Abstract
Objective
To determine whether or not detailed cystic feature analysis on CT scans can assist in the differential diagnosis of pancreatic ductal adenocarcinoma (PDAC) from serous cystadenoma (SCN), mucinous cystadenoma (MCN), and a pseudocyst.
Materials and Methods
This study received Institutional Review Board approval and informed patient consent was waived. Electronic radiology and pathology databases were searched to identify patients with PDAC (n = 19), SCN (n = 26), MCN (n = 20) and a pseudocyst (n = 23) who underwent pancreatic CT imaging. The number, size, location, and contents of cysts, and the contour of the lesions were reviewed, in addition to the wall thickness, enhancement patterns, and other signs of pancreatic and peripancreatic involvement. Diagnosis was based on lesion resection (n = 82) or on a combination of cytological findings, biochemical markers, and tumor markers (n = 6). Fisher's exact test was used to analyze the results.
Results
A combination of the CT findings including irregular contour, multiple cysts, mural nodes, and localized thickening, had a relatively high sensitivity (74%) and specificity (75%) for differentiating PDAC from SCN, MCN, and pseudocysts (p < 0.05). Other CT findings such as location, greatest dimension, or the presence of calcification were not significantly different.
Conclusion
The CT findings for PDAC are non-specific, but perhaps helpful for differentiation. PDAC should be included in the general differential diagnosis of pancreatic cystic neoplasms.
Keywords: Computed tomography (CT), Pancreatic ductal adenocarcinoma with cystic features, Serous cystadenoma, Mucinous cystadenoma, Pseudocyst
INTRODUCTION
Because of improvements in the field of radiology as well as increased awareness, cystic neoplasms are increasingly diagnosed. Other factors include the optimization of the timing of contrast agent injection and the imaging during the appropriate vascular phase after contrast agent injection. Cystic tumors of the pancreas accounts for about 10-15% of all cystic pancreatic lesions (1) and are composed of a variety of neoplasms with a wide range of malignant potential.
Ductal adenocarcinoma is the most common of pancreatic neoplasms, accounting for 90% (2). Although the tumors are predominantly solid, cystlike features (cystic degeneration, retention cysts, and attached pseudocysts) were found at histologic analysis in 8% of ductal adenocarcinomas (3-6). Moreover, pancreatic ductal adenocarcinomas with cystic features (PDAC) are sometimes radiographically similar to some other cystic pancreatic lesions (2, 7, 8), including pancreatic serous cystadenoma (SCN), mucinous cystadenoma (MCN), pseudocyst, and so on. Serous cystadenoma are benign tumors, and in asymptomatic patients, do not require surgical excision. Pseudocysts represent the majority of cystic lesions and require different management, whereas most mucinous cystadenomas have malignant potential that warrants surgery but has a better prognosis than that of ductal adenocarcinomas (9, 10). Hence, accurate preoperative characterization of the lesions aids in prognostication and guides therapeutic decision making.
The role of the pancreatic cyst biopsy is debated. A particular concern is that the biopsy of a malignant fluid-containing lesion may lead to leakage of the fluid from the cyst, hence spreading malignant cells. Moreover, a histological analysis of needle aspirates and chemical analysis of cyst fluid produces a questionable diagnostic yield. Hence, a non-invasive method of assessment is a significant clinical need. Multi-detector row computed tomography (MDCT) allows thin-section scanning of the pancreas and has become the preferred imaging modality for both the initial detection and characterization of pancreatic cysts (11, 12). But until now, no systematic investigation about radiographic appearances of PDAC has been reported.
Thus, the purpose of our study was to compare the CT appearances of pancreatic ductal adenocarcinoma with cystic features, of serous cystadenomas, mucinous cystadenoma, and of pseudocysts by reviewing the cases of a relatively large number of patients to determine if there are CT findings that may assist in the differential diagnosis.
