Abstract
Objective
We wanted to evaluate the status of self-expandable nitinol stents implanted in the P2 and P3 segments of the popliteal artery in Korean patients.
Materials and Methods
We retrospectively analyzed 189 consecutive patients who underwent endovascular treatment for stenoocclusive lesions in the femoropopliteal artery from July 2003 to March 2009, and 18 patients who underwent stent placement in popliteal arterial P2 and P3 segments were finally enrolled. Lesion patency was evaluated by ultrasound or CT angiography, and stent fracture was assessed by plain X-rays at 1, 3, 6 and 12 months and annually thereafter.
Results
At the 1-month follow-up, stent fracture (Type 2) was seen in one limb (up to P3, 1 of 18, 6%) and it was identified in seven limbs at the 3-month follow-up (Type 2, Type 3, Type 4) (n = 1: up to P2; n = 6: P3). At the 6-month follow-up, one more fracture (Type 1) (up to P3) was noted. At the 1-year follow-up, there were no additional stent fractures. Just four limbs (up to P2) at the 2-year follow-up did not have stent fracture. The primary patency was 94%, 61% and 44% at 1, 3 and 6 months, respectively, and the group with stent implantation up to P3 had a higher fracture rate than that of the group that underwent stenting up to P2 (p < 0.05).
Conclusion
We suggest that stent placement up to the popliteal arterial P3 segment and over P2 in an Asian population can worsen the stent patency owing to stent fracture. It may be necessary to develop a stent design and structure for the Asian population that can resist the bending force in the knee joint.
Keywords: Artery, Stent, Intervention, Fluoroscopy, Fracture, Angioplasty
INTRODUCTION
The technical and clinical success rates of endovascular treatment for stenoocclusive lesions in the femoropopliteal artery have reached over 90% due to the improvements in the new generation devices (1, 2). However, the long-term patency of endovascular treatment remains an unsolved issue. In particular, balloon angioplasty alone and stainless-steel stent placement in the femoropopliteal artery have been far from satisfactory, and their primary patencies over 1-year follow-up are below 60% (3-6). New nitinol stents have recently been introduced and these have been proven to be superior to balloon angioplasty (7, 8) or a stainless-steel stent (9) in the superficial femoral artery in terms of the long-term patency. Therefore, endovascular treatment with nitinol stents has been widely applied for treating femoropopliteal stenoocclusive lesions. However, despite the improved primary patency, stent fracture after the deployment of nitinol stents has been recognized as an adverse event in the femoropopliteal artery and this can be considered as a risk factor for in-stent restenosis and reocclusion in a femoropopliteal arterial stent. Although the factors that have an influence on femoropopliteal arterial stent fracture have not been fully determined, stent fracture is believed to occur due to external compression caused by muscle activity, by pulsatile blood flow that seems to negatively affect the stent structures, by overlapping long stents or by mechanical stress at the articulation sites (7, 10-13). In particular, popliteal arterial segments P2 and P3 were previously avoided for stent placement due to the articulation sites where a stent could easily be bent (13, 14). Yet in practice, it is inevitable that a stent is placed in the popliteal arterial segments P2 and P3 in the case of critical limb ischemia or for bail-out after balloon angioplasty. Unfortunately, there has been little data regarding the status of stents placed in the popliteal arterial segments P2 and P3 and especially in Asian patients who often find it necessary to fully flex the knee joints in order to perform routine activities such as kneeling, squatting and sitting with both legs crossed. Therefore, the purpose of this study is to evaluate the primary patency and morphological status of self-expandable nitinol stents in the popliteal arteries, and especially the P2 and P3 segments, in Korean patients.
MATERIALS AND METHODS
From July 2003 to March 2009, the patients who presented to the Departments of Vascular Surgery, Thoracic and Cardiovascular Surgery and Orthopedic Surgery with intermittent claudication or critical limb ischemia (Rutherford category 2-6) in the unilateral or bilateral lower extremities underwent CT angiography and Ankle-Brachial Index (ABI) measurement. We retrospectively analyzed the medical and radiological records of a total of 52 patients out of the 189 patients who underwent endovascular treatment for femoropopliteal stenoocclusive lesions and who had lesions that included the popliteal arterial P2 and P3 segments. Twenty-nine limbs had stenosis, 13 limbs had an occlusion and 10 limbs had a mixed lesion of both stenosis and occlusion.
