Abstract
Calcium-transporting ATPases (Ca2+ pumps) are major players in maintaining calcium homeostasis in the cell and have been detected in all cellular organisms. Here, we report the identification of two putative Ca2+ pumps, M535L and C785L, encoded by chlorella viruses MT325 and AR158, respectively, and the functional characterization of M535L. Phylogenetic and sequence analyses place the viral proteins in group IIB of P-type ATPases even though they lack a typical feature of this class, a calmodulin-binding domain. A Ca2+ pump gene is present in 45 of 47 viruses tested and is transcribed during virus infection. Complementation analysis of the triple yeast mutant K616 confirmed that M535L transports calcium ions and, unusually for group IIB pumps, also manganese ions. In vitro assays show basal ATPase activity. This activity is inhibited by vanadate, but, unlike that of other Ca2+ pumps, is not significantly stimulated by either calcium or manganese. The enzyme forms a 32P-phosphorylated intermediate, which is inhibited by vanadate and not stimulated by the transported substrate Ca2+, thus confirming the peculiar properties of this viral pump. To our knowledge this is the first report of a functional P-type Ca2+-transporting ATPase encoded by a virus.
INTRODUCTION
P-type ion-transporting ATPases are essential molecules in eukaryotes and in most eubacteria and archaea. These ATP-hydrolysing enzymes are responsible for the primary transport of charged substrates, generally cations, across membranes. Typical of the P-type ATPase superfamily is the temporary conservation of ATP energy in the form of a phosphorylated enzyme intermediate (hence P-type) formed between the γ-phosphate of hydrolysed ATP and an invariant Asp-residue in a highly conserved sequence: SDKTGT[L/I/V/M][T/I/S] (Brini & Carafoli, 2009). This large family of primary transporters is divided into five major groups (I–V) on the basis of phylogenetic analyses and substrate specificity (Axelsen & Palmgren, 1998, 2001). All Ca2+ pumps described to date belong to group II, subgroups A and B. Type IIA Ca2+ pumps are localized primarily in endomembranes, have short N- and C-cytosolic termini and are not stimulated by calmodulin (CaM). They are found in eubacteria, archaea and eukaryotes (Axelsen & Palmgren, 2001). Type IIB Ca2+ pumps are localized both in the plasma membrane and in endomembranes and are stimulated by CaM binding to C- or N-cytosolic domains. They only exist in eukaryotes.
This manuscript describes two Ca2+-ATPase IIB members encoded by chlorella viruses, a group of viruses belonging to the family Phycodnaviridae. Chlorella viruses are large, icosahedral, plaque-forming, dsDNA viruses that replicate in certain unicellular, eukaryotic chlorella-like green algae. They contain an internal lipid bilayered membrane surrounded by an outer glycoprotein capsid (Van Etten, 2003; Wilson et al., 2009). The chlorella viruses have genomes as large as 370 kb that contain as many as 400 protein-encoding and 16 tRNA-encoding genes. Six chlorella virus genomes have been sequenced and about 80 % of the genes are common to all six viruses (Li et al., 1997; Fitzgerald et al., 2007a, b, c). Three of the sequenced viruses, PBCV-1, NY-2A and AR158, infect Chlorella NC64A; two, MT325 and FR483, infect Chlorella Pbi; and one, ATCV-1, infects Chlorella SAG 3.83 (Fitzgerald et al., 2007a, b, c). Annotation of the six genomes revealed that two viruses, MT325 and AR158, encode genes for putative Ca2+-ATPases. This manuscript describes the functional characterization of the Ca2+ pump encoded by virus MT325.
RESULTS
Sequence analysis
Annotation of six chlorella virus genomes revealed that two of them, MT325 and AR158, code for putative proteins that belong to the P-type superfamily subgroup IIB Ca2+-ATPases. Assignment to the P-type superfamily of ATPases is based on predicted membrane topology and sequence alignment of the two viral proteins with the well-known calcium pumps ACA8 from Arabidopsis thaliana (Bonza et al., 2000) and PMCA4b from Homo sapiens (James et al., 1988). The two viral proteins (Fig. 1a) are predicted to have 10 transmembrane domains (TM), a small loop between TM2 and TM3 and a large loop between TM4 and TM5 (Palmgren & Axelsen, 1998; Bonza et al., 2004). Sequence alignments of M535L and C785L with prototype Ca2+ pumps (Fig. 1b) highlight the conserved domain of the P-type superfamily, DKTGT, containing the aspartic acid residue that becomes phosphorylated during the catalytic cycle. Furthermore, M535L and C785L have several additional motifs characteristic of subgroup II (A and B) P-type ATPases. These motifs (in grey in Fig. 1b) include the PEGL sequence that plays a central role in energy transduction and the KGAPE sequence implicated in nucleotide binding (Møller, et al., 1996; Palmgren & Axelsen, 1998). Finally, a feature only found in type IIB ATPases exists in the viral proteins: one conserved putative-binding site for calcium, formed by residues E302 in TM4, N703 and D707 in TM6 (numbers refer to the M535L amino acid sequence; the residues are marked with asterisks in Fig. 1b).
Fig. 1.
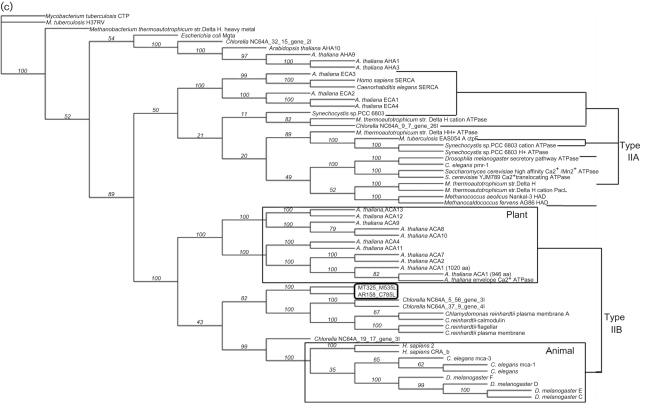
Predicted membrane topology, comparative alignment and phylogeny of chlorella virus Ca2+-transporting ATPases. (a) Hypothetical membrane topology of the viral Ca2+ pump. (b) Multiple sequence alignments performed with clustal w (1.82) of the deduced amino acid sequences of chlorella virus MT325 ORF M535L (NCBI reference no. ABT14089.1), chlorella virus AR181 ORF C785L (NCBI reference no. YP_001498866.1), and ACA8 (NCBI reference no. NP_200521.3) and PMCA4b (GenBank accession no. NM_001684) from A. thaliana and H. sapiens, respectively. Conserved residues characteristic for P-type ATPases type II are highlighted in grey. Residues corresponding to the single binding site for calcium found in type IIB Ca2+-ATPases are highlighted with asterisks. Bold lines indicate the ten transmembrane helices, TM1–TM10, predicted by the tmhmm program (www.cbs.dtu.dk/services/TMHMM/). (c) Maximum-likelihood tree of 60 P-type ATPase protein sequences. The phylogenetic tree was generated using the muscle alignment and phyml tree building programs within the Geneious Pro 4.7.5 software program. The Whelan and Goldman (WAG) amino acid substitution model was used to derive 100 bootstrap datasets (the transition/transversion ratio for DNA models and the gamma distribution parameter were estimated, proportion of invariable sites was zero and four substitution rate categories produced the illustrated unrooted tree; bootstrap values are shown).
