Abstract
Chronic wasting disease (CWD) is a prion disease that affects free-ranging and captive cervids, including mule deer, white-tailed deer, Rocky Mountain elk and moose. CWD-infected cervids have been reported in 14 USA states, two Canadian provinces and in South Korea. The possibility of a zoonotic transmission of CWD prions via diet is of particular concern in North America where hunting of cervids is a popular sport. To investigate the potential public health risks posed by CWD prions, we have investigated whether intracerebral inoculation of brain and spinal cord from CWD-infected mule deer transmits prion infection to transgenic mice overexpressing human prion protein with methionine or valine at polymorphic residue 129. These transgenic mice have been utilized in extensive transmission studies of human and animal prion disease and are susceptible to BSE and vCJD prions, allowing comparison with CWD. Here, we show that these mice proved entirely resistant to infection with mule deer CWD prions arguing that the transmission barrier associated with this prion strain/host combination is greater than that observed with classical BSE prions. However, it is possible that CWD may be caused by multiple prion strains. Further studies will be required to evaluate the transmission properties of distinct cervid prion strains as they are characterized.
INTRODUCTION
Chronic wasting disease (CWD) is a prion disease affecting free-ranging and captive cervids, including mule deer, white-tailed deer, Rocky Mountain elk and moose (Williams & Young, 1980, 1982; Williams, 2005; Baeten et al., 2007). Like all mammalian prion diseases, which include Creutzfeldt–Jakob disease (CJD), kuru and variant CJD (vCJD) in humans and bovine spongiform encephalopathy (BSE) in cattle, the central event in CWD infection is the post-translational conversion of the host-encoded, cellular prion protein (PrPC), to an abnormal isoform, designated PrPSc (Prusiner, 1998; Collinge & Clarke, 2007). Progressive accumulation of PrPSc in the central nervous system (Guiroy et al., 1993) is associated with clinical signs of CWD which include weight loss, behavioural changes, excessive salivation, difficulty swallowing, polydipsia, polyuria, and ataxia prior to death (Williams & Young, 1980, 1982; Williams, 2005). International concern over CWD is growing as infected cervids have now been reported in 14 states in North America, two Canadian provinces and in South Korea (Kim et al., 2005; Williams, 2005; Sigurdson & Aguzzi, 2007; Sigurdson, 2008). To date, CWD has not been reported in Europe, although surveillance has been limited.
The prevalence of CWD infection can reach levels of up to 30 % in free-ranging herds in North America and up to 90 % in animals housed in CWD research facilities (Williams, 2005). Infectious prions in the saliva (Mathiason et al., 2006; Haley et al., 2009; Mathiason et al., 2009), urine (Haley et al., 2009) and faeces of CWD-infected animals (Tamguney et al., 2009) may underlie the highly efficient natural transmission of CWD among cervids through environmental contamination (Mathiason et al., 2009). Protease-resistant cervid prion protein has recently been demonstrated in an environmental water sample from a CWD endemic area (Nichols et al., 2009).
Despite efficient horizontal transmission of CWD prions among cervids, to date there is no clear evidence for natural disease transmission to other species. A recent survey for transmissible spongiform encephalopathy in scavengers of white-tailed deer carcasses in a CWD endemic area of Wisconsin found no evidence for cross-species transmission (Jennelle et al., 2009). Nevertheless, the zoonotic transmission of BSE prions (Collinge et al., 1996; Hill et al., 1997; Bruce et al., 1997; Asante et al., 2002; Wadsworth & Collinge, 2007) has dramatically highlighted the potential risk posed to humans from dietary exposure to CWD prions (Belay, 2004; Sigurdson, 2008). Infectious prions are present in the blood (Mathiason et al., 2006), skeletal muscle (Angers et al., 2006) and fat (Race et al., 2009a) of CWD-infected deer and CWD prions have been shown to be experimentally transmissible after oral inoculation of elk and deer and cervid PrP expressing transgenic mice (Hamir et al., 2006; Fox et al., 2006; Trifilo et al., 2007). Consumption of hunted deer and elk is widely practised in North America and a survey conducted by the American Red Cross and other blood banking establishments has reported that ∼40 % of USA blood donors have consumed venison obtained from the wild (Belay et al., 2001). To date, however, epidemiological surveillance has not indicated any link between human disease and CWD exposure (Belay, 2004; Mawhinney et al., 2006; Anderson et al., 2007). However, incubation periods in human prion disease even in the absence of a transmission barrier can exceed 50 years (Collinge et al., 2006, 2008). Accordingly, there has been intense research interest in establishing the host range of CWD prions through experimental transmissions to laboratory animals (Tamguney et al., 2006; Raymond et al., 2007; Sigurdson et al., 2008; Heisey et al., 2010) and through the use of in vitro prion amplification systems (Raymond et al., 2000; Kurt et al., 2009).
