Abstract
The envelope glycoprotein (Env) of human immunodeficiency virus is key to viral entry of susceptible target cells and is therefore a major target for the design of vaccines and antiviral drugs. C-C chemokine receptor type 5 (CCR5)-using (R5) Env is the predominant phenotype associated with early transmission and acute infection. This study investigated the mechanism of CCR5 use and the sensitivity to CCR5 inhibitors of a panel of transmitted or early founder (T/F) Envs. The data showed that the majority of T/F Envs used CCR5 and that many also used CCR3, although less efficiently. Despite a similar ability to use wild-type CCR5, individual Envs differed significantly in their sensitivity to the CCR5 inhibitors maraviroc, CMPD-167 and SCH-412147. Inhibitor mapping experiments demonstrated that maraviroc, CMPD-167 and SCH-412147 interfered with the binding of CCR5 mAb to the C-terminal half of the second extracellular loop 2 of CCR5. Interestingly, Envs resistant to maraviroc, CMPD167 and SCH-412147 remained sensitive to TAK-779. Further studies indicated that the sensitivity of Envs to CCR5 inhibitors correlated with the molecular anatomy of CCR5 use, revealing that the inhibitor-sensitive Envs barely used the CCR5 N terminus, whereas resistant Envs showed a marked increase in its use. Taken together, these findings demonstrate that T/F R5 Envs are heterogeneous with respect to the mechanisms of CCR5 utilization. These data may have implications for therapeutic and prophylactic use of CCR5-based antiretrovirals.
INTRODUCTION
Human immunodeficiency virus type 1 (HIV-1) entry is mediated through a complex sequence of interactions between the gp120 subunit of the envelope glycoprotein (Env), the cellular receptor CD4 and co-receptors C-C chemokine receptor type 5 (CCR5) or CXCR4, which leads to activation of gp41 and fusion of the viral envelope with the plasma membrane. The importance of CCR5 in HIV transmission and ongoing infection, as well as the limited impact on health of a loss of CCR5 function seen in homozygous Δ32 allele individuals, make CCR5 inhibitors attractive candidates for both prevention and treatment. The small-molecule CCR5 antagonist maraviroc (UK-427857) is the first CCR5 inhibitor licensed for clinical use (Gulick et al., 2008). Another CCR5 inhibitor, vicriviroc (SCH-417690 or SCH-D), is currently in phase II and phase III clinical trials (Gulick et al., 2007; Schürmann et al., 2007).
In the absence of a protective vaccine, multi-drug combination therapy is currently the main strategy for HIV-1 management. However, long-term treatment of infection has been, and continues to be, complicated by drug resistance due to the high potential of HIV-1 for the generation of escape mutations. HIV resistance in vitro and in vivo to classic antiretroviral drugs (targeted at key viral enzymes) and to the gp41 entry inhibitor enfuvirtide has been intensively investigated. More recent studies have addressed the mechanism of HIV-1 resistance to the CCR5 inhibitors maraviroc (Westby et al., 2007) and vicriviroc (Anastassopoulou et al., 2009; Berro et al., 2009; Ogert et al., 2009) using in vitro-generated drug-resistant strains. Furthermore, the emergence of maraviroc- or vicriviroc-resistant HIV-1 in clinical trials has also been reported (Gulick et al., 2008; Tsibris et al., 2008). However, the frequency of natural resistance to CCR5 inhibitors in transmitted or early founder (T/F) viruses has not been characterized. This may have particular importance when considering the development of CCR5 antagonists for use as topical microbicides to prevent sexual transmission of HIV-1 (Klasse et al., 2008; Shattock & Moore, 2003), or for therapy in acute infection. Therefore, it is crucial to understand the sensitivity of primary HIV-1 strains to CCR5 inhibitors, in particular that of T/F viruses. A previous study reported that, compared with Envs from chronically infected subjects, T/F Envs demonstrated equivalent or modestly enhanced resistance to the fusion inhibitor T1249 and broadly neutralizing antibodies (Keele et al., 2008). In the current study, we investigated the molecular anatomy of co-receptor (Hu et al., 2000b) utilization by T/F Envs. In addition, we characterized the sensitivity of T/F Envs to the small-molecule CCR5 inhibitors maraviroc, CMPD-167 and SCH-412147, and explored the mechanisms of inhibition and natural resistance to this class of entry inhibitors.
RESULTS
Co-receptor utilization of T/F HIV-1 Envs
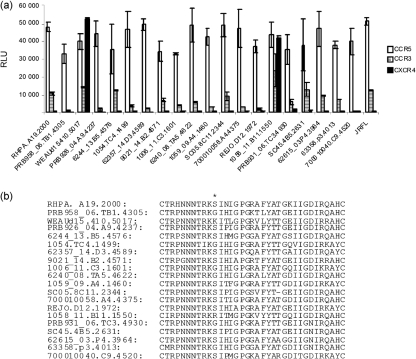
A previous study by others did not reveal a natural resistance to a small CCR5 inhibitor in primary CCR5-using (R5) isolates (Dorr et al., 2005). In this study, we focused exclusively on the assessment of T/F Envs. The genetic and phenotypic characterization of T/F HIV-1 Envs used in this study, derived by single-genome amplification from subjects with acute clade B viral infection, has been described previously (Keele et al., 2008). These T/F Envs revealed a consistent pattern of CCR5 dependence. In this study, using a well-established Env-mediated cell–cell fusion system (Hu et al., 2000a), we further characterized CCR3 utilization and its association with the use of CCR5 and CXCR4. Amongst the 20 tested T/F env clones, all used CCR5, and two clones, WEAUd15.410.5017 and 1058_11.B11.1550, also used CXCR4 (Fig. 1a). These two R5X4 Envs also demonstrated good fusogenic activity with CCR3. In addition, many of the R5 Envs were able to use CCR3, although less efficiently, and several showed comparable use of CCR3 to the two R5X4 clones. As the V3 loop is the major determinant for co-receptor utilization, we compared the V3 amino acid sequences. The two R5X4 sequences had positively charged lysine (K) or arginine (R) at position 306 (Fig. 1b), whereas all the R5 sequences had a serine (S) or glycine (G). It is known that the overall positive charge of the V3 loop is correlated with the negatively charged surface of the extracellular domains of CXCR4. Therefore, a positively charged K or R at position 306 may account for the R5X4 phenotype. In contrast, there was no discernible motif predicting the efficacy of CCR3 utilization.