M535L and C785L sequences are 64 % identical and 82 % similar to each other, 37 and 33 % identical, 56 and 50 % similar to ACA8 and PMCA4b, respectively. The cytosolic termini of the viral proteins are short (27 aa at the N terminus and 4–6 aa at the C terminus) and lack a CaM-binding domain typically located at the N terminus of plant members and at the C terminus of animal members.
Molecular phylogenetic analyses of the AR158_C785L and MT325_M535L gene products also support the relatedness of these molecules to members of the P-type IIB Ca2+-transporting ATPases (Drummond et al., 2008) (Fig. 1c). IIB Ca2+ pumps fall into two clades, one in higher plants and the other in animals (Axelsen & Palmgren, 1998; Bonza et al., 2004; Marchler-Bauer et al., 2009). The viral pumps clearly reside in the algal clade containing Chlorella and Chlamydomonas, more closely related to the animal than to the plant clade. Given the high similarity of the two viral proteins, further analysis was conducted only on M535L.
Presence of the m535l gene in other viruses and its expression during host infection
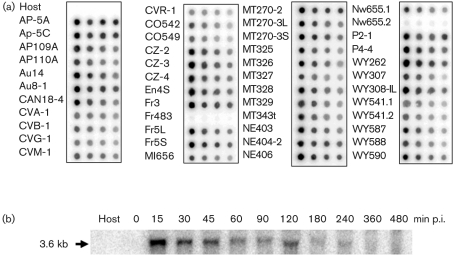
To determine if the Ca2+ pump gene is common among the chlorella viruses, genomic DNAs from 47 Chlorella Pbi viruses from diverse geographical regions were hybridized with an m535l probe (Fig. 2a). The probe hybridized strongly to 45 of 47 viruses. Virus Nw655.2 hybridized poorly with the probe and no hybridization occurred with virus Fr483. Virus Fr483 has been sequenced (Fitzgerald et al., 2007a) and it lacks a Ca2+-transporting ATPase homologue. The strength of the hybridization signal differs among the 45 positive viruses (Fig. 2a), suggesting there are significant nucleotide substitutions among these genes. The m535l probe did not hybridize to the virus MT325 host, Chlorella Pbi DNA.
Fig. 2.
Presence of the Ca2+ ATPase gene in other chlorella viruses and transcription pattern of m535l during virus MT325 infection of its host Chlorella Pbi. (a) Dot blot hybridization of m535L to DNA isolated from 47 viruses that infect Chlorella Pbi. The spots contain 1.0, 0.5, 0.25 and 0.125 μg DNA (left to right). The radioactive probe includes ∼300 bp of the large loop preceding the TM5 of the m535l gene (Fig. 1a). Host: Chlorella Pbi. No hybridization signal was detected with viruses Fr483 and Nw655.2. The virus Fr483 genome has been sequenced and it lacks a Ca2+-ATPase homologuous gene (Fitzgerald et al., 2007a). (b) Transcription of m535l in the host Chlorella Pbi infected with virus MT325. Total RNA isolated from uninfected (Host) and infected Chlorella Pbi sampled at the indicated times (min p.i.) were hybridized with the m535l probe used in Fig. 2(a).
To determine if the m535l gene is expressed during viral infection and at which stage, the probe was hybridized to total RNA extracted from MT325-infected cells. The probe hybridizes to a single transcript of ∼3.6 kb that is an appropriate size for a 871 aa protein (Fig. 2b). Hybridization is strongest at 15 min post-infection (p.i.) and decreases slowly with time (Fig. 2b). Assuming the replication cycle of virus MT325 resembles that of the prototype chlorella virus PBCV-1 (Van Etten, 2003), m535l is an early gene, i.e. it is expressed prior to virus DNA synthesis. Proteomic analyses did not detect M535L or C785 in their respective virions (D. D. Dunigan and others, unpublished data). This result is consistent with the finding that m535l is an early gene because proteins packaged in nascent virions are usually transcribed at later stages of infection. The fact that the calcium transporter gene is present in most of the Pbi viruses and that it is transcribed during virus infection suggests the protein might serve a function in virus replication. Consequently, we tested the gene product for functional activity.
Heterologous expression of m535l in Saccharomyces cerevisiae triple mutant K616
The viral m535l gene was expressed in S. cerevisiae mutant K616 that lacks all endogenous Ca2+-ATPases (Cunningham & Fink, 1994). Consequently, K616 does not grow in Ca2+-depleted medium unless it is transformed with a gene encoding a fully active Ca2+ pump (Geisler et al., 2000; Bonza et al., 2004; Baekgaard et al., 2006). The m535l gene was cloned into yeast vector pYES2-NTC, which adds an N-terminal His tag to the recombinant protein. Protein expression in high calcium, a non-selective condition, was evaluated by Coomassie staining and Western blot and compared to that of control yeast transformed with the empty vector. Coomassie staining of the proteins in the microsomal fraction reveals a strong band with the expected molecular mass of the M535L polypeptide (96.3 kDa) (data not shown). Western blot analysis with antiserum against the His-tag clearly identifies this band as the m535l gene product (data not shown).
