Abstract
Understanding why we eat and the motivational factors driving food choices is important to addressing the epidemics of obesity, diabetes and cardiovascular disease. Eating behavior is a complex interplay of physiologic, psychological, social, and genetic factors that influence meal timing, quantity of food intake, and food preference. Here we review the current and emerging knowledge of the genetic influences of eating behavior and how these relate to obesity with particular emphasis on the genetics of taste, meal size and selection, and the emerging use of functional magnetic resonance imaging to study neural reactions in response to food stimuli in normal, overweight and obese individuals.
Keywords: Eating behavior, Genetics, Obesity
INTRODUCTION
Understanding why we eat and the motivational factors driving food choices is important to addressing the epidemics of obesity, diabetes and cardiovascular disease, as food intake is a significant factor impacting the development and treatment of these disorders. Eating behavior is a complex interplay of physiologic, psychological, social and genetic factors that influence meal timing, quantity of food intake, food preference, and food selection. Active research involving the genetics of taste, food preference, pathological eating behaviors, meal size, and meal selection is rapidly expanding our understanding of how and why we eat. More recently, neural imaging modalities, specifically functional magnetic resonance imaging (FMRI), has emerged as a modality to effectively study eating behavior and genetics in fascinating ways. Here we review the current knowledge of the genetic influences of eating behavior with particular emphasis on the genetics of taste, meal size and selection, and the emerging use of FMRI as it applies to imaging neurophysiologic response to food stimuli. In this review, we focus primarily on obesity as a consequence of eating behavior, but other pathological disorders of eating behavior, including anorexia nervosa and bulimia nervosa, also have strong genetic, psychological, and environmental components.1
The rapid rise in obesity and associated co-morbidities (metabolic syndrome, coronary artery disease, sleep apnea, skeletal disorders, hyperlipidemia, and hypertension) over the past 30 years has led to the urgency of coming to a more complete understanding of the pathophysiology of obesity. The study of eating behavior attempts to define eating patterns and food preferences, and to explain why there is gravitation toward specific behaviors and food choices, and aims to develop approaches to bring about effective changes in modifiable behaviors. Knowledge of the biological mechanisms guiding eating behavior can provide effective treatment targets for obesity and associated disorders.
Rare monogenic genetic disorders involving hyperphagia and obesity have been identified.2 Resulting from a deletion of the 11-13q region of chromosome 15, Prader Willi (PW) is characterized by hypotonia and poor feeding in early infancy, cognitive, motor and behavioral impairment, followed by insatiable hunger and the development of morbid obesity and diabetes during childhood.3 PW patients rarely survive beyond the age of 25 to 30 years; the cause of death is often related to diabetes and cardiac failure. Monosomy 1p36 has also been associated with obesity and hyperphagia in a PW negative cohort.4 Individuals with loss of function mutations of the leptin (LEP) gene on chromosome 7q31.3, or its receptor (LEPR) also display abnormal eating behavior and develop early-onset morbid obesity.5–6 Leptin replacement can improve satiety and promote weight loss in leptin deficient individuals.7 Leptin promotes α-melanocyte stimulating hormone(α-MSH) synthesis which promotes satiety.8 α-MSH is bound by the melanocortin 4 receptor (MCR4) protein. MC4R mutations are associated with early onset obesity.9–10 Discoveries of the genes and their respective proteins involved in these rare forms of obesity helps shed light on the pathways involved in regulating eating behavior and energy homeostasis. Although important, monogenic forms of obesity account for less than ten percent of today’s obesity epidemic.11
Although rare genetic mutations cause dramatic hyperphagia, most common genetic variants have smaller effect sizes. The risk of obesity, metabolic syndrome, and other complications is increased by a variety of common genetic variants, and many of these are associated with specific eating behaviors. Research tools used to measure eating behavior include food logs, observation, food preference flash cards, labeled scaling, and more recently FMRI. A widely used research tool known as the Three-Factor Questionnaire (TFQ) has been used to quantify eating behaviors in normal-weight, obese, and in individuals with eating disorders.12 This questionnaire uses a series of questions to measure three patterns of behavior: restraint, disinhibition, and hunger. Both high restraint and disinhibition scores are positively correlated with BMI.13–14 Restraint is characterized by intentional avoidance of certain foods in order to control body weight, and is measured by response to questions on the TFQ such as “I avoid certain foods because they make me fat.” Disinhibition is the tendency to overeat when surrounded by others who are overeating. Hunger measures the subjective sense of an individual’s need to eat. Heritability and linkage analysis of eating behavior measured by the TFQ provides evidence that these behavior traits are heritable.15–16 Although much remains to be understood about the genes regulating these behaviors, genetic influence of disinhibition has been linked to neuromedin, a factor mediating satiety, in a French Canadian cohort and to TAS2R38, a bitter taste receptor, in a cohort of Amish women.16–17 GAD (glutamic acid decarboxylase) has also been linked to eating behavior. GAD decarboxylates glutamate into GABA (γ-aminobutyric acid), a major inhibitory neurotransmitter in the brain. Two specific GAD variants, rs7908975 and rs992990 have been reported to be associated with disinhibition and disordered food intake, specifically increased carbohydrate intake, in women.18
GENETICS OF TASTE
Taste affects food preference and food intake thereby directly influencing eating behavior. However, not all humans perceive taste in exactly the same way. The density of taste papillae on the tongue, genetic differences in taste receptors or sensitivity of taste receptors, constituents of saliva, and other factors all contribute to an individual’s taste perception and subsequent food preferences.19 Differences in taste papillae density impacts taste sensitivity and are thought to be genetically determined20; however, the gene or genes responsible for this trait have yet to be identified. Differences in taste perception and preference influence food choices and have significant impact on nutrient and caloric intake.
Five tastes are recognized by humans: sweet, bitter, sour, salty, and umami— described as the taste of glutamate or the taste of amino acids and proteins. Food preference and intake is influenced by sweet and bitter taste. For example, individuals who possess enhanced perception of bitter taste tend to avoid certain foods, including specific fruit and vegetables.21 Preference for sweet and high-fat food has been reported to decrease with increasing perception of bitter taste.21–24 Evidence suggest bitter tasting ability may be related to body mass index (BMI), adiposity, and risk factors for CVD,25–26 while the perceived sweetness of foods has been shown to be inversely correlated with BMI.27 Bitter taste sensitivity has also been linked to variation in height among children, suggesting this trait may influence food selection and impact growth rate.28–29 Individuals who are particularly sensitive to bitter compounds tend to avoid the bitter taste of beer and alcohol, and avoid cigarette smoking as well.25,30 Bitter taste as well as preference for sweet and fat guide ingestive behaviors, and have been linked to obesity, and these food preference traits may in part be genetically determined.
