Abstract
We recently demonstrated that Sirt1, a NAD+ dependent histone deacetylase, was overexpressed in prostate cancer (PCa) and its inhibition resulted in a significant anti-proliferative response in human PCa cells. Studies have suggested a link between Sirt1 and circadian rhythms, the disruption of which has been linked to cancer. Interestingly, a decreased production of the pineal melatonin has been shown to deregulate the circadian rhythm machinery and increase cancer risk. Further, disruption in melatonin production and circadian rhythmicity has been associated with aging. Here, we challenged our hypothesis that melatonin will impart anti-proliferative response against PCa via inhibiting Sirt1. We demonstrated that melatonin significantly inhibited Sirt1 protein and activity in vitro in multiple human PCa cell lines and melatonin-mediated Sirt1 inhibition was accompanied with a significant decrease in the proliferative potential of PCa cells, but not of normal cells. Forced overexpression of Sirt1 partially rescued the PCa cells from melatonin’s anti-proliferative effects, suggesting that Sirt1 is a direct target of melatonin. Employing TRAMP mice, we also demonstrated that oral administration of melatonin, at human achievable doses, significantly inhibited PCa tumorigenesis as shown by decreases in (i) prostate and genitourinary (GU) weight, (ii) serum insulin-like growth factor-1 (IGF-1)/IGF-binding protein-3 (IGFBP3) ratio, (iii) mRNA and protein levels of the proliferation markers (PCNA, Ki-67). This anti-PCa response was accompanied with a significant decrease in Sirt1 in TRAMP prostate. Our data identified melatonin as a novel inhibitor of Sirt1 and suggest that melatonin can inhibit PCa growth via Sirt1 inhibition.
Keywords: sirtuins, prostate cancer, melatonin, TRAMP
Introduction
The likelihood of prostate cancer (PCa) diagnosis in men increases with age. In a recent study, we demonstrated that Sirt1, a class III histone deacetylases (HDAC) known to be involved in aging, was overexpressed in human PCa cells and tissues and Sirt1 inhibition resulted in FoxO1 activation-mediated decreased in the growth and viability of human PCa cells [1]. In a follow-up study, we provided evidence that Sirt1 inhibition caused an increase in apoptosis in a p53-independent fashion and an increase in senescence in a p53-dependent fashion [2]. These studies and several other reports [3–5] in many experimental models suggested that Sirt1 inhibition could be an interesting target for management of certain age-related cancers including PCa. Recent studies have shown that Sirt1 regulates circadian clock gene expression [6] and modulates Clock-mediated chromatin remodeling and circadian control [7]. The mammalian circadian rhythm is controlled by a number of metabolic and physiological core components and the expression of clock genes is a characteristic feature of the central rhythm generator and peripheral oscillators. It is composed of a central pacemaker in the suprachiasmatic nucleus (SCN) of the brain which acts to synchronize clock component genes such as Clock, Bmal1, Periods (Pers), and Cryptochromes (Crys) [8].
Interestingly, recently studies have connected the hormone melatonin (N-Acetyl-5-methoxytryptamine) with the regulation of circadian rhythm and clock apparatus [9,10]. Melatonin is a small molecule secreted by the pineal gland and its synthesis shows a circadian pattern as levels are low during light hours and high in dark hours. Of note is the fact that both melatonin levels and the rhythmicity of the circadian clockwork circuitry diminish and deteriorate gradually in the aged population. Therefore, it has been suggested that loss of melatonin in the elderly may lead to a disruption of circadian rhythm components resulting in a decrease in overall health and an increase in cancer susceptibility or progression [11]. Further, studies have suggested that melatonin possesses anti-proliferative, chemopreventive, oncostatic and tumor inhibitory effects in a variety of in vitro and in vivo experimental cancer models [12]. Here we report that melatonin is a novel Sirt1 inhibitor and it imparts anti-proliferative effects in vitro in human PCa cells as well as in vivo in TRAMP (TRansgenic Adenocarcinoma of Mouse Prostate) mice via inhibiting Sirt1.
Methods and Materials
Cell Culture
The human prostate carcinoma cells viz. LNCaP, 22Rν1, DU145, and PC3 (American Type Culture Collection, ATCC, Manassas, VA, USA), and human kidney epithelial cells, HEK 293T (Invitrogen, Carlsbad, CA, USA) were maintained in vendor-recommended media supplemented with FBS and antibiotics (penicillin/streptomycin). Normal human prostate epithelial (PrEC) cells (Cambrex, East Rutherford, NJ, USA) were maintained in PrEBM media with growth factors and supplements as recommended by the vendor. All cells were maintained at standard cell culture conditions.
