Abstract
Genetically modified cells of haematopoietic and lymphocytic lineages could provide potentially curative treatments for a wide range of inherited and acquired diseases. However, this application is limited in mouse models by the low efficiency of lentiviral vectors. To facilitate the rapid production of high-titre helper-free retroviral vectors for enhanced gene delivery, multiple modifications to a prototype moloney murine leukemia virus (MoMLV)-derived vector system were made including adaptation of the vector system to simian virus 40 ori/T antigen-mediated episomal replication in packaging cells, replacement of the MoMLV 5′ U3 promoter with a series of stronger composite promoters and addition of an extra polyadenylation signal downstream of the 3′ long terminal repeat. These modifications enhanced vector production by 2–3 logs. High-titre vector stocks were tested for their ability to infect a variety of cells derived from humans and mice, including primary monocyte-derived macrophage cultures. Whilst the lentiviral vector was significantly restricted at the integration level, the MoMLV-based vector showed effective gene transduction of mouse cells. This high-titre retroviral vector system represents a useful tool for efficient gene delivery into human and mouse haematopoietic and lymphocytic cells, with particular application in mice as a small animal model for novel gene therapy tests.
INTRODUCTION
Retroviral vector-mediated gene therapy is a promising approach for treating human diseases. Initial attention has centred on candidate diseases affecting bone marrow, such as haemoglobinopathies and severe combined immunodeficiencies (Culver et al., 1991). Theoretically, diseases aside from those affecting bone marrow could also be treated with haematopoietic cells transduced with therapeutic genes. For instance, the appearance and activation of macrophages are thought to be rapid events in the development of many pathological lesions. This has prompted recent attempts to use macrophages as novel cellular vehicles for gene therapy (Dou et al., 2006, 2009). Macrophages are genetically modified ex vivo and reintroduced into the body in the hope that a significant portion will then migrate to the afflicted site (Burke et al., 2002). Alternatively, lymphocytes have several features that make them attractive, as they are readily available and easily manipulated in tissue culture, which permits time for selection and testing for gene expression before infusion into the patient (Culver et al., 1991). As a result, there is an increasing interest in the development of efficient methods for gene delivery into haematopoietic and lymphocytic cells such as primary macrophages, lymphocytes and derivative cell lines (Clay et al., 1999; Gough & Raines, 2003; Okasora et al., 2008; Roszkowski et al., 2005; Wilson & Kluth, 2003). Retroviral vectors have been tailored for over 20 years as a means of corrective therapy, as well as tools for research (Cockrell & Kafri, 2003). In recent decades, however, low transduction efficiencies have limited the clinical application of most transduction protocols, and results of the majority of human haematopoietic- and lymphoid-cell gene therapies have been largely disappointing (Ayuk et al., 2001; Hawley, 2001), paralleled with the limited success achieved in preclinical gene transfer studies in non-human primates and human haematopoietic stem cells in xenogenic transplant assays (Donahue et al., 1996). Fortunately, gene therapy has recently had some important successes in treating severe inherited diseases (Aiuti et al., 2009; Cartier et al., 2009; Fischer & Cavazzana-Calvo, 2008; Miller, 2008) after years of scepticism from the scientific community and neglect by the pharmaceutical industry (Naldini, 2009). Two trials have indicated successful clinical testing using retroviral (Aiuti et al., 2009) and lentiviral (Cartier et al., 2009) vectors, respectively, in haematopoietic stem cell-based gene therapy.
Murine primary cells are poorly permissive to lentiviral vector infection (Noser et al., 2006). Therefore, it would be of particular interest to establish a high-titre retroviral vector system for the development of mouse models for gene therapy tests. In this report, multiple strategies aimed at the production of high-titre Moloney murine leukemia virus (MoMLV)-based retroviral vectors were tested and evaluated. Vectors derived from the modified system were tested on a wide array of cell types and indicated improved efficiency of gene delivery into cells from both humans and mice.
RESULTS
Enhanced vector titres by multiple modifications
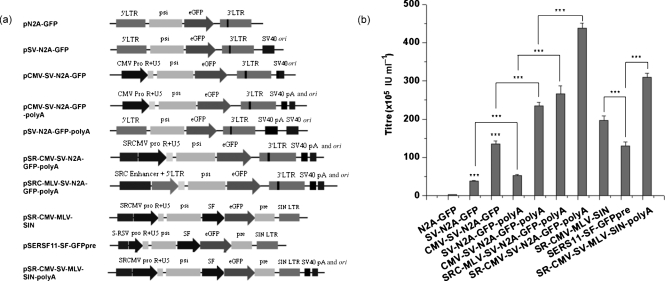
To boost vector titres from a prototype MoMLV-based vector system so that effective transduction of target cells could be achieved, multiple modifications to the transfer plasmids were tested and evaluated (see Methods). As shown in Fig. 1(a), these modifications included adaptation of an SV40 ori into the backbone, replacement of the 5′ native MoMLV U3 promoter with a series of heterologous and/or composite promoters, and the addition of an extra SV40 polyadenylation signal downstream of the 3′ long terminal repeat (LTR). The adaptation of SV40 ori not only raised the vector titre by more than tenfold [from 2.8×105 to 3.8×106 infection units (IU) ml−1, Fig. 1b, P<0.001], but also effectively extended the temporal vector production to more than 10 days compared with 4 days for the original construct (data not shown). Furthermore, addition of the SV40 poly(A) alone roughly doubled the vector titres, whilst the human cytomegalovirus (CMV) promoter replacement alone increased the vector titre by fourfold. Together, these two modifications resulted in an approximately tenfold increase in vector production, with an absolute titre of more than 3.0×107 IU ml−1 (Fig. 1b).
Fig. 1.
