Abstract
The anchor cell/ventral uterine precursor cell (AC/VU) decision in Caenorhabditis elegans is a canonical example of lin-12/Notch-mediated lateral specification. Two initially equivalent cells interact via the receptor LIN-12 and its ligand LAG-2, so that one becomes the AC and the other a VU. During this interaction, feedback loops amplify a small difference in lin-12 activity, limiting lin-12 transcription to the presumptive VU and lag-2 transcription to the presumptive AC. Here, we find that hlh-2 appears to be required for the VU fate and directly activates lag-2 transcription in the presumptive AC. HLH-2 appears to accumulate selectively in the presumptive AC prior to differential transcription of lin-12 or lag-2, and is therefore the earliest detectable difference between the two cells undergoing the AC/VU decision. The restricted accumulation of HLH-2 to the presumptive AC reflects post-transcriptional down-regulation of HLH-2 in the presumptive VU. Our observations suggest that hlh-2 is regulated as part of the negative feedback that down-regulates lag-2 transcription in the presumptive VU. Finally, we show that the AC/VU decision in an individual hermaphrodite is biased by the relative birth order of the two cells, so that the first-born cell is more likely to become the VU. We propose models to suggest how birth order, HLH-2 accumulation, and transcription of lag-2 may be linked during the AC/VU decision.
Keywords: HLH-2, LIN-12, Notch, birth order, LAG-2, Delta
Receptors of the LIN-12/Notch family mediate many cell-cell interactions that specify cell fate during animal development (for review, see Greenwald 1998). LIN-12/Notch is activated by binding of a ligand of the DSL (Delta/Serrate/LAG-2) family. Ligand binding induces proteolytic cleavages of LIN-12/Notch that culminate in the release of the intracellular domain, which translocates to the nucleus. The intracellular domain forms a complex with a sequence-specific DNA-binding protein named LAG-1 in Caenorhabditis elegans, Su(H) in Drosophila, and CBF1 or RBP-J in mammals, and activates the transcription of target genes.
LIN-12/Notch proteins have been studied widely for their roles in mediating interactions between equivalent cells. A canonical example of this kind of interaction, known as “lateral inhibition” or “lateral specification”, occurs during C. elegans gonadogenesis. Two initially equivalent cells, named Z1.ppp and Z4.aaa, interact with each other, so that one becomes the anchor cell (AC), a terminally differentiated cell that organizes vulval development, whereas the other becomes a ventral uterine precursor cell (VU), which divides to produce descendants that contribute to the uterus (Kimble and Hirsh 1979; Kimble 1981; Seydoux and Greenwald 1989). In a given hermaphrodite, which of the two cells becomes the AC and which becomes the VU has been thought to be random (Kimble and Hirsh 1979). For these cells, lin-12 activity is necessary and sufficient to specify the VU fate, in that the absence of lin-12 activity causes both Z1.ppp and Z4.aaa to become ACs, whereas elevated lin-12 activity causes both cells to become VUs (Greenwald et al. 1983). Both Z1.ppp and Z4.aaa initially express the ligand LAG-2 and the receptor LIN-12, but an apparently stochastic small difference in the level of lin-12 activity is amplified by a feedback mechanism, so that the presumptive AC ultimately expresses only lag-2, whereas the presumptive VU expresses only lin-12 (Seydoux and Greenwald 1989; Wilkinson et al. 1994). In the presumptive VU, continued transcription of lin-12 appears to be maintained by a positive, autoregulatory feedback loop involving activated LIN-12 (Wilkinson et al. 1994). The molecular basis for the negative regulation of lag-2 expression in the presumptive VU has not been described previously, and is the subject of this study.
In Drosophila, specification of the sense organ precursors (SOPs) that give rise to the microchaete bristles appears to be analogous to the AC/VU decision. Each SOP is generated from a small proneural cluster of equivalent cells that have the potential to adopt either a neural (SOP) or epidermal fate. Interactions among these cells, mediated by the ligand Delta and the receptor Notch, ensures that only one will become an SOP; the cell with lower Notch activity becomes the SOP, and the cells with higher Notch activity become epidermal (Hartenstein and Posakony 1990; Simpson 1990). Genetic evidence suggests that positive and negative feedback loops analogous to those operating in the AC/VU decision exist (Heitzler and Simpson 1991), and a feedback loop that leads from Notch activity to the down-regulation of Delta transcription has been described (Heitzler et al. 1996).
Activation of Notch in a cell of the proneural cluster leads to transcription of a set of basic helix-loop-helix (bHLH) transcription factors encoded by the Enhancer of split complex [E(spl)-C] (Bailey and Posakony 1995; Lecourtois and Schweisguth 1995). bHLH proteins have been assigned to different classes on the basis of binding specificities and sequence features (Massari and Murre 2000). The E(spl) bHLH proteins belong to Class VI, and form a complex with the corepressor Groucho (Gro) to repress transcription of the achaete-scute complex (AS-C) genes (Oellers et al. 1994; Paroush et al. 1994). The AS-C genes encode four partially redundant Class II bHLH proteins, which together with their Class I bHLH dimerization partner, Daughterless, are required for transcription of Delta (Murre et al. 1989; Kunisch et al. 1994). Thus, activation of Notch leads to expression of repressors that lead to loss of the transcription factors that promote Delta expression, thereby constituting a negative feedback loop.
Daughterless is the only Class I bHLH protein in Drosophila (Ruvkun and Hobert 1998; Ledent and Vervoot 2001), and as the obligate dimerization partner of Class II bHLH proteins, it has been assumed to be present in all cells of the proneural cluster and its derivatives. However, there is evidence that in the ovary, despite its apparent ubiquitous expression (Cronmiller and Cummings 1993), daughterless transcription is not constitutive, but rather is dynamically regulated (Smith and Cronmiller 2001). It is therefore possible that transcriptional regulation of daughterless also plays a role in SOP specification, but at this time, this possibility has not been investigated.
In C. elegans, as in Drosophila, there are numerous Class II bHLH proteins and a single Class I bHLH protein, HLH-2, the apparent ortholog of Daughterless (Krause et al. 1997). HLH-2 is expressed in a dynamic and restricted pattern during C. elegans development, including in the AC (M. Krause, pers. comm.). The AC expression and the potential parallel with the involvement of bHLH proteins in regulating Delta expression led us to investigate hlh-2 as a potential regulator of lag-2 transcription in the AC/VU decision.
Here, we describe evidence that HLH-2 directly activates transcription of lag-2 in the presumptive AC and that post-transcriptional down-regulation of HLH-2 in the presumptive VU can account for loss of lag-2 expression in response to LIN-12 activation. Our analysis has also revealed that a difference in HLH-2 accumulation is the earliest detectable difference to date between the presumptive AC and VU. In trying to determine the factors that might influence this difference, we made the surprising finding that relative birth order of Z1.ppp and Z4.aaa strongly correlates with the outcome of the AC/VU decision. We propose models that incorporate how birth order, HLH-2 accumulation, and transcription of lag-2 may be linked during the AC/VU decision.
Results
hlh-2 functions in the AC/VU decision
Existing weak hlh-2 alleles show no obvious phenotype on their own (Portman and Emmons 2000), and a null allele of hlh-2 has eluded isolation, possibly because of haploinsufficiency (M. Krause, pers. comm.). We therefore used RNA-mediated interference (RNAi) to assess whether loss or reduction of gene activity alters the number of ACs formed. Wild-type hermaphrodites have one AC, whereas mutants with reduced lin-12 or lag-2 activity have two ACs (Greenwald et al. 1983; Lambie and Kimble 1991). If hlh-2 functions in lin-12 -mediated signaling during the AC/VU decision, reducing hlh-2 activity may cause a 2 AC phenotype.
Delivery of double-stranded RNA (dsRNA) to embryos or L1 larvae results in phenotypes that preclude evaluation of the AC/VU decision, including lethality (Krause et al. 1997) and highly penetrant gonadal abnormalities that occur prior to the AC/VU decision (X. Karp and I. Greenwald, unpubl.). We therefore placed hermaphrodites at or near the beginning of the L2 stage, at the onset of the AC/VU decision, onto plates containing bacteria expressing hlh-2 dsRNA (see Materials and Methods). We refer to such hermaphrodites as “hlh-2(RNAi-L2)” hermaphrodites. Under these conditions, we observed a 2 AC phenotype (Fig. 1). Although the 2 AC phenotype occurs at a low penetrance in hlh-2(RNAi-L2) hermaphrodites, it was consistently observed in repeated experiments and in RNAi experiments using two different parts of the gene (Materials and Methods) and never observed in controls (Fig. 1; data not shown). We believe that the low penetrance of the 2 AC phenotype in hlh-2(RNAi-L2) hermaphrodites is likely to reflect the relatively short amount of time that the animals are exposed to the dsRNA prior to the resolution of the AC/VU decision, as longer exposure to dsRNA for hlh-2 has a highly penetrant effect on lag-2 expression (see below); in addition, under the same conditions, dsRNA for lag-2 does not cause a 2 AC phenotype, although longer exposure does (Fig. 1; data not shown). These results suggest that hlh-2 functions in the AC/VU decision to promote the VU fate.