All the endovascular treatments were performed in the interventional procedure room. Written informed consent was obtained from all the study subjects and this retrospective study was approved by the Institutional Review Board of our institution. Standardized heparin 3000-5000 IU was administered systematically via the introducer sheath at the beginning of the intervention. The indication for endovascular treatment for femoropopliteal lesions included > 70% of vessel diameter stenosis without inflow lesions after obtaining the initial diagnostic angiograms of the lower limb. The approaches for endovascular treatment for the femoropopliteal lesions were determined at the operator's discretion. In general, after inserting a 6-Fr or 7-Fr sheath, either a 0.035- or a 0.018-inch guide wire supported by a differently shaped diagnostic catheter was used to cross the lesion. The modalities for endovascular treatment were as follows: Primary stent placement was performed for the stenoocclusive lesion in the SFA and the P1 segment of the popliteal artery. Balloon angioplasty or primary stent placement was performed in the popliteal arterial P2 and P3 segments and bail-out stenting was performed in the patients with unsuccessful primary balloon angioplasty, which was defined as a residual stenosis > 30% or the presence of a flow-limiting dissection. Balloon catheters or stents between 5 to 8 mm were used depending on the target lesion. A variety of balloon catheters (UT: Boston Scientific, Natick, MA; Powerflex: Cordis, Miami, FL; Rider: Bolton, Barcelona, Spain) and a single self-expandable nitinol stent (SMART: Cordis, Miami, FL) were used. Dual antiplatelet therapy with aspirin (100 mg/day) and clopidogrel (75 mg/day) was started immediately after the procedure and this was continued until the 1-month follow-up for clopidogrel and the duration of treatment was life-long for aspirin.
Stent fracture was defined as clear interruption of the stent struts as identified by plain X-rays from at least two projections, with resulting kink or misalignment along the axial length of the stent. The morphology of the stent fracture was classified based on a report by Rocha-Singh et al. (15). Single-strut fracture was defined as type 1, multiple-strut fractures was defined as type 2, stent fracture with preserved alignment of the components fractures was defined as type 3, stent fracture with mal-alignment of the components fractures was defined as type 4 and stent fracture in a trans-axial spiral configuration fractures was defined as type 5. Lesion patency was evaluated by ultrasound or CT angiography, and stent fracture was assessed by X-rays at 1, 3, 6 and 12 months and annually thereafter.
Endovascular procedures were considered technically successful when all the treated lesions had < 20% residual stenosis on completion of angiography. Primary patency was defined as the absence of restenosis or occlusion in the treated arterial segment. Restenosis was defined as more than 50% of the vessel diameter at the treated segments as observed on CT angiography or the absence of flow or a focal increase of the peak systolic velocity ratio of ≥ 2.5 on color Duplex ultrasound. Additionally, lesion patency according to the type of stent fracture was analyzed, and the difference in stent fracture according to stent implantation until P2 or until P3 and over P2 was also analyzed.
Statistical analysis was performed using SPSS. The Chi-square test was used to compare the fractures between the groups with stent implantation until P2 and until P3 over P2, P2 with the level of statistical significance set at p values < 0.05. Primary patency was determined by performing Kaplan-Meier survival analysis.
RESULTS
Technical success was achieved in 100% of the patients who underwent stent placement in the popliteal arterial P2 or P3 segments (lesion length: 8-27 cm, mean lesion length: 16.5 cm). Forty-one of 52 limbs underwent balloon angioplasty to recanalize the stenoocclusive lesions and among them, seven limbs underwent stent placement in the popliteal arterial P2 and P3 segments for bail-out because of inappropriate results of balloon angioplasty such as elastic recoil (n = 1) or flow-limiting dissection (n = 6). Of them, two limbs underwent bail-out stent placement at up to the P2 segment and five limbs underwent bail-out stent placement at up to the P3 segment and over the P2 segment. The remaining eleven of 52 limbs underwent primary stenting in the popliteal arterial P2 and P3 segments due to severe calcification or critical limb ischemia. Eight limbs underwent stent placement up to the P2 segment and 3 limbs underwent stent placement up to the P3 and over the P2 segment.
