb, 2008; Watkins et al., 2008) and limited successes (Rerks-Ngarm et al., 2009) of HIV-1 vaccines to date, strategies to optimize induction of mucosal anti-HIV-1 immunity (Holmgren & Czerkinsky, 2005; Schoenly & Weiner, 2008) are needed. Approaches that have been explored include immunization via mucosal routes (e.g. IN), targeting antigens to lymph nodes (Finerty et al., 2001; Hinkula et al., 2008; Koopman et al., 2007; Lehner et al., 1999) and administering vaccines with mucosal adjuvants (Connell, 2007; Glenn et al., 2007; Stevceva & Ferrari, 2005).
While DNA prime–protein boost regimens have been evaluated for induction of systemic immunity, little is known about their effect on mucosal responses. We therefore hypothesized that a multimodal DNA prime–protein boost approach using parenteral and mucosal adjuvanted-protein delivery would maximize both mucosal and systemic immunity. To this end, two promising new adjuvants (Advax-M and Advax-P), specifically designed for mucosal and parenteral administration, were tested with gp120 protein. Similar, but unrelated, vaccine strategies have shown induction of long-term HIV-1-specific IgA responses in mucosal secretions and serum when mice were immunized intranasally with DNA encoding gp160 and gp41 peptides (Devito et al., 2000).
Our previous studies have demonstrated the utility of DNA priming prior to protein boost for induction of a strong systemic anti-gp120 antibody response (Cristillo et al., 2006; Pal et al., 2006; Wang et al., 2006, 2008). Those studies used QS-21 adjuvant, which has been found to be potent but is associated with reactogenicity in humans (Kennedy et al., 2008; Petrovsky, 2008). Extending these earlier findings, we now show the importance of the prime–boost regimen for induction of IgA. This was evidenced by the fact that strategies that failed to elicit anti-gp120 IgA responses in vaginal wash (Advax-P1 group) or serum (Advax-P1 group) samples following protein immunization alone induced anti-gp120 IgA following combined prime–boost vaccination (Fig. 2). While parenteral DNA immunization has previously been shown to elicit mucosal and systemic IgA antibodies, responses were weak and/or short-lived (Lai et al., 2007). To enhance such mucosal responses, DNA has been administered mucosally (Bertley et al., 2004; Manrique et al., 2009; Raska et al., 2008; Wang et al., 2004) and/or adjuvants such as GM-CSF (Lai et al., 2007), polyethyleneimine (Huang et al., 2007) or QS-21 (Sasaki et al., 1998) have been used. However, in the current study, naked DNA was administered parenterally followed by adjuvanted-protein boost delivered by mucosal, parenteral or combination routes. This alternate multimodal approach elicited robust and persistent mucosal IgA and systemic humoral and cellular immunity.
The importance of HIV-specific IgA has been demonstrated by studies showing that serum and mucosal IgA from highly exposed, persistently seronegative individuals inhibited HIV-1 transcytosis (Devito et al., 2000; Mazzoli et al., 1999) and could neutralize primary isolates of many subtypes (Devito et al., 2002). More recently, HIV-specific serum IgA from long-term survivors of HIV infection was shown to neutralize genetically diverse HIV-1 strains (Planque et al., 2010). For IgA characterization in mucosal samples, we did not discriminate between monomeric or secretory (dimeric or multimeric) IgA, nor did we determine whether the origin of IgA was the mucosa or sera. However, given the effectiveness of IgA from both sera and mucosa, such discrimination might not be critical in this instance.
The mechanism(s) by which Advax adjuvants enhanced serum and mucosal IgA levels following DNA priming has yet to be characterized. Other related carbohydrate adjuvants (e.g. γ-inulin) have been shown to enhance B-cell differentiation and antibody production, in part via activation of the alternative complement pathway (Carroll, 1998; Silva et al., 2004). In addition, galactosyl ceramide has been shown to enhance mucosal IgA production via CXCL16–CXCR6-dependent induction of IL-4 by mucosal NKT-cells (Kamijuku et al., 2008). Mucosal adjuvants, such as cholera toxin B subunit, promote IgA via transforming growth factor β1 (Kim et al., 1998) whereas CpG and polyinosinic:polycytidynic acid promote IgA class switching through induction of APRIL (a proliferation-inducing ligand) (Barone et al., 2009; Hardenberg et al., 2007; He et al., 2007; Shang et al., 2008). Future studies will investigate whether Advax adjuvants function via these mechanisms.
Combination mucosal–parenteral delivery strategies using protein alone have been reported to elicit robust mucosal antibody and T-cell responses in mice (Goodsell et al., 2008; Srivastava et al., 2008) and macaques (Barnett et al., 2008). Consistent with these reports, the current study also found induction of strong mucosal immunity following prime–boost immunization (Fig. 4). It is likely that protein immunization via the mucosal route expands DNA-primed Bmem and triggers specific homing to mucosal compartments. Subsequent parenteral protein boost further activates Bmem in spleen and bone marrow, thereby boosting systemic IgG production. Such distinct compartmentalization of mucosal and systemic immune responses has been described previously (Kantele et al., 1997, 1999; Qadri et al., 1998; Quiding-Järbrink et al., 1997). Future studies will examine whether a coordinated, compartment-driven homing of DNA-primed T- and B-cells occurs following protein immunization in non-human primates.
Involvement of specific IgG subclasses, including IgG2 (Ngo-Giang-Huong et al., 2001) and IgG3 (Scharf et al., 2001), in neutralizing HIV-1 and controlling viraemia have been described. In this study, an adjuvant-dependent broadening of the IgG-subclass response was noted following DNA prime–protein boost immunization that was not seen following administration of DNA or protein alone. It is likely that such broadening of IgG responses by our vaccine regimen may ultimately provide better control of viraemia in non-human primate efficacy studies.