Treatment of Cells with Melatonin
Cells were grown to 60% confluency and then treated with 10nM - 2mM melatonin (Enzo Life Sciences, Plymouth Meeting, PA, USA). Melatonin was dissolved in ≤ 200μL of 96% ethanol (final ethanol concentration of ≤ 1%) and then diluted with PBS to the desired melatonin concentration. Cells were incubated with melatonin, or 1% ethanol vehicle control, for 24–48 hours or up to 14 days after which they were used for subsequent experiments.
Trypan Blue Exclusion, Clonogenic and Soft Agar Assays
Trypan blue exclusion assay was performed as described previously [1]. Colony formation was assessed as previously described [13]. For soft-agar assay, a base agar layer containing 1.0% agar and 20% FBS supplemented media was prepared and allowed to solidify. Following treatment with melatonin, the PCa cells were collected by trypsinization and added to the top agar layer containing 0.7% agar and 20% FBS supplemented media. The mixture was allowed to solidify and the plates were incubated at 37°C, 5% CO2 in a humidified incubator for 28 days. Cells were then stained with 0.005% crystal violet (in methanol: H2O; 1:1) for one hour at room temperature followed by a PBS wash. Pictures were captured and colonies were counted.
CytoSelect Transformation Assay
PCa cells were suspended in an agar matrix and then plated on top of a base agar matrix. Cells were then treated with melatonin (10nM-10μM) supplemented media for 7 days after which the agar matrix was solubilized and quantitated with MTT solution according to the vendor’s protocol (Cell Biolabs, San Diego, CA, USA).
Preparation of Protein Lysates and Western Blot Analysis
Protein lysates and Western blot analysis were performed as described earlier [1]. For the in vivo experiments, prostatic tissues (10–100mg) from each animal were homogenized in 1X RIPA lysis buffer with freshly added phenylmethylsulfonyl fluoride, 100mM sodium orthovanadate, protease inhibitor cocktail and phosphatase inhibitor cocktail. Protein lysates prepared from prostate tissue from each animal and then pooled (n=4) into three samples and then averaged for densitometric analysis. Immunoblot analysis was performed using primary antibodies: anti-Sirt1, (Cell Signaling, Danvers, MA, USA), anti-Ki-67, anti-PCNA, anti-SV40 T Ag, and anti-actin (Santa Cruz, Santa Cruz, CA, USA) and secondary antibodies: goat anti-rabbit and goat anti-mouse HRP-conjugated antibodies (Millipore, Billerica, MA, USA), donkey anti-goat HRP-conjugated antibody (Santa Cruz, Santa Cruz, CA, USA).
Immunoprecipitation and Sirt1 Enzyme Activity Assay
Immunoprecipitation of Sirt1 protein and Sirt1 activity assay were done as described previously [1].
Quantitative Real Time Reverse Transcriptase-PCR
RNA was isolated from cells, pooled (n=4) into three different samples and quantitative RT-PCR (qRT-PCR) was performed in triplicate as described previously [1]. The Ct values of pooled samples were averaged for relative fold change calculations. Primer sequences are as follows: mKi-67 Forward: CAGTACTCGGAATGCAGCAA, mKi-67 Reverse: CAGTCTTCAGGGGCTCTGTC, mPCNA Forward: GGGTTGGTAGTTGTCGCTGT, mPCNA Reverse: AGCACCTTCTTCAGGATGGA, mIGFBP3 Forward: CAACCTGCTCCAGGAAACAT, mIGFBP3 Reverse: AACTTTGTAGCGCTGGCTGT, mIGF-1 Forward: TCGTCTTCACACCTCTTCTA, mIGF-1 Reverse: AAGCAACACTCATCCACAAT, mSirt1 Forward: CAGACCCTCAAGCCATGTTT, mSirt1 Reverse: GATCCTTTGGATTCCTGAAA, mGAPDH Forward: AACTTTGGCATTGTGGAAGG, mGAPDH Reverse: ACACATTGGGGGTAGGAACA. Purity of product was checked by dissociation curve analysis as well as running the samples on 3% agarose gel.