Complementation of the K616 phenotype
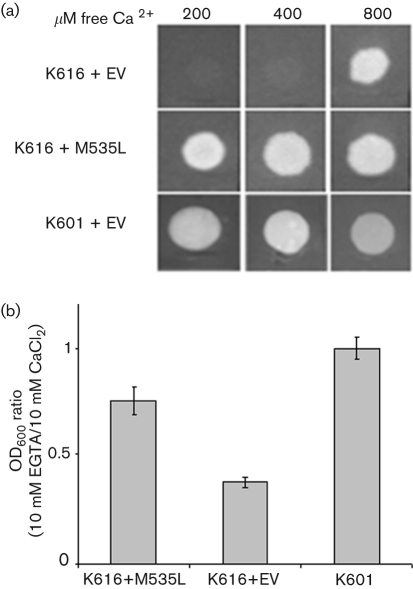
To determine if the expressed protein was functional, we incubated K616 transformed with m535l in low external calcium concentrations. When the test was performed on solid medium (Fig. 3a), yeast growth occurred at calcium concentrations as low as 200 μM, a non-permissive condition for the negative control (K616 transformed with the empty vector). As a positive control, we used yeast K601, which grows in low (nM) external calcium concentrations. Even though m535l clearly supports K616 growth in micromolar calcium concentrations, we never observed complementation at nanomolar concentrations, a result expected for high-affinity calcium pumps (Geisler et al., 2000; Kabala & Klobus, 2005). Therefore, we used a different complementation test in which yeast growth was evaluated in liquid culture that provides better aeration of the medium. Protein expression was induced in mid-exponential cultures by substituting fresh medium containing either 10 mM CaCl2 or 10 mM EGTA; the latter medium contains nominal nanomolar concentrations of free calcium. Yeast growth was evaluated by OD600 measurements 24 h after induction and the results plotted as the ratio of OD600 in EGTA over CaCl2 (Fig. 3b). The M535L protein clearly supports growth in nanomolar calcium, allowing yeast cells to grow almost as fast as in millimolar calcium (OD600 ratio EGTA/CaCl2=0.75±0.07). In contrast, poor growth occurs in the negative control at low calcium concentrations (OD600 ratio EGTA/CaCl2=0.37±0.02). From these experiments we conclude that M535L forms a functional high-affinity calcium transporter in S. cerevisiae.
Fig. 3.
Complementation of the K616 phenotype by m535l. (a) Complementation on solid medium. The Ca2+ ATPase-deficient yeast strain K616 transformed with pYES2-m535l and with the empty pYES2 vector (EV) were grown at increasing free Ca2+ concentrations (200–800 μM). The K601 strain transformed with empty pYES2 vector served as a positive control. Results are from one of three independent experiments. (b) Complementation in liquid culture. Cells were grown 24 h in solutions containing 10 mM EGTA or 10 mM CaCl2 (selective and non-selective medium, respectively). Results are expressed as the ratio of OD600 of the two cultures (10 mM EGTA/10 mM CaCl2) and are the mean of four independent experiments; bars represent the standard error.
Selectivity of M535L
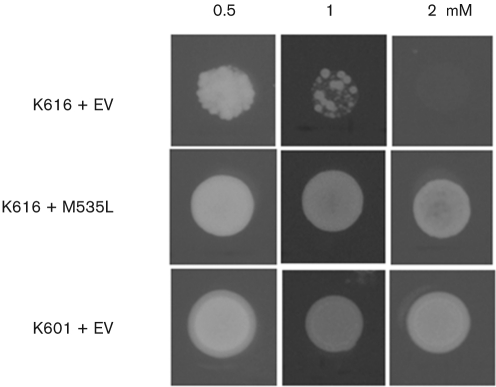
To determine the ion selectivity of M535L, we tested the ability of the protein to rescue the K616 phenotype on solid medium supplemented with either 0.5, 1.0 or 2.0 mM Mn2+; at these concentrations this ion is toxic to K616 that lacks the Ca2+/Mn2+ pump Pmr1, which removes excess Mn2+ from the cytoplasm (Cunningham & Fink, 1994). While K616 cells transformed with the empty vector barely grew on 1 mM Mn2+ and died on 2 mM Mn2+, cells expressing M535L survived in Mn2+ concentrations as high as 2 mM and their growth was indistinguishable from the control strain K601 (Fig. 4). The finding that M535L protects K616 from excess Mn2+ suggests that the viral protein transports manganese in addition to calcium. This behaviour differs from other IIB type Ca2+ pumps and resembles IIA Ca2+ pumps ECA1 or ECA3 from A. thaliana or LCA1 from tomato that confer tolerance to Mn2+ (Wu et al., 2002; Mills et al., 2008; Johnson et al., 2009). However, in contrast to ECA1 and ECA3, M535L does not reverse the toxic effect of Zn2+ (results not shown).
Fig. 4.
M535L expression restores growth of yeast strain K616 on Mn2+-supplemented medium. K616 cells transformed with pYES2-m535l and with the empty pYES2 vector (EV) were grown on solid media containing increasing concentrations of MnCl2 (0.5–2 mM). Strain K601 transformed with empty pYES2 vector served as a positive control. Results are from one of four independent experiments.
ATPase activity
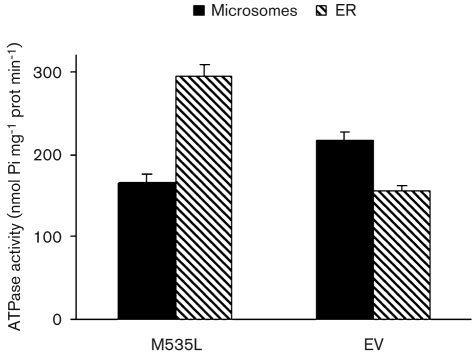
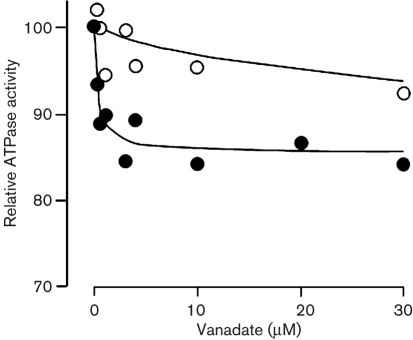
ATPase activity was assayed by monitoring Ca2+-dependent ATP hydrolysis in yeast membrane fractions (Bonza et al., 2004). We initially measured M535L Ca2+-dependent ATPase activity in crude microsomal membranes but, under these conditions, activity was barely detectable. The low Ca2+-dependent ATPase activity of the viral transporter did not increase by systematically modifying the assay conditions including: substrate concentrations, pH and addition of CaM (results not shown). To improve the specific activity of M535L, microsomes from K616 cells expressing M535L were fractionated on a sucrose density gradient to separate the viral protein from endogenous ATPases, such as the yeast plasma membrane proton pump. A strong 95 kDa band corresponding to the viral protein was detected at the 18–33 % sucrose interface (Fig. 5a), which corresponds to the ER-enriched fraction. In contrast, the endogenous yeast plasma membrane H+-ATPase was located primarily in a heavier fraction (33–45 %) corresponding to the plasma membrane (Fig. 5b). P-type ATPases overexpressed in yeast and in particular Ca2+-ATPases overexpressed in K616 cells are typically located in the endoplasmic reticulum (ER) fraction (Geisler et al., 2000; Sze et al., 2000; Bonza et al., 2004; Fusca et al., 2009). The ER-enriched fraction was then assayed for ATPase activity. Although, no Ca2+- or Mn2+-dependent activity was detected (results not shown), a 70 % increase in ATPase-specific activity occurred between the ER fraction purified from cells expressing M535L versus microsomes (Fig. 6). This enrichment parallels the strong M535L accumulation detected by Western blotting (Fig. 5a). In contrast, ATPase activity in the ER fraction from yeast transformed with the empty vector decreased by 30 %, a result that can be explained by the separation of the plasma membrane, containing the H+-ATPase, from the ER membranes. The ATPase activity of ER-enriched membranes expressing m535l was inhibited by vanadate about three times more than that from control membranes purified from cells transformed with the empty vector (Fig. 7).
