Abstract
Eukaryotic cells respond to DNA damage and stalled replication forks by activating protein kinase-mediated signaling pathways that promote cell cycle arrest and DNA repair. A central target of the cell cycle arrest program is the Cdc25A protein phosphatase. Cdc25A is required for S-phase entry and dephosphorylates tyrosine-15 phosphorylated Cdk1 (Cdc2) and Cdk2, positive regulators of cell division. Cdc25A is unstable during S-phase and is degraded through the ubiquitin-proteasome pathway, but its turnover is enhanced in response to DNA damage. Although basal and DNA-damage-induced turnover depends on the ATM-Chk2 and ATR-Chk1 pathways, how these kinases engage the ubiquitin ligase machinery is unknown. Here, we demonstrate a requirement for SCFβ-TRCP in Cdc25A turnover during an unperturbed cell cycle and in response to DNA damage. Depletion of β-TRCP stabilizes Cdc25A, leading to hyperactive Cdk2 activity. SCFβ-TRCP promotes Chk1-dependent Cdc25A ubiquitination in vitro, and this involves serine 76, a known Chk1 phosphorylation site. However, recognition of Cdc25A by β-TRCP occurs via a noncanonical phosphodegron in Cdc25A containing phosphoserine 79 and phosphoserine 82, sites that are not targeted by Chk1. These data indicate that Cdc25A turnover is more complex than previously appreciated and suggest roles for an additional kinase(s) in Chk1-dependent Cdc25A turnover.
Keywords: Cdc25A, Chk1, DNA damage, protein turnover, SCF ubiquitin ligase
In response to DNA damage and DNA replication interference, cells activate an elaborate network of signaling pathways collectively called the DNA damage stress response pathway (Zhou and Elledge 2000). The central conduits of this network are two parallel but partially overlapping protein kinase cascades, the ATM-Chk2 and ATR-Chk1 kinase modules, that transduce the damage signal to downstream effectors involved in cell cycle control, DNA repair, and apoptosis (Zhou and Elledge 2000; Shiloh 2003). ATM primarily responds to DNA double-strand breaks, whereas ATR responds to agents that interfere with DNA replication in addition to double-strand breaks (Zou and Elledge 2003).
The branches of these pathways used in arresting the cell cycle are called checkpoints. In many eukaryotes, the targets of checkpoint pathways are the cyclin-dependent kinases, Cdks, which control multiple cell cycle transitions including the G1/S transition, late origin firing during S phase, and the G2/M transition, each of which is inhibited in response to DNA damage. Cells have evolved multiple mechanisms to inhibit Cdk activity. In mammals, the Cdk inhibitor p21Cip1 is induced in response to DNA damage through activation of the p53 transcription factor, which is activated and stabilized in response to DNA damage (Giaccia and Kastan 1998; Zhou and Elledge 2000). In addition, levels of cyclin D1 rapidly decrease in response to DNA damage during G1, leading to redistribution of p21Cip1 from Cdk4 to Cdk2 (Agami and Bernards 2000). Cdks are also regulated by inhibitory phosphorylation on tyrosine, which is altered in response to DNA damage (Jin et al. 1997; Rhind et al. 1997; for review, see Takizawa and Morgan 2000; Donzelli and Draetta 2003).
The regulatory mechanisms controlling Cdk phosphorylation have been extensively studied. Tyrosine phosphorylation is regulated by the opposing activities of the tyrosine kinases Wee1 and Myt1, and a group of tyrosine phosphatases known as Cdc25A, Cdc25B, Cdc25C (for review, see Morgan 1997). A direct connection between checkpoint signaling and Cdc25 was established when it was found that the checkpoint kinase Chk1, and later Chk2, could phosphorylate Cdc25C on a site relevant to its checkpoint function in vivo (Peng et al. 1996; Sanchez et al. 1996; Furnari et al. 1997; Matsuoka et al. 1998). Furthermore, these kinases were shown to phosphorylate all three Cdc25 family members, suggesting they were general targets of DNA damage stress response pathways (Sanchez et al. 1996; Matsuoka et al. 1998). Analysis of Chk regulation of Cdc25 from several systems showed that phosphorylation of Cdc25C both inhibited kinase activity (Blasina et al. 1999; Furnari et al. 1999) and maintained Cdc25C in the cytoplasm, where it cannot access Cdk/cyclin complexes efficiently (Zeng et al. 1998; Kumagai and Dunphy 1999; Lopez-Girona et al. 1999; Zeng and Piwnica-Worms 1999). However, mice lacking Cdc25C grow normally and have intact checkpoint responses (Chen et al. 2001), suggesting that other family members may play more prominent roles in Cdk regulation.
A second family member implicated in the damage response is Cdc25A. Cdc25A is capable of removing inhibitory tyrosine phosphorylation from both Cdk1 and Cdk2 kinases to promote entry into and progression through S phase and mitosis (Hoffmann et al. 1994; Vigo et al. 1999; for review, see Donzelli and Draetta 2003). Cdc25A has also been shown to be a phosphorylation target of Chk kinases (Sanchez et al. 1996) and to be regulated by Chk kinases in response to DNA damage (for review, see Donzelli and Draetta 2003). In contrast to regulation of Cdc25C, Cdc25A is destroyed in response to ionizing radiation (IR) and ultraviolet (UV) light through a process involving ubiquitin-mediated proteolysis. During G1, UV treatment leads to Chk1-dependent elimination of Cdc25A (Mailand et al. 2000) and persistent Cdk2 Y15 phosphorylation. During an unperturbed S phase, Cdc25A is unstable and this instability requires Cdc25A phosphorylation by Chk1 (Falck et al. 2001; Sorensen et al. 2003). IR during S phase leads to accelerated Cdc25A phosphorylation by Chk1 with a concomitant increase in turnover. Defects in this intra-S-phase checkpoint lead to radio-resistant DNA synthesis (RDS; Falck et al. 2001; Xiao et al. 2003). Whereas depletion of Chk1 leads to an RDS phenotype, expression of a Cdk2 mutant that is resistant to inhibitory tyrosine phosphorylation overcomes IR-dependent S-phase arrest (Falck et al. 2001), implicating elimination of Cdc25A in the intra-S-phase checkpoint.
Recent studies indicate that Cdc25A turnover through the ubiquitin pathway involves at least two temporally distinct components (Donzelli et al. 2002; Donzelli and Draetta 2003). During mitotic exit and early G1, Cdc25A stability is controlled by the anaphase-promoting complex in conjunction with Cdh1. During interphase, however, Cdc25A turnover is dependent on Cul1 (Donzelli et al. 2002), a central component of the SCF (Skp1/Cul1/F-box protein) ubiquitin ligase (Feldman et al. 1997; Skowyra et al. 1997). Precisely how Cul1 promotes turnover of Cdc25A is unknown. In SCF complexes, Cul1 together with the RING-H2 finger protein Rbx1 forms the core ubiquitin ligase that binds ubiquitin-conjugating enzymes (for review, see Deshaies 1999; Koepp et al. 1999). Specificity in these reactions is achieved by a substrate-binding module composed of Skp1 and a member of the F-box family of proteins. F-box proteins interact with Skp1 through the F-box motif (Bai et al. 1996) and with substrates through C-terminal protein interaction domains, including WD40 propellers (Skowyra et al. 1997; Wu et al. 2003; Orlicky et al. 2003). Frequently, association of SCF targets with the requisite F-box protein requires that the substrate be modified, typically by phosphorylation to produce a short peptide motif displaying properties of a phosphodegron (Winston et al. 1999a; Koepp et al. 2001; Nash et al. 2001; Wu et al. 2003; Orlicky et al. 2003).
Here we report that constitutive and DNA-damage-induced turnover of Cdc25A occurs via the SCFβ-TRCP ubiquitin ligase. Depletion of β-TRCP by shRNA stabilizes Cdc25A, leading to inappropriately high levels of Cdk2 kinase activity characteristic of a checkpoint defect. Cdc25A ubiquitination by SCFβ-TRCP in vitro involves Chk1-dependent phosphorylation principally at S76, consistent with the requirement for Chk1 in vivo. However, Chk1-mediated phosphorylation of Cdc25A does not appear to be sufficient to generate the requisite phosphodegron for β-TRCP recruitment. We find that residues 79-84 of Cdc25A constitute a phosphodegron for recognition by β-TRCP and implicate S79 and S82 as phosphoacceptor sites in this motif. Indeed, S82 is in a sequence context (Asp-Ser-Gly-Phe) reminiscent of previously identified phosphodegrons in the β-TRCP substrates IκBα and β-catenin. We suggest that Chk1-mediated phosphorylation of S75 may promote Cdc25A turnover by facilitating the phosphorylation of the adjacent phosphodegron targeted by β-TRCP.