High-titre vector production boosted by multiple modifications. (a) Maps of transfer vectors with multiple modifications. SIN vectors contained 3′ self-inactivating LTRs as a result of the deletion of the U3 promoter. eGFP, Enhanced GFP; pA poly(A); pre, post-transcriptional regulatory element; pro, promoter; psi, Ψ domain; R, repeat region of the LTR; SF, spleen focus-forming virus U3 promoter; SRC, combination of enhancers from SV40, SRV and CMV promoters; SRCMV, hybrid promoter with CMV promoter strengthened with enhancers from SV40 and RSV promoters; S-RSV, hybrid promoter with RSV promoter strengthened with enhancer from SV40 promoter. (b) Vector titres derived from the plasmids in (a). Supernatants were collected on day 3 post-transfection and titrated in CEM-SS cells. The titres presented were derived from representative experiments with three replicates (mean±sd). ***, P≤0.001.
To maximize vector titres further, two composite hybrid promoters were constructed and evaluated. As shown in Fig. 1(a), two strategies were employed. One was to strengthen the CMV promoter with two enhancers from SV40 and human respiratory syncytial virus (RSV) promoters; the other was to strengthen the MoMLV U3 promoter with a combination of three enhancers from SV40, RSV and CMV promoters. Titres derived from the modified plasmids indicated that strengthening the MoMLV U3 promoter with enhancers from SV40, RSV and CMV promoters increased the titre from 5.47×106 to 2.32×107 IU ml−1, a greater than fourfold increase, whilst strengthening the CMV promoter with enhancers from SV40 and RSV promoters elevated vector production from 2.89×107 to 4.52×107 IU ml−1 (Fig. 1b, P<0.001). Overall, the transfer plasmid containing the CMV promoter strengthened with enhancers from SV40 and RSV promoters demonstrated the highest titre vector production (P<0.001). Based on this plasmid, two self-inactivating (SIN) vectors (Fig. 1a) were generated and tested. As shown in Fig. 1(b), compared with the parental pSERSF11-SF-GFPpre plasmid, pSR-CMV-MLV-SIN increased the titre from 1.31×107 to 1.97×107 IU ml−1, and pSR-CMV-SV-MLV-SIN-polyA further boosted the titre to 3.09×107 IU ml−1. Overall, these modifications stimulated titres nearly three times higher than the original SIN plasmid.
Vector concentration and biosafety assay
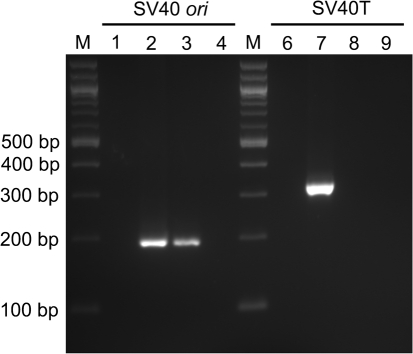
As shown in Table 1, MoMLV-based vector titres were increased significantly by over 200 times (from 1.57±0.11×107 IU ml−1 to 4.22±0.22×109 IU ml−1) following a one-step ultracentrifugation, and titration tests demonstrated a recovery efficiency of approximately 90 %, which is comparable to that of human immunodeficiency virus type 1 (HIV-1)-based vectors (Zeng et al., 2006). Through this concentration protocol, a helper virus assay was performed with no detection of any replication-competent virus. To meet the US Food and Drug Administration requirement that clinical vector material generated in 293T cells should be tested for whether there is transfer of SV40 sequences to the target cells, PCR-based tests specific for the SV40 T antigen (SV40T) and SV40 ori sequences showed that there was a detectable amount of transfer of SV40 ori into the supernatant collected from the producer cells, but this sequence was not further transferred into target cells. In addition, there was no detection of the SV40T sequence in either the supernatant or the target cells (Fig. 2).
Table 1.
Vector concentration by single-step ultracentrifugation
Vector titres were determined on 293T cells.
| Vector | Unconcentrated (IU ml−1)* | Concentrated (IU ml−1) | % Recovery |
|---|---|---|---|
| MoMLV | (1.57±0.11)×107 | (4.22±0.22)×109 | 89.48±3.08 |
| DHIV-101 | (1.55±0.10)×107 | (4.34±0.27)×109 | 93.33±0.89 |
| HR-cPPT | (5.86±0.41)×106 | (1.49±0.13)×109 | 84.46±1.39 |
*Vector-containing supernatant was collected every 24 h for 3 days post-transfection from a representative transfection experiment, which was performed in triplicate for each vector type.
Fig. 2.
PCR-based detection of transfer of SV40 ori and SV40T sequences. Lane M, 100 bp DNA ladder (New England Biolabs); lanes 1 and 6, negative controls using ddH2O; lanes 2 and 7, templates of 50 ng DNA from transfected 293T producer cells as positive controls; lanes 3 and 8, 1 μl supernatant collected from 293T producer cells used as template to detect SV40 sequences; lanes 4 and 9, templates of 50 ng DNA extracted from transduced CEM-SS cells to detect the transfer of SV40 sequences into target cells.
Differential transduction of human- and mouse-derived cells
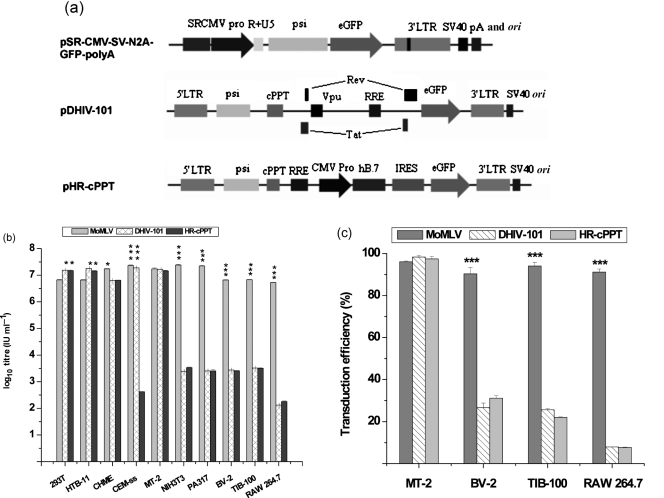
Based on high-titre vector preparations from the multi-modified system, the titration sensitivity of the modified vector was tested in various cell types originating from humans and mice, with two types of HIV-1-based lentiviral vectors titrated in parallel for comparison (Fig. 3a). As shown in Fig. 3(b), HIV-1-based vectors showed high efficiencies in transducing cell lines derived from humans, except for pHR-cPPT in CEM-SS cells, which may be due to differences in the usage of promoters. However, both HIV-1-based vectors were found to be much less efficient at transducing mouse cells. When the same vector preparation was titrated, HIV-1-based vectors showed an infectious titre of >107 IU ml−1 in human cells, whilst the infectious titre of this vector stock dropped to 104 IU ml−1 in mouse cells, with the lowest vector titre of 103 IU ml−1 in RAW 264.7 cells. In sharp contrast, the modified MoMLV-based system showed comparable titres when used to infect these same human and mouse cells. As shown in Fig. 3(b), CEM-SS, NIH3T3 and PA317 cells were the most susceptible to MoMLV-based vectors, whilst RAW 264.7 cells were the least, although the difference in apparent titres was less than tenfold.