Persistence of T- and B-cell responses will be of key importance to an effective HIV vaccine, especially for developing countries, where adherence to vaccination schedules may be an issue. In this study, the prime–boost vaccine regimen demonstrated persistent antibody and cell mediated immune (CMI) response over a 23-week period following final immunization. This led to the generation of memory B-cell as well as central and effector memory T-cells (Fig. 6). Recent studies have suggested that effector memory T-cells, which are the predominant memory T-cell population in mucosal compartments, may play a critical role in protection against SIV infection (Hansen et al., 2009). Therefore, such long-lived responses may be important for the containment of HIV-1.
Although a detailed characterization of neutralizing antibodies is difficult to perform in mice, due to limited availability of immune serum, preliminary neutralization assays were performed with pooled sera from each immunized group. While induction of homologous neutralizing antibodies was clearly noted following immunization, titres against live virus (SHIVBa-L) were higher than those against pseudovirus (HIV-1BaL.26). This finding may be due, in part, to the sequence heterogeneity amongst these two isolates. Neutralizing titres noted in the IN/IM group were found to be higher than those noted in the IM/IN group. Interestingly, the IN/IM group also demonstrated increased memory B-cell responses compared with the IM/IN group. Such findings, although preliminary, suggest that our vaccine, delivered via the IN/IM route, may have the potential to elicit high-titre neutralizing antibodies and may induce long-term memory B-cell responses. No heterologous neutralization was detected in any of the immunized groups. This finding was not surprising, as the HIV-1Ba-L Env antigen used for this study has been shown previously to elicit type-specific neutralization. The purpose of this selected antigen was primarily to show Env-specific-binding antibody responses in the periphery and in mucosal compartments. Future studies will include Env antigens, based on sequence and/or structural analyses, that yield an immunogen poised to induce broadly neutralizing antibodies.
One area relevant to HIV vaccine development that was not addressed in this study is the efficacy of the candidate vaccine against a defined challenge virus. Specific differences between the mucosal immune systems of rodents and primates limit the use of rodent models for the detailed evaluation of efficacy (Kunisawa et al., 2005; Vajdy & Singh, 2005). While humanized mice models have been developed recently for HIV transmission studies (Denton & Garcia, 2009), the immune responses in these models have not been characterized extensively, thereby limiting their use in vaccine-efficacy studies. Thus, the non-human primate model represents a better alternative for testing the efficacy of this vaccine regimen and is the subject of ongoing research. Given that neutralization was noted against SHIVBa-L with the vaccine regimen tested, it is probable that protection against a homologous challenge would be noted in non-human primate studies, as has been observed previously with polyvalent vaccines.
METHODS
Antigens and adjuvants.
Codon-optimized HIV-1 env gene encoding subtype B (HIV-1Ba-L) plasmid DNA and recombinant gp120 vaccine components were prepared as described (Cristillo et al., 2006; Pal et al., 2005, 2006; Wang et al., 2006). Recombinant gp120 was formulated with Advax-M and Advax-P1 adjuvants provided by Vaxine Pty Ltd by simple mixing prior to administration.
Murine immunizations.
For the first study, BALB/c mice (5–7 week-old females) were vaccinated with either recombinant gp120 alone or by DNA prime–protein boost immunization. For protein-only immunizations, mice were injected at weeks 0, 2 and 5 with recombinant gp120 (25 μg) either intramuscularly, formulated in Advax-P1 (1 mg) adjuvant, or intranasally, formulated in Advax-M (2 μg) adjuvant. For prime–boost immunizations, mice were immunized intramuscularly with DNA (100 μg) at weeks 0, 2 and 4, and with 25 μg adjuvant-formulated protein at weeks 9 and 11. At 2 weeks post-final immunization, mice were sacrificed and serum, vaginal washes, saliva, faecal pellets and splenocytes were collected to evaluate humoral and cellular immune responses.
For the second study, BALB/c mice (5–7 week-old females) were vaccinated using DNA prime–protein boost combination strategies. Mice were immunized intramuscularly with DNA (100 μg) at weeks 0, 2 and 4, and with adjuvanted gp120 protein (25 μg) at weeks 9 and 11. Alternatively, combination delivery strategies were tested in which protein was administered via the IM route at week 9 and via the IN route at week 11, or vice versa. At 2 and 23 weeks post-final protein immunization, mice were sacrificed and serum, vaginal washes, saliva and splenocytes were collected for analyses.
Peptides.
For the murine study, 79 HIV-1 Env (BaL) peptides (15-mers) with 11 overlapping residues were synthesized (Infinity Biotech Research and Resource) that spanned the gp120 Env sequence. These were resuspended in one peptide pool. For T-cell immune assays, cells were stimulated using a final per peptide concentration of 1 μg ml−1.
IFN-γ ELISPOT.
The IFN-γ ELISPOT assay was performed using splenocytes and according to the manufacturer's protocol (U-CyTech) as described previously (Cristillo et al., 2006, 2008a; Pal et al., 2005).
CBA.
CBA (BD Biosciences) was performed to quantify secreted Th1/Th2 cytokines from splenocytes, as described previously by Cristillo et al. (2008a, b).
Measuring gp120-specific IgG responses in serum by ELISA.
Sera were assayed for anti-gp120-specific IgG antibodies using an ELISA as described previously by Pal et al. (2002). Titres were determined, by ELISA, as the highest dilution of immune serum that produced A450 readings greater than or equal to two times the signal detected with a corresponding dilution of pre-immune serum.
Measuring gp120-specific IgG isotype responses in serum.