At the 1-month follow-up, stent fracture (Type 2) was seen in one limb that underwent primary stenting up to the popliteal arterial P3 segment and over the P2 segment (1 of 18, 6%); stent fracture was identified in seven limbs at 3-month follow-up (Type 1, Type 2, Type 3 and multiple; n = 1, n = 4, n = 1 and n = 1, respectively), including one of 10 limbs that underwent stent placement up to the P2 segment (Type 1) (Fig. 1) and six limbs that underwent stent placement up to the P3 and over the P2 segment (Type 2, Type 3 and multiple) (Figs. 2, 3). At the 6-month follow-up, one more fracture (Type 2) was noted in a residual limb that underwent stent placement up to the P3 segment. Therefore, all the limbs (n = 8) that underwent stent placement up to the popliteal arterial P3 segment had a stent fracture at the 6-month follow-up and just one limb (1 of 10, 10%) had a fracture in the stent placed up to the P2 segment. At the 1-year follow-up there was no additional stent fracture in the 12 limbs that were available for follow-up. Just four limbs were available for the 2-year follow-up; each of them had undergone stent placement up to the P2 segment and none had developed new stent fracture.
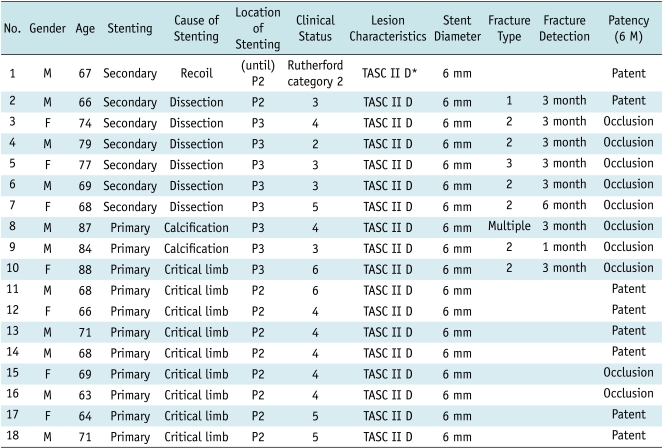
Fig. 1.