Bitter, sweet and umami tastes are mediated by G-protein-coupled receptors (GPCR). Bitter taste receptors are encoded by 25–30 TAS2R genes, located on chromosomes 12p13, 7q34 and 5p15.31. The ligand specificity of TAS2Rs appears to be quite broad, consistent with their roles in detecting thousands of bitter-tasting compounds.31 One of these, TAS2R38 has been extensively characterized in vitro, in vivo and in human populations, and is responsive to the bitter stimuli phenylthiocarbamide (PTC), propylthiouracil (PROP), and to thiocyanates—bitter compounds found in brassia vegetables such as brussels sprouts and broccoli. Two common haplotypes of TAS2R38 have been shown to influence perception of bitter taste and are significantly related to differences in bitter taste sensitivity,32 preference for sucrose and sweet tasting foods and beverages, and to modestly lower risk of type 2 diabetes among participants of the British Women’s Heart and Health Study.33–34 While studies are not all in complete agreement, individuals most sensitive to the taste of PROP more often dislike bitter fruits and vegetables, such as grapefruit and kale. These low energy foods may be replaced by more energy dense foods among individuals more sensitive to bitter taste.35 TAS2R38 haplotype has been suggested to be predictive of obesity,36 however, to date, studies involving large cohorts have failed to demonstrate convincing evidence for a direct relationship between TAS2R38 and BMI in spite of evidence that polymorphisms in this gene influence ingestive behavior.37 The majority of TAS2R38 studies have been conducted in Caucasian populations, therefore further research is necessary to determine how well current findings can be generalized to other ethnic populations.
TAS2R5, another bitter receptor, may be an important regulator of ingestive behavior. This gene resides in a region of chromosome 7 that is significantly associated with a quantitative phenotypic marker of alcohol dependence called ttth 1. Furthermore, a single nucleotide polymorphism (SNP) located within a linkage disequilibrium block that includes TAS2R5 accounts for this association.38 A SNP in another chromosome 7 gene, TAS2R16, has been linked to alcohol dependence as well.39 These findings suggest that genetic variation in TAS2R genes may be involved in regulating ingestive behaviors.
The receptors for sweet and umami taste are encoded by three TAS1R genes located on chromosome 1p36. Heteromeric TAS1R2:TAS1R3 taste receptors respond to sweet-tasting compounds such as sugars, high-potency sweeteners, and some D-amino acids, while TAS1R1:TAS1R3 heteromers comprise an umami taste receptor sensitive to L-amino acids.31 Both subunits of the sweet taste receptor bind sugar ligands, though they do so with distinct affinities and ligand-dependent conformational changes.40–41 Although variability in both sweet and umami taste have been described, these traits are not as well defined as those of PROP tasting, and specific genetic variants responsible for variation in sweet and umami taste remain to be identified.
TAS1Rs and TAS2Rs are expressed in diverse tissue including brain, adrenal gland, pancreas, small intestine, retina, skeletal muscle, salivary gland and tongue.42–44 Of particular interest is the observation that TAS1R and TAS2R receptors, as well as other proteins involved in taste transduction, are expressed in the gastrointestinal mucosa, where they modulate responses to ingested nutrients via glucagon like peptide-1 (GLP-1), cholecystokinin (CCK), and gastric inhibitory polypeptide (GIP).44–45 GIP, GLP-1 and CCK regulate gut motility and appetite. Therefore, TAS1R and TAS2Rs may be integral to modulating both taste and ingestive behavior via mediating enteroendocrine secretion. Dotson et al demonstrated TAS2R9 to be involved in GLP-1 secretion, with a loss of function mutation in the gene resulting in attenuated GLP-1 response to agonist.46 In another study, Dotson et al have also shown genetic variation in TAS2R38 to be associated with eating behavior in a cohort of Amish women.17 Genetic variation in TAS1R and TAS2Rs may impact eating behavior via altered taste perception as well as via alteration in neuroendocrine signals impacting satiety. The observation that these receptors are involved in both taste and secretion of hormones involved in satiety tell us that these processes may be biologically entwined. Table 1 summarizes gene variants linked to eating behavior and taste.
Table 1.
Common variants associated with variation taste and ingestive behavior
| Tastes | Chromosome | Gene | Influence on Ingestive behavior | Reference |
|---|---|---|---|---|
| Sweet | 1p36 | TAS1R2, TAS1R3 | Unknown | Nie et al. (2005)41, Nie et al. (2006)40, Scott et al. (2005)31 |
| Umani | 1p36 | TAS1R1, TAS1R3 | Unknown | Scott et al. (2005)31 |
| Bitter | 12p13, 7q34, 5p15.31 | TAS2Rs: TAS2R38, TAS2R5, TAS2R16 |
Vegetable avoidance, increased fat and sweet intake, disinhibited eating behavior among women Alcohol dependence |
Kim et al. (2003)32 Drewnowski et al. (1997)21 Mennella et al. (2005)33 Timpson et al. (2005)34 Dotson et al. (2008)46 Lin (2005)38, Hinrichs (2006)39 |
MEAL SELECTION AND SIZE
Research into meal size and selection is especially complex as socioeconomic environment, learned eating behaviors, physiologic conditions such as depression, and even medical treatments can all influence appetite and food selection, independent of genetics; however meal quantity, frequency, and timing are thought to be at least in part under genetic control. The study of genetic variants in digestive neuroendocrine hormones, such as CCK, leptin and ghrelin, are providing new insights into how these hormones and their genetic variants may be involved in pathways regulating appetite and eating behavior.