MATERIALS AND METHODS
Institutional Review Board approval was obtained for this retrospective study and informed patient consent was not required. Patients whose medical and imaging data had been entered into our electronic radiology database from January 1, 2003 through October 31, 2009 were identified by means of a search for the term "pancreatic cyst" among the dictated CT examination reports. One hundred seventysix subjects were identified. Our pathology database was then used to determine whether a resection or aspiration of the cystic lesion had been performed. The patients who had got SCN, MCN, pseudocyst and PDAC on CT were included in the study. PDAC could be defined as pancreatic ductal adenocarcinoma with cyst-like features such as intratumoral cystic degeneration or necrosis, retention cysts induced by ductal obstruction, and attached pseudocysts (6, 8). As such, intraductal papillary-mucinous neoplasms (IPMN), mucinous cystadenocarcinomas, and other solid neoplasms sometimes undergo cystic changes and leads to the results being confused with PDAC radiographically and causes difficulty in differentiation. In view of all these entities requiring surgical resection, their preoperative differentiation may not be important in a clinical setting.
In addition, if the communication between a peripherally based cystic pancreatic lesion and the ductal system can be established at imaging, the diagnosis of a side branch intraductal papillary mucinous neoplasm can be made (13, 14). As a result, all these cases were excluded from the study. Finally, 88 patients were available for analysis, and these patients constituted our study population.
There were 26 patients with SCN (M:F = 7:19; age range, 29-70 years; mean age, 53 years). The masses were detected incidentally (n = 12) or with symptoms (n = 14) such as abdominal pain, abdominal distention or fever. A total of 20 patients with MCN (M:F = 7:13; age range, 18-77 years; mean age, 45 years). The lesions were detected because of abdominal pain, abdominal distention or jaundice (n = 16), or by incidental detection (n = 4). Only one of the patients with SCN and MCN respectively had a history of pancreatitis, whereas there were a total of 23 patients with a pseudocyst (16 men; age range, 30-54 years; mean age, 44 years). Of the 23 patients, 21 had a history of pancreatitis. All patients were symptomatic (abdominal pain, n = 19; abdominal distention, n = 15) except for one, who was detected incidentally. Finally, there were 19 patients with PDAC (M:F = 8:11; age range, 42-76 years; mean age, 61 years). Three of these lesions were incidentally found. The other 16 patients were symptomatic with nausea, emesis, weight loss, and jaundice, while four patients had a history of pancreatitis. Elevated tumor markers and blood sugar were found in nine patients.
CT Protocols
Different imaging protocols and equipment were used during the 6-year study period. Helical CT was performed with multi-detector row unit (16 or 64 detector rows) (GE LightSpeed; GE Medical Systems, Milwaukee, WI) in all patients. For routine abdominal CT scanning, 80-120 mL of nonionic contrast material (1.5 mL/kg) was injected at a rate of 2.0-3.0 mL/sec, and images were acquired at a 5 mm section thickness after a 60-65 second delay. The field of view was adjusted according to the size of the patient, while 32 patients received conventional scanning (16 on 16 detector rows, 16 on 64 rows).
In patients with known or suspected to have a pancreatic lesion, a dedicated pancreatic CT angiography was used as either the initial or the follow-up study modality. After nonenhanced CT acquisitions in the liver and pancreas were performed, 80-120 mL of nonionic contrast material (1.5 mL/kg) was injected at a rate of 2.5-3 mL/sec, and two acquisitions were performed. Pancreatic phase imaging was performed 40 seconds after contrast material injection by obtaining 3.75 mm or larger sections through the pancreas. Portal venous phase imaging with 3.75 mm thick sections followed at 65 seconds after contrast material injection. The reconstruction was performed in the pancreatic phase and the portal phase (section thickness, 2.5 mm; pitch, 1.25). A total of 56 patients accepted the CT angiography operations (20 on 16 detector rows, 36 on 64 rows).
Image Analysis
The CT images were retrospectively reviewed by consensus between two radiologists (more than 10 years experience), who were aware of the diagnosis of the cystic pancreatic lesion but were blinded to the specific diagnosis and clinical information. The number, size, location (head, neck, body or tail, diffuse), lesion contour (round or ovoid, lobulated or irregular), content (homogeneous, heterogeneous), and the morphologic features of the cysts such as the presence or absence of calcifications, septa, or mural nodules on CT images, were recorded. "Lobulation" was defined as the presence of rounded contours that could not be described as the borders of the same circle. "Irregular" was defined as the mass or lesion not having clear dimensions that can be measured. The wall of the cyst was considered to be thick if it was more than 2 mm in diameter for at least 25% of the lesion circumference. The presence or absence of enhancement of the wall was identified on images obtained after contrast enhancement (12).