66-year-old man had symptoms of severe intermittent claudication in his left leg. He had chronic total occlusion in distal superficial artery and popliteal artery. He underwent stent placement up to P2 segment for bail-out after failed balloon angioplasty. Type 1 stent fracture (arrow) was detected on fluoroscopy at 3-month follow-up.
Fig. 2.
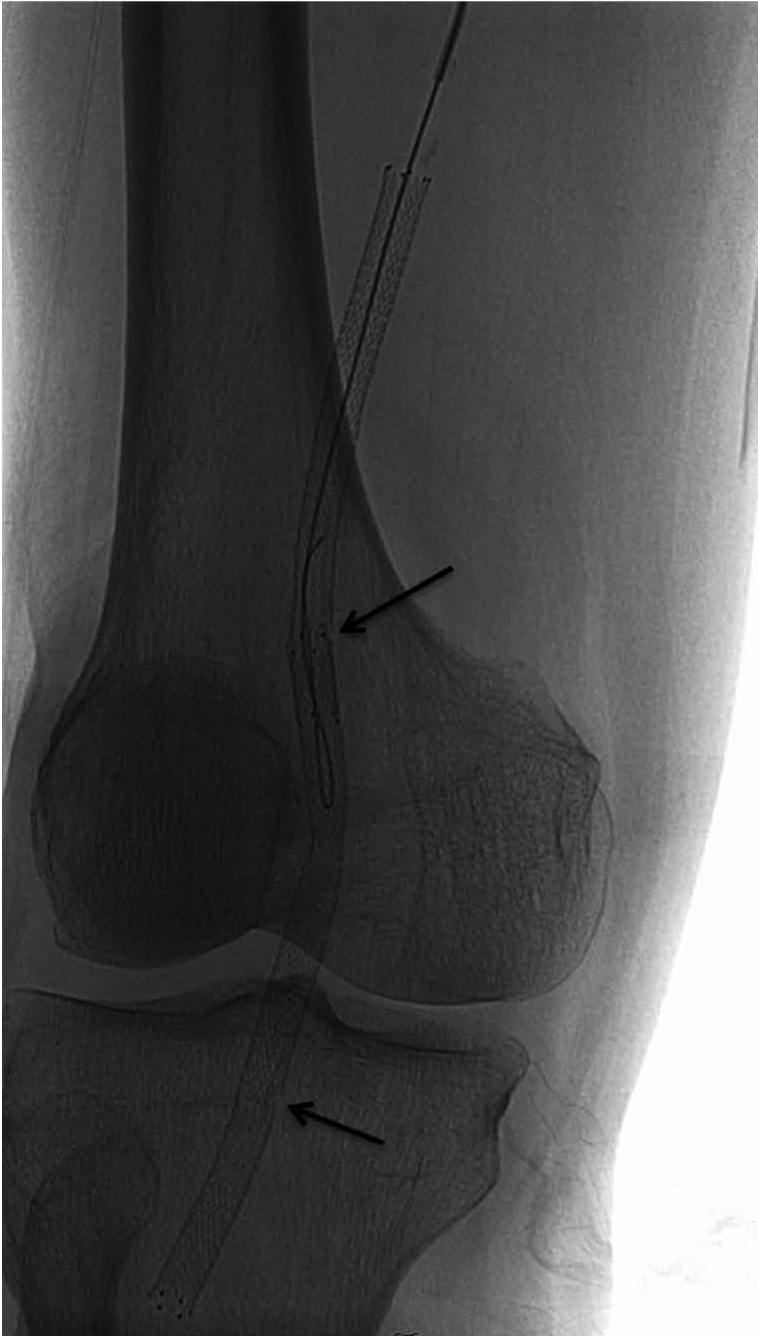
74-year-old man had diabetes with resting pain in his right foot. He had chronic total occlusion in distal superficial femoral artery and popliteal artery. He underwent stent placement up to P3 segment for bail-out after failed balloon angioplasty. At 3-month follow-up, his symptoms had recurred and type 2 stent fracture (arrows) was detected on fluoroscopy.
Fig. 3.
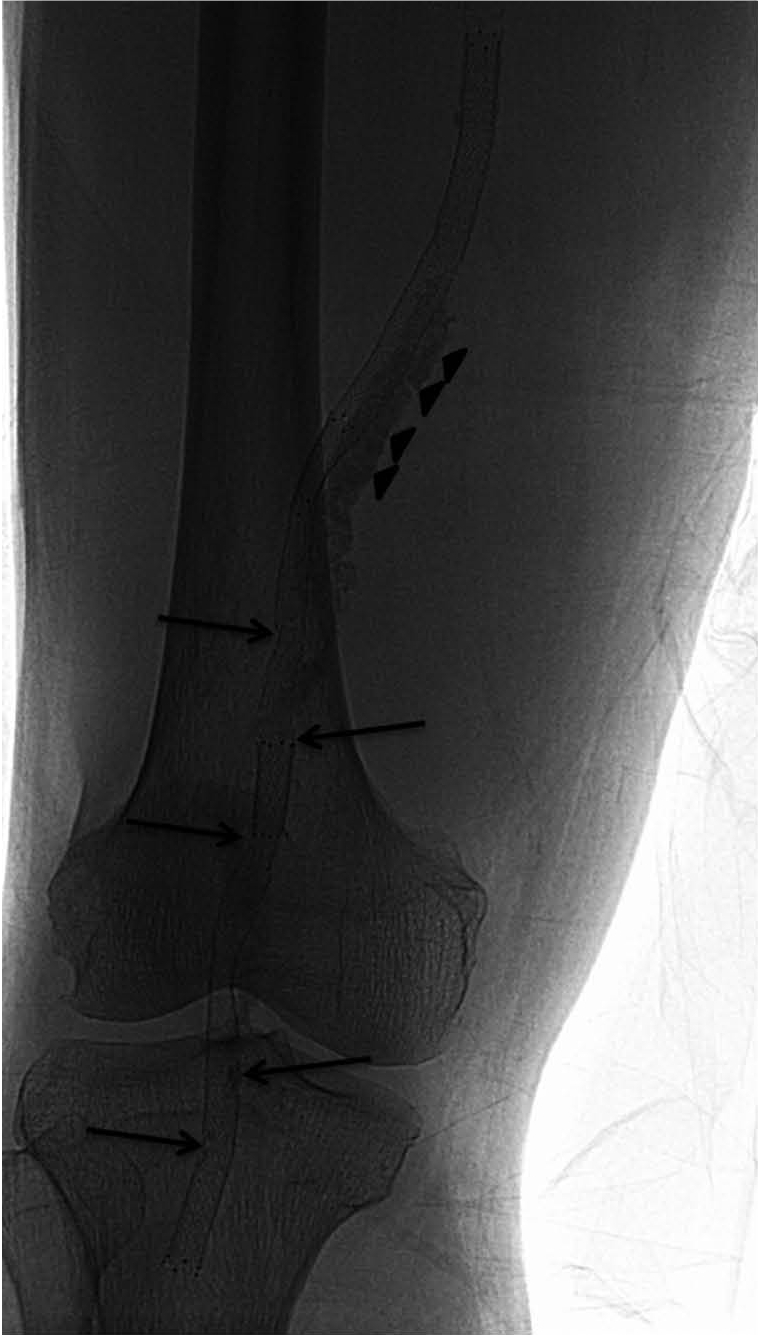
87-year-old man had diabetes with resting pain in his right foot. He had chronic total occlusion from mid-superficial femoral artery to popliteal arterial P3 segment with massive calcifications (arrowheads). Therefore, he underwent primary stenting there. However, at 3-month follow-up, his symptoms had recurred and type 2, 3 and 4 fractures (arrows) were detected on fluoroscopy.
A total of nine limbs experienced overt stent fracture during the follow-up period; among them, type 2 fractures were identified in six limbs, type 1 fracture was identified in one limb and type 3 fracture was identified in one limb. One limb had a multiple-type of fracture such as combined type 2, type 3 and type 4. The blood flow through the stent was completely occluded at the time of detection of stent fracture in all the cases of type 2, 3 and 4 fractures, but the stent patency was well preserved in the case of type 1 fracture. In addition, all the cases that underwent stent placement up to the popliteal arterial P3 segment and over the P2 segment demonstrated stent fractures with complete occlusion of blood flow; however, the cases that underwent stent placement up to the P2 segment included just one case of stent fracture without evidence of stenosis or occlusion. Therefore, the group with stent implantation up to the P3 segment had a higher fracture rate than did the group that was stented up to the P2 segment (p < 0.05). The overall primary patency was 94%, 61% and 44% at 1, 3 and 6 months, respectively (Table 1).
Table 1.
Patients' and Lesions' Characteristics
Note.- TASC II D* = Chronic total occlusions of common femoral artery or superficial femoral artery (> 20 cm, involving popliteal artery). Chronic total occlusion of popliteal artery and proximal trifurcation vessels (25).
There were no peri-procedural complications and all of the patients were discharged within seven days of their procedure.