Results
Involvement of the SCF pathway in DNA-damage-induced Cdc25A turnover
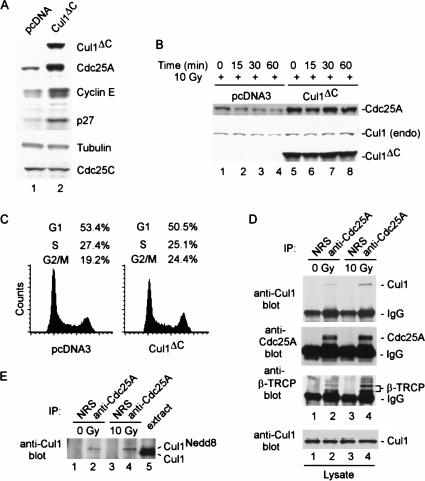
The precise pathways involved in interphase and DNA-damage-mediated turnover of Cdc25A and the role of phosphorylation in this process are unknown. To address these issues, we examined whether DNA-damage-dependent elimination of Cdc25A, like its interphase counterpart, also involves the SCF pathway. Cells were transiently transfected with a vector expressing a dominant-negative version of Cul1, Cul1DN, which binds to Skp1 but does not associate with the essential ring-finger protein Rbx1 (Donzelli et al. 2002). As expected, Cul1DN expression resulted in accumulation of Cdc25A, as well as other SCF substrates including p27 and cyclin E, in the absence of DNA damage (Fig. 1A). In control transfected cells, ionizing radiation (IR) induced a time-dependent decrease in the abundance of Cdc25A (Fig. 1B, lanes 1-4). However, expression of Cul1DN led to increased Cdc25A abundance throughout the time course (Fig. 1B, lanes 5-8), implicating an SCF-like complex in Cdc25A turnover in response to DNA damage, as well as during interphase. Flow cytometry (Fig. 1C) indicated that, under these conditions, expression of Cul1DN has only a minor influence on the cell cycle distribution in these cells. Thus, the dramatic stabilization of Cdc25A by Cul1DN does not appear to be caused by an indirect effect of cell cycle position.
Figure 1.
In vivo association of Cdc25A with the SCFβ-TRCP ubiquitin ligase. (A) Disruption of the Cul1 ubiquitin ligase pathway leads to accumulation of Cdc25A. 293T cells were transfected with vectors expressing an N-terminal fragment of Cul1 (residues 1-452; Cul1DN) or empty vector (3 μg) and after 48 h, lysates were subjected to immunoblotting with the indicated antibodies. (B) Disruption of the Cul1 pathway blocks DNA-damage-dependent elimination of Cdc25A. 293T cells were transfected with 3 μg of pCMV-Cul1DN or empty vector subjected to ionizing radiation (10 Gy) prior to analysis of total Cdc25A by immunoblotting. (C) Effect of Cul1DN on cell cycle control. 293T cells were collected 48 h after transfection with either pcDNA3 or pcDNA3-Cul1DN as described in B and then subjected to flow cytometry after staining with propidium iodide. (D) Cdc25A associates with endogenous Cul1 and β-TRCP in the presence and absence of DNA damage. 293T cells were treated with IR (10 Gy). Then, 30 min later, cells were lysed and Cdc25A immune complexes were prepared from 1 mg of extract prior to immunoblotting for Cul1. The blot was stripped and reprobed for β-TRCP. The relative levels of Cdc25A, Cul1, and β-TRCP were determined by densitometry. After normalizing for a small (17%) increase in the amount of Cdc25A in the presence of damage, we determined a 1.85- and 2.5-fold increase in Cdc25A-associated β-TRCP and Cul1 levels in response to DNA damage. (E) Cdc25A associates specifically with the neddylated and activated form of Cul1. Immune complexes were prepared as in D and subjected to electrophoresis together with crude extract (25 μg) to visualize the position of neddylated and unneddylated Cul1. As expected, neddylated Cul1 represents ∼5% of the total Cul1 present in crude extracts.
We next examined association of Cdc25A with Cul1 in the presence and absence of DNA damage. Consistent with previous studies (Donzelli et al. 2002), endogenous Cdc25A was found to associate with endogenous Cul1 in the absence of DNA damage (Fig. 1D, lane 2). Moreover, Cdc25A was also associated with Cul1 in the presence of IR (10 Gy), and quantification of the blot indicated an ∼twofold increase in the quantity of Cdc25A-associated Cul1 (relative to Cdc25A levels) in the presence of DNA damage (Fig. 1D, lane 4). Importantly, Cdc25A was associated exclusively with the neddylated, and therefore potentially activated, form of Cul1 (Fig. 1E).
The β-TRCP F-box protein specifically associates with Cdc25A
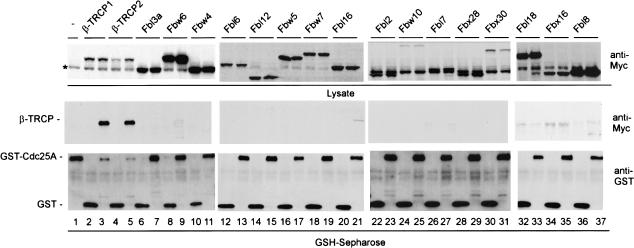
Substrate specificity in SCF-driven ubiquitination reactions is controlled by the identity of the F-box protein (Bai et al. 1996). To search for F-box proteins involved in Cdc25A ubiquitination, we cloned and expressed 18 of the 70 known human F-box proteins (Winston et al. 1999b; J. Jin and J.W. Harper, unpubl.) as Myc-tagged protein fusions (Fig. 2). These F-box protein expression plasmids were then individually cotransfected with vectors expressing either GST-Cdc25A or GST as a negative control. F-box proteins associating with GST-Cdc25A were identified by immunoblotting. Two WD40-containing F-box proteins—β-TRCP1 and β-TRCP2—were found to bind efficiently to GST-Cdc25A but not GST alone, and this binding occurred independently of addition of exogenous DNA damaging agents (Fig. 2, lanes 3,5). β-TRCP proteins are derived from distinct genes, yet are closely related in sequence and appear to interact with identical phosphodegrons in several ubiquitinated targets, including β-catenin, IκBα, and Emi1 (Latres et al. 1999; Winston et al. 1999a; Guardavaccaro et al. 2003). Using previously characterized antibodies that react with both β-TRCP1 and β-TRCP2 (Spiegelman et al. 2002), we found that β-TRCP is present in Cdc25A immune complexes and that the association is enhanced ∼twofold in the presence of ionizing irradiation (Fig. 1D, lanes 2,4). These data suggest a possible regulatory connection between β-TRCP and Cdc25A turnover.
Figure 2.
Screening a panel of F-box proteins for association with Cdc25A reveals specific association with β-TRCP1 and β-TRCP2. Vectors expressing the indicated Myc-tagged F-box proteins (1 μg) were cotransfected into 293T cells (2-cm dish) with either pCMV-GST or pCMV-GST-Cdc25A (1 μg) and association with GST-Cdc25A determined 48 h later after purification of GSH-Sepharose. A band cross reacting with anti-Myc antibodies in crude extracts is indicated by the asterisk.
Linkage of β-TRCP with elimination of Cdc25A in the presence and absence of DNA damage
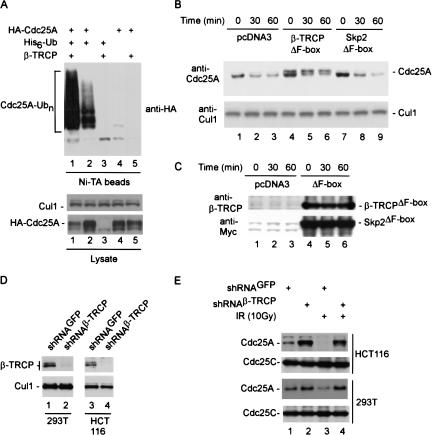
We next examined whether expression of β-TRCP can promote Cdc25A ubiquitination in tissue culture cells. 293T cells were transiently transfected with vectors expressing HA-Cdc25A, β-TRCP1, and/or His6-tagged ubiquitin. After 36 h, guanidine-denatured extracts were subjected to Ni-NTA purification, and bound proteins were immunoblotted with anti-HA antibodies. β-TRCP1 dramatically promoted the formation of high-molecular-weight Cdc25A/His6-Ub conjugates, when compared with those produced in the absence of exogenous β-TRCP (Fig. 3A, lanes 1,2). Immunoblotting for total Cdc25A in crude cell lysates indicated reduced levels of Cdc25A in cells expressing β-TRCP, when compared with control cell lysates (Fig. 3A, lower panels). Thus, the increased abundance of Cdc25A-ubiquitin conjugates is not a reflection of higher levels of total Cdc25A in this experiment. A similar dramatic reduction in the levels of Cdc25A was also seen when higher quantities of pCMV-β-TRCP were used (Fig. 2, lanes 3,5). None of the other 16 F-box proteins tested displayed an ability to reduce steady-state Cdc25A levels when coexpressed, indicating a high degree of specificity for β-TRCP in this regard.
Figure 3.