Subjective visual criteria were used in place of region-of-interest measurements. The common bile duct was considered abnormally dilated if the diameter was more than 6 mm (12); whereas, the main pancreatic duct was considered abnormally dilated if the diameter was more than 3 mm at the pancreatic head and 2 mm in the body and tail. The presence and distribution of pancreatic intraductal calcifications (defined as areas of hyperattenuation on nonenhanced images) were recorded. Peripancreatic abnormalities, including lymphadenopathy, vascular involvement, peripancreatic fat infiltration, and metastasis were noted. Peripancreatic lymphadenopathy was diagnosed when ovoid or round extravisceral masses were identified that had short-axis diameters of 10 mm or more and attenuation less than or equal to that of skeletal muscle. The peripancreatic adipose tissue was considered infiltrated if visually perceptible increased attenuation relative to that of subcutaneous fat was present.
Histopathologic Analysis
Color slides of the cut surface of the resected gross specimen or the paraffin-embedded tissue section were reviewed by an experienced pathologist. Enzyme levels, biochemical markers, immunohistochemical markers, and tumor markers were measured in cyst fluids. The maximal serum reference values for the normal range for amylase, CA199, CA125, carcinoembryonic antigen (CEA) and CA 72-4 were < 5000 U/L, < 37 U/mL, < 35 U/mL, < 10 ng/mL (5 µg/L), and < 40 U/mL, respectively.
From each case, one or two tissue blocks were analyzed immunohistochemically by using the primary antisera (MUC1, MUC2, MUC5AC, MUC6, p53). Cytologic examination was performed in all cases. Cuboidal epithelial cells containing cytoplasmic glycogen were characteristic of serous cystadenoma. Epithelial cells with cytoplasmic mucin led to the diagnosis of mucinous cystadenoma. The presence of acute inflammation and histiocytes was suggestive of a pseudocyst. The epithelial lining of these cysts was generally positive for MUC5AC, and CEA, MUC1, MUC6, or p53 were considered to be ductal adenocarcinomas with cystic changes (6). The combination of the typical features of the biochemical analysis, tumor markers, immunohistochemical markers, and cytologic analysis was considered sufficient to establish a specific diagnosis.
Statistical Analysis
Differences in numbers between PDAC and the other three groups were compared by using the Fisher's exact test. A p value less than 0.05 was considered to be statistically significant difference. The sensitivity and specificity values of the CT criteria were calculated.
RESULTS
Histopathologic Findings
Eighty-two patients underwent lesion resections, while the remaining six patients accepted a cyst fluid analysis. Twenty-four of the 26 patients with SCN underwent surgical resections. For the two remaining patients, a cyst fluid analysis demonstrated low tumor marker levels. Of the 20 patients with mucinous cystadenoma, 19 of the diagnoses were based on resections and histopathologic confirmations, while the one remaining diagnosis was based on a cyst fluid analysis. A pancreatic resection was performed in 21 of the 23 patients with a pseudocyst, and a histopathologic evaluation demonstrated the cystic lesion without lining epithelium. A cyst fluid analysis was performed in the two remaining patients with pseudocyst, and the analysis demonstrated high amylase levels (> 5000 U/L), abundant acute inflammation, and absence of epithelial cells. Pancreatic resections were performed in 18 of the 19 patients with PDAC. The remaining one received chemotherapy because of metastasis. A histopathologic evaluation demonstrated a solid mass containing cystic lesions with or without lining epithelium which was generally positive for MUC5AC and CEA, MUC1, MUC6, or p53.
Imaging Findings
The average size of PDAC (4.3 cm; size range, 1.5 to 7.5 cm) was significantly smaller than a pseudocyst (9.9 cm; size range, 3.3-16.5 cm), but not different from SCN (3.1 cm; size range, 1.5 to 10 cm) or MCN (mean, 6.6 cm; size range, 2.5 to 15 cm).