Anti-gp120-specific isotype responses in sera were assayed by ELISA using isotype-specific conjugates. Briefly, recombinant gp120 was coated onto 96-well plates (100 ng per well) (Greiner) and incubated overnight at 4 °C. Plates were washed (PBS pH 7.3/0.01 % Tween) and blocked [PBS/5 % dried-milk powder (DM)] for 1 h at 37 °C. Diluted (PBS/5 % DM) samples (100 μl) were added to plates and incubated for 1 h at 37 °C. Specific IgG isotypes were detected using rat anti-mouse IgG1–HRP (BD Pharmigen), rat anti-mouse IgG2a–HRP (BD Pharmigen), goat anti-mouse IgG2b–HRP (AbD Serotec) and goat anti-mouse IgG3–HRP (AbD Serotec). Anti-mouse IgG isotype conjugates (diluted 1 : 5000 in PBS/5 % DM) were added to the plates and incubated for 1 h at 37 °C. After washing, 100 μl Enhanced K-Blue tetramethylbenzidine (TMB) substrate (Neogen) was added and the plates were incubated for 15 min at 37 °C. Reactions were stopped with 100 μl 2 M sulfuric acid (LabChem) and A450 were measured (SpectraMax Plus 384; Molecular Devices).
Measuring gp120-specific IgA responses in serum and mucosal secretions by ELISA.
Saliva and vaginal-wash samples were collected from immunized or control mice as described by Kaminski & VanCott (1999). To rule out blood contamination contributing to transudated serum IgA (Meckelein et al., 2003), all mucosal washes in our study were tested with Haemoccult and shown to be negative. Coating of plates with gp120 and blocking were performed as described above. Samples (100 μl diluted in PBS/5 % DM at a 1 : 5 ratio for saliva and a 1 : 2 ratio for vaginal washes) were added to washed plates and incubated for 1 h at 37 °C. Goat anti-mouse IgA-HRP (100 μl, diluted 1 : 5000 in PBS/5 % DM) (Southern Biotech) was added to plates and incubated for 1 h at 37 °C. Plates were developed with TMB substrate as described above.
Neutralization assay.
Neutralization activity was measured with pooled sera (for each group) using a TZM-bl assay where a reduction in luciferase gene expression was measured after a single round of infection in the presence of immune serum compared with untreated control. Viruses were incubated with serial dilutions of duplicate serum samples (50 μl) in complete Dulbecco's modified Eagle's medium (DMEM) for 1 h at 37 °C. Freshly trypsinized TZM-bl cells (10 000 cells in 50 μl complete DMEM medium with 60 μg DEAE–dextran ml−1) were added to each well. For controls, wells received either cells and virus (virus control) or cells alone (background control). After 48 h, 100 μl lysate was transferred to 96-well black plates for measurement of luminescence (Bright-Glo Luciferase assay system; Promega). Neutralization titres are defined as the dilution of serum at which the relative luminescence units (RLU) were reduced by 50 % compared with virus control wells after subtraction of background RLU.
Memory B-cell analysis.
At 23 weeks post-final immunization, vaccine-specific memory B-cell responses were evaluated in splenocytes of immunized mice using two murine-adapted stimulation protocols, as described previously (Guan et al., 2009; Slifka & Ahmed, 1996; Traggiai et al., 2004). Splenocytes (1×105, 2×104 and 4×103 cells) were stimulated for 7 days with 4 mg CpG ODN2006 ml−1 or ConA supernatant mixture, in the presence of mitomycin-treated splenocyte feeder cells, to activate murine Bmem to become antibody-secreting cells as described by Slifka & Ahmed, (1996). Following stimulation, anti-gp120 titres in the supernatants were determined by ELISA (Pal et al., 2002).
Memory T-cell analysis.
CD8 and CD4 effector (CD44hiCD62L−) and central (CD44hiCD62L+) memory T-cell responses, measured by intracellular Th1 (TNF-α, IL-2 and IFN-γ) cytokine production, were evaluated in splenocytes of immunized and naive mice at 23 weeks post-final immunization using multi-parameter flow cytometry as described by Cristillo et al. (2008b). Splenocytes (1.25×106 cells ml−1), stimulated for 24 h with 1 μg Env peptide pool ml−1 at 37 °C and 5 % CO2 (and with 1 μg GolgiPlug ml−1 for the final 6 h), were collected, washed (BD FACS wash buffer; BD Biosciences) and stained with anti-murine anti-CD3–APC–Cy7, CD4–PerCP–Cy5.5, CD8–PE–Cy7, CD44–FITC (BD Biosciences) and CD62L–PE–Texas Red (Invitrogen). Intracellular staining of cells was performed using anti-TNF-α−PE, IFN-γ−PE and IL-2–PE (BD BioSciences). Cells (20 000 each of CD44hi+, CD4+ or CD8+ memory cells) were acquired using an LSRII cytometer and data were analysed using facs diva (BD BioSciences).
Statistics.
A two-tailed Mann–Whitney non-parametric test was performed to assess the statistical significance of the ELISA and CBA data for mice immunized with Advax-M and Advax-P1 in study I. For the ELISA, ELISPOT and CBA data from study II, in which multiple groups of mice were immunized using Advax-M and Advax-P1 by combination routes, the Kruskall–Wallis non-parametric test followed by Dunn's multiple-comparison post-test was used.
Acknowledgments
The authors thank Dr Deborah Weiss for veterinary care of the animals, Frimpong Kodua for conducting immunizations and sample collections, and Sharon Orndorff and James Treece for technical coordination of the animal studies. Pseudoviruses (AC10.0, QH00692, HIV-1BaL.26) were obtained through the NIH AIDS Research and Reference Reagent Program from Drs Mascola, Montefiori and Gao. Development of Advax adjuvants was supported by the NIAID with grants U01-AI061142 and HHSN272200800039C.