Ghrelin, a 28-amino acid peptide, is primary produced by the stomach and pancreas and is involved in promoting meal intake and hunger through receptors in the hypothalamus.47 Plasma ghrelin levels rise pre-meal and are suppressed by food intake.48 GHRL is located on chromosome 3. The gene product is involved in growth hormone release, and post translational modifications yield the hormones ghrelin and obstatin. Obstatin opposes the effects of ghrelin and is responsible for satiety and decreasing food intake.49 Many studies have been devoted to investigating GHRL variants with respect to obesity. A common variant, Leu72Met has been associated with obesity,50–51 metabolic syndrome,52 and binge eating.53
Leptin and CCK work in opposition to ghrelin to promote satiety. CCK is released in response to lipids and promotes rapid post-prandial satiety in contrast to the long term action of leptin.54 In a large case-control study of 17,000 obese and normal weight women, common leptin variants, (rs4577902, rs2060736, and rs4731413), were associated with increased risk of extreme snacking behavior (top 5th percentile based on 11 question questionnaire), but not increased meal size.55 CCK variants (rs6809785, rs7611677, rs6801844, and rs6791019) were found to be more associated with extreme meal size (top 5th percentile based on estimated portion sizes using 28 picture cards) but not increased snacking behavior in the same study. This study suggests that genetic variation in genes encoding CCK and leptin may contribute to obesity risk by influencing satiety, and may have independent effects. Additional studies are needed to further clarify the role of genetic variation in these genes to provide a better understanding of how they may modulate eating behavior.
FTO, fat mass and obesity-associated gene, has been highly associated with increased risk of obesity.56 FTO, is localized to chromosome 16 and is expressed in adipocytes, the pancreas, and the hypothalamus, particularly in regions known to regulate appetite. FTO may contribute to obesity by down regulating adipocyte production of leptin.57 A common variant, rs9939609 is associated with adiposity, and possibly satiety responsiveness. den Hoed et al demonstrated the A allele of rs9939609 to be associated with reduced post-prandial satiety, and may also contribute to excess caloric intake in a study of men and women of Western European descent with BMI’s ranging from 19–31, (5 of 62 subjects had a BMI >30).56 This study also analyzed post prandial response to hunger and the interaction among variants in leptin, the leptin receptor and methyltransferase genes. The authors concluded that the effect of the rs9939609 A allele on the postprandial response in hunger appears to be mediated by an epistatic interaction involving variants in a methyltransferase gene and the leptin receptor. In another study, Scottish children who were homozygous or heterozygous for the rs9939609 A allele also demonstrated increased energy intake without associated energy expenditure. Of interest, all the children ate approximately the same weight of food, but those children with the rs9939609 A allele consumed more energy-dense foods.58 The authors of this study concluded that this FTO variant confers a predisposition to obesity and may play a role in the control of food intake and food choice, perhaps involving a hyperphagic phenotype or a preference for energy-dense foods. Tanofsky-Kraff replicated these findings in a cohort of 289 children and adolescents, suggesting that FTO may indeed contribute to preference for higher fat intake and large meal size.59 Although provoking, these findings should be interpreted with some caution, as at least one study has shown rs9939609 not to be correlated with increased risk of obesity,60 however the current accumulated evidence clearly implicates FTO as having a significant impact on food intake and obesity.
Genetic variations in FTO, leptin, the leptin receptor and ghrelin, genes involved in the neuroregulation of food intake, appear to contribute to obesity risk by influencing satiety and hunger, and may contribute to increased caloric intake. Larger and more genetically diverse cohorts need to confirm these observations. Functional studies of the impact of these variants on gene expression or action are also needed. Improving our understanding of the mechanisms whereby these genes interact and their potential molecular cross talk may provide novel targets for developing treatments for individuals with reduced satiety in response to meals. Table 2 summarizes the current knowledge with respect to genetic variants linked to meal selection and size.
Table 2.
Common variants associated with meal selection and size
| Hormone | Gene variants | Physiologic effect of gene product | Contributions to eating behavior | References |
|---|---|---|---|---|
| CCK | rs6809785, rs7611677, rs6801844 | Rapid post-prandial satiety | Extreme meal size | de Krom et al. (2007)55 |
| Leptin | rs4577902, rs2060736, rs4731413 | Promote satiety | Extreme snacking behavior | de Krom et al. (2007)55 |
| Ghrelin | Leu72Met, 51GLN | Promote meal intake and hunger Metabolic Syndrome Obesity |
Binge Eating | Monteleone et al. (2007)53 Hinney et al. (2002)51 Korbonits et al. (2002)50 Steinle et al. (2005)52 |
| FTO | rs9939609 | Downregulates leptin, suppress satiety | Reduced post-prandial satiety, increased caloric intake | den Hoed et al.(2009)56 Cecil et al. (2008)58 Tanofsky-Kraff et al. (2009)59 |
| GAD | rs7908975, rs992990 | Promote GABA, regulate food intake | Increased carbohydrate intake | Choquette et al. (1998)18 |
Functional Magnetic Resonance Imaging
A variety of cognitive pathways are involved in motivation and control of eating behavior. The new use of neuroimaging techniques, specifically functional magnetic resonance imaging (FMRI), to demonstrate specific neural reactions in response to food stimulus is revolutionizing the study of eating behavior. Other imaging techniques, such as position emission tomography (PET) studies have been previously used to investigate neural responses to taste and identify neural pathways involved in eating behavior.61 FMRI has previously been well-established in identify pathology in studies of schizophrenia,62 Alzheimer’s disease,63 and many other areas of neuroscience research. Nearly all FMRI studies utilize blood oxygen level dependence (BOLD) to identify areas in the brain that demonstrate increased glucose uptake and therefore increased activity in response to specific stimuli. Eating behavior research utilizing FMRI has focused on BOLD changes in specific brain regions in obese compared to normal weight individuals.