Figure 1.
Genetic evidence for hlh-2 function in the AC/VU decision. Wild-type hermaphrodites carrying the AC marker arIs51[cdh-3::gfp] were subject to RNAi for either lacZ (A,B), or hlh-2 (C,D). The number of ACs was assessed by morphology (A,C) and GFP expression (B,D). An arrow indicates an AC. In this and subsequent figures, anterior is to the left; views are lateral (ventral down), unless otherwise indicated. (A,B) lacZ(RNAi-L2) negative control hermaphrodites. (C,D) hlh-2(RNAi-L2) hermaphrodites with 2 ACs. Note that the nucleus of only one of the two ACs is in focus in these pictures. However, GFP expression in both ACs is visible in this focal plane, because GFP is localized in the cytoplasm as well as the nucleus. (E) RNAi data. Each bar represents a separate experiment. Numbers above the bars represent number of animals with 2 ACs over the total number of animals. All controls were done in parallel with hlh-2(RNAi-L2) experiments. Controls were either empty vector (first bar), lacZ (second and third bar), or lag-2 (fourth bar - under this RNAi condition, lag-2 does not cause a 2 AC phenotype; see text).
HLH-2 protein accumulates specifically in the presumptive AC
HLH-2 accumulates in the differentiated AC (M. Krause, pers. comm.; X. Karp and I. Greenwald, unpubl.) and the RNAi data indicate that hlh-2 functions during the AC/VU decision. The pattern of HLH-2 protein accumulation with respect to the progression of the AC/VU decision may illuminate the role of hlh-2 during the decision.
The progression of the AC/VU decision may be monitored by lacZ reporter genes for lag-2 and lin-12 transcription (Wilkinson et al. 1994). In the early L2 stage, lag-2::lacZ and lin-12::lacZ are expressed in both Z1.ppp and Z4.aaa. Subsequently, expression of lag-2::lacZ becomes restricted to the presumptive AC and expression of lin-12::lacZ becomes restricted to the presumptive VU. The reciprocal change in lag-2 and lin-12 expression is maintained after commitment, with lag-2::lacZ expression evident in the AC and lin-12::lacZ in the VU (Wilkinson et al. 1994).
We examined the accumulation of HLH-2 protein by costaining lag-2::lacZ or lin-12::lacZ transgenic hermaphrodites with anti-HLH-2 and anti-LacZ. We classified them as to whether one or both cells express the lacZ marker, and which, if any, of the cells express HLH-2. We identified two categories of hermaphrodites that express lag-2::lacZ or lin-12::lacZ in both Z1.ppp and Z4.aaa, and thus, are at an early point in the AC/VU decision; those that do not display HLH-2 accumulation in either cell (Fig. 2A,B), and those that display HLH-2 accumulation in one of the two cells (Fig. 2C,D). We interpret these results as reflecting a temporal sequence, such that cells that display HLH-2 accumulation have progressed further toward resolving the AC/VU decision than cells that do not. In hermaphrodites in which the AC/VU decision has advanced even further, lag-2::lacZ expression is restricted to the presumptive AC or lin-12::lacZ is restricted to the presumptive VU. In these hermaphrodites, HLH-2 accumulation is evident only in the presumptive AC (Fig. 2E,F).
Figure 2.
Dynamic pattern of HLH-2 protein accumulation during the AC/VU decision. (A-F) Confocal micrographs of L2 hermaphrodite gonads. Hermaphrodites stained with antibodies against HLH-2 (green) and LacZ (red); cells in which both antibodies are detected appear yellow. Small yellow arrows indicate Z1.ppp and Z4.aaa. Larger white arrows indicate HLH-2 expression in the presumptive AC. Distal tip cells are marked with an asterisk. Photomicrographs are arranged in an inferred temporal sequence, on the basis of the observation that lin-12::lacZ and lag-2::lacZ are initially expressed in both Z1.ppp and Z4.aaa, but become restricted to the presumptive AC (lag-2::lacZ expressing) or presumptive VU (lin-12::lacZ expressing). Only (and all) animals that showed HLH-2 expression in both distal tip cells as well as lin-12::lacZ expression in all six vulval precursor cells (Wilkinson and Greenwald 1995) or lag-2::lacZ expression in both distal tip cells (Henderson et al. 1994) were counted, to be certain that the staining procedure had worked well in each animal scored. (A,C,E) lin-12::lacZ animals (full genotype: smg-1(r861) unc-54(r293); arIs11[lin-12::lacZ]). A total of 36 animals were observed with lin-12::lacZ in both Z1.ppp and Z4.aaa. Of these, 20/36 animals showed no HLH-2 accumulation in either cell (A), and 16/36 showed HLH-2 accumulation in one cell (C). Seven animals were observed in which lin-12::lacZ expression was already restricted to the presumptive VU. Of these, 7/7 showed HLH-2 accumulation in the other cell, the presumptive AC (E). (B,D,F) lag-2::lacZ animals (full genotype: smg-1(r861) unc-54(r293); arIs13[lag-2::lacZ]). Seven animals were observed with lag-2::lacZ expressed in both Z1.ppp and Z4.aaa. Of these, 2/7 animals showed no HLH-2 accumulation in either cell (B), and 5/7 showed HLH-2 accumulation in one cell (D). A total of 21 animals were observed in which lag-2::lacZ expression was restricted to the presumptive AC. Of these, 21/21 showed HLH-2 accumulation costaining the presumptive AC (F).
The presence of HLH-2 in the presumptive AC suggests that HLH-2 may directly regulate lag-2 transcription during the AC/VU decision. We experimentally address this possibility in the next section. Furthermore, because a difference in HLH-2 accumulation precedes the reciprocal change in transcription of lag-2 and lin-12 reporter genes, it may somehow be linked to the stochastic event that creates an initial difference between Z1.ppp and Z4.aaa. We will consider this possibility in subsequent sections.
HLH-2 regulates lag-2 transcription in the presumptive AC
hlh-2 activity is required for the AC/VU decision and HLH-2 accumulates in the presumptive AC, which also expresses lag-2. As lag-2 contains 11 potential HLH-2-binding sequences (called E-boxes, see below) in its 5′- and 3′-flanking regions (Fig. 3A), a simple hypothesis that is consistent with these observations is that HLH-2 positively and directly regulates lag-2 transcription in the presumptive AC.
Figure 3.
HLH-2 regulates lag-2 transcription in the presumptive AC. (A) The 5′- and 3′-flanking regions of lag-2 contains 11 E-boxes (CANNTG) of the type predicted to bind HLH-2 (see text). (B) A lag-2::lacZ transcriptional reporter was expressed in the AC in 64/68 gfp(RNAi) negative control hermaphrodites that showed lacZ expression in both distal tip cells (B, top). In contrast, the AC expression was lost in 39/49 hlh-2(RNAi-L2) animals that still showed lacZ expression in both distal tip cells (B, bottom). Expression in both distal tip cells served as an internal control for the staining procedure, as we had ascertained previously that under these conditions, lag-2::lacZ expression in the distal tip cells did not appear to be affected in hlh-2(RNAi-L2); lag-2::lacZ hermaphrodites (data not shown). (C) Expression of lag-2::lacZ constructs with wild-type and mutated E-boxes. Each bar represents an independent transgenic line. Numbers above each bar indicate the number of animals in which lag-2::lacZ expression was observed in the AC over the total number of animals. Three lines (black bars) carry transgenes with the wild-type lag-2 sequence driving lacZ expression (the same construct used in B); six lines (hatched bars) carry transgenes in which the 11 E-boxes were mutated from CANNTG to AANNAG. (D) Rescue of the 2 AC defect of lag-2(q420ts) at 25°C by lag-2 genomic constructs. Each bar indicates an independent transgenic line. Numbers above each bar indicate the number of animals with 1 AC (i.e., rescued) over the total. Four lines (black bars) carry transgenes formed from wild-type lag-2 genomic sequence; five lines (hatched bars) carry transgenes formed from a lag-2 genomic sequence, in which each of the 11 E-boxes was mutated from CANNTG to AANNAG; and two lines (gray bars) carried transgenes formed from the genomic sequence of lag-2 with the E-boxes mutated, but with lacZ inserted such that the only protein made will be β-Galactosidase and not LAG-2. These latter lines verify that the transgenes are showing rescue due to production of the LAG-2 protein and not due to the presence of the lag-2 DNA itself.