References
- Ahmed, R. K., Biberfeld, G. & Thorstensson, R. (2005). Innate immunity in experimental SIV infection and vaccination. Mol Immunol 42, 251–258. [DOI] [PubMed] [Google Scholar]
- Alving, C. R. & Rao, M. (2008). Lipid A and liposomes containing lipid A as antigens and adjuvants. Vaccine 26, 3036–3045. [DOI] [PubMed] [Google Scholar]
- Asahi-Ozaki, Y., Yoshikawa, T., Iwakura, Y., Suzuki, Y., Tamura, S., Kurata, T. & Sata, T. (2004). Secretory IgA antibodies provide cross-protection against infection with different strains of influenza B virus. J Med Virol 74, 328–335. [DOI] [PubMed] [Google Scholar]
- Barnett, S. W., Srivastava, I. K., Kan, E., Zhou, F., Goodsell, A., Cristillo, A. D., Ferrai, M. G., Weiss, D. E., Letvin, N. L. & other authors (2008). Protection of macaques against vaginal SHIV challenge by systemic or mucosal and systemic vaccinations with HIV-envelope. AIDS 22, 339–348. [DOI] [PubMed] [Google Scholar]
- Barone, F., Patel, P., Sanderson, J. D. & Spencer, J. (2009). Gut-associated lymphoid tissue contains the molecular machinery to support T-cell-dependent and T-cell-independent class switch recombination. Mucosal Immunol 2, 495–503. [DOI] [PubMed] [Google Scholar]
- Belyakov, I. M., Kuznetsov, V. A., Kelsall, B., Klinman, D., Moniuszko, M., Lemon, M., Markham, P. D., Pal, R., Clements, J. D. & other authors (2006). Impact of vaccine-induced mucosal high-avidity CD8+ CTLs in delay of AIDS viral dissemination from mucosa. Blood 107, 3258–3264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertley, F. M., Kozlowski, P. A., Wang, S. W., Chappelle, J., Patel, J., Sonuyi, O., Mazzara, G., Montefiori, D., Carville, A. & other authors (2004). Control of simian/human immunodeficiency virus viremia and disease progression after IL-2-augmented DNA-modified vaccinia virus Ankara nasal vaccination in nonhuman primates. J Immunol 172, 3745–3757. [DOI] [PubMed] [Google Scholar]
- Bradac, J. & Dieffenbach, C. W. (2009). HIV vaccine development: lessons from the past, informing the future. IDrugs 12, 435–439. [PubMed] [Google Scholar]
- Bradney, C. P., Sempowski, G. D., Liao, H. X., Haynes, B. F. & Staats, H. F. (2002). Cytokines as adjuvants for the induction of anti-human immunodeficiency virus peptide immunoglobulin G (IgG) and IgA antibodies in serum and mucosal secretions after nasal immunization. J Virol 76, 517–524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenchley, J. M., Schacker, T. W., Ruff, L. E., Price, D. A., Taylor, J. H., Beilman, G. J., Nguyen, P. L., Khoruts, A., Larson, M. & other authors (2004). CD4+ T cell depletion during all stages of HIV disease occurs predominantly in the gastrointestinal tract. J Exp Med 200, 749–759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll, M. C. (1998). The role of complement and complement receptors in induction and regulation of immunity. Annu Rev Immunol 16, 545–568. [DOI] [PubMed] [Google Scholar]
- Chase, A. J., Sedaghat, A. R., German, J. R., Gama, L., Zink, M. C., Clements, J. E. & Siliciano, R. F. (2007). Severe depletion of CD4+ CD25+ regulatory T cells from the intestinal lamina propria but not peripheral blood or lymph nodes during acute simian immunodeficiency virus infection. J Virol 81, 12748–12757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connell, T. D. (2007). Cholera toxin, LT-I, LT-IIa and LT-IIb: the critical role of ganglioside binding in immunomodulation by type I and type II heat-labile enterotoxins. Expert Rev Vaccines 6, 821–834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper, P. D. (1995). Vaccine adjuvants based on gamma inulin. Pharm Biotechnol 6, 559–580. [DOI] [PubMed] [Google Scholar]
- Cooper, P. D., McComb, C. & Steele, E. J. (1991). The adjuvanticity of Algammulin, a new vaccine adjuvant. Vaccine 9, 408–415. [DOI] [PubMed] [Google Scholar]
- Couch, R. B. (2004). Nasal vaccination, Escherichia coli enterotoxin, and Bell's palsy. N Engl J Med 350, 860–861. [DOI] [PubMed] [Google Scholar]
- Cristillo, A. D., Wang, S., Caskey, M. S., Unangst, T., Hocker, L., He, L., Hudacik, L., Whitney, S., Keen, T. & other authors (2006). Preclinical evaluation of cellular immune responses elicited by a polyvalent DNA prime/protein boost HIV-1 vaccine. Virology 346, 151–168. [DOI] [PubMed] [Google Scholar]
- Cristillo, A. D., Weiss, D., Hudacik, L., Restrepo, S., Galmin, L., Suschak, J., Draghia-Akli, R., Markham, P. & Pal, R. (2008a). Persistent antibody and T cell responses induced by HIV-1 DNA vaccine delivered by electroporation. Biochem Biophys Res Commun 366, 29–35. [DOI] [PubMed] [Google Scholar]
- Cristillo, A. D., Galmin, L., Restrepo, S., Hudacik, L., Suschak, J., Lewis, B., Draghia-Akli, R., Aziz, N., Weiss, D. & other authors (2008b). HIV-1 Env vaccine comprised of electroporated DNA and protein co-administered with Talabostat. Biochem Biophys Res Commun 370, 22–26. [DOI] [PubMed] [Google Scholar]