Tables 1 and 2 summarized the different imaging features observed in patients with PDAC, SCN, MCN, and a pseudocyst. The location of PDAC had no specificity and was significantly difference when compared to SCN. However, a statistical difference did exist for the comparison with MCN, which predominantly involved in the body and tail of the pancreas (16 of 20) (p < 0.05). Many pseudocysts (10 of 23) occurred diffusely, which differed from PDAC (p < 0.001). The contour of SCN, MCN, and pseudocyst was mainly round or ovoid, which was significantly different from that of PDAC, and irregular (specificity of 74% for the diagnosis of PDAC) (p < 0.01). Further more, the lobulated contour was predominantly observed in SCN (9 of 26). The cystic type was divided into solitary, oligocystic or polycystic. The cystic type of PDAC was mainly multiple (16 of 19), similar to that of SCN (10 of 26) (p > 0.05), but different from MCN and a pseudocyst (p < 0.05). Because the cystic type of the latter two was mainly solitary. This gave a 75% specificity for the diagnosis of PDAC. The content of cysts in PDAC and MCN was mainly heterogeneous, which was significantly different with SCN (p < 0.05) and pseudocyst (p < 0.001). The linear or curvilinear features could be observed in most patients with PDAC (18 of 19) (Fig. 1), similar to SCN (15 of 26), and MCN (19 of 20) and conversely to pseudocysts (8 of 23). The central stellate was observed exclusively in SCN (5 of 26). Moreover, the mural nodules and localized thickening of the wall were predominantly observed in patients with PDAC (Fig. 2), or for patients who showed a statistically significant difference compared to patients with SCN (p < 0.001) and pseudocysts (p < 0.0001), but showed a similarities for patients with MCN, which yielded a specificity of 62% and 61% for PDAC respectively. Proximal atrophy could often be observed in patients with PDAC (10 of 19), MCN (15 of 20) and a pseudocyst (7 of 23), except for patients with SCN (2 of 26). The dilation of the biliary system and main pancreatic duct for PDAC showed a statistically significant difference compared to cases of MCN and pseudocysts (p < 0.05), but was not statistically significance when compared to SCN. This result yielded a specificity of 81% for the diagnosis of PDAC. Lymphadenopathy was observed in patients with PDAC and MCN, but not for patients with SCN (p < 0.05) or a pseudocyst (p < 0.05). The presence of peripancreatic fat infiltration and vascular or peripheral tissue involvement was observed in many patients with PDAC, which is similar to a pseudocyst, but significantly different from SCN (p < 0.05) and MCN (p < 0.05), which led to a specificity of 74% for the diagnosis of PDAC.
Table 1.
CT Features in 88 Cystic Pancreatic Lesions
Note.- †CT features with statistical significance (p < 0.05). All compared with pancreatic ductal adenocarcinom with cystic features. Data are number of patients. MCN = mucinous cystadenoma, PDAC = pancreatic ductal adenocarcinom with cystic features, SCN = serous cystadenoma, ... = not significant
Table 2.
Other CT Features in 88 Cystic Pancreatic Lesions
Note.- †CT features with statistical significance (p < 0.05). All compared with pancreatic ductal adenocarcinom with cystic features. Data are number of patients. MCN = mucinous cystadenoma, PDAC = pancreatic ductal adenocarcinom with cystic features, SCN = serous cystadenoma, ... = not significant
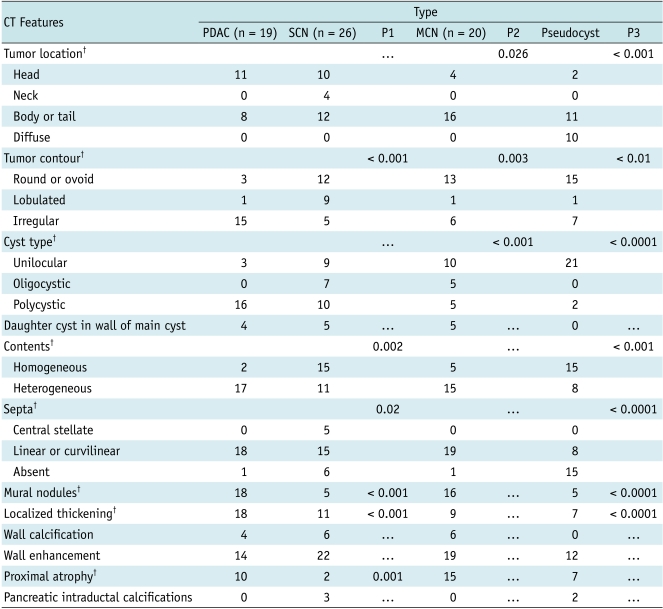
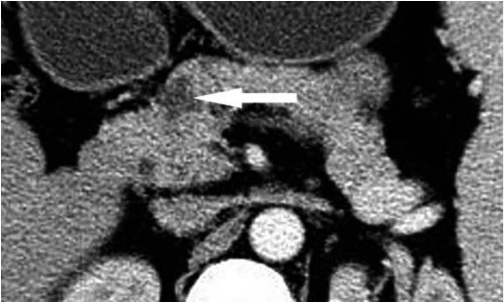
Fig. 1.