To determine whether HLH-2 positively regulates lag-2 transcription, we asked whether expression of the lag-2::lacZ reporter depends upon hlh-2 activity. We had technical problems with detecting β-galactosidase activity in individual staged hlh-2 (RNAi-L2); lag-2::lacZ hermaphrodites at the L2 stage, and our anti-LacZ antibody staining protocol is not readily adapted to staining individuals (see Materials and Methods). However, we were able to detect β-galactosidase activity in older (L3) hermaphrodites, after the AC/VU decision has occurred and the AC has fully differentiated, so we asked whether hlh-2 activity is required for lag-2 transcription at this stage. We found that delivery of hlh-2 dsRNA beginning in the L2 stage was highly effective at abolishing lag-2::lacZ expression in the AC of hermaphrodites scored in the L3 stage (Fig. 3B), indicating that hlh-2 activity is required for lag-2 transcription in the AC.
To determine whether HLH-2 regulation of lag-2 may be direct, we asked whether intact E-boxes are required for lag-2 expression. The E-box is a well-studied binding site for bHLH proteins, and has the consensus sequence CANNTG. Daughterless, the Drosophila ortholog of HLH-2, has been found to bind preferentially in vitro to a subset of possible E-boxes, described as CAGC/GTG (Ohsako et al. 1994), or A/GCAGNTGN (Van Doren et al. 1991). In C. elegans, Krause et al. (1997) have shown that HLH-2 is capable of binding in vitro to the sequence GCAGGTG, which fits both Daughterless consensus sequences. Krause et al. (1997) have further shown that the binding is specific, as HLH-2 no longer binds the mutated site GAAGGAG. Subsequently, three additional C. elegans groups have all shown specific in vitro binding of HLH-2 to similar sites: GCAGGTG (Portman and Emmons 2000), ACAGNTG (Thellmann et al. 2003), A/GCAGGTG (Hwang and Sternberg 2004).
The 11 E-boxes that fit either or both of the Daughterless consensus-binding sequences were mutated from CANNTG to AANNAG. When we examined the consequence of mutating these E-boxes on expression of the lag-2::lacZ reporter in the AC, in most lines, we observed a decrease in lag-2::lacZ expression in the AC relative to wild-type controls (Fig. 3C), suggesting that the E-boxes are important for lag-2 transcription. As described above, we could not reliably detect lacZ activity in individual L2 hermaphrodites during the AC/VU decision. Therefore, to examine the requirement for E-boxes during the AC/VU decision, we examined the effect of mutating the E-boxes on the ability of a genomic fragment of lag-2 to rescue the highly penetrant 2 AC defect of a lag-2 temperature-sensitive allele, lag-2(q420) at the nonpermissive temperature (Lambie and Kimble 1991; Fig. 3D). We found that mutation of the E-boxes significantly impaired the ability of the genomic fragment to rescue the 2 AC phenotype as compared with an unmutated control (Fig. 3D), indicating that the E-boxes are necessary for efficient lag-2 expression during the AC/VU decision in vivo. The low level of residual lag-2 activity could be explained by low-affinity binding of HLH-2 to noncanonical sites, such as has been seen in Drosophila (Yang et al. 2001). We note that mutation of the E-boxes does not appear to impair the ability of the genomic fragment to rescue the larval lethality resulting from other aberrant cell-fate decisions, suggesting that HLH-2 is not a general factor for lag-2 transcription.
Post-transcriptional down-regulation of HLH-2 in the presumptive VU restricts HLH-2 accumulation to the presumptive AC
HLH-2 accumulates specifically in the presumptive AC. A simple expectation is that this pattern of accumulation reflects differential transcription of hlh-2 in the presumptive AC. Krause et al. (1997) showed that in the C. elegans embryo, HLH-2 accumulates in a dynamic and restricted pattern that reflects the transcriptional pattern of the hlh-2 gene.
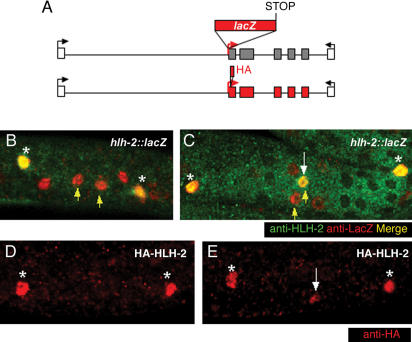
An hlh-2::lacZ transcriptional reporter containing just 5′-flanking sequence (Krause et al. 1997) is not expressed in Z1.ppp and Z4.aaa or in the distal tip cells, suggesting that important gonadal regulatory elements are missing (data not shown). We therefore designed an hlh-2::lacZ reporter containing introns and 3′-flanking region (Fig. 4A). This reporter construct has the same basic design as lin-12::lacZ (Wilkinson et al. 1994), and requires a smg-1 background for expression. When we examined the expression of this hlh-2::lacZ transcriptional reporter (Fig. 4), we found that hlh-2 appears to be transcribed in both Z1.ppp and Z4.aaa, even though HLH-2 protein is never detected in more than the presumptive AC (Fig. 4B,C). The observation that the transcriptional reporter is expressed in the presumptive VU, in which HLH-2 protein accumulation is not detected, suggests that HLH-2 protein accumulation may be post-transcriptionally regulated.
Figure 4.
Post-transcriptional regulation of HLH-2 during the AC/VU decision. (A) Schematic depiction of the hlh-2::lacZ transcriptional reporter and the HA-HLH-2 translational reporter. Coding regions are shown as boxes, with noncoding regions as lines. Red indicates that the region will be translated, whereas gray coding regions should not be translated, as they follow a stop codon. The reporters include the region from the end of the upstream-most gene (left white box) to the beginning of the downstream most gene (right white box). (B,C) Confocal photomicrographs, small yellow arrows indicate Z1.ppp and Z4.aaa. Larger white arrows indicate HLH-2 expression in the presumptive AC. Distal tip cells are marked with an asterisk. An hlh-2::lacZ transcriptional reporter consistently shows expression in both Z1.ppp and Z4.aaa and their sisters (LacZ-positive cells on either side of Z1.ppp and Z4.aaa) in two independent transgenic lines carrying complex arrays (full genotypes: smg-1(r861) unc-54(r293); pha-1 (e2123); arEx445 and smg-1(r861) unc-54(r293); pha-1 (e2123); arEx467). All animals (and only animals) with both distal tip cells expressing hlh-2::lacZ were counted; 39/40 arEx445 animals and 27/30 arEx467 animals had detectable LacZ expression in both Z1.ppp and Z4.aaa. The level of LacZ appeared to be the same in Z1.ppp, Z4.aaa, and their sisters. Post-transcriptional regulation is inferred because the patterns of LacZ and endogenous HLH-2 accumulation are not coincident. Of animals with detectable LacZ expression in both Z1.ppp and Z4.aaa, 39/39 arEx445 animals and 27/27 arEx467 animals showed HLH-2 accumulation in neither B or only one C of the two cells (B is a dorsolateral view and C is a ventral view). In addition, HLH-2 was not observed in the sisters of Z1.ppp and Z4.aaa, although hlh-2::lacZ is expressed there. (D,E) To examine HLH-2 accumulation under conditions that are directly comparable with the hlh-2::lacZ transcriptional reporter, we generated complex arrays using the identical regulatory region (A), in which HLH-2 was tagged with HA. In lines carrying these arrays [smg-1(r861) unc-54(r293); pha-1 (e2123); arEx431 (shown), smg-1(r861) unc-54(r293); pha-1 (e2123); arEx425], HA-HLH-2, detected with an anti-HA antibody, displayed the same pattern of accumulation as endogenous HLH-2; when we could be confident of scoring Z1.ppp and Z4.aaa, HA-HLH-2 was absent from both Z1.ppp or Z4.aaa, 15/28 arEx425 animals and 18/45 arEx431 animals (D), or present in only one of the two cells, 13/28 arEx425 animals and 27/45 arEx431 animals (E). We also generated lines in which HA-HLH-2 and hlh-2::lacZ were present in the same transgenic array; however, we were unable to find staining conditions in which both antigens could be visualized simultaneously (data not shown).