Concern that CWD prions might be transmissible to humans was heightened in 2005 by the finding that squirrel monkeys can be infected by intracerebral inoculation with CWD mule deer brain homogenate (Marsh et al., 2005). However, a more recent study has shown that cynomolgus macaques (that are evolutionarily closer to humans) differ significantly from squirrel monkeys with respect to their susceptibility to infection with CWD prions, with no evidence for clinical disease in macaques at 70 months post-inoculation (Race et al., 2009b). Crucially however, because prion transmission barriers and prion strains are intimately related by conformational selection (Collinge, 1999; Collinge & Clarke, 2007) the ability of CWD prions to propagate in humans cannot be inferred by studying the interaction of CWD prions with distinct (albeit highly conserved) PrP sequences from other species. To date, two studies have reported that transgenic mice expressing human PrP with methionine at polymorphic residue 129 are resistant to intracerebral challenge with CWD prions. The first of these studies used two lines of transgenic mice expressing human PrP at either one or two times the endogenous level of mouse brain. After inoculation with CWD-infected elk brain homogenate, none of these transgenic mice showed clinical signs of prion disease or detectable accumulation of abnormal PrP by either immunohistochemistry or immunoblotting (Kong et al., 2005). Although these mice are susceptible to infection with atypical BSE prions, their susceptibility to classic BSE prions or vCJD prions has not been reported (Kong et al., 2008). The second study used hemizygous transgenic mice expressing human PrP at two times the endogenous level of murine PrP expression in mouse brain. No evidence of clinical prion disease was observed following intracerebral challenge with CWD-infected elk, mule deer or white-tailed deer brain homogenate; however, importantly subclinical infection was not excluded (Tamguney et al., 2006). Susceptibility of these mice to infection with BSE or vCJD prions has not been reported. Here, to investigate further the potential risks for transmission of cervid prions to humans, we have transmitted mule deer CWD prions to lines of transgenic mice overexpressing human PrP two- to sixfold with either methionine or valine at polymorphic residue 129 in which we have extensive experience of transmission of a wide range of human acquired, sporadic and inherited prion disease isolates, including kuru and multiple vCJD cases (Collinge et al., 1995a, b, 1996; Hill et al., 1997; Wadsworth et al., 2008a). Extensive comparative data are available on transmission of multiple cattle BSE isolates (Hill et al., 1997; Asante et al., 2002, 2006; Wadsworth et al., 2004) as well as BSE experimentally passaged or naturally transmitted to multiple mammalian species and these mice are therefore suitable for comparative assessment of the zoonotic potential of CWD prions.
RESULTS
Immunoblot analysis of CWD-infected brain and spinal cord
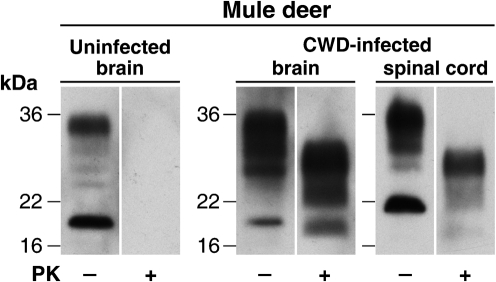
CWD-infected mule deer brain (from animal D10) and spinal cord (from animal D08) originated from captive animals housed at the Colorado Division of Wildlife, Wildlife Research Centre, Colorado, USA. Homogenates (10 % w/v) of these tissues were prepared in PBS and examined for proteinase K (PK)-resistant PrP by immunoblotting. Both samples showed a high level of cervid PrPSc (Fig. 1) with a PrP glycoform ratio that showed a dominant diglycosylated conformer, typical of that associated with CWD prions (Race et al., 2002). In contrast, identical analysis of brain homogenates prepared from uninfected mule deer showed no detectable PK-resistant PrP (Fig. 1 and data not shown).
Fig. 1.
Detection of PrPSc in the brain and spinal cord from CWD-infected mule deer. Immunoblots show the analysis of 5 μl aliquots of 10 % (w/v) homogenates of uninfected mule deer brain or CWD-infected mule deer brain or spinal cord, before (−) or after (+) digestion with PK. Immunoblots were analysed by enhanced chemiluminescence with anti-PrP monoclonal antibody ICSM35.
CWD prions do not transmit prion disease to transgenic mice overexpressing human prion protein
PrPSc-positive CWD-infected brain and spinal cord homogenates were used to prepare inocula for transmission studies in transgenic mice overexpressing human PrP with either methionine or valine at polymorphic residue 129. 129MM Tg35, 129MM Tg45 and 129VV Tg152 transgenic mice overexpress human PrP in brain at levels of two, four and six times that of human brain, respectively (Collinge et al., 1995b, 1996; Hill et al., 1997; Asante et al., 2002). These lines of mice have been extensively used by us for over 15 years and have proven susceptibility to infection with human or BSE prions (Collinge et al., 1995b, 1996; Hill et al., 1997; Asante et al., 2002, 2006; Wadsworth et al., 2004, 2007, 2008a). Following intracerebral inoculation with CWD brain or spinal cord, groups of 10 transgenic mice were observed throughout their life time for clinical signs of prion disease. As reported in Table 1, we observed no clinical prion disease in any inoculated mouse, including those with post-inoculation intervals greater than 700 days (Table 1). Accordingly, brains from mice culled as a result of inter-current illness or senescence were examined for subclinical prion transmission. In all cases examined, pathological PrP accumulation in brain was undetectable by either immunoblotting (Fig. 2, Table 1) or immunohistochemistry (Fig. 3, Table 1). Futhermore, neuropathological examination of CWD-inoculated transgenic mouse brain, showed no evidence of spongiform change or gliosis consistent with prion disease and their appearance was indistinguishable from the brain of age matched control mice inoculated with normal mule deer brain (Fig. 3 and data not shown). In summary, we conclude that intracerebral challenge of these transgenic mice with CWD prions caused no clinical or subclinical prion infection, indicating that both methionine and valine 129 polymorphs of human PrP are refractory to pathological conversion by CWD prions.