Fig. 5.
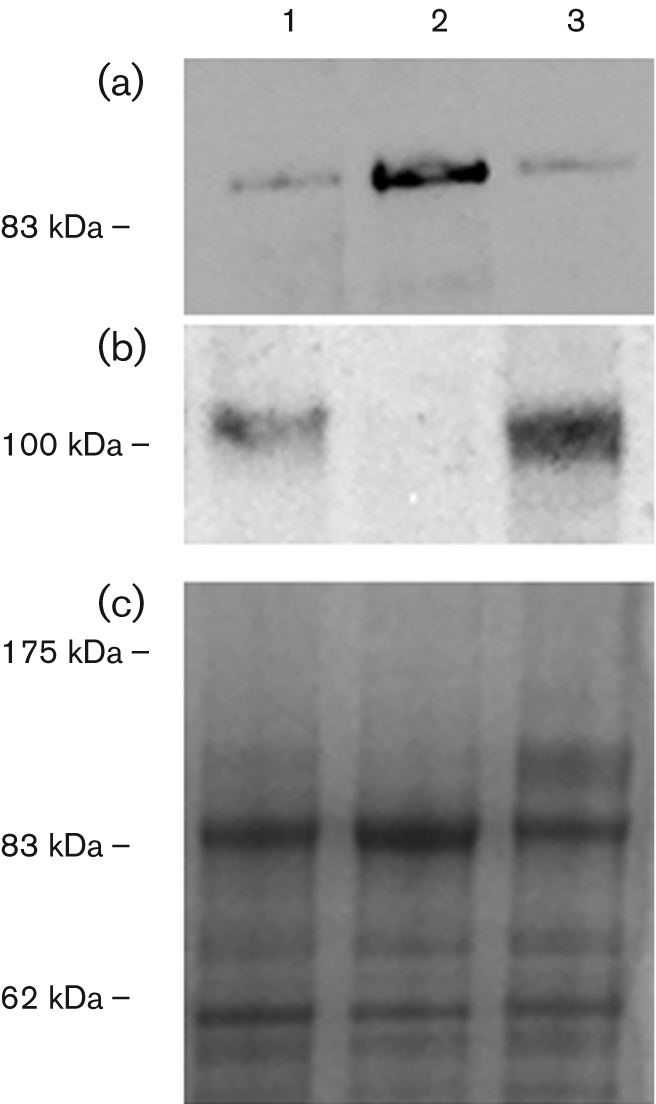
M535L protein enrichment in the ER fraction. Western blot analysis of different membrane fractions obtained from K616 cells expressing M535L. Microsomes isolated from K616 cells expressing M535L (lane 1) were loaded onto a sucrose step gradient. After overnight centrifugation, fractions corresponding to the 18–33 % interface (lane 2) and 33–45 % interface (lane 3) were collected and subjected to SDS-PAGE. Western analysis was performed with antisera against the His-tag (panel a, 4 μg proteins) or against the PM H+-ATPase of Neurospora crassa (panel b, 2 μg proteins). (c) Coomassie blue staining of the proteins.
Fig. 6.
Total ATPase activity in microsomes (black bars) and in ER fractions purified from cells expressing m535l or from cells transformed with the empty vector (dashed bars). Results (±sem) are from three gradients performed on different yeast cultures.
Fig. 7.
Vanadate inhibition of ATPase activity from an ER-enriched fraction purified from K616 cells either expressing M535L (closed symbols) or transformed with the empty pYES2 vector (open symbols). ATPase activity, assayed in the presence of increasing concentrations of vanadate, is expressed as a per cent of the activity measured in the absence of vanadate (350 nmol Pi mg−1 protein min−1 for M535L and 160 nmol Pi mg−1 protein min−1 for empty vector).
Since vanadate specifically inhibits formation of the phosphorylated intermediate during the catalytic cycle of P-type ATPases (Møller et al., 1996), we looked for such an intermediate in M535L-expressing cells. ER-enriched membranes from control and M535L-expressing yeast cells were exposed to [32P]ATP in the presence and in the absence of vanadate. The predicted M535L phosphorylated intermediate was detected on an acidic SDS-PAGE (Fig. 8). Two bands are clearly visible: a band, at about 100 kDa, which most likely is the plasma membrane yeast proton pump and is also present in control membranes (Fig. 8, lane 4). A second band, migrating at about 95 kDa, is about the expected size of the viral protein; this band only appears in membranes expressing M535L (Fig. 8, lanes 1–3). The three lanes show the level of phosphorylation obtained with [32P]ATP in the presence of Mg2+ and Ca2+ (Fig. 8, lane 1), Mg2+, Ca2+ and vanadate (Fig. 8, lane 2), and Mg2+ alone (Fig. 8, lane 3). The formation of the phosphorylated intermediate is strongly inhibited by vanadate; surprisingly, the formation of the phosphorylated intermediate does not require Ca2+ (Fig. 8, compare lanes 1 and 3). This result is unique among Ca2+-transporting ATPases and agrees with our inability to measure a Ca2+-dependent ATPase activity in vitro.
Fig. 8.
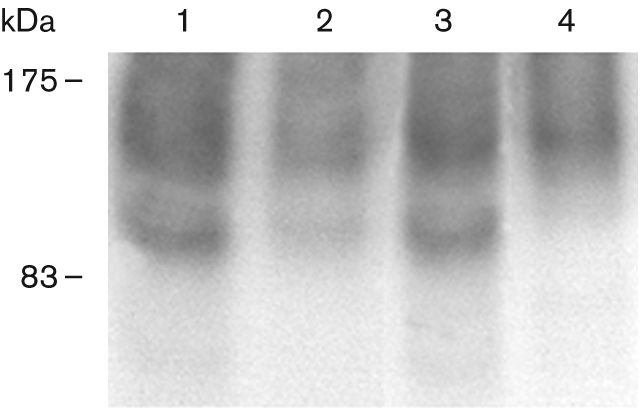
M535L forms a phosphorylated intermediate during its catalytic cycle. Phosphorylation with [γ-32]ATP of ER-enriched membranes from K616 transformed with m535l was performed in the presence of either MgCl2 and CaCl2 (lane 1), MgCl2, CaCl2 and vanadate (lane 2) or MgCl2 alone (lane 3). As a negative control, ER membrane proteins from K616 transformed with the empty vector were used (lane 4). Acidic SDS-PAGE, all lanes were loaded with 40 μg protein. Numbers on the left indicate the size of the molecular mass marker in kDa.