DISCUSSION
Endovascular intervention in the peripheral circulation has proven to be problematic. Although generally effective in the relatively large inflow arteries of the extracranial cerebrovascular and iliac circulations, endovascular manipulation of the infrainguinal arteries is technically more challenging and the outcome less durable (7, 16-20). There are many possible explanations for the discouraging results of infrainguinal endovascular intervention. The infrainguinal circulation is characterized by long conduits with a heavy plaque burden, high impedance outflow with low mean flow rates, prolonged fractions of the cardiac cycle with stagnant flow and a tendency toward exaggerated bending and twisting with skeletal movement (21). The latter phenomenon contributes to stent fracture of self-expanding stents in the femoropopliteal arteries. Indeed, self-expanding stents in the SFA have shown an alarming tendency to fracture, with rates as high as 65% in one clinical report (22); another recent clinical study of 40 consecutive patients treated with the Luminexx stent documented strut fracture in 28%, with more frequent occurrence in the patients who were vigorously active and ambulatory (23). There have been many studies of stent patency and stent fracture in the femoropopliteal arteries. In our study, stent fracture worsened the patency during the first two years, but it did not apparently affect the patency beyond two years. Although type 2 stent fracture revealed poor outcomes, neither type 1 nor type 3 stent fractures affected the primary patency compared with those stents without stent fracture (7, 8). In our study, all the cases with type 2 stent fractures had complete occlusion of blood flow, while good stent patency was identified with type 1 stent fracture; these results were similar to those of previous studies. However, contrary to the previous studies, the cases of type 3 stent fracture in our study had complete occlusion of stent patency, although one case had a mixed lesion that included type 2 and type 4 fractures. Therefore, this study cannot give us a full answer regarding the correlation between stent patency and the types of stent fracture because of the small number of type 3 stent fractures (n = 2) and the small total number of cases with stent fracture (n = 9).
In general, stent implantation in the femoropopliteal arteries has been performed in the SFA and proximal popliteal artery (P1 segment), and one study recommended that stent implantation be avoided in the middle or distal third of the popliteal artery (the P2 and P3 segments) (13). While those previous studies observed stent fracture in the femoropopliteal arteries, they did not analyze the stent status in the bending portion of the popliteal arterial P2 and P3 segments. In addition, although it is well known that stent implantation in the popliteal arterial P2 and P3 segments can be considered harmful, it is very difficult to find results regarding the stent structural problems such as fracture after implantation in the popliteal arterial P2 and P3 segments. Although our study involved a small study population, it will be valuable to analyze the stent status in the popliteal arterial P2 and P3 segments, which are more vulnerable to mechanical forces such as bending than are the SFA and the popliteal arterial P1 segment.
Iida et al. (24) suggested in their study that the incidence of stent fracture was lower than that observed in the FESTO (Femoral Stenting in Obstructions) trial (12), and this result may be attributed to the differences of the life-styles and physique in the Eastern population. They assumed that the body mass index in the patients included in that study was approximately 22 kg/m2 and it may be interesting to speculate that stent fracture is adversely affected by muscle volume through compression and expansion mechanisms. However, that study did not mention the stent status in the middle or distal popliteal artery. Full flexion is often necessary in Asian culture to perform key routine activities such as kneeling, squatting and sitting with both legs crossed; in response to such demand, efforts have been made to achieve greater flexion of stents in the knee joint of the Asian population than that in the Western population. Therefore, we assumed that stent placement in the popliteal arterial P2 and P3 segments in the Asian population may result in more structural problems of stents, such as fracture, than that in the Western population and in fact, all of our patients who underwent stent placement over P2 and up to the P3 segment of the popliteal artery had stent fracture accompanied by complete occlusion.
Our study has some limitations and shortcomings. First, this study was retrospectively conducted. Second, the number of cases was few and the follow-up data over six months was incomplete. Third, our study did not collect data regarding re-intervention in the case of stent fracture with complete occlusion of blood flow because all the patients refused the second intervention and some of them moved to other hospitals. Fourth, only one type of stent was used during the period of this study; therefore, it may be difficult to apply our results to other stents. Fifth, our study did not analyze the clinical symptomatic improvement after technically successful stent placement due to the lack of medical records regarding this.
In conclusion, we suggest that self-expandable stent placement up to the popliteal arterial P3 segment and over the P2 segment can worsen the preservation of stent patency with a high incidence of stent fracture, and especially in the Korean population. It is necessary to develop a stent design and structure that can resist the bending force in the knee joint.