Table 1.
Primary transmission of CWD and BSE prions to transgenic mice
Data for BSE transmissions have been published previously (Hill et al., 1997; Asante et al., 2002).
| Transgenic line | CWD brain | CWD spinal cord | BSE brain |
|---|---|---|---|
| Attack rate* | Attack rate* | Attack rate* | |
| 129VV Tg152 | 0/8† | 0/6‡ | 10/26 |
| 129MM Tg45 | 0/7§ | 0/6|| | 9/12 |
| 129MM Tg35 | 0/9¶ | 0/5# | 14/49 |
*All mice were inoculated with 30 μl of 1 % (w/v) tissue homogenate. Attack rate is defined as the total number of both clinically affected and subclinically infected mice as a proportion of the number of inoculated mice. Subclinical prion infection was assessed by sodium phosphotungstic acid precipitation of 250 μl 10 % brain homogenate and analysis for PrPSc by immunoblotting and/or immunohistochemical examination of brain.
†Mice culled at 274, 316, 321, 436, 517, 517, 587 and 781 days post-inoculation.
‡Mice culled at 354, 364, 463, 541, 704 and 724 days post-inoculation.
§Mice culled at 322, 322, 395, 400, 529, 656 and 736 days post-inoculation.
||Mice culled at 275, 345, 396, 462, 462 and 532 days post-inoculation.
¶Mice culled at 341, 559, 662, 662, 680, 707, 707, 747 and 748 days post-inoculation.
#Mice culled at 392, 414, 542, 699 and 732 days post-inoculation.
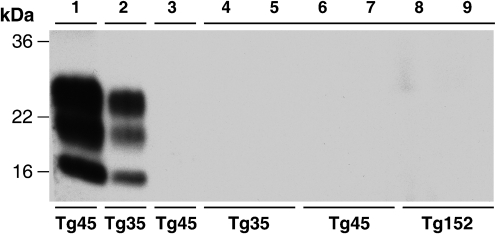
Fig. 2.
Failure to detect PrPSc in the brain of CWD prion-inoculated transgenic mice. The high sensitivity immunoblot using anti-PrP monoclonal antibody 3F4 shows PK-digested sodium phosphotungstic acid pellets recovered from 10 % (w/v) transgenic mouse brain homogenates. Lanes 1 and 2, positive controls showing efficient recovery of PrPSc after spiking 2 μl 10 % (w/v) BSE-inoculated 129MM Tg45 and 129MM Tg35 transgenic mouse brain homogenates (Asante et al., 2002) into 100 μl 10 % (w/v) uninfected 129MM Tg45 and 129MM Tg35 mouse brain homogenates, respectively. Lane 3, PK-digested sodium phosphotungstic acid pellet from 250 μl 10 % (w/v) brain homogenate from a 129MM Tg45 mouse inoculated with normal mule deer brain. Lanes 4–9, PK-digested sodium phosphotungstic acid pellets from 250 μl 10 % (w/v) brain homogenates from 129MM Tg35, 129MM Tg45 and 129VV Tg152 mice inoculated with CWD-infected mule deer brain.
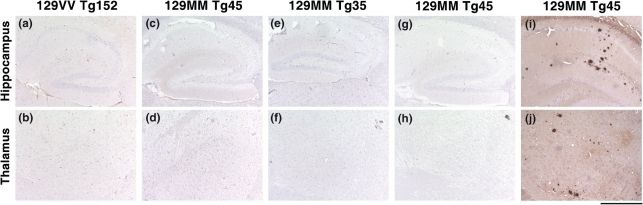
Fig. 3.
Failure to detect abnormal PrP deposition in the brain of CWD prion-inoculated transgenic mice. Representative PrP immunohistochemistry using anti-PrP monoclonal antibody ICSM35. Panels (a–f) show no abnormal PrP deposition in either the hippocampus or thalamus of 129VV Tg152, 129MM Tg45 or 129MM Tg35 mice inoculated with CWD-infected brain homogenate. These mice were culled 517, 529 and 559 days post-inoculation, respectively. Panels (g) and (h) show hippocampus and thalamus from an age matched control 129MM Tg45 mouse brain inoculated with 10 % (w/v) uninfected mule deer brain homogenate. In contrast, extensive deposition of abnormal PrP is seen in the hippocampus and thalamus of a BSE-infected 129MM Tg45 mouse with subclinical prion disease (panels i and j) (Asante et al., 2002). Bar, 500 μm.