- Denton, P. W. & Garcia, J. V. (2009). Novel humanized murine models for HIV research. Curr HIV/AIDS Rep 6, 13–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devito, C., Broliden, K., Kaul, R., Svensson, L., Johansen, K., Kiama, P., Kimani, J., Lopalco, L., Piconi, S. & other authors (2000). Mucosal and plasma IgA from HIV-1-exposed uninfected individuals inhibit HIV-1 transcytosis across human epithelial cells. J Immunol 165, 5170–5176. [DOI] [PubMed] [Google Scholar]
- Devito, C., Hinkula, J., Kaul, R., Kimani, J., Kiama, P., Lopalco, L., Barass, C., Piconi, S., Trabattoni, D. & other authors (2002). Cross-clade HIV-1-specific neutralizing IgA in mucosal and systemic compartments of HIV-1-exposed, persistently seronegative subjects. J Acquir Immune Defic Syndr 30, 413–420. [DOI] [PubMed] [Google Scholar]
- Finerty, S., Stokes, C. R., Gruffydd-Jones, T. J., Hillman, T. J., Barr, F. J. & Harbour, D. A. (2001). Targeted lymph node immunization can protect cats from a mucosal challenge with feline immunodeficiency virus. Vaccine 20, 49–58. [DOI] [PubMed] [Google Scholar]
- Fujii, S., Shimizu, K., Hemmi, H., Fukui, M., Bonito, A. J., Chen, G., Franck, R. W., Tsuji, M. & Steinman, R. M. (2006). Glycolipid α-C-galactosylceramide is a distinct inducer of dendritic cell function during innate and adaptive immune responses of mice. Proc Natl Acad Sci U S A 103, 11252–11257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glenn, G. M., Flyer, D. C., Ellingsworth, L. R., Frech, S. A., Frerichs, D. M., Seid, R. C. & Yu, J. (2007). Transcutaneous immunization with heat-labile enterotoxin: development of a needle-free vaccine patch. Expert Rev Vaccines 6, 809–819. [DOI] [PubMed] [Google Scholar]
- Goodsell, A., Zhou, F., Gupta, S., Singh, M., Malyala, P., Kazzaz, J., Greer, C., Legg, H., Tang, T. & other authors (2008). β7-Integrin-independent enhancement of mucosal and systemic anti-HIV antibody responses following combined mucosal and systemic gene delivery. Immunology 123, 378–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan, Y., Sajadi, M. M., Kamin-Lewis, R., Fouts, T. R., Dimitrov, A., Zhang, Z., Redfield, R. R., DeVico, A. L., Gallo, R. C. & Lewis, G. K. (2009). Discordant memory B cell and circulating anti-Env antibody responses in HIV-1 infection. Proc Natl Acad Sci U S A 106, 3952–3957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen, S. G., Vieville, C., Whizin, N., Coyne-Johnson, L., Siess, D. C., Drummond, D. D., Legasse, A. W., Axthelm, M. K., Oswald, K. & other authors (2009). Effector memory T cell responses are associated with protection of rhesus monkeys from mucosal simian immunodeficiency virus challenge. Nat Med 15, 293–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardenberg, G., Planelles, L., Schwarte, C. M., van Bostelen, L., Le Huong, T., Hahne, M. & Medema, J. P. (2007). Specific TLR ligands regulate APRIL secretion by dendritic cells in a PKR-dependent manner. Eur J Immunol 37, 2900–2911. [DOI] [PubMed] [Google Scholar]
- He, B., Xu, W., Santini, P. A., Polydorides, A. D., Chiu, A., Estrella, J., Shan, M., Chadburn, A., Villanacci, V. & other authors (2007). Intestinal bacteria trigger T cell-independent immunoglobulin A2 class switching by inducing epithelial-cell secretion of the cytokine APRIL. Immunity 26, 812–826. [DOI] [PubMed] [Google Scholar]
- Hinkula, J., Hagbom, M., Wahren, B. & Schroder, U. (2008). Safety and immunogenicity, after nasal application of HIV-1 DNA gagp37 plasmid vaccine in young mice. Vaccine 26, 5101–5106. [DOI] [PubMed] [Google Scholar]
- Holmgren, J. & Czerkinsky, C. (2005). Mucosal immunity and vaccines. Nat Med 11, S45–S53. [DOI] [PubMed] [Google Scholar]
- Huang, X., Xu, J., Qiu, C., Ren, L., Liu, L., Wan, Y., Zhang, N., Peng, H. & Shao, Y. (2007). Mucosal priming with PEI/DNA complex and systemic boosting with recombinant TianTan vaccinia stimulate vigorous mucosal and systemic immune responses. Vaccine 25, 2620–2629. [DOI] [PubMed] [Google Scholar]
- Kamijuku, H., Nagata, Y., Jiang, X., Ichinohe, T., Tashiro, T., Mori, K., Taniguchi, M., Hase, K., Ohno, H. & other authors (2008). Mechanism of NKT cell activation by intranasal coadministration of alpha-galactosylceramide, which can induce cross-protection against influenza viruses. Mucosal Immunol 1, 208–218. [DOI] [PubMed] [Google Scholar]
- Kaminski, R. W. & VanCott, T. C . (1999). Collection and processing of mucosal secretions from mice. Methods Mol Med 17, 329–339. [DOI] [PubMed] [Google Scholar]
- Kantele, A., Kantele, J. M., Savilahti, E., Westerholm, M., Arvilommi, H., Lazarovits, A., Butcher, E. C. & Makela, P. H. (1997). Homing potentials of circulating lymphocytes in humans depend on the site of activation: oral, but not parenteral, typhoid vaccination induces circulating antibody-secreting cells that all bear homing receptors directing them to the gut. J Immunol 158, 574–579. [PubMed] [Google Scholar]
- Kantele, A., Westerholm, M., Kantele, J. M., Makela, P. H. & Savilahti, E. (1999). Homing potentials of circulating antibody-secreting cells after administration of oral or parenteral protein or polysaccharide vaccine in humans. Vaccine 17, 229–236. [DOI] [PubMed] [Google Scholar]