DISCUSSION
In this manuscript we provide evidence that some chlorella viruses encode P-type ATPases that are expressed during virus replication and we show that one of them, M535L from virus MT325, is functional. Phylogenetic analysis indicates that the viral proteins belong to the IIB subgroup of Ca2+-transporting ATPases. Sequence comparison with plant and animal proteins identified several conserved motifs and only one putative Ca2+-binding site, characteristic of IIB Ca2+ pumps (Brini & Carafoli, 2009). The two viral proteins have short cytosolic termini and lack the regulatory domains present at the N- or C-termini of plant and animal IIB Ca2+-transporting ATPases (Kabala & Klobus 2005; Di Leva et al., 2008). The M535L protein is functional because it complements mutant yeast K616 growth on Ca2+-depleted medium. Complementation of the K616 phenotype clearly indicates that M535L is a fully active Ca2+ pump. In fact, only expression of non-autoinhibited plant Ca2+-ATPases support K616 growth on Ca2+-depleted medium; expression of functional, but autoinhibited IIB pumps do not (Geisler et al., 2000; Sze et al., 2000; Fusca et al., 2009). M535L also complements K616 growth in toxic concentrations of Mn2+, indicating that the protein transports both calcium and manganese. These apparently conflicting observations can be reconciled because transformation of yeast cells with the functional viral pump allows cell survival in low external Ca2+ and high external Mn2+ for different reasons. Expression of a functional calcium pump, presumably in the ER, allows the yeast mutant to survive low external Ca2+ because it provides a high-affinity mechanism for pumping the (very low) Ca2+ ions present in the cytosol into its internal stores. Replenishing stores with Ca2+ is essential for yeast cell survival and is related to signal transduction. On the other hand, when the yeast cells are grown in high external Mn2+, the presence of a pump in the ER that transports Mn2+ ions efficiently allows the yeast cells to survive because it removes the toxic Mn2+ ions from the cytosol and accumulates them in the internal stores.
The ability to transport Mn2+, in addition to Ca2+, is a property of pumps in the secretory pathway (SPCA) (Brini & Carafoli, 2009). In plants that lack SPCA pumps, only Ca2+-ATPases belonging to type IIA subgroup, such as ECA1 and ECA3 from A. thaliana and LCA1 from tomato, function as Ca2+/Mn2+ pumps (Wu et al., 2002; Mills et al., 2008; Li et al., 2008; Johnson et al., 2009). The best characterized Ca2+/Mn2+-transporting pump is PMR1, a yeast protein localized in the Golgi apparatus. In PMR1, the Mn2+ selectivity is defined by residues Q783 in TM6 and V335 in TM4 (Brini & Carafoli, 2009). These residues are not conserved in M535L; furthermore, the viral protein has slightly less resemblance to PMR1 (30 % aa identity, 49 % similarity) than to plant and animal IIB Ca2+-ATPases (see results). Therefore, to our knowledge, this is the first report of a type IIB pump that transports both calcium and manganese.
No convincing Ca2+-dependent or Mn2+-dependent ATPase activity was detected in the in vitro experiments. However, vanadate-sensitive ATPase activity was enriched about 70 % in the ER fraction purified from yeast cells expressing m535l and this enrichment paralleled M535L accumulation in the ER, the typical cellular location of plant Ca2+-ATPases expressed in yeast K616 (Bonza et al., 2004). Moreover, M535L forms a vanadate-sensitive phosphorylated intermediate. The formation of the phosphorylated intermediate also occurs in the presence of MgCl2 alone, suggesting that M535L also transports magnesium. Since the presence of Mg2+ is unavoidable in the ATPase assay, this could explain the independence of M535L ATPase activity from exogenous Ca2+ or Mn2+. The presumption that M535L is a functional protein is supported by the finding that the gene is expressed during viral replication. In addition, the gene is present in 45 of 47 viruses that infect Chlorella Pbi. The common, but not universal, presence of the gene in the chlorella viruses suggests that the function of the protein is auxiliary, but not essential for virus infection/replication. The conclusion of an auxiliary function is supported by the fact that the gene is not present in all chlorella viruses that infect different hosts. Out of four other sequenced viruses that infect either Chlorella NC64A (three viruses) or Chlorella SAG 3.83 (one virus), only one contained the gene (virus AR158 that infects Chlorella NC64A). Again these data suggest that the activity of the protein is not essential; presumably the predecessor chlorella virus had the gene and some of the viruses lost the gene with time without losing their infectivity. However, we cannot eliminate the possibility that the gene was acquired independently by the viruses on two occasions.
The evidence that M535L is functional together with its transcription during infection suggests that the protein functions as an ATP-driven ion pump during the virus infection/replication cycle. Altering ion conducting membrane proteins during virus replication is a common mechanism to change the ion milieu in host cells or change the electrical properties of membranes. Several genes encoding membrane transport proteins have been described in the chlorella viruses, including ion channels, aquaglyceroporins and transporters (Plugge et al., 2000; Kang et al., 2003; Gazzarrini et al., 2004, 2006). While the role(s) of the aquaglyceroporin and of the Ca2+-transporting ATPase are still unknown, there is circumstantial evidence that the potassium ion channel is required early during virus infection. The present study does not define the role of the virus ATPase. However, expression studies indicate that the gene is transcribed early during virus replication, suggesting the protein fulfils an important function in Ca2+ homeostasis during infection. This speculation is not unreasonable because Ca2+ is an important messenger in cells and many enzymes in eukaryotes are sensitive to Ca2+. M535L function could be important under the non-physiological conditions which probably occur during infection, e.g. altered ion concentrations, an alkaline pH, etc.