Fig. 3.
Titration sensitivity test for MoMLV-based vectors in comparison with HIV-1-based counterparts. (a) Maps of vector constructs used for the test. pSR-CMV-SV-N2A-GFP-polyA, Modified MoMLV vector; pDHIV-101, defective HIV-1-based transfer construct expressing Tat, Rev and Vpu accessory proteins; pHR-cPPT, fully gutted HIV-1-based transfer construct using an internal CMV promoter (CMV Pro) for transgene expression, with eGFP mediated by an internal ribosome entry site element. cPPT, Central polypurine tract; RRE, Rev response element; for other abbreviations, see Fig. 1 legend. (b) Titres in ten cell lines from human and mouse. (c) Transduction efficiency in representative cell lines using concentrated vector. *, 0.01<P≤0.05; ***, P≤0.001.
This dramatic difference in the transduction of mouse cells was further confirmed using concentrated vectors for transduction. As shown in Fig. 3(c), MT-2, BV-2, TIB-100 and RAW 264.7 cells were tested in parallel with these three types of vector using the same m.o.i. of 100. Transduction efficiencies were determined on day 3 post-infection (p.i.). MT-2 cells were confirmed to be the most susceptible to all three vectors, whilst the other three mouse cell lines were susceptible to MoMLV-based vectors but refractory to HIV-1-based vectors. As evaluated by the percentage of green fluorescent protein (GFP)-positive cells, the MoMLV-based vector demonstrated over 90 % transduction efficiency in all cell types tested; HIV-1-based vectors, however, showed a high transduction efficiency only in the human-derived MT-2 cells, and lower transduction efficiencies in the mouse-derived BV-2 and RAW 264.7 cells, ranging from 32 % in BV-2 cells to 8 % in RAW 264.7 cells (Fig. 3c).
Characterization of transduction with semi-quantitative PCR
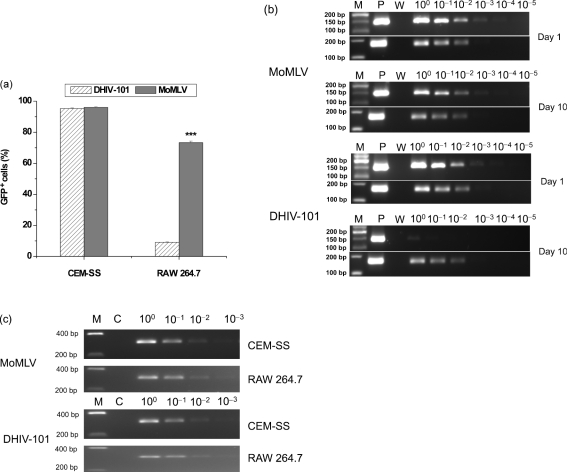
To examine the potential cause making mouse-derived cells less susceptible to stable transduction with HIV-1-based lentiviral vectors, a semi-quantitative PCR assay was performed. As shown in Fig. 4(a), infection of the human lymphocyte-derived cell line CEM-SS resulted in a transduction efficiency of greater than 95 %, as indicated by the percentage of GFP-positive cells, using both the MoMLV- and HIV-1-based vectors. In the mouse-derived macrophage cell line, however, infection with the MoMLV-based vector showed 74.25 % transduction efficiency, but the HIV-1-based lentiviral vector showed only 9.32 % GFP-positive cells. To examine the possibility that the decreased transduction efficiency by the lentiviral vector was caused by inhibition of genome conversion following infection, DNA was extracted from infected cells and analysed by semi-quantitative PCR. As shown in Fig. 4(b), DNAs from RAW 264.7 cells infected with both types of vector had similar levels of proviral DNA, as indicated by the GFP gene, on day 1 p.i. However, following a longer period of cultivation up to day 10 p.i., only DNAs extracted from the MoMLV-based vector-infected cells maintained similar levels of the GFP gene. Whilst there was no apparent difference in viability, morphology or growth of the transduced cells with either vector type, extended cultivation led to a dramatic decrease of approximately 1000-fold (Fig. 4b) in the GFP gene from the HIV-1-based vector-infected cells. Although MoMLV-based vector-infected cells also showed some degree of decline in the level of the GFP gene at 10 days p.i., the decrease was less than tenfold.
Fig. 4.
Vector transduction characterized by semi-quantitative PCR. (a) Differential transduction efficiencies with MoMLV- and HIV-1-based vectors. Cells were infected as described in Methods and the percentage of GFP-positive cells was determined on day 10 p.i. ***, P≤0.001. (b) Semi-quantitative PCR detection of DNA from RAW 264.7 cells infected with MoMLV- and HIV-1-based vectors. The upper panels are GFP-specific amplifications, whilst the lower panels show β-actin-specific amplification as a control of the amount of template. Lane M, 50 or 100 bp ladder (NEB); lane P, positive control; lane W, negative control (H2O). (c) Semi-quantitative PCR detection of 2-LTR circle junctions. DNAs from CEM-SS or RAW 264.7 cells infected with MoMLV- or HIV-1-based vectors were diluted and used as templates for PCRs as described in Methods. Lane M, HyperLadder I (Bioline); lane C, negative control with 20 ng DNA from mock-transduced cells.