Fig. 1.
Co-receptor use of T/F HIV-1 Envs. (a) Fusogenic activity of T/F HIV-1 Envs. QT6 effector cells were prepared by infection with vTF1.1 for 1 h, followed by transfection with Env expression constructs. Target QT6 cells were transfected with CD4 and candidate co-receptor in pcDNA3, and a construct encoding luciferase under the transcriptional control of the T7 promoter. The effector and target cell populations were mixed at 16–18 h following transfection, and luciferase activity of cell lysates was determined approximately 8 h later. Fusogenic activity was shown as relative light units (RLU). Data are representative of three independent experiments, with each determination performed in triplicate (mean±sd). (b) Alignment of V3 loop sequences of T/F Envs. V3 loop sequences were aligned using BioEdit 7.0. Amino acid position 306 is indicated by an asterisk.
Sensitivity of T/F HIV-1 Envs to small-molecule CCR5 inhibitors
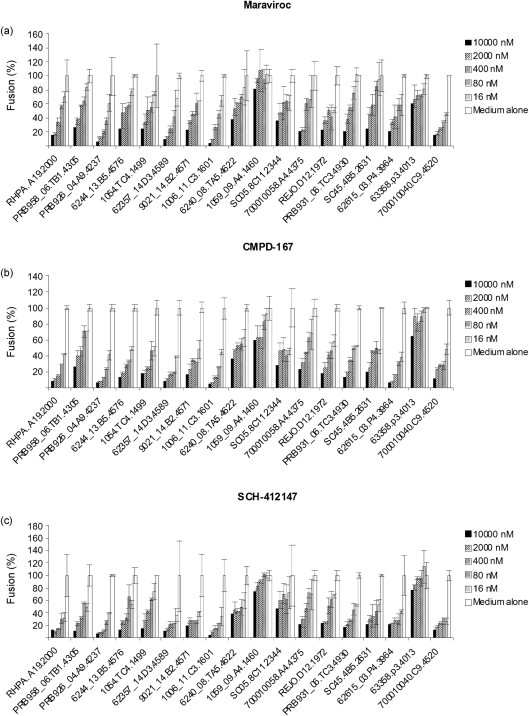
In addition to being used as therapeutic drugs for treatment, CCR5 inhibitors could be used prophylactically to prevent HIV transmission. Understanding whether T/F Envs are sensitive to CCR5 inhibitors may provide important information for topical microbicide development and treatment of acute infection. We therefore conducted experiments to test the sensitivity of T/F Envs to the CCR5 antagonists maraviroc, CMPD-167 and SCH-412147 in a widely used cell–cell fusion assay. Maraviroc inhibited the fusogenic activity of the majority of R5 Envs in a dose-dependent manner (Fig. 2a). Of interest, several Envs, particularly 1059_09.A4.1460 and 63358.p3.4013, were significantly resistant to maraviroc, and complete inhibition was not achieved even at 10 μM drug concentration, whilst others were inhibited with an IC50 range from 0.059 to 4.23 μM. Similar patterns were observed when CMPD-167 and SCH-412147 were tested against these same Envs (Fig. 2b, c).
Fig. 2.
Sensitivity to small-molecule CCR5 inhibitors of T/F HIV-1 Envs in cell–cell fusion. QT6 effector cells were prepared by infection with vTF1.1 for 1 h followed by transfection with Env expression constructs. Target QT6 cells were transfected with CD4 and CCR5 in pcDNA3, and a construct encoding luciferase under the transcriptional control of T7 promoter. At 16–18 h post-transfection, target cells were pre-treated with serially diluted CCR5 inhibitor maraviroc (a), CMPD-167 (b) or SCH-412147 (c) for 30 min before mixing with effector cells. The luciferase activity of cell lysates was determined approximately 8 h later. The fusogenic activity in medium alone was arbitrarily set to 100 % for each Env. Data are representative of three independent experiments, with each determination performed in triplicate (mean±sd).