Transverse CT scan obtained in 62-year-old man with pancreatic ductal adenocarcinoma with cystic features.
Image was obtained after intravenous injection of contrast material demonstrated irregular multicystic lesion (long arrow) in head of pancreas. Wall is thick and enhancing on this contrast-enhanced image. Note septum (short arrow).
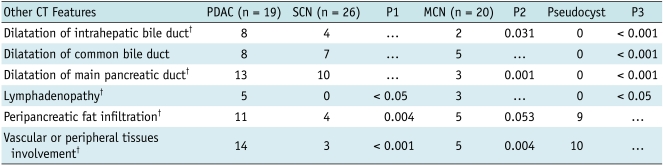
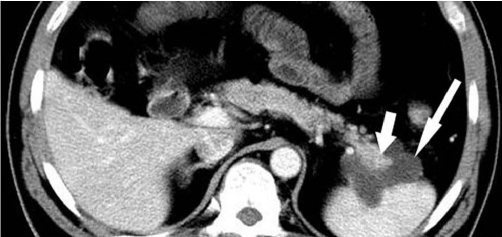
Fig. 2.
Transverse CT scans obtained in 62-year-old man with pancreatic ductal adenocarcinoma with cystic features.
A. Image obtained without intravenous contrast material demonstrates round cystic lesion in tail of pancreas. Wall of lesion was localized and found to be thick (arrow). B. Image obtained after intravenous injection of contrast material reveals enhancement of wall (short arrow). Note varicose veins of spleen (long arrow).
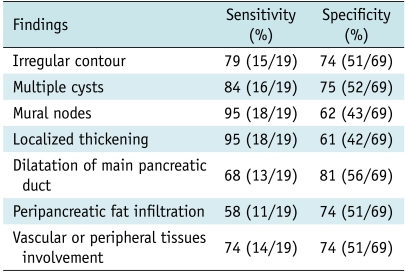
Table 3 summarized the sensitivity and specificity values for the diagnosis of pancreatic ductal adenocarcinoma with cystic features. The imaging features of irregular contour, multiple cysts, mural nodes, localized thickening, dilatation of main pancreatic duct, peripancreatic fat infiltration, and vascular or peripheral tissue involvement may be useful for the diagnosis of PDAC with a relatively high sensitivity (> 50%) and specificity (> 50%). In particular, the sensitivity of mural nodes and localized thickening could be as high as 95%. A combination of the formal four CT findings could have 79% sensitivity and 75% specificity for the diagnosis of PDAC. The other features such as homogeneity of the lesion, presence of septa, proximal atrophy, lymphadenopathy, and dilatation of the common bile duct, were not helpful for lesion characterization.
Table 3.
Sensitivity and Specificity Values for CT Findings for Pancreatic Ductal Adenocarcinoma with Cystic Features
Note.- Data in parentheses correspond to number of patients.
Our study results suggest that CT findings, which include irregular contour, multiple cysts, mural nodes, localized thickening, as well as the dilatation of the main pancreatic duct, peripancreatic fat infiltration, and vascular or peripheral tissue involvement, were helpful for the characterization of PDAC, especially the former four features.