Retroviral Production and Creation of Stable Cell Lines
CaPO4 transfections were performed using HEK 293T cells to produce retroviruses. Briefly, 6μg GAG-pol, 5μg VSV-G,8μg pYESir (Plasmid 1769, Addgene, Cambridge, MA, USA) or pBABE (empty vector) and sterile ddH20 were combined and then 50μl of 2.5M CaCl2 was added. 500μl of 2X HBS buffer (pH-7.05) was then bubbled in a 15ml conical tube and DNA plasmids/CaCl2 were added. The cells were incubated overnight, medium was replaced and incubated overnight again. After 48 and 72 hours, the supernatant from the HEK 293T cells was collected and filtered (0.45μm filter). The supernatant was used to infect target cells. The cells were infected three times daily with retrovirus supernatant (pYESir2 or pBABE) in the presence of 6μg/ml of polybrene (Sigma-Aldrich, MO). After 48 hours, the viral supernatant was replaced with regular cell medium and incubated overnight. Transfected cells were selected with 2μg/ml Puromycin (Sigma-Aldrich, St. Louis, MO, USA). The surviving cells were then allowed to propagate, checked for Sirt1 overexpression via Western blot analysis and used for subsequent experiments.
Study Design and Melatonin Supplementation in TRAMP mice
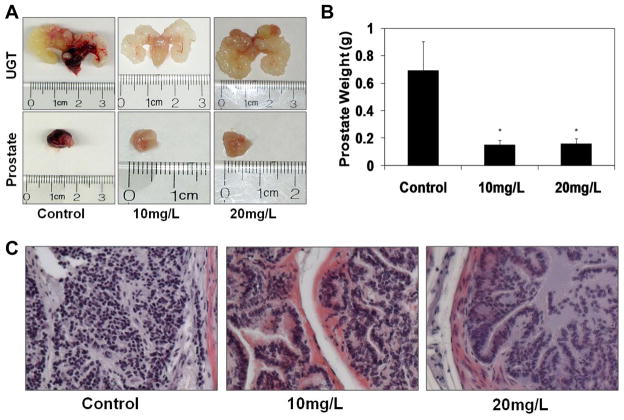
Sixteen weeks old TRAMP mice (on C57BL/6 background), obtained from The Jackson Laboratory (Bar Harbor, ME, USA), were divided into three groups of 12 animals each where the mice were given melatonin (Enzo Life Sciences, Plymouth Meeting, PA, USA) in tap water at two doses; 10 and 20 mg/L, or no treatment for 18 weeks. Melatonin was dissolved in ≤ 200μL of 96% ethanol (final ethanol concentration of ≤ 1%) and then diluted with tap water to the desired concentration. A fresh melatonin solution was prepared daily and was given ad libitum to the animals in dark colored bottles between 6 PM and 9 AM when lights were off in the animal facility and then replaced with regular tap water during day (lighted) hours (9 AM to 6 PM). As previously reported, this method of melatonin administration in drinking water has not been found to alter the amount of water consumed or the drinking rhythm of mice [14]. All animals received AIN-76-B 40 diet (ICN Biochemicals, Cleveland, OH, USA) ad libitum and were subjected to 12 hours of light-12 hours of dark. This dose regimen and protocol was based on a thorough search of the literature where similar protocols were found to be effective. Additionally, as calculated by the FDA guidelines and our published studies [15], this dosing regimen is clinically relevant to human as it is equivalent to an approximate dosage of 4.86–9.72mg for a 60kg human (Supplementary Fig. 1). The animal’s weight was recorded weekly to monitor signs of toxicity. Blood was obtained from each mouse every three weeks, late in the day at similar times, via saphenous/metatarsal vein puncture. Blood was then stored at 4°C overnight followed by serum separation via centrifugation 24 hours later, and stored at −80°C. After 18 weeks of melatonin administration, the experiment was terminated and mice were euthanized by cervical dislocation, and the urogenital tract (UGT) containing prostate and seminal vesicles was removed.
Preparation and Analysis of Tissue
The UGT containing the prostate was excised, weighed and pictures were captured. The prostate was then surgically removed, weight recorded, and pictures captured. The prostate tissue was then divided into small portions for subsequent experiments.
Determination of IGF-1 and IGFBP3 Levels in Serum
As mice do not produce prostate specific antigen (PSA), PCa progression is often monitored by following the serum levels of IGF-1 and IGFBP3. Studies have shown that elevated levels of IGF-1 with concomitant lowering of IGFBP3 levels in serum is associated with PCa risk and is sometimes used to predict PCa progression [16]. We have earlier shown the utility of serum IGF-1, and IGFBP3 levels in monitoring PCa progression in TRAMP mice [17]. The IGF-1 and IGFBP3 levels were determined by enzyme-linked immunosorbent assay (ELISA) as described by the vendor (Diagnostic Systems, Webster, TX, USA).
Statistical Analysis
Statistical analyses were performed using either Student’s t test or analysis of variance (ANOVA) followed by Tukey’s test. In either case the data are expressed as means ± SD unless specified otherwise. Statistically significant p-values are provided for each individual experiment.