The presence of a Ca2+-ATPase in some chlorella viruses is also interesting from an evolutionary point of view. To date only a few green algae have been sequenced and annotated, including one of the virus hosts, Chlorella NC64A. Chlorella NC64A and another green alga, Chlamydomonas reinhardtii, as well as others not listed in Fig. 1(c), encode several IIB type Ca2+ pumps and all of them, like the viral ones, group with the animal clade. This is surprising because green algae are considered to be ancestors of higher plants. One possible explanation for this unexpected finding is that higher plants have lost these genes through evolution. Another explanation is that algae acquired these genes after separation from higher plants. The latter hypothesis is not unreasonable because presumably the family Phycodnaviridae of viruses exclusively infect algae; higher plants are not hosts. Hence, the phycodnaviruses or their ancestors may have shuttled genes between the animal and plant kingdoms. This is reasonable because the phycodnaviruses have a common ancestor with several other large DNA viruses including poxviruses, iridoviruses, asfarviruses, ascoviruses and mimiviruses, referred to as nuclear, cytoplasmic and large DNA viruses (NCLDV). Accumulating evidence indicates that the NCLDVs have a long evolutionary history, possible dating from the time eukaryotes diverged from prokaryotes (2–3 billion years ago) (Yutin et al., 2009).
METHODS
Phylogenetic analyses.
A blastp search with the MT325_M535L (ABT14089.1) amino acid sequence was conducted using the NCBI non-redundant protein sequence database with the default settings. In addition, the bait sequence was blasted against genomes of A. thaliana (taxid: 3701), Escherichia coli (taxid: 5620), Mycobacterium tuberculosis (taxid: 1773), Synechocystis PCC6803 (taxid: 1148), Methanobacterium thermoautotrophicum str. Delta H (taxid: 187420), Methanococci (taxid: 183939), S. cerevisiae (taxid: 4932), Caenorhabditis elegans (taxid: 6239), Drosophila melanogaster (taxid: 7227) and H. sapiens (taxid: 9606). Similar analyses were carried out using the homologous sequence from AR158_C785L (YP_001498866.1). An unrooted maximum-likelihood tree of 60 P-type ATPase amino acid sequences from the above organisms was generated based on sequence alignment by using muscle and phyml in the Geneious Pro 4.7.5 software program (Drummond et al., 2008). The Whelan and Goldman (WAG) amino acid substitution model was used to derive 100 bootstrap datasets [the transition/transversion ratio for DNA models and the gamma distribution parameter were estimated, proportion of invariable sites was zero and four substitution rate categories produced the tree in Fig. 1(c); bootstrap values are shown].
Dot blot hybridization.
DNA was isolated from Chlorella Pbi and 47 viruses that infect Chlorella Pbi, transferred to nylon membrane (Osmonics) and cross-linked by UV light as described previously (Graves et al., 2001). A 281 bp highly conserved domain in m535l, located from 1744 to 2024 bp, was amplified by PCR as a hybridization probe. The probe was labelled with Random Primers DNA labelling kit (Invitrogen). The dot blot membrane was pre-hybridized in 6× SSC (standard sodium citrate), 5× Denhardt's reagent, 0.5 % SDS and denatured salmon sperm DNA at 68 °C for 1 h. The denatured dsDNA probe, labelled with [32P]dATP was added to the membrane, and hybridized at 68 °C for 1 h. The membrane was washed in 2× SSC, 0.5 % SDS at room temperature for 5 min, 2× SSC, 0.1 % SDS at room temperature for 15 min, twice, and 0.1× SSC, 0.5 % SDS at 65 °C for 2 h, and finally subjected to signal detection with a Storm Phosphorimager and ImageQuant software (Molecular Dynamics).
Northern hybridization.
Three×109 Chlorella Pbi cells were collected at various times after virus MT325 infection (m.o.i. of 5), frozen in liquid nitrogen, and stored at −80 °C. RNA was extracted with TRIzol reagent (Invitrogen), denatured with formaldehyde, separated on a 1.5 % agarose gel, and then transferred to a nylon membrane. [32P]dATP labelled probe was prepared as in the dot blot hybridization experiment. The membrane was pre-hybridized in 20 ml Church's buffer (1 mM EDTA, pH 8.0, 0.5 M NaPO4, 7 % SDS) for 1 h at 65 °C, hybridized with fresh Church's buffer and denatured probe for 16 h. After hybridization, the membrane was washed twice with 0.1× SSC, 0.1 % SDS, first time for 30 min, second time for 15 min. The signal detection was the same as in the dot blot hybridization.
Cloning.
Amplification of the m535l gene from virus MT325 DNA and addition of XhoI and XbaI restriction sites were done by standard PCR methods. Forward primer: 5′-CAACTCGAGTAAAAGATGTCCGCGTTTAAAGC-3′, reverse primer: 5′-CAATCTAGATTATTAGATGTCATCATTGTTGA-3′. The 2643 bp PCR product was cloned into XhoI and XbaI sites in a modified version of the yeast expression vector pYES2-NTC (Invitrogen). This vector has a shorter version (MGHHHHHH) of the original N-terminal tag and contains a uracil gene for selection and a galactose inducible promoter upstream of the multiple cloning site for induction of protein expression in S. cerevisae.
Yeast transformation and growth media.
S. cerevisiae strain K616 [MATα pmr1 : : HIS3 pmc1 : : TRP1 cnb1 : : LEU2, ade2, ura3 (Cunningham & Fink, 1994)] was used to express M535L (Bonza et al., 2004). K616, transformed with an empty pYES2 vector, served as a negative control. Yeast strain K601/W3031A (MATα leu2, his3, ade2 and ura3) transformed with the empty pYES2 vector served as a positive control. The transformants were selected for uracil prototrophy on a synthetic medium lacking uracil (SC-URA) supplemented with 2 % (w/v) glucose. For complementation studies, single URA-positive colonies were grown in SC-URA medium containing 2 % (w/v) glucose and 10 mM CaCl2, pelletted and washed twice with sterile water prior to protein induction in selective conditions. All media were supplemented with 50 mM succinic acid/Tris-Cl pH 5.5 and 0.7 % (w/v) yeast nitrogen base.
Complementation in liquid culture at nM Ca2+ concentrations.
Cells were diluted fourfold with either SC-URA medium, 2 % (w/v) galactose, 1 % (w/v) raffinose, 10 mM EGTA or SC-URA medium, 2 % (w/v) galactose, 1 % (w/v) raffinose, 10 mM CaCl2 (selective and non-selective medium, respectively) and then grown for 24 h by shaking at 30 °C.
Complementation on solid medium.
Five microlitre drops of yeast glucose culture (A600=1) was spotted on solid SC-URA plates containing either 2 % (w/v) galactose, 1 % (w/v) raffinose and 5 mM EGTA with increasing free Ca2+ concentrations (200–800 μM) or without EGTA but supplemented with increasing concentrations of MnCl2 (0.5, 1 or 2 mM) and incubated at 30 °C for 3–5 days. The free Ca2+ concentrations were calculated using the Kd Ca2+/EGTA at pH 5.5 (7.12×10−4).
Isolation of microsomes and ER-enriched fraction.