- Kennedy, J. S., Co, M., Green, S., Longtine, K., Longtine, J., O'Neill, M. A., Adams, J. P., Rothman, A. L., Yu, Q. & other authors (2008). The safety and tolerability of an HIV-1 DNA prime–protein boost vaccine (DP6–001) in healthy adult volunteers. Vaccine 26, 4420–4424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, P. H., Eckmann, L., Lee, W. J., Han, W. & Kagnoff, M. F. (1998). Cholera toxin and cholera toxin B subunit induce IgA switching through the action of TGF-β1. J Immunol 160, 1198–1203. [PubMed] [Google Scholar]
- Kobayashi, E., Motoki, K., Uchida, T., Fukushima, H. & Koezuka, Y. (1995). KRN7000, a novel immunomodulator, and its antitumor activities. Oncol Res 7, 529–534. [PubMed] [Google Scholar]
- Koopman, G., Bogers, W. M., van Gils, M., Koornstra, W., Barnett, S., Morein, B., Lehner, T. & Heeney, J. L. (2007). Comparison of intranasal with targeted lymph node immunization using PR8-Flu ISCOM adjuvanted HIV antigens in macaques. J Med Virol 79, 474–482. [DOI] [PubMed] [Google Scholar]
- Kunisawa, J., Fukuyama, S. & Kiyono, H. (2005). Mucosa-associated lymphoid tissues in the aerodigestive tract: their shared and divergent traits and their importance to the orchestration of the mucosal immune system. Curr Mol Med 5, 557–572. [DOI] [PubMed] [Google Scholar]
- Lai, L., Vodros, D., Kozlowski, P. A., Montefiori, D. C., Wilson, R. L., Akerstrom, V. L., Chennareddi, L., Yu, T., Kannanganat, S. & other authors (2007). GM-CSF DNA: an adjuvant for higher avidity IgG, rectal IgA, and increased protection against the acute phase of a SHIV-89.6P challenge by a DNA/MVA immunodeficiency virus vaccine. Virology 369, 153–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehner, T., Wang, Y., Ping, L., Bergmeier, L., Mitchell, E., Cranage, M., Hall, G., Dennis, M., Cook, N. & other authors (1999). The effect of route of immunization on mucosal immunity and protection. J Infect Dis 179, S489–S492. [DOI] [PubMed] [Google Scholar]
- Lobigs, M., Pavy, M., Hall, R. A., Lobigs, P., Cooper, P., Komiya, T., Toriniwa, H. & Petrovsky, N. (2010). An inactivated Vero cell-grown Japanese encephalitis vaccine formulated with Advax, a novel inulin-based adjuvant, induces protective neutralizing antibody against homologous and heterologous flaviviruses. J Gen Virol 91, 1407–1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manrique, M., Micewicz, E., Kozlowski, P. A., Wang, S. W., Aurora, D., Wilson, R. L., Ghebremichael, M., Mazzara, G., Montefiori, D. & other authors (2008). DNA-MVA vaccine protection after X4 SHIV challenge in macaques correlates with day-of-challenge antiviral CD4+ cell-mediated immunity levels and postchallenge preservation of CD4+ T cell memory. AIDS Res Hum Retroviruses 24, 505–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manrique, M., Kozlowski, P. A., Wang, S. W., Wilson, R. L., Micewicz, E., Montefiori, D. C., Mansfield, K. G., Carville, A. & Aldovini, A. (2009). Nasal DNA-MVA SIV vaccination provides more significant protection from progression to AIDS than a similar intramuscular vaccination. Mucosal Immunol 2, 536–550. [DOI] [PubMed] [Google Scholar]
- Matyas, G. R., Wieczorek, L., Beck, Z., Ochsenbauer-Jambor, C., Kappes, J. C., Michael, N. L., Polonis, V. R. & Alving, C. R. (2009). Neutralizing antibodies induced by liposomal HIV-1 glycoprotein 41 peptide simultaneously bind to both the 2F5 or 4E10 epitope and lipid epitopes. AIDS 23, 2069–2077. [DOI] [PubMed] [Google Scholar]
- Mazzoli, S., Lopalco, L., Salvi, A., Trabattoni, D., Lo Caputo, S., Semplici, F., Biasin, M., Bl, C., Cosma, A. & other authors (1999). Human immunodeficiency virus (HIV)-specific IgA and HIV neutralizing activity in the serum of exposed seronegative partners of HIV-seropositive persons. J Infect Dis 180, 871–875. [DOI] [PubMed] [Google Scholar]
- Meckelein, B., Externest, D., Schmidt, M. A. & Frey, A. (2003). Contribution of serum immunoglobulin transudate to the antibody immune status of murine intestinal secretions: influence of different sampling procedures. Clin Diagn Lab Immunol 10, 831–834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ngo-Giang-Huong, N., Candotti, D., Goubar, A., Autran, B., Maynart, M., Sicard, D., Clauvel, J. P., Agut, H., Costagliola, D. & Rouzioux, C. (2001). HIV type 1-specific IgG2 antibodies: markers of helper T cell type 1 response and prognostic marker of long-term nonprogression. AIDS Res Hum Retroviruses 17, 1435–1446. [DOI] [PubMed] [Google Scholar]
- Ozawa, Y., Suda, T., Nagata, T., Hashimoto, D., Nakamura, Y., Enomoto, N., Inui, N., Koide, Y., Nakamura, H. & Chida, K. (2009). Mucosal vaccine using CTL epitope-pulsed dendritic cell confers protection for intracellular pathogen. Am J Respir Cell Mol Biol 41, 440–448. [DOI] [PubMed] [Google Scholar]