To characterize further the step in the transduction process at which a block is encountered following reverse transcription in mouse cells, episomal 2-LTR circles (a by-product of HIV-1 infection) were analysed as markers for nuclear import of the pre-integration complex. Following comparable levels of reverse transcription (Fig. 4b), levels of 2-LTR circles were found to be similar in comparison. This suggested efficient nuclear import of the HIV-1-based vector pre-integration complex in mouse cells relative to the human counterpart, and that the processes of reverse transcription and nuclear import were not blocked in the tested mouse cells.
High-efficiency transduction of murine bone marrow-derived monocytes/macrophages
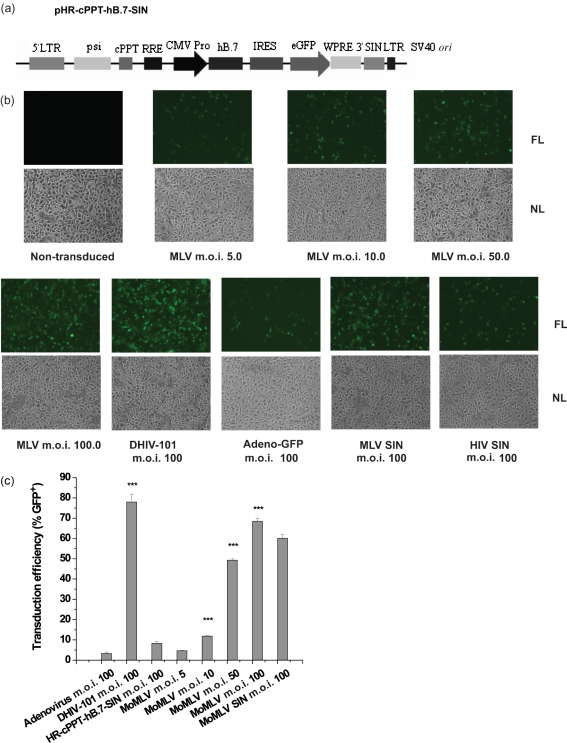
To facilitate the use of laboratory mice as a small animal model for gene therapy studies, concentrated MoMLV-based vector preparations, together with two of the newly constructed MoMLV- and HIV-1-based SIN vectors (Fig. 1a and Fig. 5a), were tested for their efficiency in transducing primary cultures of mouse monocyte-derived macrophages (MDMs). Another defective HIV-1 construct, pDHIV-101, and an adenovirus-based vector were also used as controls. As shown in Fig. 3(a), pDHIV-101 contained three of the HIV-1 accessory genes. However, rather than the other fully gutted HIV-1-based SIN vector, pHR-cPPT-hB.7-SIN (Fig. 5a), it was this first-generation vector construct that indicated efficient transduction of mouse MDMs (Fig. 5b, c). As shown in Fig. 5(b, c), the efficiency of MoMLV-based vector-mediated transduction of primary mouse MDMs was directly correlated to vector m.o.i. At an m.o.i. of 5, approximately 4.67 % of the cells appeared to be GFP-positive, whilst at an m.o.i. of 100, up to 68 % of the cells appeared to be GFP positive from a single infection. This efficiency was comparable to that of the first-generation lentiviral control, which was 77.95 % for DHIV-101 at an m.o.i. of 100. When the adenovirus-based vector was applied at the same m.o.i., however, only 3.34 % of the cells were GFP positive, showing that primary mouse MDMs are much less sensitive to infection through the adenoviral vector.
Fig. 5.
High-efficiency transduction of primary monocytes/macrophages. (a) Map of the SIN lentiviral construct used for transduction; maps for the other transfer constructs are presented in Fig. 1(a), and the adenovirus vector was derived as described in Methods. CMV Pro, CMV promoter; WPRE, woodchuck hepatitis virus post-transcriptional regulatory element; for other abbreviations, see Fig. 1 and Fig. 3 legends. (b) Transduction of mouse primary MDMs derived from bone marrow at various m.o.i. as indicated, along with HIV-1- and adenovirus-based vectors as indicated. MLV SIN, pSR-CMV-SV-MLV-SIN-polyA; HIV SIN, pHR-cPPT-hB.7-SIN. Representative microphotographs were taken on day 3 p.i. FL, Fluorescent light; NL, normal light. (c) Transduction efficiencies from the infections described in (b). The percentage of GFP-positive cells was counted on day 3 p.i., and means±sd are presented.
Similarly, the MoMLV-based SIN vector showed high transduction efficiency in the mouse MDMs, with 60.13 % GFP-positive cells at an m.o.i. of 100. The HIV-1-based SIN vector, however, resulted in only 8.33 % GFP-positive cells when infected at an m.o.i. of 100, which was significantly lower than that of the MoMLV-based vector counterpart.
DISCUSSION
In this study, standard molecular biology techniques were used to achieve enhanced production of high-titre retroviral vectors, and improved vector preparations were tested for high-efficiency gene transduction of various cell types from human and mouse. Overall, the described modifications gradually increased the vector titres by 2–3 logs, as well as giving high-titre production of SIN vectors. Collectively, the described modifications imply that vector production could be much less laborious and more economically effective, as well as providing large quantities of high-quality vectors for certain demanding applications, such as the in vivo transfer of genes into haematopoietic cells (McTaggart & Al-Rubeai, 2002). Furthermore, this modified system supported improved vector production for up to 10 days without the apparent death of the producer cells, thus minimizing the release of toxic factors from dying cells into the supernatant. Consequently, this minimizes the negative effect on the target cell populations. As demonstrated in Fig. 5(b), the primary mouse MDMs had no apparent effect on cellular viability or morphology after extended cultivation following infection, indicating that the vector preparations used were free of notable cytotoxic contaminating factors, which supports the potential use of these vectors for in vivo studies. Moreover, high-efficiency concentration, together with no detectable generation of replication-competent virus or transfer of SV40 sequences into the target cells, indicates that this vector system would be a suitable tool for protocols utilizing high titres, as well as satisfying the requirements for high biosafety standards, such as for in vivo applications.