DISCUSSION
Ductal adenocarcinoma is the most common (15) and most lethal tumor of the pancreas, with a 5-year survival rate of less than 3%. The growth pattern of these tumors is usually infiltrative and results in the invasion of adjacent vasculature. Although the tumors are predominantly solid, they occasionally also have an associated cystic component or may undergo degeneration and can mimic a cystic neoplasm at imaging. However, many radiologists believed that the pancreatic ductal adenocarcinomas with cystic features are rare (2, 7), these cyst-like features (cystic degeneration, retention cysts, and attached pseudocysts) have been reported by many pathological studies (3, 6, 8, 16, 17). They thought the pancreatic ductal adenocarcinomas and their variants with cystic features are neither rare, nor do they form a uniform group (6), and account for 7% of all cases on cystic neoplasms and lesions of the pancreas (16). However, the study results about the image findings of PDAC cannot be validated without further study to increase the sample size.
The imaging features of pancreatic adenocarcinomas are well described in the literature. The poorly vascularized, infiltrative soft-tissue lesion shows delayed enhancement and causes ductal obstruction at an early stage of its development. Complex cystic areas representing adjacent pseudocysts, internal tumor necrosis, or side-branch ductal obstruction often may be seen within or adjacent to the primary soft-tissue lesion (2). Sahani et al. (7) believed that adenocarcinomas with an associated cystic component or are undergoing degeneration can mimic a cystic neoplasm at imaging. The intratumoral cystic lesions of PDACs on MRI reported by Yoon SE et al, were classified as either neoplastic mucin cysts with smooth margins and eccentric locations or cystic necroses with irregular margins and centric locations (8). Because of the overlap between the imaging findings of PDAC and other pancreatic cystic lesions, there is still exists a challenge in accurately characterizing lesions, and misdiagnoses still occur from time to time (3, 4).
In our study, although no specific CT features were observed in PDAC, some characteristics of image finding could also be useful for the differentiation. The typical SCN appearance as a multilocular cystic tumor consisting of innumerable small cysts filled with clear fluid and separated by radiating and interlacing connective tissue of variable thickness produces a honeycomb appearance in our study, which is also found in previous studies (18, 19). SCN could be differentiated from PDAC by the characteristics of the mural nodes, localized thickening of the wall, irregular contour, and a vascular invasion. However, it is still difficult to differentiate PDAC with intratumoral cystic degeneration (Fig. 3). Clinical history may be helpful. For PDAC and MCN, though they share many features in common such as focal thickening of the wall, heterogeneous content, and mural nodules, there still are some features that are useful for differentiation. For instance, MCN occurred more frequently in the body and tail of the pancreas while the location of PDAC was non-specific in our study , which is similar to other reports (12). Besides, the image findings of multiple cysts, irregular contour, and the dilatation of the main pancreatic duct for PDAC were all different from those of MCN.
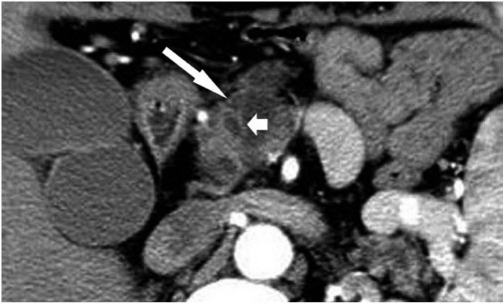
Fig. 3.
Transverse CT scan obtained during portal venous phase in 46-year-old woman with pancreatic ductal adenocarcinoma with cystic degeneration. Image shows lobulated cystic lesion (arrow) in head of pancreas, which was surrounded by thin nonenhancing wall.
The recognition of a pancreatic pseudocyst resulting from chronic pancreatitis is usually easy when there are associated stigmata of chronic pancreatitis such as parenchymal calcifications or ductal stones, ductal dilatation, and atrophy of the parenchyma. However, without these findings, pseudocysts will be a little difficult to distinguish from PDAC (Figs. 2, 4). In addition, a history of prior pancreatitis is not always available in cases of proven pseudocyst. At imaging evaluation of PDAC in our study, a number of findings had been found to be useful for differentiating. The PDAC cases in our study usually appeared as irregular and multiple, as well as different from pseudocysts which are for the most part round or ovoid and unilocular. Moreover, the findings of mural nodes and localized thickening of the wall occurred more frequently in PDAC than those of pseudocysts.
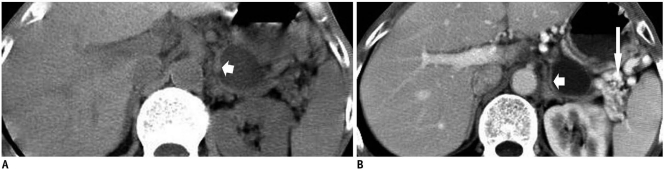
Fig. 4.