Results
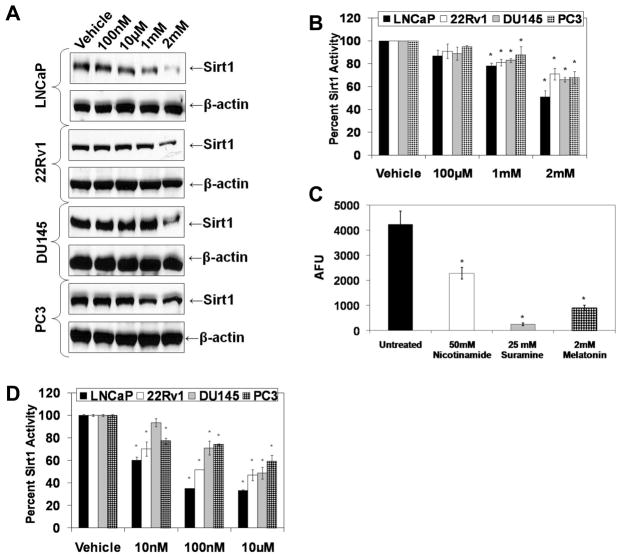
To test our hypothesis, we first assessed whether or not melatonin is an inhibitor of Sirt1 in PCa cells. We determined the effect of melatonin (100nM-2mM; for 48 or 72 hours) on Sirt1 in a panel of four PCa cell lines (LNCaP, 22Rν1, DU145 and PC3). As shown in Fig. 1A, melatonin (1mM and 2mM; 48 hours) was found to significantly inhibit Sirt1 protein in all four PCa cell lines. Further, employing a Sirt1 activity assay kit that used a fluorogenic peptide (encompassing residues 379 to 382 of p53, acetylated on lysine 382), we also demonstrated that melatonin (1mM and 2 mM; 48 hours) caused a significant decrease in Sirt1 activity in all the cell lines (Fig. 1B). In addition, we compared the Sirt1 inhibitory effect of melatonin with two known chemical Sirt1 inhibitors, nicotinamide and suramine. As shown in Fig. 1C, melatonin (2mM; 72 hours) was found to have a comparable or better Sirt1 inhibitory effect when compared to nicotinamide (50mM) and suramine (25mM) in LNCaP cells. This suggested that melatonin is a novel Sirt1 inhibitor with a significant Sirt1 inhibitory response comparable to other known Sirt1 inhibitors. Further, because we observed Sirt1 inhibition with high acute doses of melatonin, for a translational significance of our data, we determined if chronic melatonin (at low doses) possesses a Sirt1 inhibitory effect in PCa cells. Interestingly, chronic melatonin at much lower concentrations (10nM-10μM; for 14 days) was found to significantly inhibit Sirt1 activity in PCa cells (Fig. 1D), suggesting that melatonin inhibits Sirt1 at physiologically achievable concentrations.
Fig. 1.
Effect of melatonin treatment on sirtuin protein and activity levels in PCa cells. A) Effect of melatonin on Sirt1 protein levels: Following treatment of cells with melatonin (100nM-2mM; 48 hours), protein levels were detected by Western blot analysis. Equal loading was confirmed by re-probing the blot for β-actin. Data represent three experiments with similar results; B) Effect of high acute melatonin treatment on Sirt1 activity: Following treatment of cells, Sirt1 protein was immunoprecipitated and the enzyme activity was assessed using Sirt1 activity assay kit (AK-555; Biomol, PA). The data are expressed as relative percent inhibition compared to controls; C) Sirt1 inhibition by melatonin compared to other known Sirt1 chemical inhibitors: Following treatments of cells with nicotinamide, suramine or melatonin at specified concentrations (for 72 hours), Sirt1 protein was immunoprecipitated and enzyme activity was assessed. The data are expressed as relative fold change in AFU normalized to control; D) Effect of low chronic melatonin treatment on Sirt1 activity: Following treatment of cells with melatonin (10nM-10μM melatonin; 14 days), Sirt1 protein was immunoprecipitated using a Sirt1 antibody and enzyme activity was determined. The data were calculated as relative percent inhibition compared to controls. The data in Fig. 1 are expressed as mean ± SE of three experiments (p-value ≤ 0.01*).