Linkage of β-TRCP to elimination of Cdc25A in the presence and absence of DNA damage. (A) Expression of β-TRCP promotes Cdc25A ubiquitination in vivo. 293T cells in 6-cm dishes were transfected with vectors expressing HACdc25A (2 μg), β-TRCP1 (1 μg), and/or His6-Ub (4 μg). After 36 h, cells were lysed in buffers containing 6 M guanidinium-HCl and His6-Ub tagged proteins purified on Ni-NTA beads. Proteins were separated by SDS-PAGE and immunoblotted with anti-HA antibodies. Cells from a parallel transfection were lysed using conventional NP-40-containing buffers (see Materials and Methods) and extracts subjected to immunoblotting (lower panels) using anti-HA to determine the total abundance of HA-Cdc25A and anti-Cul1 as a loading control. (B,C) Accumulation of Cdc25A after DNA damage in the presence of dominant-negative β-TRCP1. 293T cells in 10-cm dishes were transfected with 5 μg of pCMV, pCMV-β-TRCPΔF-box, and pCMV-Skp2ΔF-box. After 48 h, cells were subjected to IR (10 Gy) and lysates were examined by immunoblotting (panel B) at the indicated times with anti-Cdc25A and anti-Cul1 antibodies as a loading control. For analysis of Cdc25A, extracts were subjected to a modified SDS-PAGE procedure that provides larger separation of phosphoryated forms of Cdc25A seen previously (Zhao et al. 2002). The expression of β-TRCPΔF-box and Skp2ΔF-box was confirmed by immunoblotting (panel C). (D,E) Knock-down of β-TRCP by shRNA blocks constitutive and DNA-damage-dependent elimination of Cdc25A. The indicated cells were infected with a single retroviruses expressing shRNA targeting both β-TRCP1 and β-TRCP2 as well a retrovirus expressing shRNA against GFP as a negative control. Then, 48 h later, cells were lysed prior to analysis of β-TRCP and Cul1 levels by immunoblotting (panel D; see Materials and Methods). (E) shRNA-expressing cells were either subjected to ionizing radiation (10 Gy) or left untreated. Cells were lysed 30 min later and 25 μg of extract was subjected to immunoblotting with anti-Cdc25A antibodies. Blots were reprobed with anti-Cdc25C as a loading control.
To examine whether β-TRCP is required for Cdc25A turnover, we initially examined the effects of a dominant negative β-TRCP protein lacking the F-box motif. 293T cells were transfected with vectors expressing β-TRCPΔF-box or Skp2ΔF-box as a negative control and then subjected to irradiation (Fig. 3B,C). In this experiment, a modified SDS-PAGE system was used to enhance the separation of phosphorylated and unphosphorylated forms of Cdc25A seen previously (Zhao et al. 2002; Materials and Methods). Cdc25A was eliminated in cells expressing Skp2ΔF-box at a rate similar to that found with vector-only control cells (Fig. 3B, lanes 1-3, 7-9), while the levels of Cul1 used as a loading control were constant. In contrast, the levels of the more slowly migrating phosphorylated forms of Cdc25A were elevated in cells expressing β-TRCPΔF-box both in the absence of irradiation and throughout the time course after DNA damage (Fig. 3B, lanes 4-6), suggesting that β-TRCP is important for turnover of Cdc25A in response to its phosphorylation. Rapid turnover of the more rapidly migrating Cdc25A isoforms in this experiment likely reflects a population of cells that did not receive the β-TRCPΔF-box plasmid, as the efficiency of transfection under these conditions is <80% (data not shown). The levels of dominant-negative β-TRCP and Skp2 were maintained throughout the time course (Fig. 3C).
To extend these results, we used vectors expressing shRNA against β-TRCP proteins. The targeting sequence used represents a sequence fully conserved in both β-TRCP1 and β-TRCP2 and has been demonstrated to effectively knock down expression of both isoforms (Fong and Sun 2002), a result confirmed here in 293T and HCT116 cells (Fig. 3D). Expression of β-TRCP shRNA in both 293T and HCT116 cells resulted in accumulation of Cdc25A both in unirradiated cells (Fig. 3E, lane 2) and in cells exposed to IR (lane 4), when compared with control cells expressing shRNA against GFP (lanes 1,3). These data are consistent with a role for β-TRCP in Cdc25A turnover.
Depletion of β-TRCP stabilizes Cdc25A and increases cyclin E/Cdk2 activity in the presence and absence of DNA damage
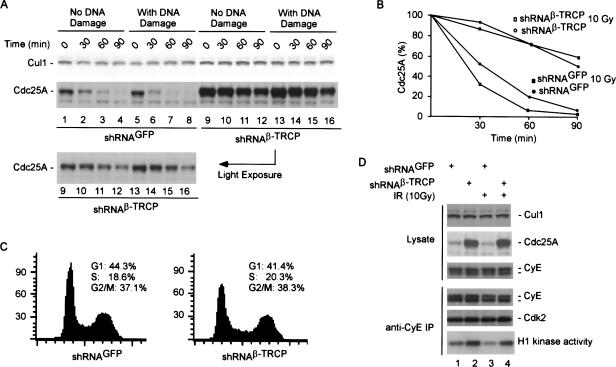
To examine Cdc25A turnover directly, we determined Cdc25A levels in the presence of cyclohexamide with or without depletion of β-TRCP by shRNA and in the presence or absence of DNA damage (Fig. 4A). In 293T cells expressing a control shRNA in the presence or absence of DNA damage, Cdc25A was eliminated with an apparent half-life of 30 min or less (Fig. 4A, lanes 1-8; Fig. 4B). As expected, DNA damage accelerated Cdc25A turnover. In contrast, Cdc25A was dramatically stabilized upon depletion of β-TRCP in both the presence and absence of DNA damage, with an apparent half-life of ∼90 min (Fig. 4A, lanes 1-8; Fig. 4B). In addition, the total abundance of Cdc25A was greatly elevated by depletion of β-TRCP, when compared with Cul1 used as a loading control (Fig. 4A, lanes 1-8 vs. lanes 9-16). One explanation for altered Cdc25A turnover is that β-TRCP depletion leads to cell cycle arrest at a point in the cell cycle where Cdc25A is more stable. To examine this possibility, cells from the experiment shown in Figure 4A were processed for flow cytometry in parallel. As shown in Figure 4C, β-TRCP depletion had a negligible effect on the cell cycle distribution of 293T cells when compared with control cells expressing shRNAGFP. Thus, β-TRCP is required for Cdc25A turnover in the presence and absence of DNA damage.
Figure 4.
Stabilization of Cdc25A by depletion of β-TRCP in the presence or absence of DNA damage leads to deregulated cyclin E/Cdk2 activity. (A,B) Turnover of Cdc25A requires β-TRCP. 293T cells were transfected with pSUPER-shRNAGFP or pSUPER-shRNAβ-TRCP using lipofectamine 48 h after dual transfection and then either left untreated or subjected to DNA damage (10 Gy). Translation was immediately blocked by addition of cyclohexamide, and cells lysed at the indicated time points prior to SDS-PAGE. (A) Blots were probed with anti-Cdc25A, stripped, and reprobed with anti-Cul1 antibodies. (B) Blots of comparable intensity for shRNAGFP and shRNAβ-TRCP were quantified by densitometry. (C) Depletion of β-TRCP does not alter cell cycle progression. Cells from A were subjected to flow cytometry after staining with propidium iodide. (D) Increased cyclin E/Cdk2 kinase activity in cells depleted of β-TRCP. 293T cells were transfected with vectors expressing shRNA against GFP as control or β-TRCP using the dual-transfection protocol. After 48 h, cells were lysed and cyclin E immune complexes assayed for activity using histone H1 as a substrate. Cyclin E immune complexes were generated using a rabbit polyclonal antibody (C-19 from Santa Cruz Biotechnology). Parallel immunoblots were probed for Cdk2 and cyclin E to demonstrate equal loading. The cyclin E immunoblot was probed with a monoclonal antibody (HE12, Santa Cruz Biotechnology). Controls demonstrated a dramatic accumulation of Cdc25A in response to depletion of β-TRCP whereas Cul1 levels remained unchanged.
Previous studies have demonstrated that depletion of Chk1 leads to increased abundance of Cdc25A with a concomitant increase in the activity of cyclin E/Cdk2, reflecting inappropriate dephosphorylation of Y15 by Cdc25A (Falck et al. 2001; Sorensen et al. 2003). Consistent with this, we find that depletion of β-TRCP leads to increased levels of cyclin E associated kinase activity in both the presence and absence of DNA damage, while the total level of cyclin E-associated Cdk2 is unaffected (Fig. 4D). These data are consistent with a role for both Chk1 and β-TRCP in controlling Cdc25A and cyclin E/Cdk2 activity.
Chk1-dependent Cdc25A ubiquitination by SCFβ-TRCP in vitro
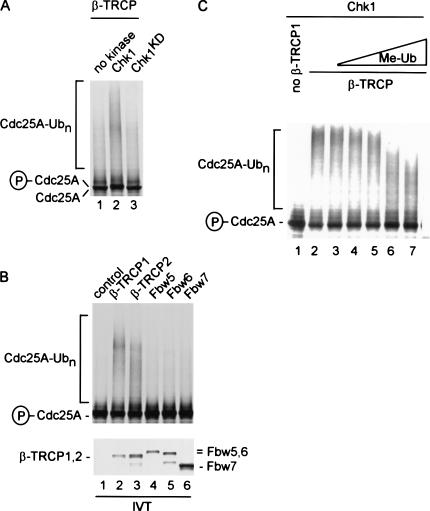
We next asked whether SCFβ-TRCP1 can function as a ubiquitin ligase for Cdc25A in vitro and whether this required phosphorylation of Cdc25A by Chk1. SCFβ-TRCP1 complexes were assembled by translation of β-TRCP1 in reticulocyte extracts. Such complexes have been shown to be proficient in ubiquitination of IκBα (Wu et al. 2003). SCFβ-TRCP1 complexes were then supplemented with E1, Ubc5, ubiquitin, ATP, and 35S-methionine-labeled Cdc25A added in the presence of wild-type or kinase-dead (KD) Chk1 (Fig. 5A). Cdc25A formed high-molecular-weight products in the presence of wild-type Chk1 (Fig. 5A, lane 2) but not in the presence of Chk1KD or samples lacking kinase (lanes 1,3), and this correlated with a shift in the mobility of Cdc25A in the presence of Chk1 indicative of phosphorylation (lane 2). Although β-TRCP2 was also capable of promoting Chk1-dependent Cdc25 ubiquitination, three other WD40-containing F-box proteins present at equivalent levels (Fbw5, Fbw6, and Fbw7) failed to promote Cdc25A ubiquitination, demonstrating specificity for β-TRCP proteins (Fig. 5B). Addition of the polyubiquitin chain terminator methyl ubiquitin led to the generation of shorter conjugates, demonstrating that the formation of high-molecular-weight Cdc25A species reflected ubiquitination (Fig. 5C). Taken together, these data indicate Cdc25A can be ubiquitinated by SCFβ-TRCP and this process is dependent on Cdc25A phosphorylation by Chk1.