- Pal, R., Venzon, D., Letvin, N. L., Santra, S., Montefiori, D. C., Miller, N. R., Tryniszewska, E., Lewis, M. G., VanCott, T. C. & other authors (2002). ALVAC-SIV-gag-pol-env-based vaccination and macaque major histocompatibility complex class I (A*01) delay simian immunodeficiency virus SIVmac-induced immunodeficiency. J Virol 76, 292–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pal, R., Taylor, B., Foulke, J. S., Woodward, R., Merges, M., Praschunus, R., Gibson, A. & Reitz, M. (2003). Characterization of a simian human immunodeficiency virus encoding the envelope gene from the CCR5-tropic HIV-1 Ba-L. J Acquir Immune Defic Syndr 33, 300–307. [DOI] [PubMed] [Google Scholar]
- Pal, R., Wang, S., Kalyanaraman, V. S., Nair, B. C., Whitney, S., Keen, T., Hocker, L., Hudacik, L., Rose, N. & other authors (2005). Polyvalent DNA prime and envelope protein boost HIV-1 vaccine elicits humoral and cellular responses and controls plasma viremia in rhesus macaques following rectal challenge with an R5 SHIV isolate. J Med Primatol 34, 226–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pal, R., Kalyanaraman, V. S., Nair, B. C., Whitney, S., Keen, T., Hocker, L., Hudacik, L., Rose, N., Mboudjeka, I. & other authors (2006). Immunization of rhesus macaques with a polyvalent DNA prime/protein boost human immunodeficiency virus type 1 vaccine elicits protective antibody response against simian human immunodeficiency virus of R5 phenotype. Virology 348, 341–353. [DOI] [PubMed] [Google Scholar]
- Pandrea, I. V., Gautam, R., Ribeiro, R. M., Brenchley, J. M., Butler, I. F., Pattison, M., Rasmussen, T., Marx, P. A., Silvestri, G. & other authors (2007). Acute loss of intestinal CD4+ T cells is not predictive of simian immunodeficiency virus virulence. J Immunol 179, 3035–3046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrovsky, N. (2006). Novel human polysaccharide adjuvants with dual Th1 and Th2 potentiating activity. Vaccine 24, S2-26–S2-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrovsky, N. (2008). Freeing vaccine adjuvants from dangerous immunological dogma. Expert Rev Vaccines 7, 7–10. [DOI] [PubMed] [Google Scholar]
- Planque, S., Salas, M., Mitsuda, Y., Sienczyk, M., Escobar, M. A., Mooney, J. P., Morris, M. K., Nishiyama, Y., Ghosh, D. & other authors (2010). Neutralization of genetically diverse HIV-1 strains by IgA antibodies to the gp120–CD4-binding site from long-term survivors of HIV infection. AIDS 24, 875–884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qadri, F., Makela, P. H., Holmgren, J., Albert, M. J., Mannoor, K., Kantele, A., Saha, D., Salam, M. A. & Kantele, J. M. (1998). Enteric infections in an endemic area induce a circulating antibody-secreting cell response with homing potentials to both mucosal and systemic tissues. J Infect Dis 177, 1594–1599. [DOI] [PubMed] [Google Scholar]
- Quiding-Järbrink, M., Nordström, I., Granström, G., Kilander, A., Jertborn, M., Butcher, E. C., Lazarovits, A. I., Holmgren, J. & Czerkinsky, C. (1997). Differential expression of tissue-specific adhesion molecules on human circulating antibody-forming cells after systemic, enteric, and nasal immunizations. A molecular basis for the compartmentalization of effector B cell responses. J Clin Invest 99, 1281–1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raska, M., Moldoveanu, Z., Novak, J., Hel, Z., Novak, L., Bozja, J., Compans, R. W., Yang, C. & Mestecky, J. (2008). Delivery of DNA HIV-1 vaccine to the liver induces high and long-lasting humoral immune responses. Vaccine 26, 1541–1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rerks-Ngarm, S., Pitisuttithum, P., Nitayaphan, S., Kaewkungwal, J., Chiu, J., Paris, R., Premsri, N., Namwat, C., de Souza, M. & other authors (2009). Vaccination with ALVAC and AIDSVAX to prevent HIV-1 infection in Thailand. N Engl J Med 361, 2209–2220. [DOI] [PubMed] [Google Scholar]
- Robb, M. L. (2008). Failure of the Merck HIV vaccine: an uncertain step forward. Lancet 372, 1857–1858. [DOI] [PubMed] [Google Scholar]
- Sasaki, S., Sumino, K., Hamajima, K., Fukushima, J., Ishii, N., Kawamoto, S., Mohri, H., Kensil, C. R. & Okuda, K. (1998). Induction of systemic and mucosal immune responses to human immunodeficiency virus type 1 by a DNA vaccine formulated with QS-21 saponin adjuvant via intramuscular and intranasal routes. J Virol 72, 4931–4939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scharf, O., Golding, H., King, L. R., Eller, N., Frazier, D., Golding, B. & Scott, D. E. (2001). Immunoglobulin G3 from polyclonal human immunodeficiency virus (HIV) immune globulin is more potent than other subclasses in neutralizing HIV type 1. J Virol 75, 6558–6565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoenly, K. A. & Weiner, D. B. (2008). Human immunodeficiency virus type 1 vaccine development: recent advances in the cytotoxic T-lymphocyte platform “spotty business”. J Virol 82, 3166–3180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shang, L., Fukata, M., Thirunarayanan, N., Martin, A. P., Arnaboldi, P., Maussang, D., Berin, C., Unkeless, J. C., Mayer, L. & other authors (2008). Toll-like receptor signaling in small intestinal epithelium promotes B-cell recruitment and IgA production in lamina propria. Gastroenterology 135, 529–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shattock, R. J., Haynes, B. F., Pulendran, B., Flores, J. & Esparza, J. (2008). Improving defences at the portal of HIV entry: mucosal and innate immunity. PLoS Med 5, e81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva, D. G., Cooper, P. D. & Petrovsky, N. (2004). Inulin-derived adjuvants efficiently promote both Th1 and Th2 immune responses. Immunol Cell Biol 82, 611–616. [DOI] [PubMed] [Google Scholar]