References
- 1.Dormandy JA, Rutherford RB TASC Working Group. TransAtlantic Inter-Society Consensus (TASC) Management of peripheral arterial disease (PAD) J Vasc Surg. 2000;31:S1–S296. [PubMed] [Google Scholar]
- 2.Norgren L, Hiatt WR, Dormandy JA, Nehler MR, Harris KA, Fowkes FG, et al. Inter-society consensus for the management of peripheral arterial disease. Int Angiol. 2007;26:81–157. [PubMed] [Google Scholar]
- 3.Cejna M, Thurnher S, Illiasch H, Horvath W, Waldenberger P, Hornik K, et al. PTA versus Palmaz stent placement in femoropopliteal artery obstructions: a multicenter prospective randomized study. J Vasc Interv Radiol. 2001;12:23–31. doi: 10.1016/s1051-0443(07)61397-9. [DOI] [PubMed] [Google Scholar]
- 4.Conroy RM, Gordon IL, Tobis JM, Hiro T, Kasaoka S, Stemmer EA, et al. Angioplasty and stent placement in chronic occlusion of the superficial femoral artery: technique and results. J Vasc Interv Radiol. 2000;11:1009–1020. doi: 10.1016/s1051-0443(07)61331-1. [DOI] [PubMed] [Google Scholar]
- 5.Gordon IL, Conroy RM, Arefi M, Tobis JM, Stemmer EA, Wilson SE. Three-year outcome of endovascular treatment of superficial femoral artery occlusion. Arch Surg. 2001;136:221–228. doi: 10.1001/archsurg.136.2.221. [DOI] [PubMed] [Google Scholar]
- 6.Johnston KW. Femoral and popliteal arteries: reanalysis of results of balloon angioplasty. Radiology. 1992;183:767–771. doi: 10.1148/radiology.183.3.1294068. [DOI] [PubMed] [Google Scholar]
- 7.Schillinger M, Sabeti S, Loewe C, Dick P, Amighi J, Mlekusch W, et al. Balloon angioplasty versus implantation of nitinol stents in the superficial femoral artery. N Engl J Med. 2006;354:1879–1888. doi: 10.1056/NEJMoa051303. [DOI] [PubMed] [Google Scholar]
- 8.Schillinger M, Sabeti S, Dick P, Amighi J, Mlekusch W, Schlager O, et al. Sustained benefit at 2 years of primary femoropopliteal stenting compared with balloon angioplasty with optional stenting. Circulation. 2007;115:2745–2749. doi: 10.1161/CIRCULATIONAHA.107.688341. [DOI] [PubMed] [Google Scholar]
- 9.Sabeti S, Schillinger M, Amighi J, Sherif C, Mlekusch W, Ahmadi R, et al. Primary patency of femoropopliteal arteries treated with nitinol versus stainless steel self-expanding stents: propensity score-adjusted analysis. Radiology. 2004;232:516–521. doi: 10.1148/radiol.2322031345. [DOI] [PubMed] [Google Scholar]
- 10.Duda SH, Bosiers M, Lammer J, Scheinert D, Zeller T, Tielbeek A, et al. Sirolimus-eluting versus bare nitinol stent for obstructive superficial femoral artery disease: the SIROCCO II trial. J Vasc Interv Radiol. 2005;16:331–338. doi: 10.1097/01.RVI.0000151260.74519.CA. [DOI] [PubMed] [Google Scholar]
- 11.Duda SH, Pusich B, Richter G, Landwehr P, Oliva VL, Tielbeek A, et al. Sirolimus-eluting stents for the treatment of obstructive superficial femoral artery disease: six-month results. Circulation. 2002;106:1505–1509. doi: 10.1161/01.cir.0000029746.10018.36. [DOI] [PubMed] [Google Scholar]
- 12.Scheinert D, Scheinert S, Sax J, Piorkowski C, Braunlich S, Ulrich M, et al. Prevalence and clinical impact of stent fractures after femoropopliteal stenting. J Am Coll Cardiol. 2005;45:312–315. doi: 10.1016/j.jacc.2004.11.026. [DOI] [PubMed] [Google Scholar]