Figure 5.
Chk1-dependent ubiquitination of Cdc25A by SCFβ-TRCP in vitro. (A) Activation of Cdc25A ubiquitination by SCFβ-TRCP requires active Chk1. In vitro translated and 35S-methionine-labeled Cdc25A was subjected to ubiquitination of SCFβ-TRCP complexes assembled in reticulocyte extracts in the absence of kinase or in the presence of active or inactive Chk1 (100 ng) made in insect cells as described in Materials and Methods. Phosphorylation of Cdc25A by Chk1 leads to a small decrease in electrophoretic mobility, as indicated. (B) Both β-TRCP1 and β-TRCP2, but not other WD40-containing F-box proteins, can ubiquitinate Cdc25A in vitro. Assays were performed as in A in the presence of Chk1 with the indicated in vitro translated F-box proteins. (C) Polyubiquitination of Cdc25A by SCFβ-TRCP is attenuated in the presence of methyl ubiquitin. Chk1-dependent Cdc25A ubiquitination reactions were performed in the presence of constant amounts of SCFβ-TRCP and increasing concentrations of methyl ubiquitin. The methyl ubiquitin concentrations used were 0.25, 1, 2.5, 25, and 100 μg/mL.
Role of Chk1-dependent phosphorylation in Cdc25A ubiquitination by SCFβ-TRCP
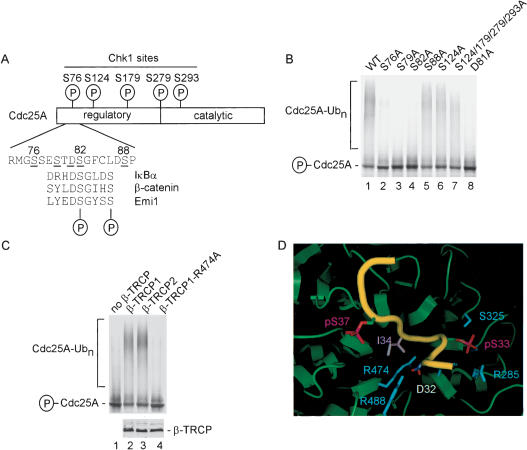
Given that Chk1 promotes Cdc25A turnover in response to DNA damage in vivo (Falck et al. 2001; Sorensen et al. 2003) and that Chk1 is required for Cdc25A ubiquitination by SCFβ-TRCP in vitro, we explored the role of Cdc25A phosphorylation in the ubiquitination process. Chk1 is known to phosphorylate Cdc25A on at least five sites: S76, S124, S179, S279, and S293 (Fig. 6A). By mass spectrometry, we demonstrated phosphorylation of S76 and S124 in Cdc25A phosphorylated by our recombinant Chk1 in vitro (Table 1). Early studies indicated a prominent role for phosphorylation of S124 in DNA-damage-dependent Cdc25A turnover (Falck et al. 2001; Sorensen et al. 2003), but more recent work suggests the key involvement of S76 in controlling Cdc25A turnover, particularly in response to ultraviolet radiation (Goloudina et al. 2003; Hassepass et al. 2003). Indeed, DNA damage leads to increased S76 phosphorylation as determined using a phosphospecific antibody to this site (Goloudina et al. 2003). Using our in vitro Cdc25A ubiquitination assay and Cdc25A mutants in which particular phosphorylated serine residues are replaced by alanine, we found that S124 phosphorylation is not required for Cdc25A ubiquitination in vitro (Fig. 6B, lane 6). Moreover, a quadruple mutant lacking S124, S179, S279, and S293 was ubiquitinated by SCFβ-TRCP, although we did consistently find that the quadruple mutant produced smaller ubiquitin conjugates than were generated with the wild-type protein (Fig. 6B, lanes 1,7). In contrast, replacement of S76 with alanine greatly reduced SCFβ-TRCP-driven Cdc25A ubiquitination in this assay (Fig. 6B, lane 2). Thus, our biochemical data reveal a critical role for Chk1-mediated phosphorylation of S76 in promoting Cdc25A ubiquitination and is consistent with the involvement of this phosphorylation event in promoting Cdc25A turnover in vivo (Goloudina et al. 2003; Hassepass et al. 2003).
Figure 6.
Chk1 phosphorylation sites in Cdc25A are required for SCFβ-TRCP-mediated ubiquitination but do not appear to constitute the major Cdc25A phosphodegron. (A) Schematic representation of Chk1 phosphorylation sites in Cdc25A and comparison of a putative phosphodegron in Cdc25A with the IκBα, β-catenin, and Emi1 phosphodegron recognized by β-TRCP. The Cdc25A residues are designated based on GenBank accession number AAH18642. (B) Identification of residues in Cdc25A important for Chk1-dependent ubiquitination. The indicated Cdc25A mutants were used in SCFβ-TRCP-driven ubiquitination reactions in the presence of Chk1. (C) Arg 474 in β-TRCP1 is required for Chk1-dependent Cdc25A ubiquitination. Ubiquitination of Chk1-phosphorylated Cdc25A was performed in the presence of β-TRCP1 or an R474A mutant. An aliquot of each β-TRCP synthesis reaction was supplemented with 35S-methionine to demonstrate equal expression of β-TRCP proteins (lower panel). (D) Three-dimensional structure of β-TRCP bound to the phosphodegron of β-catenin depicting the interaction of D32 and phosphoserine-33 (pS33) in the phosphodegron with R474 and R285 in β-TRCP. Graphics were generated using Pymol.
Table 1.
Mass spectral analysis of Cdc25A phosphorylation by Chk1
| Tryptic fragment | Residues | Number of phosphates | Calculated average massb (D) | Measured average massb (D) |
|---|---|---|---|---|
| 6a | 73-94 | 1, S76c | 2217.4 | 2218 |
| 12-13a | 121-148 | 1, S124d | 3320.4 | 3321 |
| 12-15 | 121-152 | 1 | 3801.1 | 3801 |
| 35-40 | 323-377 | 1 | 6119.0 | 6120 |
These phosphopeptides were sequenced with LC/MS/MS.
Average mass of dephosphorylated form.
Phosphorylation of S77 in addition to S76 was also detected at lower levels.
Phosphorylation of S126 in addition to S124 was also detected at lower levels.
Identification of a novel phosphodegron in Cdc25A
Degrons are considered to be minimal sequences that support recognition of cognate E3s and may possess relevant lysine side chains for ubiquitination. The finding that F-box proteins such as β-TRCP and Cdc4 frequently interact with short phosphopeptide motifs has led to the use of the term “phosphodegron” to refer to short sequences capable of interacting specifically with particular F-box proteins (Nash et al. 2001), although these sequences may not themselves contain relevant lysines for ubiquitination. The β-propeller of β-TRCP is known to interact with a phosphodegron containing the sequence DpSGΦXpS, where pS is phospho-serine and Φ is a hydrophobic amino acid (Spencer et al. 1999; Winston et al. 1999a; Wu et al. 2003). Both the aspartate and glycine residues in this motif are required for high-affinity interactions. However, none of the known Chk1 phosphorylation sites in Cdc25A, including S76, would be expected to create a classical β-TRCP recognition motif. This suggested that either β-TRCP is recognizing phosphorylated Cdc25A through a nonclassical phosphodegron or that additional phosphorylation events are involved. Scanning the Cdc25A sequence, we identified a single region (DSGFCLDSP, residues 81-89) that displayed characteristics of the classical β-TRCP recognition motif and is located adjacent to S76 (Fig. 6A). In particular, the first four residues of this motif conform to a portion of the known β-TRCP recognition motif or phosphodegron (Fig. 6A).
To examine the possible involvement of this region, we first determined the effect of mutation of S82 to alanine. This residue corresponds to phospho-S33 in β-catenin, which interacts with a network of hydrogen bonds involving R285 and S325 in β-TRCP (Fig. 6D). Interestingly, Cdc25AS82A was not ubiquitinated by SCFβ-TRCP in vitro, suggesting the involvement of this motif in Cdc25A ubiquitination (Fig. 6B, lane 4). We next considered the possibility that S88 may potentially be involved in recognition, in which case, β-TRCP would need to accommodate alternative spacing between phospho-serine residues in the phosphodegron. However, we found that Cdc25AS88A was ubiquitinated with an efficiency similar to that found with the wild-type protein (Fig. 6B, lane 5). We also noted that Cdc25A contains an additional serine residue at residue 79, which could potentially be part of the phosphodegron. We found that mutation of S79 to alanine abolished Cdc25A ubiquitination by SCFβ-TRCP (Fig. 6B, lane 3).