Eating behavior FMRI studies have shown that a fasting state increases cortical activation among lean individuals,64 increases preference for high calorie foods in obese individuals,64–66 and that obese men have attenuated post-prandial brain reactions to satiety which may explain excess caloric intake.67 Ghrelin infusion in normal weight volunteers produced increased BOLD response to food pictures in the amygdala, orbitofrontal cortex, anterior insula, and striatum, areas of the brain involved in activating ingestive behavior, and elicited increased self-reports of hunger.68 Likewise, patients with lower leptin levels secondary to weight loss or secondary to genetic leptin deficiency have increased BOLD activity in brain areas involved in emotional, cognitive, and sensory control of food intake in response to food stimuli, which subsequently normalize with leptin infusion.7,69
Neurophysiologic processing in response to food is largely accomplished in the left hemisphere, specifically in the dorsal and ventral striatum, fusiform gyri and insula— the latter two known as the “primary gustatory complex.”64 Feeding is associated with dopamine release,70 and the amount of dopamine release positively correlates with perceived food pleasure.71 Obese individuals have lower striatal concentrations of the D2 dopamine receptor,71 findings suggesting that the lower concentrations of this G protein coupled receptor may evoke overeating in obese individuals in order to produce a reward response. An alternative interpretation is that dopamine receptors may be downregulated in response to excessive food stimuli. Martin et al demonstrated that brain regions involved in pathways of food reward in obese individuals exhibited increased BOLD activation, specifically the limbic region and prefrontal region, both which have high concentrations of dopamine receptors.72 Obese individuals also had greater memory for foods in the fasted state. Fasted obese individuals have also been shown to exhibit higher pre-meal activation of the anterior cingulated cortex and medial prefrontal cortex, areas of the brain implicated in motivational processing.72 These findings have been supported by Haase et al who also noted increased BOLD activation in the prefrontal and limbic regions in response to taste stimuli among fasting obese individuals.73 Although these studies are promising, they remain limited by small numbers of participants and many of these studies have focused largely on obese women. Because MRI equipment can withstand limited study subject body weight, these studies are restricted to the study of subjects whose body weights can be accommodated by the MRI equipment. Despite these limitations, FMRI has been used successfully to shed light into pathways involved in eating behavior and to demonstrate important functional difference in brain imaging among obese individuals.
Of particular interest are innovative studies combining genetic studies with FMRI to investigate eating behavior. Combining these modalities may help uncover inter-relationships among genetics and the neurophysiologic pathways involved in food response and eating behavior. Felsted et al hypothesized that polymorphisms in genes involved in the neurophysiology of feeding and reward processing would demonstrate differential responses in brain regions known to be involved in food reward.74 They chose to investigate a particular variant, TAQ1A,75 a restriction fragment length polymorphism located on ANKK1 (ankyrin repeat and protein kinase domain-containing protein 1), a regulatory gene downstream of the dopamine D2 receptor, and to perform functional magnetic resonance imaging to measure neural response to the ingestion of palatable and caloric milkshakes in 26 healthy subjects (24 women and 2 men). The TAQ1A variant has previously been implicated in having a role in obesity and eating behavior, particularly with respect to the relationship between neural response to food and prospective weight gain.71 Individuals with the A1/A1 or A1/A2 allele of TAQ1A are more likely to be obese, and have 30–40% fewer dopamine receptors.71 In the Felsted study, either a milkshake or a tastless, odorless liquid was randomly dripped into the mouths of the subjects while subjects rested within the MRI device. The investigators attempted to control for confounding variables that may influence food response. Participants were all matched for BMI, hunger rating, and for psychological factors such as impulsivity, addiction, and eating style assessed through a variety of psychological and food intake surveys. No subject reported taking prescription or over-the-counter medications. This study elegantly demonstrated that individuals possessing the A1 TAQ1A allele had decreased BOLD response to a milkshake in midbrain, thalamus, and orbital frontal cortex regions, regions of the brain involved in regulating eating behavior, even though all participants rated similarly the perceived pleasantness and familiarity of the milkshake.74 These findings suggest that individuals possessing the A1 allele might be predisposed to overeating as they experience attenuated neural reward response to food. Whether this variation in response to food stimulus is due to diminished number of dopamine receptors is yet to be determined. This study is the first of our knowledge to directly demonstrate that individuals with a specific genetic variant have measurable neural changes directly correlated with eating behavior in responsiveness to a food stimulus. These findings provide hope for the future development of treatment aimed at modulating food induced sensitivity to pleasure and satiety centers in genetically susceptible individuals.
CONCLUSION
Clinical applications and future directions
Eating behavior is a complex trait with both genetic and environmental influences. While sequencing an individual’s entire genome is expensive, this process will become less costly and more rapid in the future. Personalized medicine, tailoring pharmacologic and behavioral therapy to an individual’s genetic code, is an emerging practice. Applications of research of the genetics of eating behavior may lead to individualizing therapies targeting specific genetic mutations and behavioral interventions addressing eating behaviors. For example, once the role of specific gene variants in pathways involved in specific behaviors or food responses are well established, treatment could be individualized toward modifying these behaviors (for example carbohydrate craving, unrestrained or binge eating, comfort eating, food addiction) and pharmacologic modalities developed to modify molecular pathways involved. Individuals with a TAS2R38 variant associated with enhanced bitter taste might be counseled to select healthy foods that might be more palatable or instructed regarding methods of food preparation to make bitter vegetables more palatable. While current research is limited, preliminary studies hold promise toward these ends. Authors of a nutra-genomic study involving customized treatment with a nutraceutical based on the study subjects’ genetic profile report improvement in weight loss, sugar craving reduction, appetite suppression, snack reduction, reduction of late night eating among study participants who received therapy tailored toward their genetic profile.76
The risk of obesity, metabolic syndrome, and related complications is increased by a variety of common genetic variants, and many of these are associated with specific eating behaviors. Although rare genetic mutations cause dramatic hyperphagia, common genetic variants usually are responsible for smaller effect sizes. It is likely, however, that genetic susceptibility toward aberrant eating behavior and obesity may be overcome by practicing healthy behaviors. This principle was demonstrated among individuals harboring the common TCF7L2 variant which is associated with increased risk of developing type 2 diabetes mellitus (T2DM). In the Diabetes Prevention Study, individuals with the at risk variant who were randomized to intense lifestyle interventions including prudent diet, weight loss and physical activity, demonstrated reduced progression to T2DM in spite of their genetic predisposition.77 Studies involving FTO variants also demonstrate that genetic predispositions to obesity can be overcome by prudent diet and exercise.78–79 With additional research individuals and physicians will have more tools to help identify susceptible individuals and to guide therapy and treatment. The study of the genetics of eating behavior and its interplay with obesity is undergoing rapid progress, and new techniques, including FMRI, are changing how behavioral research is performed and providing new insights into mechanisms of eating behavior. While it is premature to know if developing pharmacologic therapy targeting the A1 allele of TAQ1A or other alleles discussed in this review will contribute to substantial changes in eating behavior or weight loss treatment, at this point in time, it may be helpful for an individual to be aware of his or her genetic susceptibilities of becoming overweight and to practice prudent nutritional behaviors before becoming overweight or obese.