DISCUSSION
In this study, we have shown that transgenic mice overexpressing human PrP of both residue 129 polymorphic forms, known to be susceptible to a wide range of human and other prions, are highly resistant to infection with mule deer CWD prions. These findings agree with those of others who have previously reported an inability of CWD prions to transmit disease to transgenic mice expressing human PrP 129 methionine (Kong et al., 2005; Tamguney et al., 2006) or a poor ability of human PrP to act as a substrate for CWD prions in in vitro conversion assays (Raymond et al., 2000; Kurt et al., 2009). Importantly, the transgenic mice used in our study have proven susceptibility to infection with BSE prions [Hill et al., 1997; Asante et al., 2002, 2006; Wadsworth et al., 2004 (Table 1)]. The negative transmissions that we report here therefore strongly support the conclusion that the transmission barrier associated with the interaction of human PrP and these CWD prions is greater than that associated with interaction of human PrP and the prion strain causing epizootic BSE in cattle.
The failure to show propagation of CWD prions using human PrP as a substrate either in vivo in transgenic mice or in vitro in biochemical conversion assays suggests that potential zoonotic threat from CWD is low. However, an important caveat in this regard is that the number of prion strains propagated in CWD is currently unknown (Browning et al., 2004; Raymond et al., 2007; Green et al., 2008; Angers et al., 2010). Because prion strains can adapt and mutate on passage in new species (Collinge & Clarke, 2007; Beringue et al., 2008; Castilla et al., 2008; Collinge, 2010), and also within species as a result of PrP polymorphisms and other genetic factors (Asante et al., 2002; Lloyd et al., 2004; Wadsworth et al., 2004; Mead et al., 2009; Lloyd et al., 2009), the risk that each prion strain poses to public health must be evaluated directly. There is now growing evidence that polymorphisms of cervid PrP may dictate prion strain selection (O'Rourke et al., 2004; Meade-White et al., 2007; Green et al., 2008; Angers et al., 2010). Thus, while the available experimental data appear reassuring, further transmission studies will be of vital importance to evaluate the properties of distinct cervid prion strains as they are isolated.
METHODS
Mule deer tissues.
Importation, storage and use of CWD-infected tissues was performed under licence granted by Defra under the terms of the Importation of Animal Pathogens Order 1980. CWD-infected mule deer brain (from animal D10) and spinal cord (from animal D08) originated from naturally infected captive animals from Colorado, USA that had clinical signs consistent with terminal stages of prion disease. CWD-infection in these animals was confirmed by the presence of histopathological lesions in the brain, including spongiform degeneration of the perikaryon, by immunohistochemical or immunoblot detection of disease-related PrP and by positive transmission of prion disease to transgenic mice expressing cervid PrP (Browning et al., 2004; Angers et al., 2006; Green et al., 2008). Brain from uninfected mule deer fawns (FPS 6.98 and FPS 3.98) was used as negative controls.
Transgenic mice.
Transgenic mice homozygous for a human PrP 129V transgene array and murine PrP null alleles (Prnpo/o) designated Tg(HuPrP129V+/+ Prnpo/o)-152 mice (129VV Tg152 mice) or homozygous for a human PrP 129M transgene array and murine PrP null alleles (Prnpo/o) designated Tg(HuPrP129M+/+ Prnpo/o)-35 mice (129MM Tg35 mice) or Tg(HuPrP129M+/+ Prnpo/o)-45 mice (129MM Tg45 mice) have been described previously (Collinge et al., 1995b, 1996; Hill et al., 1997; Asante et al., 2002, 2006; Wadsworth et al., 2004, 2007, 2008a).
Transmission studies.
All procedures were carried out in a microbiological containment level 3 facility with strict adherence to safety protocols. Care of mice was according to institutional guidelines. Mule deer tissues were prepared as 10 % (w/v) homogenates in sterile PBS lacking Ca2+ and Mg2+ ions by serial passage through needles of decreasing diameter, and subsequently diluted to 1 % (w/v) in PBS. Following intracerebral inoculation with 30 μl of 1 % (w/v) tissue homogenate as described previously (Asante et al., 2002, 2006; Wadsworth et al., 2004), mice were examined daily and were killed if exhibiting signs of distress or once a diagnosis of clinical prion disease was established. Brains from inoculated mice were analysed by PrP immunoblotting or immunohistochemistry and by neuropathological examination.
Immunoblotting.
All procedures were carried out in a microbiological containment level 3 facility with strict adherence to safety protocols. Tissue homogenates (10 % w/v) were prepared in PBS lacking Ca2+ or Mg2+ ions. PK digestion (50 or 100 μg ml−1 final protease concentration, 1 h, 37 °C), electrophoresis and immunoblotting was performed as described previously (Wadsworth et al., 2001, 2008b). Immunoblot detection was performed using anti-PrP monoclonal antibody ICSM35 (D-Gen) for cervid PrP or 3F4 (Kascsak et al., 1987) for human PrP in transgenic mice. Brain homogenates scored negative for PrPSc after analysis of 10 μl 10 % (w/v) brain homogenate were re-analysed by sodium phosphotungstic acid precipitation of PrPSc (Safar et al., 1998) from 250 μl of 10 % (w/v) brain homogenate as described previously (Wadsworth et al., 2001).