Additional mutagenesis experiments indicate the direct involvement of a phosphodegron containing phospho-S82 in Cdc25A recognition by β-TRCP. Mutation of D81 in Cdc25A abolished ubiquitination by β-TRCP in vitro (Fig. 6B, lane 8). D81 corresponds to D32 in β-catenin, which is frequently mutated in stabilized alleles of β-catenin (see Wu et al. 2003). In the β-catenin/β-TRCP crystal structure, D32 (which is invariant in β-TRCP substrates) is buried in the β-propeller and forms hydrogen bonds with R474 and Y488 (Fig. 6D). Previous studies indicate that replacement of R474 with alanine blocks IκBα ubiquitination by SCFβ-TRCP, revealing that this interaction is crucial for recognition of canonical β-TRCP targets (Wu et al. 2003). Likewise, we found that R474 in β-TRCP is required for Chk1-dependent Cdc25A ubiquitination (Fig. 6C, lane 4), consistent with the proposed interaction with D81 in Cdc25A.
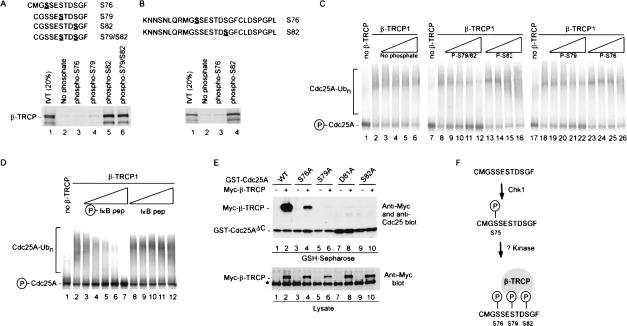
Synthetic phosphopeptides have been useful in defining phosphodegrons in cyclin E, β-catenin, IκBα, and Sic1 (Winston et al. 1999a; Koepp et al. 2001; Nash et al. 2001). Therefore, we directly examined whether sequences containing the DSG motif in Cdc25 could function as a phosphodegron. Immobilized peptides spanning this candidate phosphodegron were found to interact efficiently with β-TRCP when S82 or both S79 and S82 were phosphorylated, whereas the phospho-S79 peptide bound slightly better than the unphosphorylated control peptide (Fig. 7A). In contrast, a peptide containing phospho-S76, the site of Chk1 phosphorylation, displayed no detectable interaction with β-TRCP in this assay (Fig. 7A), consistent with the idea that Chk1-mediated phosphorylation of this site is not sufficient for β-TRCP recognition. A larger Cdc25A peptide (residues 66-92) also failed to interact with β-TRCP when phosphorylated on S76 but did associate when phosphorylated on S82 (Fig. 7B).
Figure 7.
Involvement of a novel phosphodegron in Cdc25A centered at S82 in association with β-TRCP in vivo. (A,B) A Cdc25A-derived phosphodegron containing at phospho-S79 and phospho-S82 binds β-TRCP in vitro. The indicated synthetic peptides spanning S76-S82 in Cdc25A in phosphorylated or unphosphorylated forms were immobilized on agarose beads and used in binding reactions with in vitro translated and 35S-methionine-labeled β-TRCP1. Bound proteins were separated by SDS-PAGE and visualized by autoradiography. Peptides in A were coupled to Sulfo-link agarose through an N-terminal cysteine while peptides in B were coupled to Affigel-10 through the side chain of the N-terminal lysine. (C) Cdc25A-derived phosphodegrons inhibit ubiquitination of Cdc25A by SCFβ-TRCP in vitro. Assays were performed as described in Figure 5 in the presence of the indicated peptides at 2, 10, 20, and 40 μg/mL. (D) Cdc25A ubiquitination is blocked by an IκBα phosphodegron. Phosphorylated or unphosphorylated synthetic peptides encompassing the IκBα phosphodegron were used in competition reactions with Chk1-dependent Cdc25A ubiquitination. The concentrations of peptides used were 2, 10, 20, 40, and 100 μg/mL. (E) Vectors expressing the indicated Cdc25AΔC proteins were cotransfected into 293T cells with a vector expressing Myc-tagged β-TRCP (1 μg each). After 36 h, cells were lysed and subjected to GSH-Sepharose pull-down assays. Blots were probed with anti-Myc antibodies and reprobed with andi-Cdc25A antibodies to demonstrate similar protein levels. The protein labeled with the asterisk is the endogenous c-Myc protein. (F) Model for recognition of Cdc25A by β-TRCP in response to phosphorylation by Chk1. See text for details.
To more accurately determine the relative affinities of various Cdc25A-derived phosphopeptides for β-TRCP, we developed a competition assay using β-TRCP-driven Cdc25A ubiquitination (Fig. 7C). In principle, this approach avoids the concentration and steric effects sometimes seen with immobilized peptides. Neither the unphosphorylated peptide nor the singly phosphorylated peptide containing phospho-S76 was capable of blocking Cdc25A ubiquitination in this assay (Fig. 7C, lanes 3-6, 23-26). In contrast, a doubly phosphorylated S79/S82 peptide, and to a lesser extent a singly phosphorylated S79 peptide, were capable of inhibiting Cdc25A ubiquitination by SCFβ-TRCP as judged by both the lower levels of ubiquitin conjugates and the increased abundance of unmodified Cdc25A (Fig. 7C, lanes 9-12). The peptide containing phospho-S82 reduced the extent of polyubiquitination (Fig. 7C, lanes 13-16). Interestingly, the extent of inhibition by the phospho-S79/S82 peptide was comparable to that observed with the phosphodegron from IκBα (Winston et al. 1999a) when examined in the same concentration range (Fig. 7D). Taken together, these data indicate that S79 and S82 are central components of the Cdc25A phosphodegron recognized by β-TRCP and indicate that Chk1 phosphorylation alone is not sufficient to create the requisite epitopes for β-TRCP recognition. The data also indicate that Cdc25A and IκBα use at least partially overlapping binding sites in β-TRCP.
Recognition of β-TRCP by Cdc25A in vivo requires a phosphodegron anchored by S82
To verify the involvement of residues 76 to 82 in β-TRCP recognition in vivo, GST-Cdc25AΔC containing the N-terminal regulatory domain and mutant versions in which S76, S79, D81, and S82 were individually replaced with alanine were tested for binding after expression in 293T cells (Fig. 7E). Mutation of S76 reduced binding by ∼10-fold (Fig. 7E, lane 4 vs. lane 2), whereas mutation of S79, D81, and S82 bound even less efficiently (Fig. 7E, lanes 6,8,10 vs. lane 2). Taken together, these in vitro and in vivo data strongly implicate a novel phosphodegron in Cdc25A centered at S82 as being important for its association with the β-TRCP ubiquitin ligase.
Discussion
β-TRCP is involved in a large number of seemingly unrelated processes. β-TRCP controls phosphorylation-mediated destruction or processing of several transcriptional regulators, including IκB, β-catenin, ATF-4, and p105NFκB (Latres et al. 1999; Spencer et al. 1999; Orian et al. 2000; Winston et al. 1999a; Fong and Sun 2002). Moreover, β-TRCP controls multiple cell cycle-related processes, including centrosome duplication in Drosophila and destruction of the mitotic regulator Emi1 during mitosis (Wojcik et al. 2000; Guardavaccaro et al. 2003). Our data now implicate β-TRCP1 and β-TRCP2 in control of Cdc25A turnover during both a normal cell cycle and in response to DNA damage. We have shown that endogenous Cdc25A forms complexes with SCFβ-TRCP in the presence or absence of DNA damage. Moreover, depletion of β-TRCP by shRNA stabilizes Cdc25A in the presence or absence of DNA damage, and SCFβ-TRCP can ubiquitinate Cdc25A in a Chk1-dependent manner in vitro. Several lines of biochemical evidence suggest the involvement of a novel phosphodegron in Cdc25A containing phospho-S79 and phospho-S82 in β-TRCP recognition. Chk1 appears to be required, but not sufficient, to generate this novel phosphodegron. Previous studies indicate that Cdc25A is phosphorylated by Chk1 during a normal cell cycle, probably during S phase, leading to its instability during S and G2 phases (Sorensen et al. 2003). This process is accelerated in response to DNA damage (Sorensen et al. 2003). Chk1 is essential for cell proliferation in mammals (Liu et al. 2000), and maintaining low levels of Cdc25A during S and G2 phases could represent a component of its essential functions. Inappropriately high levels of Cdc25A during DNA replication could influence the kinetics of S-phase progression and thereby affect the fidelity of DNA synthesis.
Our biochemical experiments indicate that Cdc25A turnover is more complex than previously appreciated. Early models suggested that phosphorylation of Cdc25A by Chk1 at multiple sites might be required for Cdc25A turnover. Our reconstitution studies indicate that of all the Chk1 sites in Cdc25A, only S76 phosphorylation plays a prominent role in facilitating SCFβ-TRCP-dependent Cdc25A ubiquitination in vitro, consistent with recent work on the role of this residue in Cdc25A turnover in vivo (Goloudina et al. 2003; Hassepass et al. 2003). Other known Chk1 sites in Cdc25A (S124, S179, S279, and S293) are not required for ubiquitination in vitro but could, nevertheless, be important for turnover in vivo. One possibility is that phosphorylation affects the site(s) of ubiquitination, which has recently been shown to affect the kinetics of proteasome-mediated destruction of an SCF substrate Sic1 (Petroski and Deshaies 2003). Moreover, we also note that we consistently see the formation of shorter ubiquitin conjugates with the Cdc25A mutant lacking these four Chk1 sites, which could affect the efficiency of recruitment to the proteasome.