In addition to high titres, gene delivery into a broad range of cells is important for potential applications. For this reason, vectors from the modified system were tested on a series of cell types derived from humans and mice in comparison with two HIV-1-based vectors. Interestingly, the MoMLV-based vectors were capable of efficient infection of both human and mouse cells, indicating that they were not restricted by cell type. In contrast, HIV-1-based vectors showed high efficiency in human cells but significantly reduced infectivity in mouse cells. Possible reasons for this phenomenon were examined by semi-quantitative PCR tests of the reverse transcription product, as well as its persistence in the dividing cells and the formation of 2-LTR circle junctions. The results suggested that, following HIV-1 virion entry mediated by the pan-tropic vesicular stomatitis virus G protein, reverse transcription occurred efficiently in RAW 264.7 cells, but the resultant proviral DNAs failed to persist for the extended culture time of the infected cells. In addition, a comparable level of 2-LTR circles was detected, which suggested efficient import of the HIV-1 pre-integration complex into the nucleus. These results might allow mapping of the transduction block in RAW 264.7 cells to some event following nuclear entry and after the formation of 2-LTR circle junctions, but before the successful integration event. Theoretically, high-efficiency transduction of mouse MDMs by the first-generation HIV-1 vector DHIV-101 could be facilitated by any of the three accessory genes, among which Vpu is the most likely responsible factor, as Vif and Vpu are known to counteract the antiviral effects of cellular restrictions to early and late steps in the virus replication cycle (Sharova et al., 2008). This gene, however, should be clarified, as it may not be desirable for gene therapy vectors due its side effects and biosafety concerns.
Although the MoMLV vector is considered unsuitable for transduction of dormant or resting cells, this can be overcome by recent advances, such as the present application of macrophage colony stimulating factor, or controlled cell-expansion techniques (May et al., 2009). As demonstrated in Fig. 5(b, c), MoMLV vectors proved to be effective transducers of primary MDMs in a single infection. This is particularly important, as conduction of in vivo experiments and data from animal tests depend largely on efficient transduction of primary cells. Furthermore, the ability to transduce most cells through a single infection could avoid cell selection procedures and allow immediate transplantation of transduced cells, representing another advantage of using a high-titre vector system. With encouragement from the recent advances in successful clinical trials using retroviral vectors (Aiuti et al., 2009), the development of retroviral vectors for superior gene delivery into mouse cells, especially cells of haematopoietic and lymphocytic origin, may be of particular significance. The system constructed through this study is a prime example, especially due to the high efficiency observed in human cells.
In summary, a low-titre MoMLV-based vector system was effectively improved through a series of modifications, which led ultimately to elevated vector titres of more than 4.5×107 IU ml−1. It was also demonstrated that the MoMLV vectors were highly infective to cells derived from both humans and mice. Overall, this vector system could serve as a valuable tool for efficient gene delivery into a wide range of cells, including primary murine cells, during gene therapy trials using mice as a small animal model.
METHODS
MoMLV-based transfer plasmid construction.
All parental plasmids used in this research, unless specified otherwise, for MoMLV- and HIV-1-based vector systems were from Dr Vicente Planelles (University of Utah, UT, USA). An SV40 ori-containing fragment was cut from pDHIV-3 by SmaI and PvuI, blunted using Klenow fragment and ligated into pN2A-GFP cut by HindIII and NdeI and blunted. The resultant plasmid was named pSV-N2A-GFP. The SV40 poly(A) tail was amplified by PCR using primers 5′-TTGTTGTTAACTTGTTTATTGCAG-3′ (forward) and 5′-GAGTTTGGACAAACCACAAC-3′ (reverse) from pmRFP-N (Campbell et al., 2002) and ligated into the blunted SpeI site of pSV-N2A-GFP. The resultant plasmid was named pSV-N2A-GFP-polyA. To replace the 5′ MoMLV U3 promoter with the CMV promoter, the CMV promoter was amplified from pmRFP-N1 with primers 5′-GCGCGATGCATCCGTATTACCGCCATGC-3′ (forward) and 5′- GTCAATCGGAGGACTGGCGCGGTTCACTAAACCAGC-3′ (reverse; underlined sequence overlaps the R region of the 5′ LTR). The second fragment from pN2A-GFP, including R, U5 and the packaging signal, was amplified with 5′-CGCCAGTCCTCCGATTGAC-3′ (forward) and 5′-GCGCGATGCATCGTTCCACTGAGCGTCAG-3′ (reverse). The products were used as templates for fusion PCR with the forward primer of the first PCR and the reverse primer of the second PCR. The fusion PCR product was digested with NsiI and BsrGI and ligated into pSV-N2A-GFP and pSV-N2A-GFP-polyA between NsiI and BsrGI. The resultant plasmids were named pCMV-N2A-GFP and pCMV-N2A-GFP-polyA. A fragment containing the enhancers from the SV40 and RSV promoters was amplified from pSERS11-SF-GFPpre (Schambach et al., 2006) (obtained from Dr Christopher Baum, Hannover Medical School, Germany) with 5′-GTTTGCGCAACGTTGTTGCCATT-3′ (forward) and 5′-GGGAATTCAGTGGTTCGTCCAATC-3′ (reverse). The PCR product was cloned into pCR-4-TOPO (Invitrogen), cut with FspI and EcoRI, blunted and ligated into pCMV-N2A-GFP-polyA cut with FspI and NsiI and blunted. The resultant plasmid was named pSR-CMV-SV-N2A-GFP-polyA. A fragment containing the combination of SV40, SRV and CMV enhancers was released from pSR-CMV-SV-N2A-GFP-polyA by FspI and SnaBI, and ligated with the 5938 bp fragment from pSV-N2A-GFP-polyA cut by FspI, followed by a partial cut with NheI, and blunted. The resultant plasmid was named pSRC-MLV-SV-N2A-GFP-polyA. A fragment containing the internal promoter, GFP gene and 3′ SIN LTR was released from pSERS11-SF-GFPpre by BglII and XhoI digestion and ligated into pSR-CMV-SV-N2A-GFP-polyA cut with BamHI and XhoI. The resultant plasmid was either cut with XhoI and SacI with self-ligation to generate pSR-CMV-SV-MLV-SIN-polyA, or with XhoI and BsaXI to generate pSR-CMV-MLV-SIN.