We were concerned that the difference in expression between the hlh-2::lacZ transcriptional reporter and the endogenous HLH-2 protein may be an artifact due to overexpression from the hlh-2::lacZ extrachromosomal arrays, missing repressor elements in the reporter, or other causes related to transgenes. We therefore made a translational reporter, HA-HLH-2, under the control of identical regulatory sequences as hlh-2::lacZ for comparison (Fig. 4A). Transgenic lines were generated at the same concentrations and in a smg-1 background, as for hlh-2::lacZ (see Materials and Methods). We found that HA-HLH-2 showed the same pattern of accumulation as endogenous HLH-2 protein (Fig. 4D,E). We again saw two categories of animals, those with accumulation in neither Z1.ppp nor Z4.aaa (younger animals, Fig. 4D), and those with accumulation in one cell (older animals, Fig. 4E). The similar patterns of accumulation of HA-HLH-2 and endogenous HLH-2 indicate that the difference in expression between the hlh-2::lacZ transcriptional reporter and the endogenous HLH-2 protein is real, and therefore, that the level of HLH-2 accumulation is regulated post-transcriptionally.
We note that we have also observed that HLH-2 does not accumulate in two other cells in which hlh-2 is transcribed, Z1.ppa and Z4.aap, the sisters of Z1.ppp and Z4.aaa (Fig. 4). These cells have the potential to become ACs (Seydoux et al. 1990), but in wild-type hermaphrodites, invariably become VUs (Kimble and Hirsh 1979). The apparent post-transcriptional regulation that occurs in these cells further suggests that the VU fate and down-regulation of HLH-2 are intimately connected.
Birth order influences whether Z1.ppp or Z4.aaa becomes the AC
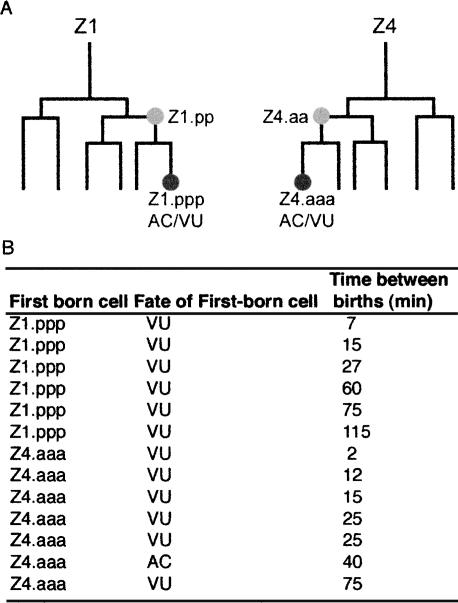
The observation that HLH-2 accumulation in the presumptive AC is the first detectable difference between Z1.ppp and Z4.aaa suggests that the activity of hlh-2 may somehow be linked to an earlier event that creates an initial difference between Z1.ppp and Z4.aaa. In considering potential early influences on the AC/VU decision, we wondered whether there might be a difference in the time of birth between Z1.ppp and Z4.aaa (which are not sisters; Fig. 5), and if so, whether birth order might influence their fates. We therefore examined the Z1 and Z4 lineages of 13 wild-type hermaphrodites, and found that there is a difference in the time at which Z1.ppp and Z4.aaa are born. This difference ranged from 2 min to 2 h. From the data shown in Figure 5, it is apparent that birth order does influence the AC/VU decision; in 12/13 hermaphrodites, the first-born cell became the presumptive VU. In the one exceptional case, the two cells were born 40 min apart (the median time difference is 30 min).
Figure 5.
Birth order influences the AC/VU decision. (A) The cell lineage of the somatic gonad founder cells Z1 and Z4 is pictured. These lineages are mirror symmetric (Kimble and Hirsh 1979), so that Z1.ppp and Z4.aaa are lineal homologs. Z1 and Z4 are not sisters, and are descended by six cell divisions from a common ancestor. (B) The cell lineages of 13 wild-type (N2) hermaphrodites were observed to determine which cell was born first, the time between cell births, and the fates each cell assumed (evident by position and morphology). The likelihood that we would observe the first-born cell adopting the VU fate in 12/13 hermaphrodites by chance, if there were no birth-order bias, is <0.5% [χ2 = (9.31,1)]. Lineage analysis was carried out as described (Sulston and Horvitz 1977).
Discussion
The AC/VU decision is a paradigm for LIN-12/Notch mediated cell-cell interactions that occur between equivalent cells to make them different. Z1.ppp and Z4.aaa are two equivalent cells that interact so that one becomes the AC and the other a VU. During the AC/VU decision, lag-2 and lin-12 are initially transcribed in both Z1.ppp and Z4.aaa, but then transcription becomes restricted, such that one cell expresses only lag-2 and becomes the AC and the other expresses only lin-12 and becomes the VU (Wilkinson et al. 1994). LIN-12 protein accumulation reflects this pattern of transcription (Levitan and Greenwald 1998). lin-12 activity in the presumptive VU leads to positive autoregulation of lin-12 transcription and to down-regulation of lag-2 transcription (Wilkinson et al. 1994). An important feature of the AC/VU decision is that it appears to be stochastic, in that in half of wild-type hermaphrodites, Z1.ppp becomes the AC, whereas in the other half, Z4.aaa becomes the AC (Kimble and Hirsh 1979).
In this study, we have focused on the function and regulation of hlh-2, the C. elegans ortholog of Drosophila Daughterless and mammalian E proteins (Krause et al. 1997). We have established a role for hlh-2 in promoting transcription of lag-2 by obtaining genetic evidence that hlh-2 activity is important for the AC/VU decision, showing that hlh-2 activity is required for lag-2 expression in the AC, and demonstrating that potential HLH-2 binding sites in the lag-2 promoter are necessary for efficient transcription during the AC/VU decision.
We also report three main additional findings that, in conjunction with the basic characterization of the role of hlh-2, lead to new insights into the AC/VU decision that will be discussed further below. First, the accumulation of HLH-2 protein is restricted to the presumptive AC prior to the reciprocal changes in expression of lag-2 and lin-12, and hence, is the earliest manifestation of a nascent difference between Z1.ppp and Z4.aaa detected to date. The inferred temporal sequence of HLH-2 accumulation suggests that hlh-2 functions after the initial phase of lag-2 expression, at the time that the stochastic difference in the level of lin-12 activity is being amplified by the feedback mechanism. Second, the absence of HLH-2 accumulation in the presumptive VU appears to reflect post-transcriptional down-regulation of HLH-2, and not transcriptional regulation. Third, the relative birth order of Z1.ppp and Z4.aaa is highly correlated with their fates; the first-born cell becomes a VU, and the second-born, the AC. Thus, birth order may be the stochastic event that influences the initial level of lin-12 activity in Z1.ppp and Z4.aaa.
HLH-2 as a component of the feedback loop that leads to transcriptional down-regulation of lag-2 in the presumptive VU
Because hlh-2 promotes transcription of lag-2 during the AC/VU decision, negative regulation of hlh-2 activity in the presumptive VU could be the proximate cause of loss of lag-2 expression in a negative feedback loop. The temporal pattern of HLH-2 accumulation and the negative regulation of HLH-2 accumulation in the presumptive VU are consistent with this possibility.
We have inferred a temporal sequence of HLH-2 accumulation during the AC/VU decision as follows: (1) the absence of detectable HLH-2 accumulation in Z1.ppp and Z4.aaa, (2) HLH-2 accumulation restricted to the presumptive AC prior to the restriction of lag-2 transcription to that cell, and (3) the restriction of both HLH-2 accumulation and lag-2 transcription to the presumptive AC. This inferred temporal sequence assumes that the LacZ moiety accurately reflects the time that lag-2::lacZ is expressed. The initial absence of detectable HLH-2 in Z1.ppp or Z4.aaa while both express lag-2::lacZ may be accounted for by proposing that another transcription factor is principally responsible for the initial phase of lag-2 transcription immediately after Z1.ppp and Z4.aaa are born, and that the role of HLH-2 is principally to promote the maintenance or up-regulation of lag-2 transcription as the AC/VU decision progresses. Alternatively, it may be that the initial phase of lag-2 transcription requires a lower level of HLH-2, below the level of detection in our experiments. The presence of HLH-2 accumulation in the presumptive AC prior to the restriction of lag-2::lacZ expression to just that cell is consistent with hlh-2 function in the presumptive AC at the time when the difference between Z1.ppp and Z4.aaa is being amplified via the activity of feedback loops that depend on lin-12 activity.