Although Chk1-mediated phosphorylation is required for Cdc25A turnover in vivo and ubiquitination by SCFβ-TRCP in vitro, it appears that Chk1 activity is not sufficient for this process. First, Chk1 kinases are known to preferentially phosphorylate R-X-X-S/T motifs (O'Neill et al. 2002). Although all of the known Chk1 sites in Cdc25A conform to this consensus sequence, none is expected to generate phosphodegrons of the type known to interact with β-TRCP. Consistent with this, peptides containing phospho-S76 failed to interact with β-TRCP in a direct binding assay. Moreover, these peptides did not efficiently inhibit Cdc25A ubiquitination in vitro. Second, mass spectral analysis of bacterial Cdc25A phosphorylated in vitro by recombinant Chk1 revealed strong phosphorylation of S76 and S124 (>90%), but no phosphorylation at S79 or S82 was detected, indicating that Chk1 cannot directly phosphorylate these two sites. Finally, bacterial Cdc25A that was previously phosphorylated by Chk1 was not a substrate for ubiquitination by purified SCFβ-TRCP complexes in vitro that are competent for IκB ubiquitination (data not shown).
We propose that β-TRCP recognizes a novel phosphodegron in Cdc25A. This sequence contains a DSG motif characteristic of all previously identified β-TRCP targets (Emi1, β-catenin, IκBα, p105NFκB). Several lines of evidence implicate this degron in β-TRCP recognition. First, point mutations in Cdc25A that remove S79 or S82 abolish ubiquitination by SCFβ-TRCP in vitro and association with β-TRCP in transfected cells. Second, point mutations in the aspartate of the DSG motif, or in its complementary ligand in the WD40 propeller of β-TRCP, R474, abolish ubiquitination by SCFβ-TRCP. Finally, synthetic Cdc25A peptides containing phospho-S79 and phospho-S82 alone can associate with β-TRCP in vitro and can block Cdc25A ubiquitination by SCFβ-TRCP in a competition assay, as can a canonical phosphodegron from IκBα.
The simplest explanation for these results is that generation of the phosphodegron in Cdc25A involves two critical steps (Fig. 7F). In the first step, Chk1 phosphorylates S76. In the second step, S76-phosphorylated Cdc25A is then phosphorylated by one or more kinases on S82 and S79 to generate the phosphodegron recognized by β-TRCP. In the case of our in vitro Cdc25A ubiquitination assay, where S76 and Chk1 are required, this second kinase activity appears to be provided by one or more kinases in the reticulocyte extract. According to this model, Chk1 may perform a priming function that facilitates modification of S79 and/or S82. Other β-TRCP substrates also use analogous priming reactions. For example, β-catenin is phosphorylated by casein kinase I on T41 and T45, and this facilitates phosphorylation of S33 and S37 by GSK3β in a sequential mechanism (Liu et al. 2002). At present, we cannot exclude the possibility that Cdc25A is constitutively phosphorylated on S82/S79 and that Chk1 in a second step induces a conformational change in the adjacent S79/S82 phosphodegron that allows it to be recognized by β-TRCP or protects these residues from rapid dephosphorylation. The finding that Cdc25AS76A displays detectable, albeit greatly reduced, association with β-TRCP in vivo, whereas Cdc25AS82A mutants display no detectable binding, suggests that S76 phosphorylation is not an absolute requirement for S79/S82 phosphorylation. A determination of the mechanism by which Chk1 promotes formation of the degron awaits identification of relevant kinases. In this regard, one candidate kinase is casein kinase Iα. However, addition of two specific casein kinase Iα inhibitors (CKI-7, US Biologicals; IC261, Calbiochem) continuously throughout Cdc25A translation, Chk1 phosphorylation, and in vitro ubiquitination assay failed to block Cdc25A ubiquitination (data not shown). It is also possible that additional cofactors may be involved in Cdc25A turnover independent of the kinases involved. For example, Cks1 is required for p27 ubiquitination by SCFSkp2 (for review, see Harper 2001).
The discovery of a role for β-TRCP in Cdc25A turnover now sets the stage for the elucidation of the interplay between regulatory mechanisms responsible for directing the destruction of Cdc25A and control of cell cycle transitions. Although Chk1 is a critical player in this regard, our studies reveal a previously unanticipated level of complexity in the targeting of Cdc25A for ubiquitination involving other participants. These new players may themselves be targets of regulation intended to control cell cycle progression. In addition to targeting Cdc25A for degradation, positive feedback mechanisms involving Cdk1 phosphorylation have been implicated in stabilization of Cdc25A to promote mitotic entry (Mailand et al. 2002; Ducruet and Lazo 2003). How these competing regulatory pathways interface with the Chk1 pathway to control Cdc25A stability remains to be determined.
Materials and methods
Plasmids
F-box proteins were generated by PCR and cloned into pUNI50 (Liu et al. 1998) prior to sequence analysis. Details of these constructs will be published elsewhere. For expression, pUNI50 constructs were recombined with pCMV-Myc-lox-RS (pJP150) using Cre recombinase, placing N-terminally Myc3-tagged F-box proteins under control of the CMV promoter. The pUNI backbone was then removed by RS-mediated recombination. Vectors for expression of CDC25A and mutants were generated using pENTR vectors and recombined into the indicated bacterial or mammalian expression construct using clonase (Invitrogen). To generate retroviral vectors expressing shRNAGFP and shRNAβ-TRCP, double-stranded oligonucleotides were cloned into a modified version of pSuper-retro (Oligoengine, Inc.) wherein the puromycin marker was replaced with an expression cassette for enhanced cyan fluorescence protein (ECFP). The sequences used were: humanized Renilla GFP, GGACTTC CCCGAGTACCACttcaagagaGTGGTACTCGGGGAAGTCCtt; β-TRCP, GTGGAATTTGTGGAACATCttcaagagaGATGTTC CACAAATTCCACtt (gene-specific sequences are in caps, and hairpin sequences are underlined). Point mutations in Cdc25A were generated using a QuickChange mutagenesis kit (Stratagene). Cdc25AΔC encodes amino acids 1-100 and was generated by PCR prior to cloning into pcDNA3. Vectors for expression of β-TRCPR474A are from a previous study (Wu et al. 2003). pCMV-β-TRCPΔF-box and pCMV-Skp2ΔF-box were provided by R. Benarous (Institut Cochin, Cochin, France) and L. Larsson (Swedish University of Agricultural Sciences, Uppsala), respectively.
Protein interactions
293T cells were cultured in DMEM supplemented with 10% fetal bovine serum (5% CO2). Transfections were performed using Lipofectamine 2000 (Invitrogen), except in the case of retrovirus production, where calcium-phosphate-mediated DNA transfer was used. At the indicated time, cells were lysed in extraction buffer (25 mM Tris-HCl at pH7.5, 150 mM NaCl, 0.5% nonidet P-40, 1 mM EDTA, 10 mM β-glycerol phosphate, 10 mM p-nitrophenyl phosphate, 0.1 μM okadaic acid, and 5 mM NaF). Extracts were subjected to direct Western blot analysis or complex purification using the indicated affinity reagent. For determining Cdc25A ubiquitination in vivo using His6-Ub, cells were lysed in buffer containing 6M guanidinium hydrochloride in 0.1 M sodium phosphate buffer (pH 8.0) prior to binding to Ni-NTA resin. Where indicated, cells were subject to γ-irradiation (10 Gy) using a Cobalt source. Antibodies against Cdc25A were from Neomarker, Inc. Anti-Cul1 was from Zymed. Anti-cyclin E and Cdc25C antibodies were from Santa Cruz Biotechnology. Anti-β-TRCP was from a previous study and has been shown to interact with both β-TRCP1 and β-TRCP2 (Spiegelman et al. 2002).
β-TRCP suppression
Knock-down of β-TRCP was performed using retroviral-mediated transfer of vectors expressing shRNA for β-TRCP1 and β-TRCP2 or GFP as a negative control in 293T or HCT116 cells. The experiments were performed at 48 h postinfection. In some experiments, lipofectamine-2000-mediated transfection of shRNA in pSUPER vectors was performed. 293T cells were transfected twice in 24 h. Then, 48 h later, cells were subjected to γ-irradiation (10 Gy) or left untreated. At the indicated time, cell extracts were generated and used for direct analysis by immunoblotting with the indicated antibodies. In some experiments, cyclohexamide (25 μg/mL) was added immediately after DNA damage. To verify β-TRCP knock-down, lysates (500 μg, 100 μL) were incubated with 20 μL of agarose-IκB phosphodegron beads prior to immunoblotting, as described (Winston et al. 1999a). This step is required to enrich for β-TRCP as available antibodies do not specifically interact with β-TRCP in crude extracts. Cell cycle analysis was performed using propidium iodide essentially as described (Ye et al. 2003). Histone H1 kinase assays were performed as described (Ye et al. 2003). With the exception of Figure 3A, all SDS-PAGE separations were performed using 8%-12% gradient minigels (Invitrogen). Figure 3A used a 15-cm 10% polyacrylamide gel, which gave higher resolution of Cdc25A isoforms observed previously (Zhao et al. 2002).