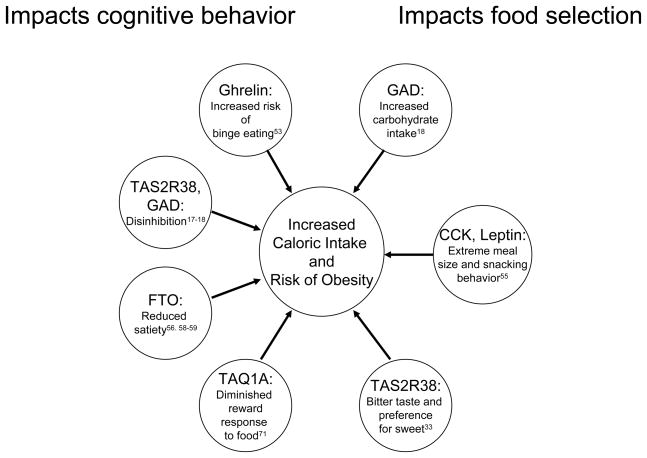
Figure 1.
Genes identified with common variants influencing eating behavior with potential impact on obesity
Acknowledgments
FUNDING
Nanette Steinle is supported by NIH R01HL076768 and NIH P30DK072488.
References
- 1.Magni P, Dozio E, Ruscica M, et al. Feeding behavior in mammals including humans. Ann NY Acad Sci. 2009;1163:221–232. doi: 10.1111/j.1749-6632.2008.03627.x. [DOI] [PubMed] [Google Scholar]
- 2.Holsen LM, Zarcone JR, Brooks WM, et al. Neural mechanisms underlying hyperphagia in Prader-Willi syndrome. Obesity. 2006;14(6):1028–1037. doi: 10.1038/oby.2006.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Greenswag LR. Adults with Prader-Willi syndrome: a survey of 232 cases. Dev Med Child Neurol. 1987;29(2):145–152. doi: 10.1111/j.1469-8749.1987.tb02129.x. [DOI] [PubMed] [Google Scholar]
- 4.D’Angelo CS, Kohl I, Varela MC, et al. Extending the phenotype of monosomy 1p36 syndrome and mapping of a critical region for obesity and hyperphagia. Am J Med Genet A. 2010;152A(1):102–110. doi: 10.1002/ajmg.a.33160. [DOI] [PubMed] [Google Scholar]
- 5.Montague CT, Farooqi IS, Whitehead JP, et al. Congenital leptin deficiency is associated with severe early-onset obesity in humans. Nature. 1997;387:903–908. doi: 10.1038/43185. [DOI] [PubMed] [Google Scholar]
- 6.Clément K, Vaisse C, Lahlou N, et al. A mutation in the human leptin receptor gene causes obesity and pituitary dysfunction. Nature. 1998;392(6674):398–401. doi: 10.1038/32911. [DOI] [PubMed] [Google Scholar]
- 7.Baicy K, London ED, Monterosso J, et al. Leptin replacement alters brain response to food cues in genetically leptin-deficient adults. Proceedings of the National Academy of Sciences of the United States of America. 2007;104(46):18276–18279. doi: 10.1073/pnas.0706481104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shimizu H, Inoue K, Mori M. The leptin-dependent and -independent melanocortin signaling system: regulation of feeding and energy expenditure. J Endocrinol. 2007;193(1):1–9. doi: 10.1677/JOE-06-0144. [DOI] [PubMed] [Google Scholar]
- 9.Hinney A, Schmidt A, Nottebom K, et al. Several mutations in the melanocortin-4 receptor gene including a nonsense and a frameshift mutation associated with dominantly inherited obesity in humans. J Clin Endocrinol Metab. 1999;84(4):1483–1486. doi: 10.1210/jcem.84.4.5728. [DOI] [PubMed] [Google Scholar]
- 10.Farooqi IS, Keogh JM, Yeo GS, Lank EJ, Cheetham T, O’Rahilly S. Clinical spectrum of obesity and mutations in the melanocortin 4 receptor gene. N Engl J Med. 2003;348:1085–1095. doi: 10.1056/NEJMoa022050. [DOI] [PubMed] [Google Scholar]
- 11.Farooqi S, O’Rahilly S. Genetics of Obesity in Humans. Endocrine Reviews. 2006;27 (7):710–718. doi: 10.1210/er.2006-0040. [DOI] [PubMed] [Google Scholar]
- 12.Stunkard AJ, Messick S. The three-factor eating questionnaire to measure dietary restraint, disinhibition and hunger. J Psychosom Res. 1985;29:71–83. doi: 10.1016/0022-3999(85)90010-8. [DOI] [PubMed] [Google Scholar]
- 13.Provencher V, Drapeau V, Tremblay A, Després JP, Lemieux S. Eating behaviors and indexes of body composition in men and women from the Québec family study. Obes Res. 2003;11(6):783–92. doi: 10.1038/oby.2003.109. [DOI] [PubMed] [Google Scholar]
- 14.Gallant AR, Tremblay A, Pérusse L, Bouchard C, Després JP, Drapeau V. The Three-Factor Eating Questionnaire and BMI in adolescents: results from the Québec Family Study. Br J Nutr. 2010 May 7;:1–6. doi: 10.1017/S0007114510001662. [DOI] [PubMed] [Google Scholar]
- 15.Steinle NI, Hsueh WC, Snitker S, et al. Eating behavior in the Old Order Amish: heritability analysis and a genome-wide linkage analysis. Am J Clin Nutr. 2002;75(6):1098–1106. doi: 10.1093/ajcn/75.6.1098. [DOI] [PubMed] [Google Scholar]
- 16.Bouchard L, Drapeau V, Provencher V, et al. Neuromedin beta: a strong candidate gene linking eating behaviors and susceptibility to obesity. Am J Clin Nutr. 2004;80(6):1478–1486. doi: 10.1093/ajcn/80.6.1478. [DOI] [PubMed] [Google Scholar]
- 17.Dotson CD, Shaw HL, Mitchell BD, Munger SD, Steinle NI. Variation in the gene TAS2R38 is associated with the eating behavior disinhibition in Old Order Amish women. Appetite. 2010;54(1):93–9. doi: 10.1016/j.appet.2009.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Choquette AC, Lemieux S, Tremblay A, et al. GAD2 gene sequence variations are associated with eating behaviors and weight gain in women from the Quebec family study. Physiol Behav. 1998;4:505–510. doi: 10.1016/j.physbeh.2009.08.004. [DOI] [PubMed] [Google Scholar]
- 19.Rolls ET. Taste, olfactory, and food texture processing in the brain, and the control of food intake. Physiol Behav. 2005;85(1):45–56. doi: 10.1016/j.physbeh.2005.04.012. [DOI] [PubMed] [Google Scholar]
- 20.Bartoshuk LM, Duffy VB, Miller IJ. PTC/PROP tasting: anatomy, psychophysics, and sex effects. Physiol BheBehav. 1994;56:1165–1171. doi: 10.1016/0031-9384(94)90361-1. [DOI] [PubMed] [Google Scholar]