- Slifka, M. K. & Ahmed, R. (1996). Limiting dilution analysis of virus-specific memory B cells by an ELISPOT assay. J Immunol Methods 199, 37–46. [DOI] [PubMed] [Google Scholar]
- Srivastava, I., Goodsell, A., Zhou, F., Sun, Y., Burke, B., Barnett, S. & Vajdy, M. (2008). Dynamics of acute and memory mucosal and systemic immune responses against HIV-1 envelope following immunizations through single or combinations of mucosal and systemic routes. Vaccine 26, 2796–2806. [DOI] [PubMed] [Google Scholar]
- Staats, H. F., Bradney, C. P., Gwinn, W. M., Jackson, S. S., Sempowski, G. D., Liao, H. X., Letvin, N. L. & Haynes, B. F. (2001). Cytokine requirements for induction of systemic and mucosal CTL after nasal immunization. J Immunol 167, 5386–5394. [DOI] [PubMed] [Google Scholar]
- Stevceva, L. & Ferrari, M. G. (2005). Mucosal adjuvants. Curr Pharm Des 11, 801–811. [DOI] [PubMed] [Google Scholar]
- Tamura, S., Samegai, Y., Kurata, H., Nagamine, T., Aizawa, C. & Kurata, T. (1988). Protection against influenza virus infection by vaccine inoculated intranasally with cholera toxin B subunit. Vaccine 6, 409–413. [DOI] [PubMed] [Google Scholar]
- Traggiai, E., Becker, S., Subbarao, K., Kolesnikova, L., Uematsu, Y., Gismondo, M. R., Murphy, B. R., Rappuoli, R. & Lanzavecchia, A. (2004). An efficient method to make human monoclonal antibodies from memory B cells: potent neutralization of SARS coronavirus. Nat Med 10, 871–875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vajdy, M. (2006). Current efforts on generation of optimal immune responses against HIV through mucosal immunisations. Drugs R D 7, 267–288. [DOI] [PubMed] [Google Scholar]
- Vajdy, M. & Singh, M. (2005). The role of adjuvants in the development of mucosal vaccines. Expert Opin Biol Ther 5, 953–965. [DOI] [PubMed] [Google Scholar]
- Vajdy, M. & Singh, M. (2006). Intranasal delivery of vaccines against HIV. Expert Opin Drug Deliv 3, 247–259. [DOI] [PubMed] [Google Scholar]
- van Ginkel, F. W., Jackson, R. J., Yuki, Y. & McGhee, J. R. (2000). Cutting edge: the mucosal adjuvant cholera toxin redirects vaccine proteins into olfactory tissues. J Immunol 165, 4778–4782. [DOI] [PubMed] [Google Scholar]
- van Ginkel, F. W., Jackson, R. J., Yoshino, N., Hagiwara, Y., Metzger, D. J., Connell, T. D., Vu, H. L., Martin, M., Fujihashi, K. & McGhee, J. R. (2005). Enterotoxin-based mucosal adjuvants alter antigen trafficking and induce inflammatory responses in the nasal tract. Infect Immun 73, 6892–6902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veldt, B. J., van der Vliet, H. J., von Blomberg, B. M., van Vlierberghe, H., Gerken, G., Nishi, N., Hayashi, K., Scheper, R. J., de Knegt, R. J. & other authors (2007). Randomized placebo controlled phase I/II trial of α-galactosylceramide for the treatment of chronic hepatitis C. J Hepatol 47, 356–365. [DOI] [PubMed] [Google Scholar]
- Wang, S. W., Bertley, F. M., Kozlowski, P. A., Herrmann, L., Manson, K., Mazzara, G., Piatak, M., Johnson, R. P., Carville, A. & other authors (2004). An SHIV DNA/MVA rectal vaccination in macaques provides systemic and mucosal virus-specific responses and protection against AIDS. AIDS Res Hum Retroviruses 20, 846–859. [DOI] [PubMed] [Google Scholar]
- Wang, S., Pal, R., Mascola, J. R., Chou, T. H., Mboudjeka, I., Shen, S., Liu, Q., Whitney, S., Keen, T. & other authors (2006). Polyvalent HIV-1 Env vaccine formulations delivered by the DNA priming plus protein boosting approach are effective in generating neutralizing antibodies against primary human immunodeficiency virus type 1 isolates from subtypes A, B, C, D and E. Virology 350, 34–47. [DOI] [PubMed] [Google Scholar]
- Wang, S., Kennedy, J. S., West, K., Montefiori, D. C., Coley, S., Lawrence, J., Shen, S., Green, S., Rothman, A. L. & other authors (2008). Cross-subtype antibody and cellular immune responses induced by a polyvalent DNA prime-protein boost HIV-1 vaccine in healthy human volunteers. Vaccine 26, 3947–3957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe, I., Hagiwara, Y., Kadowaki, S. E., Yoshikawa, T., Komase, K., Aizawa, C., Kiyono, H., Takeda, Y., McGhee, J. R. & other authors (2002). Characterization of protective immune responses induced by nasal influenza vaccine containing mutant cholera toxin as a safe adjuvant (CT112K). Vaccine 20, 3443–3455. [DOI] [PubMed] [Google Scholar]
- Watkins, D. I., Burton, D. R., Kallas, E. G., Moore, J. P. & Koff, W. C. (2008). Nonhuman primate models and the failure of the Merck HIV-1 vaccine in humans. Nat Med 14, 617–621. [DOI] [PMC free article] [PubMed] [Google Scholar]