Neuropathology and immunohistochemistry.
All steps prior to prion decontamination with formic acid were performed within a microbiological containment level 3 facility with strict adherence to safety protocols. Brain was fixed in 10 % buffered formal saline and then immersed in 98 % formic acid for 1 h and paraffin wax embedded. Serial sections of 4 μm thickness were pre-treated by boiling for 10 min in a low ionic strength buffer (2.1 mM Tris, 1.3 mM EDTA, 1.1 mM sodium citrate, pH 7.8) before exposure to 98 % formic acid for 5 min. Abnormal PrP accumulation was examined using anti-PrP monoclonal antibody ICSM35 (D-Gen) on a Ventana automated immunohistochemical staining machine (Ventana Medical Systems) using proprietary secondary detection reagents (Ventana Medical Systems) before development with 3′3-diaminobenzedine tetrachloride as the chromogen (Wadsworth et al., 2008b). Harris haematoxylin and eosin staining was done by conventional methods. Appropriate positive and negative controls were used throughout. Photographs were taken on an ImageView digital camera and composed with Adobe Photoshop.
Acknowledgments
We thank our biological service team for animal care and R. Young for the preparation of figures. We thank Michael W. Miller and the Colorado Division of Wildlife for access to the CWD-infected deer samples. This research was funded by the Medical Research Council (UK) and the European Union. Conflict of interest statement: J. C. is a Director and J. C. and J. D. F. W. are shareholders and consultants of D-Gen Limited, an academic spin-out company working in the field of prion disease diagnosis, decontamination and therapeutics. D-Gen markets the ICSM35 antibody used in this study.
References
- Anderson, C. A., Bosque, P., Filley, C. M., Arciniegas, D. B., Kleinschmidt-Demasters, B. K., Pape, W. J. & Tyler, K. L. (2007). Colorado surveillance program for chronic wasting disease transmission to humans: lessons from 2 highly suspicious but negative cases. Arch Neurol 64, 439–441. [DOI] [PubMed] [Google Scholar]
- Angers, R. C., Browning, S. R., Seward, T. S., Sigurdson, C. J., Miller, M. W., Hoover, E. A. & Telling, G. C. (2006). Prions in skeletal muscles of deer with chronic wasting disease. Science 311, 1117. [DOI] [PubMed] [Google Scholar]
- Angers, R. C., Kang, H. E., Napier, D., Browning, S., Seward, T., Mathiason, C., Balachandran, A., McKenzie, D., Castilla, J. & other authors (2010). Prion strain mutation determined by prion protein conformational compatibility and primary structure. Science 328, 1154–1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asante, E. A., Linehan, J. M., Desbruslais, M., Joiner, S., Gowland, I., Wood, A. L., Welch, J., Hill, A. F., Lloyd, S. E. & other authors (2002). BSE prions propagate as either variant CJD-like or sporadic CJD-like prion strains in transgenic mice expressing human prion protein. EMBO J 21, 6358–6366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asante, E. A., Linehan, J. M., Gowland, I., Joiner, S., Fox, K., Cooper, S., Osiguwa, O., Gorry, M., Welch, J. & other authors (2006). Dissociation of pathological and molecular phenotype of variant Creutzfeldt–Jakob disease in transgenic human prion protein 129 heterozygous mice. Proc Natl Acad Sci U S A 103, 10759–10764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baeten, L. A., Powers, B. E., Jewell, J. E., Spraker, T. R. & Miller, M. W. (2007). A natural case of chronic wasting disease in a free-ranging moose (Alces alces shirasi). J Wildl Dis 43, 309–314. [DOI] [PubMed] [Google Scholar]
- Belay, E. D. (2004). Chronic wasting disease and potential transmission to humans. Emerg Infect Dis 10, 977–984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belay, E. D., Gambetti, P., Schonberger, L. B., Parchi, P., Lyon, D. R., Capellari, S., McQuiston, J. H., Bradley, K., Dowdle, G. & other authors (2001). Creutzfeldt–Jakob disease in unusually young patients who consumed venison. Arch Neurol 58, 1673–1678. [DOI] [PubMed] [Google Scholar]
- Beringue, V., Vilotte, J. L. & Laude, H. (2008). Prion agents diversity and species barrier. Vet Res 39, 47. [DOI] [PubMed] [Google Scholar]
- Browning, S. R., Mason, G. L., Seward, T., Green, M., Eliason, G. A., Mathiason, C., Miller, M. W., Williams, E. S., Hoover, E. & other authors (2004). Transmission of prions from mule deer and elk with chronic wasting disease to transgenic mice expressing cervid PrP. J Virol 78, 13345–13350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruce, M. E., Will, R. G., Ironside, J. W., McConnell, I., Drummond, D., Suttie, A., McCardle, L., Chree, A., Hope, J. & other authors (1997). Transmissions to mice indicate that ‘new variant’ CJD is caused by the BSE agent. Nature 389, 498–501. [DOI] [PubMed] [Google Scholar]