- 21.Drewnowski A, Henderson SA, Shore AB, Barratt-Fornell A. Nontasters, tasters, and supertasters of 6-n-propylthiouracil (PROP) and hedonic response to sweet. Physiol Behav. 1997;62:649–655. doi: 10.1016/s0031-9384(97)00193-5. [DOI] [PubMed] [Google Scholar]
- 22.Duffy VB, Bartoshuk LM. Food acceptance and genetic variation in taste. J Am Diet Assoc. 2000;100(6):647–655. doi: 10.1016/S0002-8223(00)00191-7. [DOI] [PubMed] [Google Scholar]
- 23.Tepper BJ, Nurse RJ. Fat perception is related to PROP taster status. Physiol Behav. 1997;61(6):949–954. doi: 10.1016/s0031-9384(96)00608-7. [DOI] [PubMed] [Google Scholar]
- 24.Tepper BJ, Nurse RJ. PROP taster status is related to fat perception and preference. Ann N Y Acad Sci. 1998;855:802–804. doi: 10.1111/j.1749-6632.1998.tb10662.x. [DOI] [PubMed] [Google Scholar]
- 25.Duffy VB, Peterson JM, Bartoshuk LM. Associations between taste genetics, oral sensation and alcohol intake. Physiol Behav. 2004;82:435–445. doi: 10.1016/j.physbeh.2004.04.060. [DOI] [PubMed] [Google Scholar]
- 26.Goldstein GL, Daun H, Tepper BJ. Adiposity in middle-aged women is associated with genetic taste blindness to 6-n-propylthiouracil. Obes Res. 2005;13(6):1017–10. doi: 10.1038/oby.2005.119. [DOI] [PubMed] [Google Scholar]
- 27.Bartoshuk LM, Duffy VB, Hayes JE, Moskowitz HR, Snyder DJ. Psychophysics of sweet and fat perception in obesity: problems, solutions and new perspectives. Philos Trans R Soc Lond B Biol Sci. 2006;361(1471):1137–1148. doi: 10.1098/rstb.2006.1853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Golding J, Steer C, Emmett P, Bartoshuk LM, Horwood J, Smith GD. Associations between the ability to detect a bitter taste, dietary behavior, and growth: a preliminary report. Annals of the New York Academy of Sciences. 2009;1170:553–557. doi: 10.1111/j.1749-6632.2009.04482.x. [DOI] [PubMed] [Google Scholar]
- 29.Johnston FE, Hertzog KP, Malina RM. Phenylthiocarbamide taste sensitivity and its relationship to growth variation. Am J Phys Anthropol. 1966;24:253–255. doi: 10.1002/ajpa.1330240214. [DOI] [PubMed] [Google Scholar]
- 30.Enoch MA, Harris CR, Goldman D. Does a reduced sensitivity to bitter taste increase the risk of becoming nicotine addicted? Addict Behav. 2001;26:399–404. doi: 10.1016/s0306-4603(00)00117-9. [DOI] [PubMed] [Google Scholar]
- 31.Scott K. Taste recognition: food for thought. Neuron. 2005;48:455–464. doi: 10.1016/j.neuron.2005.10.015. [DOI] [PubMed] [Google Scholar]
- 32.Kim UK, Jorgenson E, Coon H, Leppert M, Risch N, Drayna D. Positional cloning of the human quantitative trait locus underlying taste sensitivity to phenylthiocarbamide. Science. 2003;299:1221–1225. doi: 10.1126/science.1080190. [DOI] [PubMed] [Google Scholar]
- 33.Mennella JA, Pepino MY, Reed DR. Genetic and environmental determinants of bitter perception and sweet preferences. Pediatrics. 2005;115:e216–222. doi: 10.1542/peds.2004-1582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Timpson NJ, Christensen M, Lawlor DA, et al. TAS2R38 (phenylthiocarbamide) haplotypes, coronary heart disease traits, and eating behavior in the British Women’s Heart and Health Study. Am J Clin Nutr. 2005;81(5):1005–1111. doi: 10.1093/ajcn/81.5.1005. [DOI] [PubMed] [Google Scholar]
- 35.Bell KI, Tepper BJ. Short-term vegetable intake by young children classified by 6-npropylthoiuracil bitter-taste phenotype. Am J Clin Nutr. 2006;84:245–251.29. doi: 10.1093/ajcn/84.1.245. [DOI] [PubMed] [Google Scholar]
- 36.Tepper BJ, Koelliker Y, Zhao L, et al. Variation in the bitter-taste receptor gene TAS2R38, and adiposity in a genetically isolated populationin Southern Italy. Obesity (Silver Spring) 2008;16:2289–2295. doi: 10.1038/oby.2008.357. [DOI] [PubMed] [Google Scholar]
- 37.Sausenthaler S, Rzehak P, Wichmann HE, Heinrich J. Lack of relation between bitter taste receptor TAS2R38 and BMI in adults. Obesity (Silver Spring) 2009 May;17(5):937–938. doi: 10.1038/oby.2009.15. [DOI] [PubMed] [Google Scholar]
- 38.Lin HF, Juo SH, Cheng R. Comparison of the power between microsatellite and single-nucleotide polymorphism markers for linkage and linkage disequilibrium mapping of an electrophysiological phenotype. BMC Genet. 2005;6 (Suppl 1):S7. doi: 10.1186/1471-2156-6-S1-S7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hinrichs AL, Wang JC, Bufe B, et al. Functional variant in a bitter-taste receptor (hTAS2R16) influences risk of alcohol dependence. Am J Hum Genet. 2006;78(1):103–111. doi: 10.1086/499253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nie Y, Hobbs JR, Vigues S, Olson WJ, Conn GL, Munger SD. Expression and purification of functional ligand-binding domains of T1R3 taste receptors. Chem Senses. 2006;31(6):505–513. doi: 10.1093/chemse/bjj053. [DOI] [PubMed] [Google Scholar]