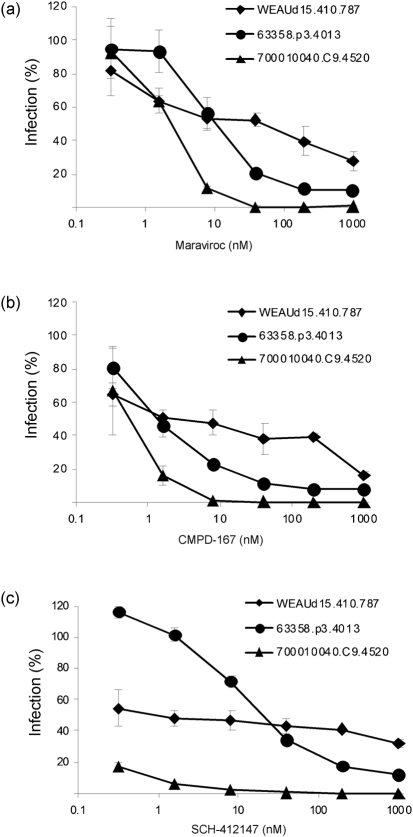
To confirm the findings in fusion assays, further experiments were performed in an infection assay using pseudotyped viruses containing the Env glycoproteins to infect TZM-bl cells. Several Envs were tested, including the R5X4 clone WEAUd15.410.5017, the resistant clones 1059_09.A4.1460 and 63358.p3.4013, and the highly sensitive clone 700010040.C9.4520. Clone 1059_09.A4.1460 was poorly infectious in the pseudotype infection system; therefore, only pseudotyped viruses with comparable infectivity were further analysed. Although the three pseudotyped viruses had similar infectivity in the absence of inhibitors, they demonstrated a range of sensitivities to the CCR5 inhibitors (Fig. 3). Not surprisingly, as TZM-bl cells express both CCR5 and CXCR4, the R5X4 Env WEAUd15.410.5017 was seen to be resistant to maraviroc, CMPD-167 and SCH-412147. In agreement with the fusogenic results, 700010040.C9.4520 was highly sensitive to all three CCR5 antagonists. In contrast, 63358.p3.4013 was significantly less sensitive to the CCR5 inhibitors. Together, these data suggested that, whilst the majority of T/F Envs were fully sensitive to CCR5 inhibitors, some individual T/F Envs can differ significantly in their sensitivity to CCR5 drugs.
Fig. 3.
Sensitivity to small-molecule CCR5 inhibitors of T/F HIV-1 Envs in pseudotyped-virus infection. Stocks of pseudotyped reporter viruses were prepared by co-transfecting 293T cells with Env expression constructs and plasmid pHIVΔEnv for 48 h and then harvested. TZM-bl cells were pre-treated with serially diluted CCR5 inhibitor maraviroc (a), CMPD-167 (b) or SCH-412147 (c) for 30 min before pseudotyped viruses were added. The luciferase activity of cell lysates was determined approximately 24 h later. The infectivity in medium alone was arbitrarily set to 100 % for each Env. Data are representative of three independent experiments, with each determination performed in triplicate (mean±sd).
Mechanisms of CCR5 inhibition and inhibitor resistance
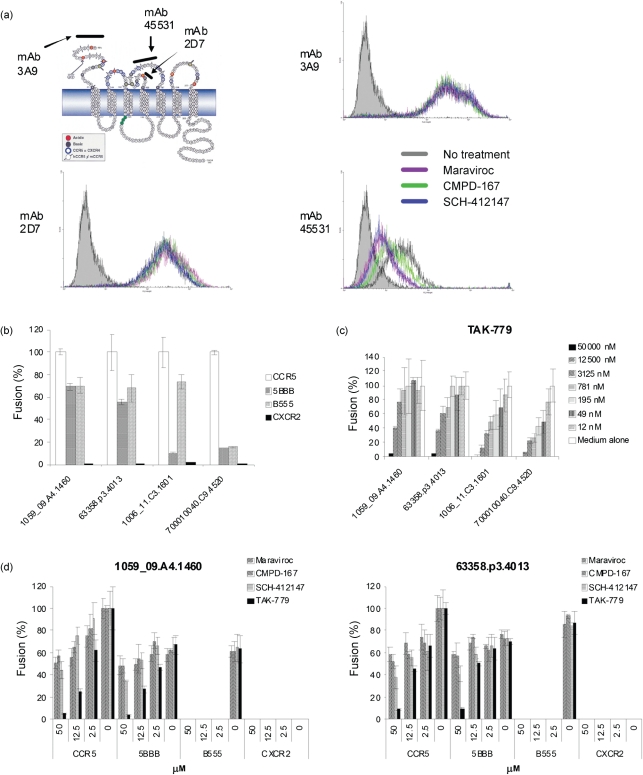
Maraviroc, CMPD-167 and the SCH-412147-related vicriviroc are thought to bind to the transmembrane domains of CCR5 (Briz et al., 2006; Kuhmann & Hartley, 2008). To explore further the mechanisms of CCR5 inhibition, we performed epitope mapping experiments using anti-CCR5 mAbs with specificity for the N terminus of CCR5 or for the secondary extracellular loop (ECL2), the two primary domains mediating HIV entry. The mAb 3A9 binds to the N terminus of CCR5, whilst mAb 2D7 binds to the N terminus of ECL2 and mAb 45531 to the C-terminal half of ECL2 (Fig. 4a) (Lee et al., 1999; Wu et al., 1997). Pre-treatment with maraviroc, CMPD-167 or SCH-412147 did not block the binding of 3A9 and 2D7 to CCR5-expressing Chinese hamster ovary (CHO) cells (Fig. 4a). In contrast, maraviroc, CMPD-167 and SCH-412147 all inhibited the binding of mAb 45531. Although these data are not a definitive indication of physical interaction between these CCR5 inhibitors and CCR5 ECL2, they do indicate that their binding to the transmembrane domains of CCR5 is sufficient to disrupt the conformation of the extracellular domain, inhibiting binding of 45531 mAb to ELC2 and utilization of CCR5 by sensitive R5 clones.