MoMLV-based packaging plasmid construction.
The MoMLV R region and a portion of the gag gene was amplified from pSVψ−-MLV-Env− (Landau & Littman, 1992), fused with the CMV promoter and cloned into pSV-N2A-GFP. The resultant plasmid, named pCMV-ψ−-N2A-GFP-polyA, was cut with BsrGI and NheI, and ligated with the gag–pol gene released from pSVψ−-MLV-Env− with BsrGI and NheI. The resultant plasmid was named pCMV-ψ−-MLV-Env−.
HIV-1-based transfer plasmid construction.
A fragment containing CMV-hB7.1-IRES-GFP elements was cut with HaeII and AccI from plasmid pHR-hB7.1-IRES-GFP, blunted and ligated into pDHIV-101 cut with EcoRI and XbaI and blunted. The resultant plasmid was named pHR-cPPT. The fragment containing the CMV-hB7.1-IRES-GFP elements was released from pHR-hB7.1-IRES-GFP with ClaI and BsrGI, blunted and ligated into pFG12 (Qin et al., 2003) (obtained from Dr David Baltimore, California Institute of Technology, CA, USA), which had been cut with XhoI and BsrGI and blunted. The resultant plasmid was named pHR-cPPT-hB.7-SIN.
Culture of cell lines.
Human embryonic kidney cells (293T), human neuroblastoma cells (HTB-11) and two mouse fibroblast cell lines (NIH3T3 and PA317) were maintained in Dulbecco's modified Eagle's medium (DMEM; Sigma) containing 1000 mg glucose l−1 and 4.0 mM l-glutamine (Sigma). Two human T-lymphocyte cell lines, CEM-SS and MT-2, were maintained in RPMI 1640 (Sigma). One human microglia cell line (CHME) and three murine cell lines [BV-2 (microglia), RAW 264.7 (macrophage) and TIB-100 (B lymphocyte)] were maintained in DMEM with 4500 mg glucose l−1 and 4.0 mM l-glutamine. All cell culture media were supplemented with 10 % heat-inactivated fetal bovine serum (HyClone), 100 U penicillin ml−1 (Sigma) and 100 μg streptomycin sulfate ml−1 (Sigma). Cells were maintained at 50–70 % confluency in a humidified 37 °C incubator with 5 % CO2 and split at a 1 : 2–4 ratio every 3–4 days.
Primary culture of monocytes and macrophages from mouse bone marrow.
Adult CD-1 mice were purchased from Charles River Laboratories and housed at the University of Hawaii at Manoa School of Medicine Animal Facility until used in experiments. All animal procedures were approved by the Animal Care and Use Committee of the University of Hawaii at Manoa. Mouse bone marrow-derived MDMs were isolated and maintained as described previously (Wirth et al., 1982; Zeng et al., 2006), with the addition of 1000 U macrophage colony-stimulating factor ml−1 (obtained from Dr Howard Gendelman, University of Nebraska, NE, USA) to the growth medium.
Vector production and concentration.
Vectors were produced as described previously (Wu & Lu, 2007). Vector-containing supernatant was collected every 24 h and either used for titration or concentrated by ultracentrifugation, as described previously (Zeng et al., 2006). The recovered vector was resuspended in serum-free DMEM, pooled, aliquotted at 100–200 μl per vial and stored at −80 °C until used. Adenovirus-based vector was prepared and aliquotted as described previously (Wu et al., 2008). Titration of the vectors was performed as described previously (Wu & Lu, 2007), except that the optimal medium for each cell type was used.
Helper virus and transfer of SV40 sequence assays.
Helper virus assays were performed as described previously (Pear et al., 1993; Soneoka et al., 1995) with minor modifications. Briefly, 200 μl concentrated vector was used to infect 2×106 NIH3T3 cells in the presence of 8.0 μg Polybrene ml−1, leading to nearly 100 % GFP-positive cells. Cells were subcultured three times at a 1 : 4 split every 3 days. Two days after the third split, approximately 100 ml supernatant was collected and concentrated into 400 μl. Fresh NIH3T3 cells were infected with the concentrated sample, maintained in growth medium and checked for GFP-positive cells on days 3 and 7 p.i. Detection of GFP-positive cells was used as an indicator for helper virus.
Detection of the transfer of SV40 sequences was performed using PCR-based methods with primers 5′-CTCGGCCTCTGCATAAAT-3′ (forward) and 5′-GATGAGTTTGGACAAACCAC-3′ (reverse) which amplified a 194 bp DNA fragment containing the SV40 ori sequence, and primers 5′-ATGGATAAAGTTTTAAACAGAGAG-3′ (forward) and 5′-CTGAGCAAAACAGGTTTTC-3′ (reverse), which amplified a 319 bp DNA fragment from the SV40T coding sequence were used for the SV40T detection. To prepare PCR templates, 293T cells were co-transfected as described, and CEM-SS cells were infected at an m.o.i. of 100 with concentrated vector. DNAs were extracted by digestion with protease K and RNase A at 56 °C for 3 h, phenol/chloroform extraction and ethanol precipitation.
Transduction of human and mouse lymphocytes and macrophages.