Pelleted yeast cells were homogenized and microsomes were harvested as reported previously (Bonza et al., 2004). Yeast membranes were purified by sucrose gradient centrifugation as described previously (Meneghelli et al., 2008). The membrane fraction was frozen at −80 °C until use. Protein concentration was determined using the Bio-Rad assay with γ-globulin as a standard.
Electrophoresis and immunoblotting analysis.
SDS-PAGE was performed as described previously (Bonza et al., 1998). Immunodetection was performed with a monoclonal anti-polyhistidine antibody (Sigma-Aldrich) or with antiserum against the PM H+-ATPase of Neurospora crassa as described previously (Hager et al., 1986).
ATPase assays.
ATPase activity was measured as MgATP hydrolysis. Samples (4 μg membrane proteins) were incubated at 25 °C for 1 h, during which the reaction proceeds linearly, in a reaction buffer containing 40 mM BTP-HEPES pH 7, 5 mM ATP, 7 mM MgSO4. Sensitivity to vanadate was tested in a reaction buffer containing 50 mM KCl and increasing concentrations of Na3VO4 (0–100 μM). Released inorganic phosphate was determined colourimetrically (De Michelis & Spanswick, 1986). Assays were performed at least three times with three replicates.
Phosphorylated intermediate formation.
The formation of 32P-phosphorylated intermediate was performed as reported in Rasi-Caldogno et al. (1995) with minor changes. The reaction mixture (0.1 ml final volume) contained 50 mM KCl, 10 mM BTP-HEPES pH 7, 0.2 μM [γ-32P]ATP [250 μCi (9.25 MBq) nmol−1], 12 μM MgSO4; 100 μM CaCl2 or 100 μM Na3VO4 were included as indicated in the text. The reaction was started by adding 100 μg ER-membrane proteins from K616-expressing M535L or transformed with empty pYES2 vector. After 60 s the reaction was stopped by adding 1.7 ml ice-cold 12 % trichloroacetic acid, 1 mM ATP and 50 mM NaH2PO4, incubated for 1 h at 0 °C and centrifuged for 1 h at 4 °C at 20 000 g. Pellets were resuspended with protease inhibitors, solubilized and separated by acidic SDS-PAGE on a 5.6 % polyacrylamide gel (40 μg protein) as described in Rasi-Caldogno et al. (1995). For 32P autoradiography, the dryed gel was exposed to Kodak Biomax MS film for 3 days at −80 °C.
Acknowledgments
We thank Dr Laura Luoni (Dipartimento di Biologia, Università degli Studi di Milano) for technical assistance. This investigation was supported in part by Public Health Service grant GM32441 (J. L. V. E.) and National Institutes of Health grant P20RR15635 from the COBRE program of the National Center for Research Resources (J. L. V.E.), by MAE, Italian Minister of Foreign Affairs in the frame of the USA significant bilateral projects and by EU FP7 project European Drug Initiative on Channels and Transporters (grant number 201924) (A. M.). G. L. was supported by a Ruth L. Kirschstein National Research Service Award 1 T32 AI060547 from the National Institute of Allergy and Infectious Diseases.
References
- Axelsen, K. B. & Palmgren, M. G. (1998). Evolution of substrate specificities in the P-type ATPase superfamily. J Mol Evol 46, 84–101. [DOI] [PubMed] [Google Scholar]
- Axelsen, K. B. & Palmgren, M. G. (2001). Inventory of the superfamily of P-type ion pumps in Arabidopsis. Plant Physiol 126, 696–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baekgaard, L., Luoni, L., De Michelis, M. I. & Palmgren, M. G. (2006). The plant plasma membrane Ca2+ pump ACA8 contains overlapping as well as physically separated autoinhibitory and calmodulin-binding domains. J Biol Chem 281, 1058–1065. [DOI] [PubMed] [Google Scholar]
- Bonza, C., Carnelli, A., Ida De Michelis, M. & Rasi-Caldogno, F. (1998). Purification of the plasma membrane Ca2+-ATPase from radish seedlings by calmodulin-agarose affinity chromatography. Plant Physiol 116, 845–851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonza, M. C., Morandini, P., Luoni, L., Geisler, M., Palmgren, M. G. & De Michelis, M. I. (2000). At-ACA8 encodes a plasma membrane-localized calcium-ATPase of Arabidopsis with a calmodulin-binding domain at the N terminus. Plant Physiol 123, 1495–1506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonza, M. C., Luoni, L. & De Michelis, M. I. (2004). Functional expression in yeast of an N-depleted form of At-ACA8, a plasma membrane Ca2+-ATPase of Arabidopsis thaliana, and characterization of a hyperactive mutant. Planta 218, 814–823. [DOI] [PubMed] [Google Scholar]
- Brini, M. & Carafoli, E. (2009). Calcium pumps in health and disease. Physiol Rev 89, 1341–1378. [DOI] [PubMed] [Google Scholar]
- Cunningham, K. W. & Fink, G. R. (1994). Calcineurin-dependent growth control in Saccharomyces cerevisiae mutants lacking PMC1, a homolog of plasma membrane Ca2+ ATPases. J Cell Biol 124, 351–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Michelis, M. I. & Spanswick, R. M. (1986). H-pumping driven by the vanadate-sensitive ATPase in membrane vesicles from corn roots. Plant Physiol 81, 542–547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Leva, F., Domi, T., Fedrizzi, L., Lim, D. & Carafoli, E. (2008). The plasma membrane Ca2+ ATPase of animal cells: structure, function and regulation. Arch Biochem Biophys 476, 65–74. [DOI] [PubMed] [Google Scholar]
- Drummond, A. J., Ashton, B., Cheung, M., Heled, J., Kearse, M., Moir, R., Stones-Havas, S. & Wilson, A. (2008). Geneious Pro v4.5.5. Available from http://wwwgeneious.com.