Fig. 4.
Mechanisms of CCR5 inhibition and inhibitor resistance. (a) Binding epitopes of CCR5 inhibitors. CHO-CCR5 stable transfectants were treated with 1 μM of each respective CCR5 inhibitor for 1 h, washed extensively and stained with PE-conjugated anti-CCR5 mAb 3A9, 2D7, 45531 or with isotype-matched control IgG before analysis by flow cytometry. hCCR5, human CCR5; mCCR5, mouse CCR5. (b–d) Fusogenic activity of T/F HIV-1 Envs with CCR5/CXCR2 chimeras (b), with CCR5 in the presence of CCR5 inhibitor TAK-779 (c) and with CCR5/CXCR2 chimeras in the presence of the CCR5 inhibitor maraviroc, CMPD-167, SCH-412147 or TAK-779 (d). QT6 effector cells were prepared by infection with vTF1.1 for 1 h, followed by transfection with Env expression constructs. Target QT6 cells were transfected with CD4 and CCR5 or CCR5/CXCR2 chimera in pcDNA3, and a construct encoding luciferase under the transcriptional control of the T7 promoter. The effector and target cell populations were mixed at 16–18 h after transfection, and the luciferase activity of cell lysates was determined approximately 8 h later. In experiments using CCR5 drugs, target cells were pre-treated with serially diluted CCR5 inhibitor for 30 min before mixing. The fusogenic activity with CCR5 was arbitrarily set to 100 %. Data are representative of three independent experiments, with each determination performed in triplicate (mean±sd).
Our previous studies demonstrated that R5 Envs vary in their ability to utilize the molecular anatomy of CCR5 (Hu et al., 2000b, 2005). In the current study, we observed that individual T/F Envs differed significantly in their sensitivity to CCR5 inhibitors. We hypothesized that the sensitivity to CCR5 inhibitors might be reflected by the molecular anatomy of CCR5 use. To test this hypothesis, the fusogenic activities of T/F Envs were examined using chimeras between CCR5, the primary co-receptor, and CXCR2 (also known as IL-8RB). To maintain maximal structural integrity, the hybrid 5BBB contained the first 20 aa of CCR5 fused to the body of CXCR2, whilst the hybrid B555 contained the first 34 aa of CXCR2 with the body of CCR5. We tested 1059_09.A4.1460 and 63358.p3.4013, together with two sensitive env clones, 1006_11.C3.1601 and 700010040.C9.4520. All four Envs utilized B555, the body of CCR5, although for 700010040.C9.4520 this was very inefficient (Fig. 4b). Of note, the sensitive clones, 1006_11.C3.1601 and 700010040.C9.4520, barely used 5BBB, whereas 1059_09.A4.1460 and 63358.p3.4013 retained 60–80 % of their fusogenic activity with 5BBB when compared with that of CCR5. Together, our data demonstrated that there is a significant correlation between sensitivity to CCR5 inhibitors and the use of CCR5 chimeras by Envs.
A previous study indicated that all tested T/F R5 Envs were sensitive to TAK-779 in a pseudotyped virus infection assay (Keele et al., 2008). In agreement, we found that the R5 env clones, including 1059_09.A4.1460 and 63358.p3.4013, were sensitive to TAK-779 in a dose-dependent fashion in the cell–cell fusion system (Fig. 4c), with an IC50 of 13.04 and 6.3 μM, respectively, despite TAK-779 being much less potent than the other three CCR5 antagonists. As 1059_09.A4.1460 and 63358.p3.4013 showed significant resistance to maraviroc, CMPD-167 and SCH-412147, we looked further at the mechanism of resistance using CCR5/CXCR2 chimeras. All four tested CCR5 inhibitors completely blocked the fusogenic activity of 1059_09.A4.1460 or 63358.p3.4013 with the body of CCR5 (B555) (Fig. 4d). Of great interest, only TAK-779 inhibited the fusogenic activity of 1059_09.A4.1460 or 63358.p3.4013 with the N terminus of CCR5 in a dose-dependent manner, whilst maraviroc, CMPD-167 and SCH-412147 did not achieve complete inhibition even at a high concentration. TAK-779 can suppress the binding of mAb 45531 to CCR5 (Baba et al., 1999) and the binding sites for TAK-779 on CCR5 have been suggested to be located near the extracellular surface of the receptor, within a cavity formed between transmembrane helices 1, 2, 3 and 7 (Dragic et al., 2000). TAK-779 has also been reported to bind to CCR2b and CXCR3, but there has been no evidence that it antagonizes CXCR2 (Baba et al., 1999; Gao et al., 2003). Our data suggested that the inhibitory mechanisms of maraviroc, CMPD-167 and SCH-412147 may be somewhat different to that of TAK-779.
DISCUSSION
By characterizing T/F HIV-1 Envs in cell–cell fusion and pseudotype infection assays, as well as determining their sensitivity to CCR5 inhibitors, we confirmed that the majority of T/F Envs used CCR5 and identified that some of the Envs also used CCR3, although less efficiently. In addition, T/F Envs significantly differed in their sensitivity to the CCR5 inhibitors maraviroc, CMPD-167 and SCH-412147, which can interfere with the binding of anti-CCR5 mAb to the C-terminal half but not to the N terminus of ECL2 of CCR5. Further study demonstrated that the sensitivity of Envs to CCR5 inhibitors correlated strongly with the molecular anatomy of CCR5 utilization.