- Castilla, J., Gonzalez-Romero, D., Saa, P., Morales, R., De Castro, J. & Soto, C. (2008). Crossing the species barrier by PrPSc replication in vitro generates unique infectious prions. Cell 134, 757–768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collinge, J. (1999). Variant Creutzfeldt–Jakob disease. Lancet 354, 317–323. [DOI] [PubMed] [Google Scholar]
- Collinge, J. (2010). Prion strain mutation and selection. Science 328, 1111–1112. [DOI] [PubMed] [Google Scholar]
- Collinge, J. & Clarke, A. R. (2007). A general model of prion strains and their pathogenicity. Science 318, 930–936. [DOI] [PubMed] [Google Scholar]
- Collinge, J., Palmer, M. S., Sidle, K. C. L., Gowland, I., Medori, R., Ironside, J. & Lantos, P. L. (1995a). Transmission of fatal familial insomnia to laboratory animals. Lancet 346, 569–570. [DOI] [PubMed] [Google Scholar]
- Collinge, J., Palmer, M. S., Sidle, K. C. L., Hill, A. F., Gowland, I., Meads, J., Asante, E., Bradley, R., Doey, L. J. & other authors (1995b). Unaltered susceptibility to BSE in transgenic mice expressing human prion protein. Nature 378, 779–783. [DOI] [PubMed] [Google Scholar]
- Collinge, J., Sidle, K. C. L., Meads, J., Ironside, J. & Hill, A. F. (1996). Molecular analysis of prion strain variation and the aetiology of ‘new variant’ CJD. Nature 383, 685–690. [DOI] [PubMed] [Google Scholar]
- Collinge, J., Whitfield, J., McKintosh, E., Beck, J., Mead, S., Thomas, D. J. & Alpers, M. P. (2006). Kuru in the 21st century – an acquired human prion disease with very long incubation periods. Lancet 367, 2068–2074. [DOI] [PubMed] [Google Scholar]
- Collinge, J., Whitfield, J., McKintosh, E., Frosh, A., Mead, S., Hill, A. F., Brandner, S., Thomas, D. & Alpers, M. P. (2008). A clinical study of kuru patients with long incubation periods at the end of the epidemic in Papua New Guinea. Philos Trans R Soc Lond B Biol Sci 363, 3725–3739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox, K. A., Jewell, J. E., Williams, E. S. & Miller, M. W. (2006). Patterns of PrPCWD accumulation during the course of chronic wasting disease infection in orally inoculated mule deer (Odocoileus hemionus). J Gen Virol 87, 3451–3461. [DOI] [PubMed] [Google Scholar]
- Green, K. M., Browning, S. R., Seward, T. S., Jewell, J. E., Ross, D. L., Green, M. A., Williams, E. S., Hoover, E. A. & Telling, G. C. (2008). The elk PRNP codon 132 polymorphism controls cervid and scrapie prion propagation. J Gen Virol 89, 598–608. [DOI] [PubMed] [Google Scholar]
- Guiroy, D. C., Williams, E. S., Song, K.-J., Yanagihara, R. & Gajdusek, D. C. (1993). Fibrils in brains of Rocky Mountain elk with chronic wasting disease contain scrapie amyloid. Acta Neuropathol 86, 77–80. [DOI] [PubMed] [Google Scholar]
- Haley, N. J., Seelig, D. M., Zabel, M. D., Telling, G. C. & Hoover, E. A. (2009). Detection of CWD prions in urine and saliva of deer by transgenic mouse bioassay. PLoS ONE 4, e4848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamir, A. N., Gidlewski, T., Spraker, T. R., Miller, J. M., Creekmore, L., Crocheck, M., Cline, T. & O'Rourke, K. I. (2006). Preliminary observations of genetic susceptibility of elk (Cervus elaphus nelsoni) to chronic wasting disease by experimental oral inoculation. J Vet Diagn Invest 18, 110–114. [DOI] [PubMed] [Google Scholar]
- Heisey, D. M., Mickelsen, N. A., Schneider, J. R., Johnson, C. J., Johnson, C. J., Langenberg, J. A., Bochsler, P. N., Keane, D. P. & Barr, D. J. (2010). Chronic wasting disease (CWD) susceptibility of several North American rodents that are sympatric with cervid CWD epidemics. J Virol 84, 210–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill, A. F., Desbruslais, M., Joiner, S., Sidle, K. C. L., Gowland, I. & Collinge, J. (1997). The same prion strain causes vCJD and BSE. Nature 389, 448–450. [DOI] [PubMed] [Google Scholar]
- Jennelle, C. S., Samuel, M. D., Nolden, C. A., Keane, D. P., Barr, D. J., Johnson, C., Vanderloo, J. P., Aiken, J. M., Hamir, A. N. & other authors (2009). Surveillance for transmissible spongiform encephalopathy in scavengers of white-tailed deer carcasses in the chronic wasting disease area of Wisconsin. J Toxicol Environ Health A 72, 1018–1024. [DOI] [PubMed] [Google Scholar]