Transverse CT scan obtained during portal venous phase in 47-year-old woman with pancreatic ductal adenocarcinoma with retention cysts. Image shows solid tissue surrounded (short arrow) by lobulated cystic lesion (long arrow) in tail of pancreas. Solid tissue component was easily mistaken for normal pancreatic tissue.
Although cystic neoplasms such as a solid pseudopapillary tumor or mucinous cystadenocarcinoma also may contain enhancing soft tissue and cystic components, the infiltrative pattern of the primary tumor in ductal adenocarcinoma facilitates diagnosis, especially when that pattern is combined with ductal obstruction and vascular invasion-all features that are well depicted at MR imaging (2).
Apart from the intrinsic limits of any retrospective study, several other limitations should be emphasized. First, if we had relied on the objective region of interest determinations rather than on the subjective visual assessment of wall enhancement, we might have improved the validity of our results. Considering the variety of this lesion, our evaluation must rely only on hard-copy film images. Second, we included the common benign and malignant potential cystic lesions as the characteristics for differentiation of PDAC, but we did not include lesions such as IPMN, solid pseudopapillary tumors, and so on. The typical location (uncinate process), appearance (grapelike locular appearance), and communication with the duct at endoscopic retrograde cholangiopancreatography (ERCP) usually separates IPMN from other lesions in the pancreas. In addition, this entity and other pancreatic solid tumors with cystic features all require surgical resections and the preoperative clinical value is not high. Third, some of the cystic lesions in our series were not resected, but instead, the diagnosis was established by analyzing a combination of biochemical markers, tumor markers, and cytological findings. Meanwhile, we missed some lesions which did not accept surgical resections or biopsies. This will affect our study sample. Fourth, different imaging protocols and equipments were used during this period. However, the number of patients that accepted the conventional scanning accounted for only about 35% of the whole sample and might not affect the study results. Lastly, it could be argued that our study population is small and a more extensive study would be necessary to validate the outcome of this study.
In conclusion, our study results show that certain findings of PDAC: irregular contour, multiple cysts, mural nodes, localized thickening, as well as the dilatation of the main pancreatic duct, peripancreatic fat infiltration, and vascular or peripheral tissues involvement, though not specific, may be helpful for differention. In addition, the results indicate that PDAC should be included in the differential diagnosis of pancreatic cystic neoplasms.
References
- 1.Brugge WR, Lauwers GY, Sahani D, Fernandez-del Castillo C, Warshaw AL. Cystic neoplasms of the pancreas. N Engl J Med. 2004;351:1218–1226. doi: 10.1056/NEJMra031623. [DOI] [PubMed] [Google Scholar]
- 2.Kalb B, Sarmiento JM, Kooby DA, Adsay NV, Martin DR. MR imaging of cystic lesions of the pancreas. Radiographics. 2009;29:1749–1765. doi: 10.1148/rg.296095506. [DOI] [PubMed] [Google Scholar]
- 3.Lee LY, Hsu HL, Chen HM, Hsueh C. Ductal adenocarcinoma of the pancreas with huge cystic degeneration: a lesion to be distinguished from pseudocyst and mucinous cystadenocarcinoma. Int J Surg Pathol. 2003;11:235–239. doi: 10.1177/106689690301100315. [DOI] [PubMed] [Google Scholar]
- 4.Dennis JW, Aranha GV, Greenlee HB, Hoffman JP, Prinz RA. Carcinoma masquerading as a pancreatic pseudocyst on ultrasound. Am Surg. 1984;50:334–339. [PubMed] [Google Scholar]