A previous study identified and characterized T/F Envs in primary infection from subjects including heterosexual and homosexual encounters (Keele et al., 2008). Of the 20 full-length Envs tested in the current study, WEAUd15.410.5017 and 1058_11.B11.1550 utilized CCR5 and CXCR4 as well as CCR3. Correspondingly, the R5X4 Envs had a positively charged lysine or arginine at position 306. Several R5 Envs also used CCR3, although considerably less efficiently than CCR5. We did not observe a correlation between transmission route and co-receptor usage. Although we focused exclusively on the characterization of T/F Envs in this study, previous studies have suggested that some clade B Envs can also use CCR3 (Hu et al., 2000a; Nedellec et al., 2009). Potential targets for CCR3-mediated infection include microglial cells, macrophages, eosinophils and T-helper 2 cells (Aasa-Chapman et al., 2006). The significance of CCR3 utilization by T/F Envs remains to be determined further. The T/F Envs demonstrate equivalent or modestly enhanced resistance to the fusion inhibitor T1249 and broadly neutralizing antibodies (4E10, 2F5, b12 and 2G12), and less sensitivity to sCD4 and mAb 17b (Keele et al., 2008). In the current study, we found that they varied significantly in their sensitivity to the CCR5 inhibitors maraviroc, CMPD-167 and SCH-412147. Given that maraviroc, CMPD-167 and SCH-412147 can all interfere with the binding of mAb 45531, it is not surprising that all the tested Envs showed a similar pattern of sensitivity to these compounds in cell–cell fusion and pseudotype infection assays. However, at least a 3 log higher concentration of drug was needed in the cell–cell fusion assay to achieve a similar inhibition to that seen in the infection assay. This discrepancy may reflect the high cell–cell fusion efficiency mediated by HIV-1 Env, also seen with other entry inhibitors (Reeves et al., 2002). In addition, cell-surface expression of CCR5 may also have influenced the potency of inhibition (Ketas et al., 2007). Nevertheless, our findings may have biological relevance because cell–cell spread of HIV-1 could be more efficient than virus–cell infection.
Due to the high degree of diversity amongst the T/F Envs, we are currently unable to identify specific sequence variations associated with variable sensitivity to CCR5 inhibitors. Using an in vitro resistance generation system, studies carried out by other groups have failed to reveal a common resistance pathway against small-molecule CCR5 inhibitors. Even under the selection pressure of the same drug, different strains appear to escape by mutating different Env sequences. For instance, Ogert et al. (2008, 2009) recently reported that K305R, R315Q and K319T amino acid changes in the V3 loop, along with P437S in C4, completely reproduced the vicriviroc resistance phenotype in a chimeric strain ADA envelope. Others, however, showed that the substitutions K305R, H308P, A316V and G321E in the V3 loop or changes of G516V, M518V and F519I in the gp41 fusion peptide conferred vicriviroc resistance (Anastassopoulou et al., 2009; Berro et al., 2009). In contrast, Westby et al. (2007) reported that two amino acid substitutions (A316T and I323V) in the V3 loop conferred maraviroc resistance. However, we did not observe any of these associations in our sequences.
It is well described that the N terminus and ECL2 of CCR5 both play critical and distinct roles in HIV infection. In particular, the tip of the V3 region of gp120 binds to ECL2 of CCR5, whilst the base and stem of V3 and the bridging sheets bind to the N terminus. We demonstrated previously that loss of the V3 crown β-hairpin disrupted utilization of the body of CCR5 (Hu et al., 2000b), whilst mutations in the stem of V3 abolished the utilization of the N terminus and the body of CCR5 (Hu et al., 2005). In the current study, we observed that the resistant Envs had very high fusogenic activity with the N terminus of CCR5, whereas the sensitive Envs barely used the CCR5 N terminus. This is in agreement with our epitope-mapping experiments, which demonstrated that maraviroc, CMPD-167 and SCH-412147 interfered with the binding of mAb 45531 to the C-terminal half of ECL2. As suggested by others, binding of the CCR5 small-molecule inhibitors SCH-351125 (SCH-C) and SCH-350581 (AD101) to the transmembrane domains of CCR5 may disrupt the conformation of its extracellular domains, thereby inhibiting ligand binding to CCR5 (Tsamis et al., 2003). Our findings suggest that an enhanced interaction of gp120 with the N terminus of CCR5 may account for the resistance to small-molecule CCR5 inhibitors, which target the body of CCR5. In agreement, using truncated CCR5 and mutants, Berro et al. (2009) and Ogert et al. (2009) observed that in vitro-generated vicriviroc-resistant HIV-1 and Envs derived from a clade D-infected subject who developed resistance to vicriviroc while participating in a phase II trial (Ogert et al., 2010) had increased dependency on interactions with the CCR5 N terminus.