HLH-2 becomes restricted to the presumptive AC by post-transcriptional down-regulation of HLH-2 accumulation in the presumptive VU. A transcriptional reporter for hlh-2 is expressed at a uniform level in both Z1.ppp and Z4.aaa continuously from birth throughout the AC/VU decision, and comparable expression of the transcriptional reporter is seen in both cells even when HLH-2 accumulation is evident in only one of them. The absence of HLH-2 accumulation in the presumptive VU despite hlh-2 transcription argues that the level of HLH-2 is regulated post-transcriptionally. The hlh-2::lacZ and HA-HLH-2 constructs contain identical regulatory sequences, but encode different proteins, suggesting that HLH-2 accumulation is regulated post-translationally.
Amodel for the negative feedback loop in the AC/VU decision
Our results suggest that hlh-2 functions in a negative feedback loop that operates during the AC/VU decision. We propose that activation of LIN-12 leads to expression or activation of a factor, X, that serves to down-regulate the level of HLH-2 in the presumptive VU post-transcriptionally (Fig. 6A). An interesting possibility is that the gene encoding X is a direct transcriptional target of the LAG-1-LIN-12(intra) complex.
Figure 6.
Models of negative feedback and birth order. (A) Model for the negative feedback loop. Activation of LIN-12 leads to expression of a factor, X, that causes down-regulation of HLH-2. In a simple circuit, X may be a direct transcriptional target of the LIN-12/LAG-1 complex. (B,C) Models to explain how LIN-12 activity may be higher in the first-born cell (see text for additional details). Only Z1.ppp is shown as becoming the presumptive VU and Z4.aaa, the presumptive AC, for convenience. (B) Advantage due to increased LIN-12 activity. LIN-12 is able to accumulate to a higher concentration in the first-born cell, giving the first-born cell an advantage in becoming the VU. Alternatively, LIN-12 may be activated by a ligand coming from a cell other than Z4.aaa, or Z1.ppp may receive a different signaling input that influences LIN-12 activity indirectly. (C) Advantage due to cell cycle progression. If LIN-12 signaling is effective only after progression through the cell cycle (see text; Ambros 1999), then, the first-born cell would have an advantage in getting to this phase and activating LIN-12.
A feedback loop involving post-transcriptional regulation of HLH-2 in the AC/VU decision is logically parallel to the circuit that operates during SOP specification in Drosophila, but differs in the mechanism by which a critical regulatory step occurs. In particular, our postulated factor X, a negative regulator of HLH-2 accumulation, plays the same formal role as the E(spl)/Gro complex, a negative regulator of AS-C transcription. In this context, we note that unc-37 and lin-22, the C. elegans counterparts of gro and E(spl) (Pflugrad et al. 1997; Wrischnik and Kenyon 1997), do not appear to function in the AC/VU decision (see Materials and Methods), consistent with a different mode of regulation for hlh-2.
Although we have focused here on the regulation of HLH-2 in the negative feedback loop, we note that there may also be positive feedback that leads to up-regulation of HLH-2 in the presumptive AC. Positive autoregulation of AS-C transcription underlies the positive feedback loop that operates in the presumptive SOP (Culi and Modolell 1998). However, if there is positive feedback control of HLH-2, our data would suggest that it also involves a post-transcriptional mechanism, as the level of hlh-2 transcription appears to be roughly the same in both Z1.ppp and Z4.aaa.
In cultured mammalian cells, HES genes [E(spl) homologs] have been shown to be targets of Notch signaling (Jarriault et al. 1995); HES genes also serve as negative regulators of the transcription of homologs of AS-C (Chen et al. 1997), suggesting that the same negative feedback circuit that has been defined in Drosophila is conserved. However, it has also been reported that Notch activation can lead to rapid degradation of the human Achaete-Scute homolog 1 protein as well as degradation of the HLH-2 homologs encoded by the mammalian E2A gene (Sriuranpong et al. 2002; Nie et al. 2003). Perhaps these observations reflect a role for post-translational regulation of the transcriptional activators of Delta expression during cell fate decisions in mammals.
The influence of birth order and the nature of the stochastic event
The key to understanding how the AC/VU decision is initiated is the nature of the stochastic event. At the molecular level, we have envisaged that it is a random difference in the level of ligand or receptor activity, which might reflect something as simple as a random difference in the number of ligand or receptor molecules at the cell surface (Seydoux and Greenwald 1989). Because gene expression itself is inherently stochastic, leading to heterogeneity in individual cells within a clonal population (e.g., Blake et al. 2003), it is unlikely that Z1.ppp and Z4.aaa (which are not sisters, but lineal homologs that have not shared a common cellular ancestor for many cell divisions) have the same concentration of components of the LIN-12/Notch pathway. Experiments in Drosophila suggest that as little as a 2:3 ratio in Notch gene dosage can be efficiently amplified by the feedback mechanism that operates during SOP specification (Heitzler and Simpson 1991). Thus, it would seem in principle that amplification of random differences in transcription of LIN-12/Notch pathway components by feedback mechanisms could account for the accuracy of the AC/VU decision in wild-type hermaphrodites.
However, we have identified an additional influence on the AC/VU decision, birth order. Z1.ppp and Z4.aaa are born at the end of the first phase of the Z1 and Z4 lineages, in the late L1 or early L2 stage (Kimble and Hirsh 1979). We have found that there is a stochastic difference in the time of their birth, ranging from 2 min to 2 h, and that this difference strikingly correlates with cell fate; the first-born usually becomes a VU, and the second-born usually becomes an AC. We note that the correlation is not absolute, as in one individual (of 13), the first-born cell became the AC, suggesting that birth order is biasing, rather than determining, the outcome of the AC/VU decision. As birth order appears to be random, but highly correlated with the outcome of the AC/VU decision, it appears that birth order is the stochastic event that sets the feedback loops into play.
As HLH-2 is the first detectable difference between the presumptive AC and the presumptive VU, it is tempting to try to forge a link between birth order and hlh-2 regulation. If the first-born cell has an advantage in activating LIN-12, then that may set into play the feedback loops that amplify the birth-order advantage, such as driving the accumulation of a negative regulator of HLH-2 (Factor X). Less HLH-2 would mean a disadvantage in the maintenance phase of lag-2 expression, thereby helping to bias the first-born cell against becoming a signaling cell, and therefore, toward becoming a receiving cell.
There are several different ways an advantage in activating LIN-12 in the first-born cell might be achieved. For example, a cell may begin to accumulate LIN-12 at birth, giving the first-born an advantage when the second-born cell appears and the two cells start to interact (Fig. 6B). In one variation on this theme, LIN-12, newly synthesized or even inherited from its parent, may be activated in the first-born cell by LAG-2 present on the parent of the second-born cell, or by a related ligand emanating from elsewhere (at a level that is not sufficient to promote the VU fate, but which can activate LIN-12 to a sufficient extent to account for the birth-order bias). Another way that the first-born cell might gain an advantage in activating LIN-12 - or that the second-born cell might be at a disadvantage - would be if other signal-transduction pathways act to influence LIN-12 activity. This kind of bias would be analogous to the establishment of polarity in the developing Drosophila ommatidium, in which positional cues cause a higher level of Frizzled activity in one cell of the R3/R4 pair, biasing that cell to lower Notch activity, and hence, to the R3 fate (Tomlinson and Struhl 1999).
An alternative hypothesis is that the first-born cell has an advantage because it begins its progression through the cell cycle first (Fig. 6C). This hypothesis is inspired by the work of Ambros (1999), who found evidence that passage through S phase into G2 is necessary for LIN-12 signal transduction to be manifest in the vulval precursor cells. If similar cell-cycle gating operates during the AC/VU decision, perhaps the first-born cell has a greater probability of reaching G2 first, and hence, adopting the VU fate associated with LIN-12 activation. As the VU divides a few hours after it has been specified, whereas the AC is a terminally differentiated cell (Kimble and Hirsh 1979), a potential connection between LIN-12 activation and cell-cycle progression in the presumptive VU seems plausible.