- Kascsak, R. J., Rubenstein, R., Merz, P. A., Tonna DeMasi, M., Fersko, R., Carp, R. I., Wisniewski, H. M. & Diringer, H. (1987). Mouse polyclonal and monoclonal antibody to scrapie-associated fibril proteins. J Virol 61, 3688–3693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, T. Y., Shon, H. J., Joo, Y. S., Mun, U. K., Kang, K. S. & Lee, Y. S. (2005). Additional cases of chronic wasting disease in imported deer in Korea. J Vet Med Sci 67, 753–759. [DOI] [PubMed] [Google Scholar]
- Kong, Q., Huang, S., Zou, W., Vanegas, D., Wang, M., Wu, D., Yuan, J., Zheng, M., Bai, H. & other authors (2005). Chronic wasting disease of elk: transmissibility to humans examined by transgenic mouse models. J Neurosci 25, 7944–7949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong, Q., Zheng, M., Casalone, C., Qing, L., Huang, S., Chakraborty, B., Wang, P., Chen, F., Cali, I. & other authors (2008). Evaluation of the human transmission risk of an atypical bovine spongiform encephalopathy prion strain. J Virol 82, 3697–3701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurt, T. D., Telling, G. C., Zabel, M. D. & Hoover, E. A. (2009). Trans-species amplification of PrPCWD and correlation with rigid loop 170N. Virology 387, 235–243. [DOI] [PubMed] [Google Scholar]
- Lloyd, S. E., Linehan, J. M., Desbruslais, M., Joiner, S., Buckell, J., Brandner, S., Wadsworth, J. D. & Collinge, J. (2004). Characterization of two distinct prion strains derived from bovine spongiform encephalopathy transmissions to inbred mice. J Gen Virol 85, 2471–2478. [DOI] [PubMed] [Google Scholar]
- Lloyd, S. E., Maytham, E. G., Pota, H., Grizenkova, J., Molou, E., Uphill, J., Hummerich, H., Whitfield, J., Alpers, M. P. & other authors (2009). HECTD2 is associated with susceptibility to mouse and human prion disease. PLoS Genet 5, e1000383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh, R. F., Kincaid, A. E., Bessen, R. A. & Bartz, J. C. (2005). Interspecies transmission of chronic wasting disease prions to squirrel monkeys (Saimiri sciureus). J Virol 79, 13794–13796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathiason, C. K., Powers, J. G., Dahmes, S. J., Osborn, D. A., Miller, K. V., Warren, R. J., Mason, G. L., Hays, S. A., Hayes-Klug, J. & other authors (2006). Infectious prions in the saliva and blood of deer with chronic wasting disease. Science 314, 133–136. [DOI] [PubMed] [Google Scholar]
- Mathiason, C. K., Hays, S. A., Powers, J., Hayes-Klug, J., Langenberg, J., Dahmes, S. J., Osborn, D. A., Miller, K. V., Warren, R. J. & other authors (2009). Infectious prions in pre-clinical deer and transmission of chronic wasting disease solely by environmental exposure. PLoS ONE 4, e5916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mawhinney, S., Pape, W. J., Forster, J. E., Anderson, C. A., Bosque, P. & Miller, M. W. (2006). Human prion disease and relative risk associated with chronic wasting disease. Emerg Infect Dis 12, 1527–1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mead, S., Poulter, M., Uphill, J., Beck, J., Whitfield, J., Webb, T. E., Campbell, T., Adamson, G., Deriziotis, P. & other authors (2009). Genetic risk factors for variant Creutzfeldt–Jakob disease: a genome-wide association study. Lancet Neurol 8, 57–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meade-White, K., Race, B., Trifilo, M., Bossers, A., Favara, C., LaCasse, R., Miller, M., Williams, E., Oldstone, M. & other authors (2007). Resistance to chronic wasting disease (CWD) in transgenic mice expressing a naturally occurring allelic variant of deer prion protein. J Virol 81, 4533–4539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols, T. A., Pulford, B., Wyckoff, A. C., Meyerett, C., Michel, B., Gertig, K., Hoover, E. A., Jewell, J. E., Telling, G. C. & other authors (2009). Detection of protease-resistant cervid prion protein in water from a CWD-endemic area. Prion 3, 171–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Rourke, K. I., Spraker, T. R., Hamburg, L. K., Besser, T. E., Brayton, K. A. & Knowles, D. P. (2004). Polymorphisms in the prion precursor functional gene but not the pseudogene are associated with susceptibility to chronic wasting disease in white-tailed deer. J Gen Virol 85, 1339–1346. [DOI] [PubMed] [Google Scholar]