Of interest, all the tested R5 Envs, including those resistant to maraviroc, CMPD-167 and SCH-412147, were sensitive to TAK-779. Despite these inhibitors having a similar mechanism of action and fitting in the same binding pocket formed by the transmembrane domains of CCR5 (Kondru et al., 2008; Maeda et al., 2006; Seibert et al., 2006), the impact of their binding on the conformation of CCR5 may differ between molecules. This is strengthened by the observation that only TAK-779 was able to inhibit the entry of Envs through the N terminus of CCR5. Our results are in agreement with the notion that, although small-molecule CCR5 inhibitors occupy overlapping binding pockets on the receptor and act via a similar mechanism, the conformational details of the inhibitory effect may differ between molecules (Kuhmann & Hartley, 2008).
Given that HIV Env is a principal target for the design of vaccines and antiviral drugs, molecular characterization of T/F Envs may be crucial to the design of effective prevention strategies (McMichael et al., 2010). The findings in this study demonstrate that T/F Envs display a wide range of sensitivities to small-molecule CCR5 inhibitors and this may have implications for the preventative and/or therapeutic use of this new class of antiretrovirals.
METHODS
Cells, env genes, CCR5 inhibitors and mAbs.
The Japanese quail fibrosarcoma cell (QT6) line was purchased from the ATCC. The TZM-bl cell line was obtained through the AIDS Research and Reference Reagent Program, Division of AIDS, NIH (MD, USA). The 293T cell line was purchased from Invitrogen. The CHO-CCR5 cell line has been described previously (Hu et al., 2000a, b). The T/F env clones have been previously described and were kindly provided by the Centre for HIV/AIDS Vaccine Immunology (CHAVI) (Keele et al., 2008). The CCR5 inhibitors maraviroc and SCH-412147 were provided by the International Partnership for Microbicides. CMPD-167 and TAK-779 have been described previously (Hu et al., 2004; Veazey et al., 2005). PE-conjugated mouse anti-human CCR5 mAbs 3A9, 2D7 and isotype control were purchased from BD Biosciences. PE-conjugated mouse anti-human CCR5 mAb 45531 and isotype control were purchased from R&D Systems.
Env-mediated cell–cell fusion assay.
The co-receptor utilization of the products encoded by the env genes was determined using a cell–cell fusion assay employing a luciferase reporter gene, as described previously (Hu et al., 2000a). Briefly, QT6 effector cells were prepared by infection with vTF1.1, which encodes T7 polymerase, for 1 h followed by transfection with pcDNA3 constructs containing the primary env clones. QT6 target cells were transfected with CD4 and the candidate co-receptor in pcDNA3, as well as a construct encoding luciferase under the transcriptional control of the T7 promoter. The effector and target cell populations were mixed at 16–18 h post-transfection. In inhibition experiments using CCR5 drugs, target cells were pre-treated with serially diluted CCR5 inhibitors for 30 min before mixing with effector cells. The luciferase activity of cell lysates was determined approximately 8 h later. Constructs CD4, T7–luciferase, CCR5, CXCR4, CXCR2 and CCR5/CXCR2 chimeras (5BBB and B555) have been described previously (Hu et al., 2000b, 2005).
Pseudotyped virus infection.
Stocks of pseudotyped reporter viruses were prepared by co-transfecting 293T cells with Env expression constructs and plasmid pHIVΔEnv as described previously (Hu et al., 2005). Single-cycle infection was assessed in TZM-bl cells pre-treated with serially diluted CCR5 inhibitors for 30 min. The luciferase activity of cell lysates was determined at 48 h post-transfection.
Flow cytometry.
CHO-hCCR5 cells were treated with 1 μM of the respective CCR5 inhibitor at 37 °C for 1 h and then stained with anti-CCR5 mAb. Cells were analysed on a FACScan device (Becton Dickinson) as described previously (Hu et al., 2004).
Sequence analysis.
The sequences of HIV-1 env genes have been reported previously (Keele et al., 2008). The amino acid sequences of env genes were aligned using BioEdit 7.0.
Acknowledgments
This work was supported by grants from the Hotung Trust, NIAID Cooperative Agreement (U19 AI76982), EUROPRISE (EC FP6 037611), CHAVI (NIH AI-0678501), the Ministry of Science and Technology of China (2006CB504200, 2008ZX10001-002, 2008ZX10001-015) and the National Natural Science Foundation of China (30872357).