Materials and methods
C. elegans strains and genetic analysis
Conventional genetic methods and the wild-type parent of all strains used in this study, C. elegans var. Bristol strain N2, were as described by Brenner (1974). Strains were grown at 20°C unless otherwise noted. The main alleles used in this work are as follows: LGI smg-1(r861) (Hodgkin et al. 1989). LGIII pha-1(e2123) (Schnabel and Schnabel 1990), LGIV dpy-20(e1282ts) (Honso et al. 1982), LGV lag-2(q420ts) (Lambie and Kimble 1991). Additional information about these alleles, and other markers used for facilitating genetic analysis in this work not listed here, can be found via Wormbase http://www.wormbase.org.
arIs11[lin-12::lacZ, rol-6(d)] and arIs13[lag-::lacZ, rol-6(d)], transcriptional reporters for lin-12 and lag-2, respectively, were described in Wilkinson et al. (1994). All experiments involving these transgenes were performed at 25°C to optimize the activity and stability of LacZ.
The scoring of ACs was greatly facilitated by the use of arIs51, made by attaching the extrachromosomal array in strain NL1008 dpy-20(e1362); Ex[cdh-3::gfp, dpy-20(+)] (Pettitt et al. 1996) by standard procedures (Mello and Fire 1995). arIs51 was outcrossed to N2 five times before use as a marker.
RNA-mediated-interference
Feeding RNA-mediated-interference (RNAi) performed at 20°C (Timmons and Fire 1998; Timmons et al. 2001) was carried out in animals carrying arIs51 (see above) in an otherwise wild-type background. The hlh-2(RNAi-L2) experiments and related controls were performed by treating animals at or near the L1 molt. Synchronized worms were prepared by bleaching hermaphrodite parents, allowing the eggs to hatch in the absence of food, and then arrested L1 larvae were added to plates containing OP50 bacteria (Brenner 1974) for ∼8-13 h, which we found placed most larvae at the correct stage. Some animals were spot-checked by Nomarski microscopy to ensure that most animals were near the L1 molt, and then, animals were washed from their plates and added to plates containing bacteria expressing the relevant dsRNA. Similar results were obtained using dsRNA corresponding to the full-length hlh-2 cDNA (Fig. 1), pXK74 (the 5′ half; 4/20 2 AC), and pKM1201 (the 3′ half; 2/28 2 AC).
As there has been no independent evidence confirming the supposition that lag-1 is involved in the AC/VU decision, we also examined the role of lag-1 by RNAi. Delivery of dsRNA by feeding was not effective (data not shown), but after the soaking procedure (Tabara et al. 1998), lag-1(RNAi) hermaphrodites have a 2 AC phenotype (7/48 and 25/33 animals in two separate experiments).
Transgenic lines
hlh-2::lacZ transcriptional reporter arEx445, arEx467 [pXK83 hlh-2::lacZ, 1 ng/μL (linearized with ScaI), pBX pha-1(+) 1 ng/μL (linearized with XhoI), N2 genomic DNA 75 ng/μL (cut with PvuII)]. Expression was analyzed in a smg-1; pha-1 genetic background.
HA-HLH-2 translational reporter arEx425, arEx43 [pXK66 HA-HLH-2, 1 ng/μL (linearlized with ScaI), pBX pha-1(+) 1 ng/μL (linearized with XhoI), N2 genomic DNA 75 ng/μL (cut with PvuII)]. Expression was analyzed in a smg-1; pha-1 genetic background.
lag-2::lacZ arrays arEx385, arEx386, and arEx387 [p226SPZ lag-2::lacZ(+) 10 μg/mL, pJP38 cdh-3::gfp 25 μg/mL, pMH86 dpy-20(+) 50 μg/mL], arEx366, arEx367, arEx368, arEx379, arEx380, and arEx381 [pXK60 lag-2::lacZ-E-boxes mutated 10 μg/mL, pJP38 cdh-3::gfp 25 μg/mL, pMH86 dpy-20(+) 50 μg/mL]. Expression was analyzed in a smg-1; dpy-20 background. cdh-3::gfp was used to identify animals in which the array was present in the AC. Such animals were picked individually and stained for β-Galactosidase activity.
lag-2 genomic region arrays arEx344, arEx345, arEx346, and arEx347 [pXK52 lag-2(+) 3 μg/mL, pJP38 cdh-3::gfp 25 μg/mL, pCW2.1 ceh-22::gfp 20 μg/mL], arEx373, arEx374, arEx375, arEx376, and arEx377 [pXK61 lag-2-E-boxes-mutated 3 μg/mL, pJP38 cdh-3::gfp 25 μg/mL, pCW2.1 ceh-22::gfp 20 μg/mL]. arEx351 and arEx352 [pXK34 lag2-E-boxes-mutated::lacZ 3 μg/mL, pJP38 cdh-3::gfp 25 μg/mL, pCW2.1 ceh-22::gfp 20 μg/mL]. Rescue assays were performed in a lag-2(q420) genetic background.
Plasmids
Cotransformation markers pBX pha-1(+) (Granato et al. 1994), pMH86 dpy-20(+) (Han and Sternberg 1991), pJP38 cdh-3::gfp (Pettitt et al. 1996), and pCW2.1 ceh-22::gfp (Okkema et al. 1997).
RNAi The following plasmids encode the given gene cloned into the double T7 RNAi feeding vector pPD129.36 (Timmons and Fire 1998): pPD128.110 gfp (Timmons et al. 2001), pKM1196 full-length hlh-2 cDNA (M. Krause, unpubl.), pXK74 5′ 1/2 hlh-2 cDNA (truncated at the SacI site), pKM1201 (M. Krause, unpubl.) 3′ 1/2 hlh-2 cDNA (truncated at the XhoI site), pNC41.1 lag-2 (N. Chen and I. Greenwald, unpubl.), pXK10 lacZ, pGC2 lag-1 (E.J. Hubbard, unpubl.).
lag-2 rescue pXK52 encodes the genomic region of lag-2, derived from p226SPZ (Wilkinson et al. 1994). It extends from 3.3 kb upstream of the ATG until 1 kb downstream of the stop codon, encompassing the region known to rescue lag-2 (Tax et al. 1994). pXK61 is identical to pXK52, except that the 11 E-boxes described in the text were mutated from CANNTG to AANNAG by site-directed mutagenesis (Stratagene #200514). Details are available upon request. pXK34 is similar to pXK61, except that lacZ is inserted at the ATG in the exact manner described for p226SPZ. In addition, E-box 10 was not mutated in this version.
lacZ reporters p226SPZ lag-2::lacZ (Wilkinson et al. 1994). pXK60 lag-2::lacZ E-boxes mutated. This plasmid is identical to p226SPZ, except for the same mutant E-boxes as in pXK61. pXK83 encodes the transcriptional reporter hlh-2::lacZ made in a similar manner to p226SPZ. A total of 12 kb of genomic sequence of hlh-2 was PCR amplified from N2 genomic DNA. This encompasses the entire region between the genes located upstream and downstream from hlh-2, including 8.4 kb of 5′ region, 2.7 kb of coding sequence plus introns, and 0.9 kb of 3′ region (Fig. 4). Homologous recombination (Yu et al. 2000) was used (DY330 bacterial strain kindly provided by Donald Court) to insert lacZ from pPD16.43 (Fire et al. 1990) in-frame following the ATG of hlh-2 beginning with the second codon of lacZ. The lacZ gene also contains its own stop codon, so that the only protein produced is β-Galactosidase.
HA-HLH-2 translational reporter pXK66 was made from the hlh-2::lacZ construct by removing lacZ using homologous recombination and substituting an HA tag immediately following the ATG. It is in-frame, and thus produces a full-length HLH-2 protein with the amino-terminal HA tag.
Immunofluorescence and β-galactosidase activity staining
Embryos were isolated by bleaching and allowed to grow at 25°C until the desired stage. For immunofluorescence, worms were fixed according to the modified Finney-Ruvkun procedure and stained with a rabbit anti-HLH-2 antibody at a 1:500 dilution and a mouse anti-LacZ antibody (Promega #Z3781) preadsorbed to C. elegans proteins was used at a 1:1000 or 1:500 dilution in Antibody buffer A. Secondary antibodies used were as follows: goat-anti-rabbit conjugated to Cy2, and goat-anti-mouse conjugated to Cy3 (Jackson ImmunoResearch #111225144 and #115165146, respectively). The stained animals were mounted onto an agarose pad with a drop of SlowFade (Molecular Probes #S-7461), and visualized with a Nikon Eclipse E800 microscope. Photomicrographs were acquired with a Bio-Rad MRC 1024ES laser scanning confocal attachment at ∼1200× magnification.