In vitro ubiquitination
Cdc25A ubiquitination was performed using in vitro 35S-methionine-labeled Cdc25A (2.5 μL) in the presence of insect-cell-derived Chk1 or Chk1KD (100 ng), E1 ubiquitin-activating enzyme (50 ng), Ubc5 (200 ng), ubiquitin (1 mg/mL), 1 μM ubiquitin aldehyde, 2.3 μL of in vitro translated F-box protein, and 4 mM ATP in a total volume of 10 μL (30 min, 30°C). In some experiments, methyl ubiquitin (Boston Biochemicals) was used as a chain terminator or synthetic peptides derived from the IκBα phosphodegron (Winston et al. 1999a) or Cdc25A used as a competitive inhibitor. Phosphorylated or unphosphorylated peptides encompassing the Cdc25A phosphodegron and containing a N-terminal cysteine were synthesized by Tufts Medical School Protein Core Facility or by Invitrogen and coupled to Sulfo-link agarose (Pierce) or Affigel-10 (Biorad) at 1 mg/mL. Binding reactions were performed with 10 μL of peptide agarose beads in combination with 5 μL of in vitro translated 35S-methionine-labeled β-TRCP1 in a total of 100 μL of extraction buffer. Beads were washed three times with extraction buffer prior to SDS-PAGE and autoradiography.
Phosphorylation analysis
Mass spectral analysis of bacterial Cdc25A previously phosphorylated with Chk1 was performed using established procedures. Mass spectral analysis of phosphopeptides was performed using Matrix-assisted laser desorption/ionization mass spectrometry (MALDI/TOF) with delayed extraction (Voyager-DE, Perseptive Biosystems) as described (Zhang et al. 1998). An electrospray ion trap mass spectrometer (LCQ, Finnigan) coupled on-line with a capillary high-pressure liquid chromatograph (Magic 2002) was used for identification of phosphorylation sites.
Acknowledgments
We thank S. Blacklow and G. Wu for assistance with Pymol. This work was supported by NIH grants to J.W.H. and S.J.E and by the Department of Defense (to J.W.H.). J.J. was supported by a Department of Defense Postdoctoral Fellowship. T.S. was supported by the Japanese Society for Science. S.J.E. is an Investigator of the Howard Hughes Medical Institute.
The publication costs of this article were defrayed in part by payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 USC section 1734 solely to indicate this fact.
Article published online ahead of print. Article and publication date are at http://www.genesdev.org/cgi/doi/10.1101/gad.1157503.
References
- Agami R. and Bernards, R. 2000. Distinct initiation and maintenance mechanisms cooperate to induce G1 cell cycle arrest in response to DNA damage. Cell 102: 55-66. [DOI] [PubMed] [Google Scholar]
- Bai C., Sen, P., Hofmann, K., Ma, L., Goebl, M., Harper, J.W., and Elledge, S.J. 1996. SKP1 connects cell cycle regulators to the ubiquitin proteolysis machinery through a novel motif, the F-box. Cell 86: 263-274. [DOI] [PubMed] [Google Scholar]
- Blasina A., de Weyer, I.V., Laus, M.C., Luyten, W.H., Parker, A.E., and McGowan, C.H. 1999. A human homologue of the checkpoint kinase Cds1 directly inhibits Cdc25 phosphatase. Curr. Biol. 9: 1-10. [DOI] [PubMed] [Google Scholar]
- Chen M.S., Hurov, J., White, L.S., Woodford-Thomas, T., and Piwnica-Worms, H. 2001. Absence of apparent phenotype in mice lacking Cdc25C protein phosphatase. Mol. Cell. Biol. 21: 3853-3861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deshaies R.J. 1999. SCF and Cullin/Ring H2-based ubiquitin ligases. Annu. Rev. Cell Dev. Biol. 15: 435-467. [DOI] [PubMed] [Google Scholar]
- Donzelli M. and Draetta, G.F. 2003. Regulating mammalian checkpoints through Cdc25 inactivation. EMBO Rep. 4: 671-677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donzelli M., Squatrito, M., Ganoth, D., Hershko, A., Pagano, M., and Draetta, G.F. 2002. Dual mode of degradation of Cdc25 A phosphatase. EMBO J. 21: 4875-4884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ducruet A.P. and Lazo, J.S. 2003. Regulation of Cdc25A half-life in interphase by cyclin-dependent kinase 2 activity. J. Biol. Chem. 278: 31838-31842. [DOI] [PubMed] [Google Scholar]
- Falck J., Mailand, N., Syljuasen, R.G., Bartek, J., and Lukas, J. 2001. The ATM-Chk2-Cdc25A checkpoint pathway guards against radioresistant DNA synthesis. Nature 410: 842-847. [DOI] [PubMed] [Google Scholar]
- Feldman R.M., Correll, C.C., Kaplan, K.B., and Deshaies, R.J. 1997. A complex of Cdc4p, Skp1p, and Cdc53p/cullin catalyzes ubiquitination of the phosphorylated CDK inhibitor Sic1p. Cell 91: 221-230. [DOI] [PubMed] [Google Scholar]
- Fong A. and Sun, S.C. 2002. Genetic evidence for the essential role of β-transducin repeat-containing protein in the inducible processing of NF κ-B2/p100. J. Biol. Chem. 277: 22111-22114. [DOI] [PubMed] [Google Scholar]
- Furnari B., Rhind, N., and Russell, P. 1997. Cdc25 mitotic inducer targeted by chk1 DNA damage checkpoint kinase. Science 277: 1495-1497. [DOI] [PubMed] [Google Scholar]
- Furnari B., Blasina, A., Boddy, M.N., McGowan, C.H., and Russell, P. 1999. Cdc25 inhibited in vivo and in vitro by checkpoint kinases Cds1 and Chk1. Mol. Biol. Cell 10: 833-845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giaccia A.J. and Kastan, M.B. 1998. The complexity of p53 modulation: Emerging patterns from divergent signals. Genes & Dev. 12: 2973-2983. [DOI] [PubMed] [Google Scholar]
- Goloudina A., Yamaguchi, H., Chervyakova, D.B., Appella, E., Fornace Jr., A.J., and Bulavin, D.V. 2003. Regulation of human Cdc25A stability by serine 75 phosphorylation is not sufficient to activate a S phase checkpoint. Cell Cycle 2: 473-478. [PubMed] [Google Scholar]
- Guardavaccaro D., Kudo, Y., Boulaire, J., Barchi, M., Busino, L., Donzelli, M., Margottin-Goguet, F., Jackson, P.K., Yamasaki, L., and Pagano, M. 2003. Control of meiotic and mitotic progression by the F box protein β-Trcp1 in vivo. Dev. Cell 4: 799-812. [DOI] [PubMed] [Google Scholar]
- Harper J.W. 2001. Protein destruction: Adapting roles for Cks proteins. Curr. Biol. 11: R431-R435. [DOI] [PubMed] [Google Scholar]
- Hassepass I., Voit, R., and Hoffmann, I. 2003. Phosphorylation at serine-75 is required for UV-mediated degradation of human Cdc25A phosphatase at the S-phase checkpoint. J. Biol. Chem. 278: 29824-29829. [DOI] [PubMed] [Google Scholar]
- Hoffmann I., Draetta, G., and Karsenti, E. 1994. Activation of the phosphatase activity of human cdc25A by a cdk2-cyclin E dependent phosphorylation at the G1/S transition. EMBO J. 13: 4302-4310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin P., Gu, Y., and Morgan, D.O. 1996. Role of inhibitory CDC2 phosphorylation in radiation-induced G2 arrest in human cells. J. Cell Biol. 134: 963-970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koepp D.M., Harper, J.W., and Elledge, S.J. 1999. How the cyclin became a cyclin: Regulated proteolysis in the cell cycle. Cell 97: 431-434. [DOI] [PubMed] [Google Scholar]
- Koepp D.M., Schaefer, L.K., Ye, X., Keyomarsi, K., Chu, C., Harper, J.W., and Elledge, S. 2001. Phosphorylation-dependent ubiquitination of cycin E by SCFFbw7 ubiquitin ligase. Science 294: 173-177. [DOI] [PubMed] [Google Scholar]
- Kumagai A. and Dunphy, W.G. 1999. Binding of 14-3-3 proteins and nuclear export control the intracellular localization of the mitotic inducer Cdc25. Genes & Dev. 13: 1067-1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latres E., Chiaur, D.S., and Pagano, M. 1999. The human F box protein β-Trcp associates with the Cul1/Skp1 complex and regulates the stability of β-catenin. Oncogene 18: 849-854. [DOI] [PubMed] [Google Scholar]
- Liu Q., Li, M.Z., Leibham, D., Cortez, D., and Elledge, S.J. 1998. The univector plasmid-fusion system, a method for rapid construction of recombinant DNA without restriction enzymes. Curr. Biol. 8: 1300-1309. [DOI] [PubMed] [Google Scholar]