Initially, various cell lines were used to titrate vector aliquots from the same preparation as described above. Subsequently, 2.0×105 MT-2, BV-2, TIB-100 or RAW264.7 cells were infected with concentrated vectors at an m.o.i. of 100 as described above. Cells were then seeded in 25 cm2 tissue culture flasks with growth medium, and the percentage of GFP-positive cells was counted on day 3. Primary MDMs were transduced on day 6 post-isolation using concentrated vectors derived from MoMLV, HIV-1 and adenovirus. For transduction with MoMLV-based vectors, cells were infected at m.o.i. of 5, 10, 50 and 100. For HIV-1- and adenovirus-based vectors and the SIN vectors, cells were infected at an m.o.i. of 100. Infected cells were maintained in growth medium and photographed for GFP-positive cells. The transduction efficiency was determined by counting the percentage of GFP-positive cells.
Characterization of vector transduction by semi-quantitative PCR.
Concentrated MoMLV-based and DHIV-101 vectors were pre-treated with DNase I (Promega) in 1× DNase I buffer for 30 min at 37 °C and then used to infect 2×106 CEM-SS and RAW 264.7 cells at an m.o.i. of 100. On day 1 p.i., 1.5×106 cells were collected for DNA extraction as described above. The remaining cells were cultured and another 1.5×106 cells were collected for DNA extraction on day 10 p.i. DNAs were diluted to 20 ng μl−1 and tenfold dilutions from 10−1 to 10−5 were made. PCR was performed in 25 μl reaction volumes using GFP gene-specific primers 5′-GGTGAGCAAGGGCGAGGAG-3′ (forward) and 5′-GCCGGTGGTGCAGATGAACT-3′ (reverse). One microlitre of each DNA dilution was added as template, and 2 ng pDHIV-101 in 1 μl was used as a positive control and 1 μl ddH2O as a negative control. Ten microlitres of each PCR product was separated on a 2 % agarose gel and photographed following ethidium bromide staining. As an internal control, PCRs with a pair of β-actin-specific primers [5′-TGGTGGGCATGGGTCAGAAG-3′ (forward) and 5′-ACGCAGCTCATTGTAGAAGGTGTG-3′ (reverse)] was performed under the same conditions, except that an annealing temperature of 60 °C and 1 ng of a previously obtained PCR product were used as template for positive controls.
CEM-SS and RAW 264.7 cells (1.5×106 each) were infected with the DHIV-101- or MoMLV-based vector at an m.o.i. of 100. At 24 h p.i., the cells were collected and the DNAs extracted as described above. Detection of 2-LTR circle junctions was performed with a forward primer annealing at the R region and a reverse primer annealing at U3. Primers 5′-GGGTCTCTCTGGTTAGACCAGATCT-3′ (forward) and 5′-TATCTGATCCCTGGCCCTGGT-3′ (reverse) were used for HIV-1 2-LTR detection, and primers 5′-CGCCAGTCCTCCGATTGAC-3′ (forward) and 5′-TCTTTCATTCCCCCCTTTTTCTG-3′ (reverse) for the detection of MoMLV 2-LTR. Both primer sets amplified 2-LTR circle junctions of 295 bp. DNA templates were diluted to 20 ng μl−1 and tenfold dilutions from 10−1 to 10−3 were prepared. One microlitre from each template was used for reactions in 25 μl volumes. The PCR product were separated and photographed as described above.
Statistical analysis.
Origin 6.0 professional software (OriginLab Corp.) was used for two-population t-tests or one-way ANOVA. P≤0.05 was considered statistically significant.
Acknowledgments
The authors would like to thank Drs Vicente Planelles, Christopher Baum and David Baltimore for their kind gifts of parental plasmids. This research was supported in part by grants from the Hawaii Community Foundation (20070438) and National Institute of Health (S11 NS43499-01A1 and MH079717-01A2).
References
- Aiuti, A., Cattaneo, F., Galimberti, S., Benninghoff, U., Cassani, B., Callegaro, L., Scaramuzza, S., Andolfi, G., Mirolo, M. & other authors (2009). Gene therapy for immunodeficiency due to adenosine deaminase deficiency. N Engl J Med 360, 447–458. [DOI] [PubMed] [Google Scholar]
- Ayuk, F. A., Zander, A. R. & Fehse, B. (2001). T lymphocytes as targets of gene transfer with Moloney-type retroviral vectors. Curr Gene Ther 1, 325–337. [DOI] [PubMed] [Google Scholar]
- Burke, B., Sumner, S., Maitland, N. & Lewis, C. E. (2002). Macrophages in gene therapy: cellular delivery vehicles and in vivo targets. J Leukoc Biol 72, 417–428. [PubMed] [Google Scholar]
- Campbell, R. E., Tour, O., Palmer, A. E., Steinbach, P. A., Baird, G. S., Zacharias, D. A. & Tsien, R. Y. (2002). A monomeric red fluorescent protein. Proc Natl Acad Sci U S A 99, 7877–7882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cartier, N., Hacein-Bey-Abina, S., Bartholomae, C. C., Veres, G., Schmidt, M., Kutschera, I., Vidaud, M., Abel, U., Dal-Cortivo, L. & other authors (2009). Hematopoietic stem cell gene therapy with a lentiviral vector in X-linked adrenoleukodystrophy. Science 326, 818–823. [DOI] [PubMed] [Google Scholar]
- Clay, T. M., Custer, M. C., Spiess, P. J. & Nishimura, M. I. (1999). Potential use of T cell receptor genes to modify hematopoietic stem cells for the gene therapy of cancer. Pathol Oncol Res 5, 3–15. [DOI] [PubMed] [Google Scholar]
- Cockrell, A. S. & Kafri, T. (2003). HIV-1 vectors: fulfilment of expectations, further advances, and still a way to go. Curr HIV Res 1, 419–439. [DOI] [PubMed] [Google Scholar]