- Prusiner, S. B. (1998). Prions. Proc Natl Acad Sci U S A 95, 13363–13383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Race, R. E., Raines, A., Baron, T. G., Miller, M. W., Jenny, A. & Williams, E. S. (2002). Comparison of abnormal prion protein glycoform patterns from transmissible spongiform encephalopathy agent-infected deer, elk, sheep, and cattle. J Virol 76, 12365–12368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Race, B., Meade-White, K., Race, R. & Chesebro, B. (2009a). Prion infectivity in fat of deer with chronic wasting disease. J Virol 83, 9608–9610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Race, B., Meade-White, K. D., Miller, M. W., Barbian, K. D., Rubenstein, R., LaFauci, G., Cervenakova, L., Favara, C., Gardner, D. & other authors (2009b). Susceptibilities of nonhuman primates to chronic wasting disease. Emerg Infect Dis 15, 1366–1376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raymond, G. J., Bossers, A., Raymond, L. D., O'Rourke, K. I., McHolland, L. E., Bryant, P. K., III, Miller, M. W., Williams, E. S., Smits, M. & other authors (2000). Evidence of a molecular barrier limiting susceptibility of humans, cattle and sheep to chronic wasting disease. EMBO J 19, 4425–4430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raymond, G. J., Raymond, L. D., Meade-White, K. D., Hughson, A. G., Favara, C., Gardner, D., Williams, E. S., Miller, M. W., Race, R. E. & other authors (2007). Transmission and adaptation of chronic wasting disease to hamsters and transgenic mice: evidence for strains. J Virol 81, 4305–4314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Safar, J., Wille, H., Itri, V., Groth, D., Serban, H., Torchia, M., Cohen, F. E. & Prusiner, S. B. (1998). Eight prion strains have PrPSc molecules with different conformations. Nat Med 4, 1157–1165. [DOI] [PubMed] [Google Scholar]
- Sigurdson, C. J. (2008). A prion disease of cervids: chronic wasting disease. Vet Res 39, 41. [DOI] [PubMed] [Google Scholar]
- Sigurdson, C. J. & Aguzzi, A. (2007). Chronic wasting disease. Biochim Biophys Acta 1772, 610–618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigurdson, C. J., Mathiason, C. K., Perrott, M. R., Eliason, G. A., Spraker, T. R., Glatzel, M., Manco, G., Bartz, J. C., Miller, M. W. & other authors (2008). Experimental chronic wasting disease (CWD) in the ferret. J Comp Pathol 138, 189–196. [DOI] [PubMed] [Google Scholar]
- Tamguney, G., Giles, K., Bouzamondo-Bernstein, E., Bosque, P. J., Miller, M. W., Safar, J., DeArmond, S. J. & Prusiner, S. B. (2006). Transmission of elk and deer prions to transgenic mice. J Virol 80, 9104–9114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamguney, G., Miller, M. W., Wolfe, L. L., Sirochman, T. M., Glidden, D. V., Palmer, C., Lemus, A., DeArmond, S. J. & Prusiner, S. B. (2009). Asymptomatic deer excrete infectious prions in faeces. Nature 461, 529–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trifilo, M. J., Ying, G., Teng, C. & Oldstone, M. B. (2007). Chronic wasting disease of deer and elk in transgenic mice: oral transmission and pathobiology. Virology 365, 136–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wadsworth, J. D. & Collinge, J. (2007). Update on human prion disease. Biochim Biophys Acta 1772, 598–609. [DOI] [PubMed] [Google Scholar]
- Wadsworth, J. D., Joiner, S., Hill, A. F., Campbell, T. A., Desbruslais, M., Luthert, P. J. & Collinge, J. (2001). Tissue distribution of protease resistant prion protein in variant CJD using a highly sensitive immuno-blotting assay. Lancet 358, 171–180. [DOI] [PubMed] [Google Scholar]
- Wadsworth, J. D., Asante, E., Desbruslais, M., Linehan, J., Joiner, S., Gowland, I., Welch, J., Stone, L., Lloyd, S. & other authors (2004). Human prion protein with valine 129 prevents expression of variant CJD phenotype. Science 306, 1793–1796. [DOI] [PubMed] [Google Scholar]
- Wadsworth, J. D., Joiner, S., Fox, K., Linehan, J., Desbruslais, M., Brandner, S., Asante, E. & Collinge, J. (2007). Prion infectivity in variant Creutzfeldt–Jakob disease rectum. Gut 56, 90–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wadsworth, J. D., Joiner, S., Linehan, J. M., Desbruslais, M., Fox, K., Cooper, S., Cronier, S., Asante, E. A., Mead, S. & other authors (2008a). Kuru prions and sporadic Creutzfeldt–Jakob disease prions have equivalent transmission properties in transgenic and wild-type mice. Proc Natl Acad Sci U S A 105, 3885–3890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wadsworth, J. D., Powell, C., Beck, J. A., Joiner, S., Linehan, J. M., Brandner, S., Mead, S. & Collinge, J. (2008b). Molecular diagnosis of human prion disease. Methods Mol Biol 459, 197–227. [DOI] [PubMed] [Google Scholar]
- Williams, E. S. (2005). Chronic wasting disease. Vet Pathol 42, 530–549. [DOI] [PubMed] [Google Scholar]
- Williams, E. S. & Young, S. (1980). Chronic wasting disease of captive mule deer: a spongiform encephalopathy. J Wildl Dis 16, 89–98. [DOI] [PubMed] [Google Scholar]
- Williams, E. S. & Young, S. (1982). Spongiform encephalopathy of Rocky Mountain elk. J Wildl Dis 18, 465–471. [DOI] [PubMed] [Google Scholar]