- 5.Ji Y, Lou WH, Jin DY, Kuang TT, Zeng MS, Tan YS, et al. A series of 64 cases of pancreatic cystic neoplasia from an institutional study of China. World J Gastroenterol. 2006;12:7380–7387. doi: 10.3748/wjg.v12.i45.7380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kosmahl M, Pauser U, Anlauf M, Kloppel G. Pancreatic ductal adenocarcinomas with cystic features: neither rare nor uniform. Mod Pathol. 2005;18:1157–1164. doi: 10.1038/modpathol.3800446. [DOI] [PubMed] [Google Scholar]
- 7.Sahani DV, Kadavigere R, Saokar A, Fernandez-del Castillo C, Brugge WR, Hahn PF. Cystic pancreatic lesions: a simple imaging-based classification system for guiding management. Radiographics. 2005;25:1471–1484. doi: 10.1148/rg.256045161. [DOI] [PubMed] [Google Scholar]
- 8.Yoon SE, Byun JH, Kim KA, Kim HJ, Lee SS, Jang SJ, et al. Pancreatic ductal adenocarcinoma with intratumoral cystic lesions on MRI: correlation with histopathological findings. Br J Radiol. 2010;83:318–326. doi: 10.1259/bjr/69770140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Compagno J, Oertel JE. Mucinous cystic neoplasms of the pancreas with overt and latent malignancy (cystadenocarcinoma and cystadenoma). A clinicopathologic study of 41 cases. Am J Clin Pathol. 1978;69:573–580. doi: 10.1093/ajcp/69.6.573. [DOI] [PubMed] [Google Scholar]
- 10.Buetow PC, Rao P, Thompson LD. From the Archives of the AFIP. Mucinous cystic neoplasms of the pancreas: radiologic-pathologic correlation. Radiographics. 1998;18:433–449. doi: 10.1148/radiographics.18.2.9536488. [DOI] [PubMed] [Google Scholar]
- 11.Mathieu D, Guigui B, Valette PJ, Dao TH, Bruneton JN, Bruel JM, et al. Pancreatic cystic neoplasms. Radiol Clin North Am. 1989;27:163–176. [PubMed] [Google Scholar]
- 12.Cohen-Scali F, Vilgrain V, Brancatelli G, Hammel P, Vullierme MP, Sauvanet A, et al. Discrimination of unilocular macrocystic serous cystadenoma from pancreatic pseudocyst and mucinous cystadenoma with CT: initial observations. Radiology. 2003;228:727–733. doi: 10.1148/radiol.2283020973. [DOI] [PubMed] [Google Scholar]
- 13.Choi BS, Kim TK, Kim AY, Kim KW, Park SW, Kim PN, et al. Differential diagnosis of benign and malignant intraductal papillary mucinous tumors of the pancreas: MR cholangiopancreatography and MR angiography. Korean J Radiol. 2003;4:157–162. doi: 10.3348/kjr.2003.4.3.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kosmahl M, Pauser U, Peters K, Sipos B, Luttges J, Kremer B, et al. Cystic neoplasms of the pancreas and tumor-like lesions with cystic features: a review of 418 cases and a classification proposal. Virchows Arch. 2004;445:168–178. doi: 10.1007/s00428-004-1043-z. [DOI] [PubMed] [Google Scholar]
- 15.Martin DR, Semelka RC. MR imaging of pancreatic masses. Magn Reson Imaging Clin N Am. 2000;8:787–812. [PubMed] [Google Scholar]
- 16.Sahani DV, Kadavigere R, Blake M, Fernandez-Del Castillo C, Lauwers GY, Hahn PF. Intraductal papillary mucinous neoplasm of pancreas: multi-detector row CT with 2D curved reformations--correlation with MRCP. Radiology. 2006;238:560–569. doi: 10.1148/radiol.2382041463. [DOI] [PubMed] [Google Scholar]
- 17.Kimura W, Sata N, Nakayama H, Muto T, Matsuhashi N, Sugano K, et al. Pancreatic carcinoma accompanied by pseudocyst: report of two cases. J Gastroenterol. 1994;29:786–791. doi: 10.1007/BF02349289. [DOI] [PubMed] [Google Scholar]
- 18.Itai Y, Moss AA, Ohtomo K. Computed tomography of cystadenoma and cystadenocarcinoma of the pancreas. Radiology. 1982;145:419–425. doi: 10.1148/radiology.145.2.7134446. [DOI] [PubMed] [Google Scholar]
- 19.Sheehan MK, Beck K, Pickleman J, Aranha GV. Spectrum of cystic neoplasms of the pancreas and their surgical management. Arch Surg. 2003;138:657–660. doi: 10.1001/archsurg.138.6.657. discussion 660-662. [DOI] [PubMed] [Google Scholar]