- Culver, K., Cornetta, K., Morgan, R., Morecki, S., Aebersold, P., Kasid, A., Lotze, M., Rosenberg, S. A., Anderson, W. F. & Blaese, R. M. (1991). Lymphocytes as cellular vehicles for gene therapy in mouse and man. Proc Natl Acad Sci U S A 88, 3155–3199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donahue, R. E., Byrne, E. R., Thomas, T. E., Kirby, M. R., Agricola, B. A., Sellers, S. E., Gaudernack, G., Karisson, S. & Lansdorp, P. M. (1996). Transplantation and gene transfer of the human glucocerebrosidase gene into immunoselected primate CD34+Thy-1+ cells. Blood 88, 4166–4172. [PubMed] [Google Scholar]
- Dou, H., Destache, C. J., Morehead, J. R., Mosley, R. L., Boska, M. D., Kingsley, J., Gorantla, S., Poluektova, L., Nelson, J. A. & other authors (2006). Development of a macrophage-based nanoparticle platform for antiretroviral drug delivery. Blood 108, 2827–2835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dou, H., Grotepas, C. B., McMillan, J. M., Destache, C. J., Chaubal, M., Werling, J., Kipp, J., Rabinow, B. & Gendelman, H. E. (2009). Macrophage delivery of nanoformulated antiretroviral drug to the brain in a murine model of neuroAIDS. J Immunol 183, 661–669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer, A. & Cavazzana-Calvo, M. (2008). Gene therapy of inherited diseases. Lancet 371, 2044–2047. [DOI] [PubMed] [Google Scholar]
- Gough, P. J. & Raines, E. W. (2003). Gene therapy of apolipoprotein E-deficient mice using a novel macrophage-specific retroviral vector. Blood 101, 485–491. [DOI] [PubMed] [Google Scholar]
- Hawley, R. G. (2001). Progress toward vector design for hematopoietic stem cell gene therapy. Curr Gene Ther 1, 1–17. [DOI] [PubMed] [Google Scholar]
- Landau, N. R. & Littman, D. R. (1992). Packaging system for rapid production of murine leukemia virus vectors with variable tropism. J Virol 66, 5110–5113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May, T., Butueva, M., Bantner, S., Marcusic, D., Seppen, J., Weich, H., Hauser, H. & Wirth, D. (2009). Synthetic gene regulation circuits for control of cell expansion. Tissue Eng Part A 16, 441–452. [DOI] [PubMed] [Google Scholar]
- McTaggart, S. & Al-Rubeai, M. (2002). Retroviral vectors for human gene delivery. Biotechnol Adv 20, 1–31. [DOI] [PubMed] [Google Scholar]
- Miller, J. W. (2008). Preliminary results of gene therapy for retinal degeneration. N Engl J Med 358, 2282–2284. [DOI] [PubMed] [Google Scholar]
- Naldini, L. (2009). A comeback for gene therapy. Science 326, 805–806. [DOI] [PubMed] [Google Scholar]
- Noser, J. A., Towers, G. J., Sakuma, R., Dumont, J.-M., Collins, M. K. L. & Ikeda, Y. (2006). Cyclosporine increases human immunodeficiency virus type 1 vector transduction of primary mouse cells. J Virol 80, 7769–7774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okasora, T., Jo, J. I. & Tabata, Y. (2008). Augmented anti-tumor therapy through natural targetability of macrophages genetically engineered by NK4 plasmid DNA. Gene Ther 15, 524–530. [DOI] [PubMed] [Google Scholar]
- Pear, W. S., Nolan, G. P., Scott, M. L. & Baltimore, D. (1993). Production of high-titer helper-free retroviruses by transient transfection. Proc Natl Acad Sci U S A 90, 8392–8396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin, X. F., An, D. S., Chen, I. S. & Baltimore, D. (2003). Inhibiting HIV-1 infection in human T cells by lentiviral-mediated delivery of small interfering RNA against CCR5. Proc Natl Acad Sci U S A 100, 183–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roszkowski, J. J., Lyons, G. E., Kast, W. M., Yee, C., Van Besien, K. & Nishimura, M. I. (2005). Simultaneous generation of CD8+ and CD4+ melanoma-reactive T cells by retroviral-mediated transfer of a single T-cell receptor. Cancer Res 65, 1570–1576. [DOI] [PubMed] [Google Scholar]
- Schambach, A., Mueller, D., Galla, M., Verstegen, M. M. A., Wagemaker, G., Loew, R., Baum, C. & Bohne, J. (2006). Overcoming promoter competition in packaging cells improves production of self-inactivating retroviral vectors. Gene Ther 13, 1524–1533. [DOI] [PubMed] [Google Scholar]
- Sharova, N., Wu, Y., Zhu, X., Stranska, R., Kaushik, R., Sharkey, M. & Stevenson, M. (2008). Primate lentiviral Vpx commandeers DDB1 to counteract a macrophage restriction. PLoS Pathog 4, e1000057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soneoka, Y., Cannon, P. M., Ramsdale, E. E., Griffiths, J. C., Romano, G., Kingsman, S. M. & Kingsman, A. J. (1995). A transient three plasmid expression system for the production of high titer retroviral vectors. Nucleic Acids Res 23, 628–633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson, H. M. & Kluth, D. C. (2003). Targeting genetically modified macrophages to the glomerulus. Nephron Exp Nephrol 94, e113–e118. [DOI] [PubMed] [Google Scholar]
- Wirth, J. J., Theisen, M. A. & Crowle, A. J. (1982). Culture conditions required for primary isolation and culture of mouse blood monocytes. J Reticuloendothel Soc 31, 325–327. [PubMed] [Google Scholar]
- Wu, C. & Lu, Y. (2007). Inclusion of high molecular weight dextran in calcium phosphate-mediated transfection significantly improves gene transfer efficiency. Cell Mol Biol 53, 67–74. [PMC free article] [PubMed] [Google Scholar]
- Wu, C., Nerurkar, V. R., Yanagihara, R. & Lu, Y. (2008). Effective modifications for improved homologous recombination and high-efficiency generation of recombinant adenovirus-based vectors. J Virol Methods 153, 120–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng, L., Yang, S., Wu, C., Ye, L. & Lu, Y. (2006). Effective transduction of primary mouse blood and bone marrow-derived monocytes/macrophages by HIV-1-based defective lentiviral vectors. J Virol Methods 134, 66–73. [DOI] [PMC free article] [PubMed] [Google Scholar]