- Liu Q., Guntuku, S., Cui, X.S., Matsuoka, S., Cortez, D., Tamai, K., Luo, G., Carattini-Rivera, S., DeMayo, F., Bradley, A., et al. 2000. Chk1 is an essential kinase that is regulated by Atr and required for the G2/M DNA damage checkpoint. Genes & Dev. 14: 1448-1459. [PMC free article] [PubMed] [Google Scholar]
- Liu C., Li, Y., Semenov, M., Han, C., Baeg, G.H., Tan, Y., Zhang, Z., Lin, X., and He, X. 2002. Control of β-catenin phosphorylation/degradation by a dual-kinase mechanism. Cell 108: 837-847. [DOI] [PubMed] [Google Scholar]
- Lopez-Girona A., Furnari, B., Mondesert, O., and Russell, P. 1999. Nuclear localization of Cdc25 is regulated by DNA damage and a 14-3-3 protein. Nature 397: 172-175. [DOI] [PubMed] [Google Scholar]
- Mailand N., Falck, J., Lukas, C., Syljuasen, R.G., Welcker, M., Bartek, J., and Lukas, J. 2000. Rapid destruction of human Cdc25A in response to DNA damage. Science 288: 1425-1429. [DOI] [PubMed] [Google Scholar]
- Mailand N., Podtelejnikov, A.V., Groth, A., Mann, M., Bartek, J., and Lukas, J. 2002. Regulation of G2/M events by Cdc25A through phosphorylation-dependent modulation of its stability. EMBO J. 21: 5911-5920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuoka S., Huang, M., and Elledge, S.J. 1998. Linkage of ATM to cell cycle regulation by the Chk2 protein kinase. Science 282: 1893-1897. [DOI] [PubMed] [Google Scholar]
- Morgan D.O. 1997. Cyclin-dependent kinases: Engines, clocks, and microprocessors. Annu. Rev. Cell Dev. Biol. 13: 261-291. [DOI] [PubMed] [Google Scholar]
- Nash P., Tang, X., Orlicky, S., Chen, Q., Gertler, F.B., Mendenhall, M.D., Sicheri, F., Pawson, T., and Tyers, M. 2001. Multisite phosphorylation of a CDK inhibitor sets a threshold for the onset of DNA replication. Nature 414: 514-521. [DOI] [PubMed] [Google Scholar]
- O'Neill T., Giarratani, L., Chen, P., Iyer, L., Lee, C.H., Bobiak, M., Kanai, F., Zhou, B.B., Chung, J.H., and Rathbun, G.A. 2002. Determination of substrate motifs for human Chk1 and hCds1/Chk2 by the oriented peptide library approach. J. Biol. Chem. 277: 16102-16115. [DOI] [PubMed] [Google Scholar]
- Orian A., Gonen, H., Bercovich, B., Fajerman, I., Eytan, E., Israel, A., Mercurio, F., Iwai, K., Schwartz, A.L., and Ciechanover, A. 2000. SCFβ-TrCP ubiquitin ligase-mediated processing of NF-κBp105 requires phosphorylation of its C-terminus by IκB kinase. EMBO J. 19: 2580-2591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orlicky S., Tang, X., Willems, A., Tyers, M., and Sicheri, F. 2003. Structural basis for phosphodependent substrate selection and orientation by the SCFCdc4 ubiquitin ligase. Cell 112: 243-256. [DOI] [PubMed] [Google Scholar]
- Peng C.Y., Graves, P.R., Thoma, R.S., Wu, Z., Shaw, A.S., and Piwnica-Worms, H. 1997. Mitotic and G2 checkpoint control: Regulation of 14-3-3 protein binding by phosphorylation of Cdc25C on serine 216. Science 277: 1501-1505. [DOI] [PubMed] [Google Scholar]
- Petroski M.D. and Deshaies, R.J. 2003. Context of multiubiquitin chain attachment influences the rate of Sic1 degradation. Mol. Cell 11: 1435-1444. [DOI] [PubMed] [Google Scholar]
- Rhind N., Furnari, B., and Russell, P. 1997. Cdc2 tyrosine phosphorylation is required for the DNA damage checkpoint in fission yeast. Genes & Dev. 11: 504-511. [DOI] [PubMed] [Google Scholar]
- Sanchez Y., Wong, C., Thoma, R.S., Richman, R., Wu, Z., Piwnica-Worms, H., and Elledge, S.J. 1997. Conservation of the Chk1 checkpoint pathway in mammals: Linkage of DNA damage to Cdk regulation through Cdc25. Science 277: 1497-1501. [DOI] [PubMed] [Google Scholar]
- Shiloh Y. 2003. ATM and related protein kinases: Safeguarding genome integrity. Nat. Rev. Cancer 3: 155-168. [DOI] [PubMed] [Google Scholar]
- Skowyra D., Craig, K.L., Tyers, M., Elledge, S.J., and Harper, J.W. 1997. F-box proteins are receptors that recruit phosphorylated substrates to the SCF ubiquitin-ligase complex. Cell 91: 209-219. [DOI] [PubMed] [Google Scholar]
- Sorensen C.S., Syljuasen, R.G., Falck, J., Schroeder, T., Ronnstrand, L., Khanna, K.K., Zhou, B.B., Bartek, J., and Lukas, J. 2003. Chk1 regulates the S phase checkpoint by coupling the physiological turnover and ionizing radiation-induced accelerated proteolysis of Cdc25A. Cancer Cell 3: 247-258. [DOI] [PubMed] [Google Scholar]
- Spencer E., Jiang, J., and Chen, Z.J. 1999. Signal-induced ubiquitination of IκBα by the F-box protein Slimb/β-TrCP. Genes & Dev. 13: 284-294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiegelman V.S., Tang, W., Katoh, M., Slaga, T.J., and Fuchs, S.Y. 2002. Inhibition of HOS expression and activities by Wnt pathway. Oncogene 21: 856-860. [DOI] [PubMed] [Google Scholar]
- Takizawa C.G. and Morgan, D.O. 2000. Control of mitosis by changes in the subcellular location of cyclin-B1-Cdk1 and Cdc25C. Curr. Opin. Cell Biol. 12: 658-665. [DOI] [PubMed] [Google Scholar]
- Vigo E., Muller, H., Prosperini, E., Hateboer, G., Cartwright, P., Moroni, M.C., and Helin, K. 1999. CDC25A phosphatase is a target of E2F and is required for efficient E2F-induced S phase. Mol. Cell. Biol. 19: 6379-6395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winston J.T., Strack, P., Beer-Romero, P., Chu, C.Y., Elledge, S.J., and Harper, J.W. 1999a. The SCFβ-TRCP-ubiquitin ligase complex associates specifically with phosphorylated destruction motifs in IκBα and β-catenin and stimulates IκBα ubiquitination in vitro. Genes & Dev. 13: 270-283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winston J.T., Koepp, D.M., Zhu, C., Elledge, S.J., and Harper, J.W. 1999b. A family of mammalian F-box proteins. Curr. Biol. 9: 1180-1182. [DOI] [PubMed] [Google Scholar]
- Wojcik E.J., Glover, D.M., and Hays, T.S. 2000. The SCF ubiquitin ligase protein slimb regulates centrosome duplication in Drosophila. Curr. Biol. 10: 1131-1134. [DOI] [PubMed] [Google Scholar]
- Wu G., Xu, G., Schulman, B.A., Jeffrey, P.D., Harper, J.W., and Pavletich, N.P. 2003. Structure of a β-TrCP1-Skp1-β-catenin complex: Destruction motif binding and lysine specificity of the SCFβ-TrCP1 ubiquitin ligase. Mol. Cell 11: 1445-1456. [DOI] [PubMed] [Google Scholar]
- Xiao Z., Chen, Z., Gunasekera, A.H., Sowin, T.J., Rosenberg, S.H., Fesik, S., and Zhang, H. 2003. Chk1 mediates S and G2 arrests through Cdc25A degradation in response to DNA-damaging agents. J. Biol. Chem. 278: 21767-21773. [DOI] [PubMed] [Google Scholar]
- Ye X., Wei, Y., Nalepa, G., and Harper, J.W. 2003. The cyclin E/Cdk2 substrate p220NPAT is required for S-phase entry, histone gene expression, and Cajal Body maintenance in human somatic cells. Mol. Cell. Biol. 23: 8586-8600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng Y. and Piwnica-Worms, H. 1999. DNA damage and replication checkpoints in fission yeast require nuclear exclusion of the Cdc25 phosphatase via 14-3-3 binding. Mol. Cell. Biol. 19: 7410-7419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng Y., Forbes, K.C., Wu, Z., Moreno, S., Piwnica-Worms, H., and Enoch, T. 1998. Replication checkpoint requires phosphorylation of the phosphatase Cdc25 by Cds1 or Chk1. Nature 395: 507-510. [DOI] [PubMed] [Google Scholar]
- Zhang X., Herring, C.J., Romano, P.R., Szczepanowska, J., Brzeska, H., Hinnebusch, A.G., and Qin, J. 1998. Identification of phosphorylation sites in proteins separated by polyacrylamide gel electrophoresis. Anal. Chem. 70: 2050-2059. [DOI] [PubMed] [Google Scholar]
- Zhao H., Watkins, J.L., and Piwnica-Worms, H. 2002. Disruption of the checkpoint kinase 1/cell division cycle 25A pathway abrogates ionizing radiation-induced S and G2 checkpoints. Proc. Natl. Acad. Sci. 99: 14795-14800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou B.B. and Elledge, S.J. 2000. The DNA damage response: Putting checkpoints in perspective. Nature 408: 433-439. [DOI] [PubMed] [Google Scholar]
- Zou L. and Elledge, S.J. 2003. Sensing DNA damage through ATRIP recognition of RPA-ssDNA complexes. Science 300: 1542-1548. [DOI] [PubMed] [Google Scholar]