References
- Aasa-Chapman, M. M., Seymour, C. R., Williams, I. & McKnight, A. (2006). Novel envelope determinants for CCR3 use by human immunodeficiency virus. J Virol 80, 10884–10889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anastassopoulou, C. G., Ketas, T. J., Klasse, P. J. & Moore, J. P. (2009). Resistance to CCR5 inhibitors caused by sequence changes in the fusion peptide of HIV-1 gp41. Proc Natl Acad Sci U S A 106, 5318–5323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baba, M., Nishimura, O., Kanzaki, N., Okamoto, M., Sawada, H., Iizawa, Y., Shiraishi, M., Aramaki, Y., Okonogi, K. & other authors (1999). A small-molecule, nonpeptide CCR5 antagonist with highly potent and selective anti-HIV-1 activity. Proc Natl Acad Sci U S A 96, 5698–5703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berro, R., Sanders, R. W., Lu, M., Klasse, P. J. & Moore, J. P. (2009). Two HIV-1 variants resistant to small molecule CCR5 inhibitors differ in how they use CCR5 for entry. PLoS Pathog 5, e1000548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briz, V., Poveda, E. & Soriano, V. (2006). HIV entry inhibitors: mechanisms of action and resistance pathways. J Antimicrob Chemother 57, 619–627. [DOI] [PubMed] [Google Scholar]
- Dorr, P., Westby, M., Dobbs, S., Griffin, P., Irvine, B., Macartney, M., Mori, J., Rickett, G., Smith-Burchnell, C. & other authors (2005). Maraviroc (UK-427,857), a potent, orally bioavailable, and selective small-molecule inhibitor of chemokine receptor CCR5 with broad-spectrum anti-human immunodeficiency virus type 1 activity. Antimicrob Agents Chemother 49, 4721–4732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dragic, T., Trkola, A., Thompson, D. A., Cormier, E. G., Kajumo, F. A., Maxwell, E., Lin, S. W., Ying, W., Smith, S. O. & other authors (2000). A binding pocket for a small molecule inhibitor of HIV-1 entry within the transmembrane helices of CCR5. Proc Natl Acad Sci U S A 97, 5639–5644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao, P., Zhou, X. Y., Yashiro-Ohtani, Y., Yang, Y. F., Sugimoto, N., Ono, S., Nakanishi, T., Obika, S., Imanishi, T. & other authors (2003). The unique target specificity of a nonpeptide chemokine receptor antagonist: selective blockade of two Th1 chemokine receptors CCR5 and CXCR3. J Leukoc Biol 73, 273–280. [DOI] [PubMed] [Google Scholar]
- Gulick, R. M., Su, Z., Flexner, C., Hughes, M. D., Skolnik, P. R., Wilkin, T. J., Gross, R., Krambrink, A., Coakley, E. & other authors (2007). Phase 2 study of the safety and efficacy of vicriviroc, a CCR5 inhibitor, in HIV-1-infected, treatment-experienced patients: AIDS clinical trials group 5211. J Infect Dis 196, 304–312. [DOI] [PubMed] [Google Scholar]
- Gulick, R. M., Lalezari, J., Goodrich, J., Clumeck, N., DeJesus, E., Horban, A., Nadler, J., Clotet, B., Karlsson, A. & other authors (2008). Maraviroc for previously treated patients with R5 HIV-1 infection. N Engl J Med 359, 1429–1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu, Q., Barry, A. P., Wang, Z., Connolly, S. M., Peiper, S. C. & Greenberg, M. L. (2000a). Evolution of the human immunodeficiency virus type 1 envelope during infection reveals molecular corollaries of specificity for coreceptor utilization and AIDS pathogenesis. J Virol 74, 11858–11872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu, Q., Trent, J. O., Tomaras, G. D., Wang, Z., Murray, J. L., Conolly, S. M., Navenot, J. M., Barry, A. P., Greenberg, M. L. & other authors (2000b). Identification of Env determinants in V3 that influence the molecular anatomy of CCR5 utilization. J Mol Biol 302, 359–375. [DOI] [PubMed] [Google Scholar]
- Hu, Q., Frank, I., Williams, V., Santos, J. J., Watts, P., Griffin, G. E., Moore, J. P., Pope, M. & Shattock, R. J. (2004). Blockade of attachment and fusion receptors inhibits HIV-1 infection of human cervical tissue. J Exp Med 199, 1065–1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu, Q., Napier, K. B., Trent, J. O., Wang, Z., Taylor, S., Griffin, G. E., Peiper, S. C. & Shattock, R. J. (2005). Restricted variable residues in the C-terminal segment of HIV-1 V3 loop regulate the molecular anatomy of CCR5 utilization. J Mol Biol 350, 699–712. [DOI] [PubMed] [Google Scholar]
- Keele, B. F., Giorgi, E. E., Salazar-Gonzalez, J. F., Decker, J. M., Pham, K. T., Salazar, M. G., Sun, C., Grayson, T., Wang, S. & other authors (2008). Identification and characterization of transmitted and early founder virus envelopes in primary HIV-1 infection. Proc Natl Acad Sci U S A 105, 7552–7557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ketas, T. J., Kuhmann, S. E., Palmer, A., Zurita, J., He, W., Ahuja, S. K., Klasse, P. J. & Moore, J. P. (2007). Cell surface expression of CCR5 and other host factors influence the inhibition of HIV-1 infection of human lymphocytes by CCR5 ligands. Virology 364, 281–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klasse, P. J., Shattock, R. & Moore, J. P. (2008). Antiretroviral drug-based microbicides to prevent HIV-1 sexual transmission. Annu Rev Med 59, 455–471. [DOI] [PubMed] [Google Scholar]
- Kondru, R., Zhang, J., Ji, C., Mirzadegan, T., Rotstein, D., Sankuratri, S. & Dioszegi, M. (2008). Molecular interactions of CCR5 with major classes of small-molecule anti-HIV CCR5 antagonists. Mol Pharmacol 73, 789–800. [DOI] [PubMed] [Google Scholar]