- Fitzgerald, L. A., Graves, M. V., Li, X., Feldblyum, T., Hartigan, J. & Van Etten, J. L. (2007a). Sequence and annotation of the 314-kb MT325 and the 321-kb FR483 viruses that infect Chlorella Pbi. Virology 358, 459–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald, L. A., Graves, M. V., Li, X., Feldblyum, T., Nierman, W. C. & Van Etten, J. L. (2007b). Sequence and annotation of the 369-kb NY-2A and the 345-kb AR158 viruses that infect Chlorella NC64A. Virology 358, 472–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald, L. A., Graves, M. V., Li, X., Hartigan, J., Pfitzner, A. J., Hoffart, E. & Van Etten, J. L. (2007c). Sequence and annotation of the 288-kb ATCV-1 virus that infects an endosymbiotic chlorella strain of the heliozoon Acanthocystis turfacea. Virology 362, 350–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fusca, T., Bonza, M. C., Luoni, L., Meneghelli, S., Marrano, C. A. & De Michelis, M. I. (2009). Single point mutations in the small cytoplasmic loop of ACA8, a plasma membrane Ca2+-ATPase of Arabidopsis thaliana, generate partially deregulated pumps. J Biol Chem 284, 30881–30888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gazzarrini, S., Kang, M., Van Etten, J. L., Tayefeh, S., Kast, S. M., DiFrancesco, D., Thiel, G. & Moroni, A. (2004). Chlorella virus MT325 encodes water and potassium channels that interact synergistically. J Biol Chem 279, 28443–28449.15105432 [Google Scholar]
- Gazzarrini, S., Kang, M., Epimashko, S., Van Etten, J. L., Dainty, J., Thiel, G. & Moroni, A. (2006). Chlorella virus MT325 encodes water and potassium channels that interact synergistically. Proc Natl Acad Sci U S A 103, 5355–5360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geisler, M., Frangne, N., Gomès, E., Martinoia, E. & Palmgren, M. G. (2000). The ACA4 gene of Arabidopsis encodes a vacuolar membrane calcium pump that improves salt tolerance in yeast. Plant Physiol 124, 1814–1827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graves, M. V., Bernadt, C. T., Cerny, R. & Van Etten, J. L. (2001). Molecular and genetic evidence for a virus-encoded glycosyltransferase involved in protein glycosylation. Virology 285, 332–345. [DOI] [PubMed] [Google Scholar]
- Hager, K. M., Mandala, S. M., Davenport, J. W., Speicher, D. W., Benz, E. J., Jr & Slayman, C. W. (1986). Amino acid sequence of the plasma membrane ATPase of Neurospora crassa: deduction from genomic and cDNA sequences. Proc Natl Acad Sci U S A 83, 7693–7697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James, P., Maeda, M., Fischer, R., Verma, A. K., Krebs, J., Penniston, J. T. & Carafoli, E. (1988). Identification and primary structure of a calmodulin binding domain of the Ca2+ pump of human erythrocytes. J Biol Chem 263, 2905–2910. [PubMed] [Google Scholar]
- Johnson, N. A., Liu, F., Weeks, P. D., Hentzen, A. E., Kruse, H. P., Parker, J. J., Laursen, M., Nissen, P., Costa, C. J. & other authors (2009). A tomato ER-type Ca2+-ATPase, LCA1, has a low thapsigargin-sensitivity and can transport manganese. Arch Biochem Biophys 481, 157–168. [DOI] [PubMed] [Google Scholar]
- Kabala, K. & Klobus, G. (2005). Plant Ca2+-ATPases. Acta Physiol Plant 27, 559–574. [Google Scholar]
- Kang, M., Moroni, A., Gazzarrini, S. & Van Etten, J. L. (2003). Are chlorella viruses a rich source of ion channel genes? FEBS Lett 552, 2–6. [DOI] [PubMed] [Google Scholar]
- Li, Y., Lu, Z., Sun, L., Ropp, S., Kutish, G. F., Rock, D. L. & Van Etten, J. L. (1997). Analysis of 74 kb of DNA located at the right end of the 330 kb chlorella virus PBCV-1 genome. Virology 237, 360–377. [DOI] [PubMed] [Google Scholar]
- Li, X., Chanroj, S., Wu, Z., Romanowsky, S. M., Harper, J. F. & Sze, H. (2008). A distinct endosomal Ca2+/Mn2+ pump affects root growth through the secretory process. Plant Physiol 147, 1675–1689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchler-Bauer, A., Anderson, J. B., Chitsaz, F., Derbyshire, M. K., DeWeese-Scott, C., Fong, J. H., Geer, L. Y., Geer, R. C., Gonzales, N. R. & other authors (2009). CDD: specific functional annotation with the conserved domain database. Nucleic Acids Res 37 ((Database issue), ), D205–D210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meneghelli, S., Luoni, L. & De Michelis, M. I. (2008). Heparin stimulates a plasma membrane Ca2+-ATPase of Arabidopsis thaliana. J Biochem 143, 253–259. [DOI] [PubMed] [Google Scholar]
- Mills, R. F., Doherty, M. L., López-Marqués, R. L., Weimar, T., Dupree, P., Palmgren, M. G., Pittman, J. K. & Williams, L. E. (2008). ECA3, a Golgi-localized P2A-type ATPase, plays a crucial role in manganese nutrition in Arabidopsis. Plant Physiol 146, 116–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Møller, J. V., Juul, B. & le Maire, M. (1996). Structural organization, ion transport, and energy transduction of P-type ATPases. Biochim Biophys Acta 1286, 1–51. [DOI] [PubMed] [Google Scholar]
- Palmgren, M. G. & Axelsen, K. B. (1998). Evolution of P-type ATPases. Biochim Biophys Acta 1365, 37–45. [DOI] [PubMed] [Google Scholar]
- Plugge, B., Gazzarrini, S., Nelson, M., Cerana, R., Van Etten, J. L., Derst, C., DiFrancesco, D., Moroni, A. & Thiel, G. (2000). A potassium channel protein encoded by chlorella virus PBCV-1. Science 287, 1641–1644. [DOI] [PubMed] [Google Scholar]
- Rasi-Caldogno, F., Carnelli, A. & De Michelis, M. I. (1995). Identification of the plasma membrane Ca2+-ATPase and of its autoinhibitory domain. Plant Physiol 108, 105–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sze, H., Liang, F., Hwang, I., Curran, A. C. & Harper, J. F. (2000). Diversity and regulation of plant Ca2+ pumps: insights from expression in yeast. Annu Rev Plant Physiol Plant Mol Biol 51, 433–462. [DOI] [PubMed] [Google Scholar]
- Van Etten, J. L. (2003). Unusual life style of giant chlorella viruses. Annu Rev Genet 37, 153–195. [DOI] [PubMed] [Google Scholar]
- Wilson, W. H., Van Etten, J. L. & Allen, M. J. (2009). The Phycodnaviridae: the story of how tiny giants rule the world. Curr Top Microbiol Immunol 328, 1–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu, Z., Liang, F., Hong, B., Young, J. C., Sussman, M. R., Harper, J. F. & Sze, H. (2002). An endoplasmic reticulum-bound Ca2+/Mn2+ pump, ECA1, supports plant growth and confers tolerance to Mn2+stress. Plant Physiol 130, 128–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yutin, N., Wolf, Y. I., Raoult, D. & Koonin, E. V. (2009). Eukaryotic large nucleo-cytoplasmic DNA viruses: clusters of orthologous genes and reconstruction of viral genome evolution. Virol J 6, 223. [DOI] [PMC free article] [PubMed] [Google Scholar]