For β-galactosidase activity staining, staged animals were grown for at least 12 h at 25°C prior to fixation, washed off plates with M9 buffer, and washed with filtered double-distilled water to remove all traces of food bacteria. They were then fixed and stained according to Wilkinson et al. (1994) and observed using a Zeiss Axioplan 2 at 400× or 800× magnification. We note that Wilkinson et al. (1994) were able to detect β-galactosidase activity in individual-stained L2 hermaphrodites; however, we were unable to detect β-galactosidase activity reliably at this stage. We attribute this problem to somewhat attenuated expression of the arIs13 transgene after several years, a problem that may have developed because it is derived from a simple array (see Hsieh et al. 1999).
lag-2 rescue experiments
Arrays were generated in a lag-2(q420ts) background to determine whether pXK52, pXK61, or pXK34 were able to rescue the 2 AC defect. Extrachromosomal arrays included cdh-3::gfp, to mark the AC(s). If one AC or more expresses GFP, the array is present in that cell and lag-2 should be expressed if the promoter is active. Additionally, as LAG-2 is a transmembrane ligand, expression in only one of the two cells (in the case of a rare mosaic animal) should be sufficient to rescue; the other cell should receive that signal and become a VU.
unc-37 and lin-22
In C. elegans, unc-37 is the ortholog of gro (Pflugrad et al. 1997) and lin-22 is the closest relative of the E(spl) genes (Wrischnik and Kenyon 1997; X. Karp and I. Greenwald, unpubl.). Apparent null alleles of unc-37 and lin-22 do not display defects in the AC/VU decision; 34/34 unc-37(wd17wd21) homozygotes segregating from unc-37/dpy-14 parents and 30/30 lin-22(mu2) homozygotes have one AC.
Acknowledgments
We thank Mike Krause for extraordinary generosity with advice, reagents, and discussion throughout this project. We also thank Byung Joon Hwang, Paul Sternberg, Doug Portman, and Scott Emmons for reagents and information prior to publication, and Oliver Hobert for expert advice on analyzing phylogenetic relationships. We thank Xinlan Zhou and Richard Ruiz for technical assistance, and Sophie Jarriault, Mike Krause, Dan Shaye, and Gary Struhl for helpful comments on the manuscript. X.K. was supported in part by training grant GM07088 (to the Department of Genetics and Development) and by NIH grants NS35556 and CA095389 (to I.G.). I.G. is an Investigator of the Howard Hughes Medical Institute.
The publication costs of this article were defrayed in part by payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 USC section 1734 solely to indicate this fact.
Article and publication are at http://www.genesdev.org/cgi/doi/10.1101/gad.1160803.
References
- Ambros V. 1999. Cell cycle-dependent sequencing of cell fate decisions in Caenorhabditis elegans vulva precursor cells. Development 126: 1947-1956. [DOI] [PubMed] [Google Scholar]
- Bailey A.M. and Posakony, J.W. 1995. Suppressor of Hairless directly activates transcription of Enhancer of split complex genes in response to Notch receptor activity. Genes & Dev. 9: 2609-2622. [DOI] [PubMed] [Google Scholar]
- Blake W.J., Kaern, M., Cantor, C.R., and Collins, J.J. 2003. Noise in eukaryotic gene expression. Nature 422: 633-637. [DOI] [PubMed] [Google Scholar]
- Brenner S. 1974. The genetics of Caenorhabditis elegans. Genetics 77: 71-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H., Thiagalingam, A., Chopra, H., Borges, M.W., Feder, J.N., Nelkin, B.D., Baylin, S.B., and Ball, D.W. 1997. Conservation of the Drosophila lateral inhibition pathway in human lung cancer: A hairy-related protein (HES-1) directly represses achaete-scute homolog-1 expression. Proc. Natl. Acad. Sci. 94: 5355-5360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cronmiller C. and Cummings, C.A. 1993. The daughterless gene product in Drosophila is a nuclear protein that is broadly expressed throughout the organism during development. Mech. Dev. 42: 159-169. [DOI] [PubMed] [Google Scholar]
- Culi J. and Modolell, J. 1998. Proneural gene self-stimulation in neural precursors: An essential mechanism for sense organ development that is regulated by Notch signaling. Genes & Dev. 12: 2036-2047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fire A., Harrison, S.W., and Dixon, D. 1990. A modular set of lacZ fusion vectors for studying gene expression in Caenorhabditis elegans. Gene 93: 189-198. [DOI] [PubMed] [Google Scholar]
- Granato M., Schnabel, H., and Schnabel, R. 1994. Genesis of an organ: Molecular analysis of the pha-1 gene. Development 120: 3005-3017. [DOI] [PubMed] [Google Scholar]
- Greenwald I. 1998. LIN-12/Notch signaling: Lessons from worms and flies. Genes & Dev. 12: 1751-1762. [DOI] [PubMed] [Google Scholar]
- Greenwald I.S., Sternberg, P.W., and Horvitz, H.R. 1983. The lin-12 locus specifies cell fates in C. elegans. Cell 34: 435-444. [DOI] [PubMed] [Google Scholar]
- Han M. and Sternberg, P.W. 1991. Analysis of dominant-negative mutations of the Caenorhabditis elegans let-60 ras gene. Genes & Dev. 5: 2188-2198. [DOI] [PubMed] [Google Scholar]
- Hartenstein V. and J. Posakony, W. 1990. A dual function of the Notch gene in Drosophila sensillum development. Dev. Biol. 142: 12-30. [DOI] [PubMed] [Google Scholar]
- Heitzler P. and Simpson, P. 1991. The choice of cell fate in the epidermis of Drosophila. Cell 64: 1083-1092. [DOI] [PubMed] [Google Scholar]
- Heitzler P., Bourouis, M., Ruel, L., Carteret, C., and Simpson, P. 1996. Genes of the Enhancer of split and achaete-scute complexes are required for a regulatory loop between Notch and Delta during lateral signaling in Drosophila. Development 122: 161-171. [DOI] [PubMed] [Google Scholar]
- Henderson S.T., Gao, D., Lambie, E.J., and Kimble, J. 1994. lag-2 may encode a signaling ligand for the GLP-1 and LIN-12 receptors of C. elegans. Development 120: 2913-2924. [DOI] [PubMed] [Google Scholar]
- Hodgkin J., Papp, A., Pulak, R., Ambros, V., and Anderson, P. 1989. A new kind of informational suppressor in the nematode C. elegans. Genetics 123: 301-313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honso R., Hirahara, K., Kuno, S., and Kurihara, T. 1982. Mutants of Caenorhabditis elegans with dumpy and rounded head phenotypes. J. Exper. Zool. 235: 409-421. [Google Scholar]
- Hsieh J., Liu, J., Kostas, S.A., Chang, C., Sternberg, P.W., and Fire, A. 1999. The RING finger/B-box factor TAM-1 and a retinoblastoma-like protein LIN-35 modulate context-dependent gene silencing in Caenorhabditis elegans. Genes & Dev. 13: 2958-2970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang B.J. and Sternberg, P.W. 2004. A cell-specific enhancer that specifies lin-3 expression in the C. elegans anchor cell for vulval development. Development (in press). [DOI] [PubMed]
- Jarriault S., Brou, C., Logeat, F., Schroeter, E.H., Kopan, R., and Israel, A. 1995. Signalling downstream of activated mammalian Notch. Nature 377: 355-358. [DOI] [PubMed] [Google Scholar]
- Kimble J. 1981. Alteration in cell lineage following laser ablation of cells in the somatic gonad of Caenorhabditis elegans. Dev. Biol. 87: 286-300. [DOI] [PubMed] [Google Scholar]
- Kimble J. and Hirsh, D. 1979. The post-embryonic cell lineages of the hermaphrodites and male gonads in Caenorhabditis elegans. Dev. Biol. 87: 396-417. [DOI] [PubMed] [Google Scholar]
- Krause M., Park, M., Zhang, J.-M., Yuan, J., Harfe, B., Xu, S.-Q., Greenwald, I., Cole, M., Paterson, B., and Fire, A. 1997. A C. elegans E/Daughterless bHLH protein marks neuronal but not striated muscle development. Development 124: 2179-2189. [DOI] [PubMed] [Google Scholar]
- Kunisch M., Haenlin, M., and Campos-Ortega, J.A. 1994. Lateral inhibition mediated by the Drosophila neurogenic gene Delta is enhanced by proneural proteins. Proc. Natl. Acad. Sci. 91: 10139-10143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambie E. and Kimble, J. 1991. Two homologous genes, lin-12 and glp-1, have overlapping functions. Development 112: 231-240. [DOI] [PubMed] [Google Scholar]
- Lecourtois M. and Schweisguth, F. 1995. The neurogenic Suppressor of Hairless DNA-binding protein mediates the transcriptional activation of the Enhancer of split complex genes triggered by Notch signaling. Genes & Dev. 9: 2598-2608. [DOI] [PubMed] [Google Scholar]
- Ledent V. and Vervoot, M. 2001. The basic helix-loop-helix protein family: Comparative genomics and phylogenetic analysis. Genome Res. 11: 754-770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levitan D. and Greenwald, I. 1998. LIN-12 protein expression and localization during vulval development in C. elegans. Development 125: 3101-3109. [DOI] [PubMed] [Google Scholar]
- Massari M.E. and Murre, C. 2000. Helix-loop-helix proteins: Regulators of transcription in eukaryotic organisms. Mol. Cell. Biol. 20: 429-440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mello C. and Fire, A. 1995. DNA transformation. In Caenorhabditis elegans: Modern biological analysis of an organism (eds. H.F. Epstein, and D.C. Shakes), pp. 451-482. Academic, San Diego, CA.