- Kuhmann, S. E. & Hartley, O. (2008). Targeting chemokine receptors in HIV: a status report. Annu Rev Pharmacol Toxicol 48, 425–461. [DOI] [PubMed] [Google Scholar]
- Lee, B., Sharron, M., Blanpain, C., Doranz, B. J., Vakili, J., Setoh, P., Berg, E., Liu, G., Guy, H. R. & other authors (1999). Epitope mapping of CCR5 reveals multiple conformational states and distinct but overlapping structures involved in chemokine and coreceptor function. J Biol Chem 274, 9617–9626. [DOI] [PubMed] [Google Scholar]
- Maeda, K., Das, D., Ogata-Aoki, H., Nakata, H., Miyakawa, T., Tojo, Y., Norman, R., Takaoka, Y., Ding, J. & other authors (2006). Structural and molecular interactions of CCR5 inhibitors with CCR5. J Biol Chem 281, 12688–12698. [DOI] [PubMed] [Google Scholar]
- McMichael, A. J., Borrow, P., Tomaras, G. D., Goonetilleke, N. & Haynes, B. F. (2010). The immune response during acute HIV-1 infection: clues for vaccine development. Nat Rev Immunol 10, 11–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nedellec, R., Coetzer, M., Shimizu, N., Hoshino, H., Polonis, V. R., Morris, L., Martensson, U. E., Binley, J., Overbaugh, J. & other authors (2009). Virus entry via the alternative coreceptors CCR3 and FPRL1 differs by human immunodeficiency virus type 1 subtype. J Virol 83, 8353–8363.19553323 [Google Scholar]
- Ogert, R. A., Wojcik, L., Buontempo, C., Ba, L., Buontempo, P., Ralston, R., Strizki, J. & Howe, J. A. (2008). Mapping resistance to the CCR5 co-receptor antagonist vicriviroc using heterologous chimeric HIV-1 envelope genes reveals key determinants in the C2–V5 domain of gp120. Virology 373, 387–399. [DOI] [PubMed] [Google Scholar]
- Ogert, R. A., Ba, L., Hou, Y., Buontempo, C., Qiu, P., Duca, J., Murgolo, N., Buontempo, P., Ralston, R. & other authors (2009). Structure–function analysis of human immunodeficiency virus type 1 gp120 amino acid mutations associated with resistance to the CCR5 coreceptor antagonist vicriviroc. J Virol 83, 12151–12163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogert, R. A., Hou, Y., Ba, L., Wojcik, L., Qiu, P., Murgolo, N., Duca, J., Dunkle, L. M., Ralston, R. & other authors (2010). Clinical resistance to vicriviroc through adaptive V3 loop mutations in HIV-1 subtype D gp120 that alter interactions with the N-terminus and ECL2 of CCR5. Virology 400, 145–155. [DOI] [PubMed] [Google Scholar]
- Reeves, J. D., Gallo, S. A., Ahmad, N., Miamidian, J. L., Harvey, P. E., Sharron, M., Pohlmann, S., Sfakianos, J. N., Derdeyn, C. A. & other authors (2002). Sensitivity of HIV-1 to entry inhibitors correlates with envelope/coreceptor affinity, receptor density, and fusion kinetics. Proc Natl Acad Sci U S A 99, 16249–16254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schürmann, D., Fätkenheuer, G., Reynes, J., Michelet, C., Raffi, F., van Lier, J., Caceres, M., Keung, A., Sansone-Parsons, A. & other authors (2007). Antiviral activity, pharmacokinetics and safety of vicriviroc, an oral CCR5 antagonist, during 14-day monotherapy in HIV-infected adults. AIDS 21, 1293–1299. [DOI] [PubMed] [Google Scholar]
- Seibert, C., Ying, W., Gavrilov, S., Tsamis, F., Kuhmann, S. E., Palani, A., Tagat, J. R., Clader, J. W., McCombie, S. W. & other authors (2006). Interaction of small molecule inhibitors of HIV-1 entry with CCR5. Virology 349, 41–54. [DOI] [PubMed] [Google Scholar]
- Shattock, R. J. & Moore, J. P. (2003). Inhibiting sexual transmission of HIV-1 infection. Nat Rev Microbiol 1, 25–34. [DOI] [PubMed] [Google Scholar]
- Tsamis, F., Gavrilov, S., Kajumo, F., Seibert, C., Kuhmann, S., Ketas, T., Trkola, A., Palani, A., Clader, J. W. & other authors (2003). Analysis of the mechanism by which the small-molecule CCR5 antagonists SCH-351125 and SCH-350581 inhibit human immunodeficiency virus type 1 entry. J Virol 77, 5201–5208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsibris, A. M., Sagar, M., Gulick, R. M., Su, Z., Hughes, M., Greaves, W., Subramanian, M., Flexner, C., Giguel, F. & other authors (2008). In vivo emergence of vicriviroc resistance in a human immunodeficiency virus type 1 subtype C-infected subject. J Virol 82, 8210–8214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veazey, R. S., Klasse, P. J., Schader, S. M., Hu, Q., Ketas, T. J., Lu, M., Marx, P. A., Dufour, J., Colonno, R. J. & other authors (2005). Protection of macaques from vaginal SHIV challenge by vaginally delivered inhibitors of virus–cell fusion. Nature 438, 99–102. [DOI] [PubMed] [Google Scholar]
- Westby, M., Smith-Burchnell, C., Mori, J., Lewis, M., Mosley, M., Stockdale, M., Dorr, P., Ciaramella, G. & Perros, M. (2007). Reduced maximal inhibition in phenotypic susceptibility assays indicates that viral strains resistant to the CCR5 antagonist maraviroc utilize inhibitor-bound receptor for entry. J Virol 81, 2359–2371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu, L., LaRosa, G., Kassam, N., Gordon, C. J., Heath, H., Ruffing, N., Chen, H., Humblias, J., Samson, M. & other authors (1997). Interaction of chemokine receptor CCR5 with its ligands: multiple domains for HIV-1 gp120 binding and a single domain for chemokine binding. J Exp Med 186, 1373–1381. [DOI] [PMC free article] [PubMed] [Google Scholar]