- 41.Nie Y, Vigues S, Hobbs JR, Conn GL, Munger SD. Distinct contributions of T1R2 and T1R3 taste receptor subunits to the detection of sweet stimuli. Curr Biol. 2005;15(21):1948–1952. doi: 10.1016/j.cub.2005.09.037. [DOI] [PubMed] [Google Scholar]
- 42.Dyer J, Salmon KS, Zibrik L, Shirazi-Beechey SP. Expression of sweet taste receptors of the T1R family in the intestinal tract and enteroendocrine cells. Biochem Soc Trans. 2005;33:302–305. doi: 10.1042/BST0330302. [DOI] [PubMed] [Google Scholar]
- 43.Sternini C, Anseimi L, Rozengurt E. Enteroendocrine cells: A stie of “taste” in the gastrointestinal chemosensing. Curr Opin Endocrinol Diabetes Obes. 2008;15(1):73–78. doi: 10.1097/MED.0b013e3282f43a73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wu SV, Rozengurt N, Yang M, Young SH, Sinnett-Smith J, Rozengurt E. Expression of bitter taste receptors of the T2R family in gastrointestinal tract and enteroendocrine STC-1 cells. Proc Natl Acad Sci USA. 2002;99(4):2392–2397. doi: 10.1073/pnas.042617699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gautier JF, Choukem SP, Girard J. Physiology of incretins (GIP and GLP-1) and abnormalities in type 2 diabetes. Diabetes Metab. 2008;34 (Suppl2):S65–S72. doi: 10.1016/S1262-3636(08)73397-4. [DOI] [PubMed] [Google Scholar]
- 46.Dotson CD, Zhang L, Xu H, et al. Bitter taste receptors influence glucose homeostasis. PLoS ONE. 2008;3(12):e3974. doi: 10.1371/journal.pone.0003974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nakazato M, Murakami N, Date Y, et al. A role for ghrelin in the central regulation of feeding. Nature. 2001;409:194–198. doi: 10.1038/35051587. [DOI] [PubMed] [Google Scholar]
- 48.Callahan HS, Cummings DE, Pepe MS, Breen PA, Matthys CC, Weigle DS. Postprandial suppression of plasma ghrelin level is proportional to ingested caloric load but does not predict intermeal interval in humans. J Clin Endocrinol Metab. 2004;89:1319–1324. doi: 10.1210/jc.2003-031267. [DOI] [PubMed] [Google Scholar]
- 49.Zhang JV, Ren PG, Avsian-Kretchmer O, et al. Obestatin, a peptide encoded by the ghrelin gene, opposes ghrelin’s effects on food intake. Science. 2005;310:996–999. doi: 10.1126/science.1117255. [DOI] [PubMed] [Google Scholar]
- 50.Korbonits M, Gueorguiev M, O’Grady E, et al. A variation in the ghrelin gene increases weight and decreases insulin secretion in tall, obese children. J Clin Endocr Metab. 2002;87:4005–4008. doi: 10.1210/jcem.87.8.8881. [DOI] [PubMed] [Google Scholar]
- 51.Hinney A, Hoch A, Geller F, et al. Ghrelin gene: identification of missense variants and a frameshift mutation in extremely obese children and adolescents and healthy normal weight students. J Clin Endocr Metab. 2002;87:2716–2719. doi: 10.1210/jcem.87.6.8672. [DOI] [PubMed] [Google Scholar]
- 52.Steinle N, Pollin T, O’Connell J, Mitchell B, Shuldiner A. Variants in the ghrelin gene are associated with metabolic syndrome in the Old Order Amish. J Clin Endocrinol Metab. 2005;90(12):6672–7. doi: 10.1210/jc.2005-0549. [DOI] [PubMed] [Google Scholar]
- 53.Monteleone P, Tortorella A, Castaldo E, Di Filippo C, Maj M. The Leu72Met polymorphism of the ghrelin gene is significantly associated with binge eating disorder. Psychiatric Genetics. 2007;17(1):13–16. doi: 10.1097/YPG.0b013e328010e2c3. [DOI] [PubMed] [Google Scholar]
- 54.Dockray GJ. Cholecystokinin and gut-brain signalling. Regul Pept. 2009;155(1–3):6–10. doi: 10.1016/j.regpep.2009.03.015. [DOI] [PubMed] [Google Scholar]
- 55.de Krom M, van der Schouw YT, Hendriks J, et al. Common genetic variations in CCK, leptin, and leptin receptor genes are associated with specific human eating patterns. Diabetes. 2007;56(1):276–280. doi: 10.2337/db06-0473. [DOI] [PubMed] [Google Scholar]
- 56.den Hoed M, Westerterp-Plantenga MS, Bouwman FG, Mariman EC, Westerterp KR. Postprandial responses in hunger and satiety are associated with the rs9939609 single nucleotide polymorphism in FTO. Am J Clin Nutr. 2009;90(5):1426–1432. doi: 10.3945/ajcn.2009.28053. [DOI] [PubMed] [Google Scholar]
- 57.Hoffstedt J, Eriksson P, Mottagui-Tabar S, Arner P. A polymorphism in the leptin promoter region (−2548 G/A) influences gene expression and adipose tissue secretion of leptin. Horm Metab Res. 2002;34:355–359. doi: 10.1055/s-2002-33466. [DOI] [PubMed] [Google Scholar]
- 58.Cecil JE, Tavendale R, Watt P, Hetherington MM, Palmer CN. An obesity-associated FTO gene variant and increased energy intake in children. N Engl J Med. 2008;359(24):2558–2566. doi: 10.1056/NEJMoa0803839. [DOI] [PubMed] [Google Scholar]
- 59.Tanofsky-Kraff M, Han JC, Anandalingam K, Shomaker LB, et al. The FTO gene rs9939609 obesity-risk allele and loss of control over eating. American Journal of Clinical Nutrition. 2009;90(6):1483–1488. doi: 10.3945/ajcn.2009.28439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Stutzmann F, Cauchi S, Durand E, et al. Common genetic variation near MC4R is associated with eating behaviour patterns in European populations. International Journal of Obesity. 2009;33(3):373–388. doi: 10.1038/ijo.2008.279. [DOI] [PubMed] [Google Scholar]