- Murre C., McCaw, P.S., Vassin, H., Caudy, M., Jan, L.Y., Jan, Y.N., Cabrera, C., Buskin, J.N., Hauschka, S.D., Lassar, A.B., et al. 1989. Interactions between heterologous helix-loop-helix proteins generate complexes that bind specifically to a common DNA sequence. Cell 58: 537-544. [DOI] [PubMed] [Google Scholar]
- Nie L., Xu, M., Vladimirova, A., and Sun, X.-H. 2003. Notch-induced E2A ubiquitination and degradation are controlled by MAP kinase activities. EMBO J. 22: 5780-5792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oellers N., Dehio, M., and Knust, E. 1994. bHLH proteins encoded by the Enhancer of split complex of Drosophila negatively interfere with transcriptional activation mediated by proneural genes. Mol. Gen. Genet. 244: 465-473. [DOI] [PubMed] [Google Scholar]
- Ohsako S., Hyer, J., Panganiban, G., Oliver, I., and Caudy, M. 1994. hairy function as a DNA-binding helix-loop-helix repressor of Drosophila sensory organ formation. Genes & Dev. 8: 2743-2755. [DOI] [PubMed] [Google Scholar]
- Okkema P., Ha, E., Haun, C., Chen, W., and Fire, A. 1997. The Caenorhabditis elegans NK-2 homeobox gene ceh-22 activates pharyngeal muscle gene expression in combination with pha-1 and is required for normal pharyngeal development. Development 124: 3965-3973. [DOI] [PubMed] [Google Scholar]
- Paroush Z., Finley, R.L., Kidd, T., Wainwright, S.M., Ingham, P.L., Brent, R., and Ish-Horowitz, D. 1994. Groucho is required for Drosophila neurogenesis, segmentation, and sex-determination and interacts directly with Hairy-related bHLH proteins. Cell 79: 805-815. [DOI] [PubMed] [Google Scholar]
- Pettitt J., Wood, W.B., and Plasterk, R.H. 1996. cdh-3, a gene encoding a member of the cadherin superfamily, functions in epithelial cell morphogenesis in Caenorhabditis elegans. Development 122: 4149-4157. [DOI] [PubMed] [Google Scholar]
- Pflugrad A., Meir, J.Y.-J., Barnes, T.M., and Miller, D.M. 1997. The Groucho-like transcription factor UNC-37 functions with the neural specificity gene unc-4 to govern motor neuron identity in C. elegans. Development 124: 1699-1709. [DOI] [PubMed] [Google Scholar]
- Portman D.S. and Emmons, S.W. 2000. The basic helix-loop-helix transcription factors LIN-32 and HLH-2 function together in multiple steps of a C. elegans neuronal sublineage. Development 127: 5415-5426. [DOI] [PubMed] [Google Scholar]
- Ruvkun G. and Hobert, O. 1998. The taxonomy of developmental control in Caenorhabditis elegans. Science 282: 2033-2041. [DOI] [PubMed] [Google Scholar]
- Schnabel H. and Schnabel, R. 1990. An organ-specific differentiation gene, pha-1, from C. elegans. Science 250: 686-688. [DOI] [PubMed] [Google Scholar]
- Seydoux G. and Greenwald, I. 1989. Cell autonomy of lin-12 function in a cell fate decision in C. elegans. Cell 57: 1237-1245. [DOI] [PubMed] [Google Scholar]
- Seydoux G., Schedl, T., and Greenwald, I. 1990. Cell-cell interactions prevent a potential inductive interaction between soma and germline in C. elegans. Cell 61: 939-951. [DOI] [PubMed] [Google Scholar]
- Simpson P. 1990. Lateral inhibition and the development of the sensory bristles of the adult peripheral nervous system of Drosophila. Development 109: 509-519. [DOI] [PubMed] [Google Scholar]
- Smith J.E. and Cronmiller, C. 2001. The Drosophila daughterless gene autoregulates and is controlled by both positive and negative cis regulation. Development 128: 4705-4714. [DOI] [PubMed] [Google Scholar]
- Sriuranpong V., Borges, M.W., Strock, C.L., Nakakura, E.K., Watkins, D.N., Blaumeueller, C.M., Nelkin, B.D., and Ball, D.W. 2002. Notch signaling induces rapid degradation of achaete-scute homolog 1. Mol. Cell. Biol. 22: 3129-3139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sulston J.E. and Horvitz, H.R. 1977. Post-embryonic cell lineages of the nematode Caenorhabditis elegans. Dev. Biol. 56: 110-156. [DOI] [PubMed] [Google Scholar]
- Tabara H., Grishok, A., and Mello, C.C. 1998. RNAi in C. elegans: Soaking in the genomic sequence. Science 282: 430-431. [DOI] [PubMed] [Google Scholar]
- Tax F.E., Yeagers, J.J., and Thomas, J.H. 1994. Sequence of C. elegans lag-2 reveals a cell-signaling domain shared with Delta and Serrate of Drosophila. Nature 368: 150-154. [DOI] [PubMed] [Google Scholar]
- Thellmann M., Hatzold, J., and Conradt, B. 2003. The Snail-like CES-1 protein of C. elegans can block the expression of the BH3-only cell-death activator gene egl-1 by antagonizing the function of bHLH proteins. Development 130: 4057-4071. [DOI] [PubMed] [Google Scholar]
- Timmons L. and Fire, A. 1998. Specific interference by ingested dsRNA. Nature 395: 854. [DOI] [PubMed] [Google Scholar]
- Timmons L., Court, D.L., and Fire, A. 2001. Ingestion of bacterially expressed dsRNAs can produce specific and potentgenetic interference in Caenorhabditis elegans. Gene 263: 103-112. [DOI] [PubMed] [Google Scholar]
- Tomlinson A. and Struhl, G. 1999. Decoding vectorial information from a gradient: Sequential roles of the receptors Frizzled and Notch in establishing planar polarity in the Drosophila eye. Development 126: 5725-5738. [DOI] [PubMed] [Google Scholar]
- Van Doren M., Ellis, H.M., and Posakony, J.W. 1991. The Drosophila extramacrochaetae protein antagonizes sequence-specific DNA binding by daughterless/achaete-scute protein complexes. Development 113: 245-255. [DOI] [PubMed] [Google Scholar]
- Wilkinson H.A. and Greenwald, I. 1995. Spatial and temporal patterns of lin-12 expression during C. elegans hermaphrodite development. Genetics 141: 513-526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson H.A., Fitzgerald, K., and Greenwald, I. 1994. Reciprocal changes in expression of the receptor lin-12 and its ligand lag-2 prior to commitment in a C. elegans cell fate decision. Cell 79: 1187-1198. [DOI] [PubMed] [Google Scholar]
- Wrischnik L.A. and Kenyon, C.J. 1997. The role of lin-22, a hairy/enhancer of split homolog, in patterning the peripheral nervous system of C. elegans. Development 124: 2875-2888. [DOI] [PubMed] [Google Scholar]
- Yang D., Lu, H., Hong, Y., Jinks, T.M., Estes, P.A., and Erickson, J.W. 2001. Interpretation of X chromosome dose at Sex-lethal requires non-E-box sites for the basic helix-loop-helix proteins SISB and daughterless. Mol. Cell. Biol. 21: 1581-1592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu D., Ellis, H.M., Lee, E.C., Jenkins, N.A., Copeland, N.G., and Court, D.L. 2000. An efficient recombination system for chromosome engineering in Escherichia coli. Proc. Natl. Acad. Sci. 97: 5978-5983. [DOI] [PMC free article] [PubMed] [Google Scholar]