- 13.Sabeti S, Mlekusch W, Amighi J, Minar E, Schillinger M. Primary patency of long-segment self-expanding nitinol stents in the femoropopliteal arteries. J Endovasc Ther. 2005;12:6–12. doi: 10.1583/04-1359.1. [DOI] [PubMed] [Google Scholar]
- 14.Strecker EP, Boos IB, Gottmann D, Vetter S, Haase W. Popliteal artery stenting using flexible tantalum stents. Cardiovasc Intervent Radiol. 2001;24:168–175. doi: 10.1007/s002700002526. [DOI] [PubMed] [Google Scholar]
- 15.Rocha-Singh KJ, Jaff MR, Crabtree TR, Bloch DA, Ansel G. Performance goals and endpoint assessments for clinical trials of femoropopliteal bare nitinol stents in patients with symptomatic peripheral arterial disease. Catheter Cardiovasc Interv. 2007;69:910–919. doi: 10.1002/ccd.21104. [DOI] [PubMed] [Google Scholar]
- 16.Costanza MJ, Queral LA, Lilly MP, Finn WR. Hemodynamic outcome of endovascular therapy for TransAtlantic InterSociety Consensus type B femoropopliteal arterial occlusive lesions. J Vasc Surg. 2004;39:343–350. doi: 10.1016/j.jvs.2003.07.007. [DOI] [PubMed] [Google Scholar]
- 17.Adam DJ, Beard JD, Cleveland T, Bell J, Bradbury AW, Forbes JF, et al. Bypass versus angioplasty in severe ischaemia of the leg (BASIL): multicentre, randomised controlled trial. Lancet. 2005;366:1925–1934. doi: 10.1016/S0140-6736(05)67704-5. [DOI] [PubMed] [Google Scholar]
- 18.Gray BH, Sullivan TM, Childs MB, Young JR, Olin JW. High incidence of restenosis/reocclusion of stents in the percutaneous treatment of long-segment superficial femoral artery disease after suboptimal angioplasty. J Vasc Surg. 1997;25:74–83. [PubMed] [Google Scholar]
- 19.Surowiec SM, Davies MG, Eberly SW, Rhodes JM, Illig KA, Shortell CK, et al. Percutaneous angioplasty and stenting of the superficial femoral artery. J Vasc Surg. 2005;41:269–278. doi: 10.1016/j.jvs.2004.11.031. [DOI] [PubMed] [Google Scholar]
- 20.van der Zaag ES, Legemate DA, Prins MH, Reekers JA, Jacobs MJ. Angioplasty or bypass for superficial femoral artery disease? A randomised controlled trial. Eur J Vasc Endovasc Surg. 2004;28:132–137. doi: 10.1016/j.ejvs.2004.04.003. [DOI] [PubMed] [Google Scholar]
- 21.Smouse HB, Nikanorov A, LaFlash D. Biomechanical forces in the femoropopliteal arterial segment. What happens during extremity movement and what is the effect on stenting? Endovasc Today. 2005;4:60–66. [Google Scholar]
- 22.Allie DE, Hebert CJ, Walker CM. Pressure-sensing guidewire analysis in RAS. Adapting this coronary-based technology to optimise "functional" renal artery revascularization could have significant clinical implications. Endovasc Today. 2004;1:14–26. [Google Scholar]
- 23.Iida O, Nanto S, Uematsu M, Morozumi T, Kotani J, Awata M, et al. Effect of exercise on frequency of stent fracture in the superficial femoral artery. Am J Cardiol. 2006;98:272–274. doi: 10.1016/j.amjcard.2006.01.091. [DOI] [PubMed] [Google Scholar]
- 24.Iida O, Nanto S, Uematsu M, Ikeoka K, Okamoto S, Nagata S. Influence of stent fracture on the long-term patency in the femoro-popliteal artery: experience of 4 years. JACC Cardiovasc Interv. 2009;2:665–671. doi: 10.1016/j.jcin.2009.04.014. [DOI] [PubMed] [Google Scholar]
- 25.Norgren L, Hiatt WR, Dormandy JA, Nehler MR, Harris KA, Fowkes FG. Inter-Society Consensus for the Management of Peripheral Arterial Disease (TASC II) J Vasc Surg. 2007;45:S5–S67. doi: 10.1016/j.jvs.2006.12.037. [DOI] [PubMed] [Google Scholar]