- 61.DelParigi A, Chen K, Salbe AD, Reiman EM, Tataranni PA. Sensory experience of food and obesity: a positron emission tomography study of the brain regions affected by tasting a liquid meal after a prolonged fast. Neuroimage. 2005;24(2):436–443. doi: 10.1016/j.neuroimage.2004.08.035. [DOI] [PubMed] [Google Scholar]
- 62.Edwards BG, Barch DM, Braver TS. Improving prefrontal cortex function in schizophrenia through focused training of cognitive control. Front Hum Neurosci. 2010;4:32. doi: 10.3389/fnhum.2010.00032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bokde AL, Karmann M, Born C, Teipel SJ. Altered Brain Activation During a Verbal Working Memory Task in Subjects with Amnestic Mild Cognitive Impairment. J Alzheimers Dis. 2010 Apr 22; doi: 10.3233/JAD-2010-091054. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 64.Porubska K, Veit R, Preissl H, Fritsche A, Birbaumer N. Subjective feeling of appetite modulates brain activity: an fMRI study. Neuroimage. 2006;32(3):1273–1280. doi: 10.1016/j.neuroimage.2006.04.216. [DOI] [PubMed] [Google Scholar]
- 65.Goldstone AP, de Hernandez CG, Beaver JD, et al. Fasting biases brain reward systems towards high-calorie foods. Eur J Neurosci. 2009;30(8):1625–1635. doi: 10.1111/j.1460-9568.2009.06949.x. [DOI] [PubMed] [Google Scholar]
- 66.Stoeckel LE, Weller RE, Cook EW, Twieg DB, Knowlton RC, Cox JE. Widespread reward-system activation in obese women in response to pictures of high-calorie foods. Neuroimage. 2008;41(2):636–647. doi: 10.1016/j.neuroimage.2008.02.031. [DOI] [PubMed] [Google Scholar]
- 67.Le DS, Pannacciulli N, Chen K, et al. Less activation of the left dorsolateral prefrontal cortex in response to a meal: a feature of obesity. Am J Clin Nutr. 2006;84(4):725–731. doi: 10.1093/ajcn/84.4.725. [DOI] [PubMed] [Google Scholar]
- 68.Malik S, McGlone F, Bedrossian D, Dagher A. Ghrelin modulates brain activity in areas that control appetitive behavior. Cell Metab. 2008;7(5):400–409. doi: 10.1016/j.cmet.2008.03.007. [DOI] [PubMed] [Google Scholar]
- 69.Ahima RS. Revisiting leptin’s role in obesity and weight loss. J Clin Invest. 2008;118(7):2583–2591. doi: 10.1172/JCI36284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Siep N, Roefs A, Roebroeck A, Havermans R, Bonte ML, Jansen A. Hunger is the best spice: an fMRI study of the effects of attention, hunger and calorie content on food reward processing in the amygdala and orbitofrontal cortex. Behav Brain Res. 2009;198(1):149–58. doi: 10.1016/j.bbr.2008.10.035. [DOI] [PubMed] [Google Scholar]
- 71.Stice E, Spoor S, Bohon C, Small DM. Relation between obesity and blunted striatal response to food is moderated by TaqIA A1 allele. Nutrition. 2009;25(2):132–133. doi: 10.1126/science.1161550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Martin LE, Holsen LM, Chambers RJ, et al. Neural mechanisms associated with food motivation in obese and healthy weight adults. Obesity. 2010;18(2):254–260. doi: 10.1038/oby.2009.220. [DOI] [PubMed] [Google Scholar]
- 73.Haase L, Cerf-Ducastel B, Murphy C. Cortical activation in response to pure taste stimuli during the physiological states of hunger and satiety. Neuroimage. 2009;44(3):1008–1021. doi: 10.1016/j.neuroimage.2008.09.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Felsted JA, Ren X, Chouinard-Decorte F, Small DM. Genetically determined differences in brain response to a primary food reward. J Neurosci. 2010;30(7):2428–2432. doi: 10.1523/JNEUROSCI.5483-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Neville MJ, Johnstone EC, Walton RT. Identification and characterization of ANKK1: a novel kinase gene closely linked to DRD2 on chromosomeband 11q23.1. Hum Mutat. 2004;23:540–545. doi: 10.1002/humu.20039. [DOI] [PubMed] [Google Scholar]
- 76.Waite RL, Williams L, Braverman ER, et al. LG839: anti-obesity effects and polymorphic gene correlates of reward deficiency syndrome. Advances in Therapy. 2008;25(9):894–913. doi: 10.1007/s12325-008-0093-z. [DOI] [PubMed] [Google Scholar]
- 77.Florez JC, Jablonski KA, Bayley N, et al. Diabetes Prevention Program Research Group. N Engl J Med. 2006;355(3):241–250. doi: 10.1056/NEJMoa062418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ruiz JR, Labayen I, Ortega FB. Attenuation of the effect of the FTO rs9939609 polymorphism on total and central body fat by physical activity in adolescents: the HELENA study. Arch Pediatr Adolesc Med. 2010;164(4):328–333. doi: 10.1001/archpediatrics.2010.29. [DOI] [PubMed] [Google Scholar]
- 79.Mitchell JA, Church TS, Rankinen T, Earnest CP, Sui X, Blair SN. FTO genotype and the weight loss benefits of moderate intensity exercise. Obesity. 2010;18(3):641–643. doi: 10.1038/oby.2009.311. [DOI] [PMC free article] [PubMed] [Google Scholar]