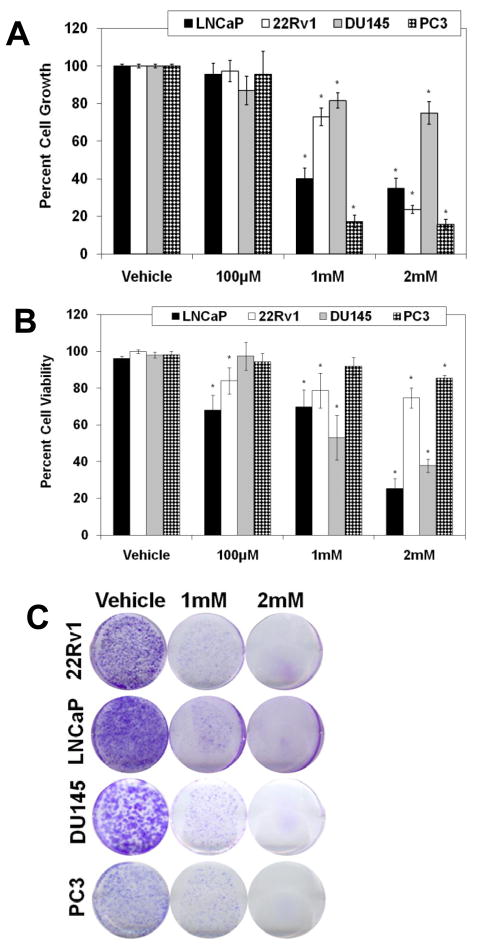
Next, employing multiple techniques, we determined the anti-proliferative potential of melatonin in human PCa cells. The PCa cells (LNCaP, 22Rν1, DU145 and PC3) were treated with melatonin (100μM to 2mM; for 48 hours) followed assessment of growth and viability. We found that melatonin caused a significant dose-dependent inhibition in growth and viability of PCa cells (Fig. 2A and B). Importantly, similar treatments did not affect the growth or viability of normal PrEC cells (Supplementary Fig. 2). Further, we also found that melatonin caused a significant inhibition in the colony formation ability of PCa cells (Fig. 2C).
Fig. 2.
Effect of melatonin treatment on the proliferation of human PCa cells and normal prostate epithelial cells. A) Effect of melatonin on cell growth in PCa cells: Following treatment of cells with melatonin (100μM-2mM; 48 hours), cell growth was assessed by Trypan Blue assay. Cell growth is expressed as percent growth (from total number of cells); B) Effect of melatonin on cell viability in PCa cells: Following treatment of cells with melatonin (100μM-2mM; 48 hours), viability was assessed by Trypan Blue assay. Cell viability is expressed as the percent viable cells out of the total number of cells. C) Effect of melatonin on colony formation ability of PCa cells: Cells were treated with melatonin (1mM or 2mM; 14 days) with fresh melatonin-supplemented medium being replaced every three days. After 14 days, colonies were fixed and stained with 0.5% crystal violet. The data presented are representative of three separate experiments with similar results. The data in Fig. 2 are expressed as mean ± SE of three experiments (p-value ≤ 0.01*).
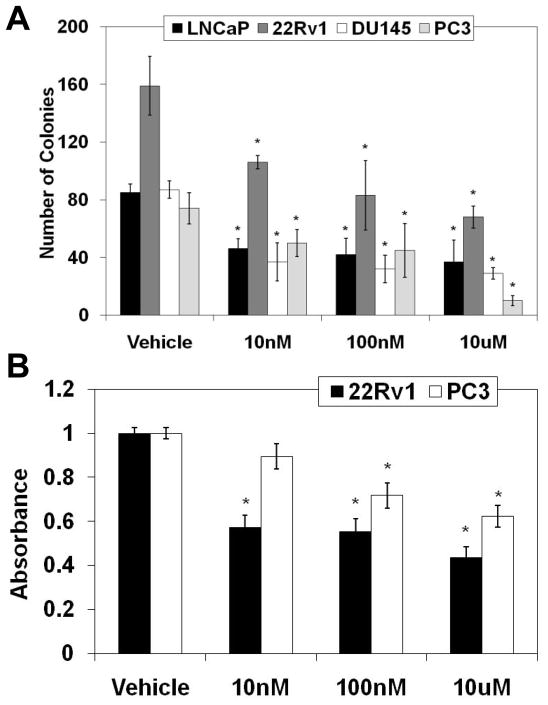
Our experiments (discussed above) used relatively high melatonin concentrations (1mM and 2mM; single exposure), which may not be physiologically relevant. Therefore, we next assessed the effects of chronic melatonin exposure with low concentrations (10nM, 100nM and 10μM; 7–28 days) on the proliferation of PCa cells. Employing a soft agar assay, we found that a chronic melatonin, even at the lowest concentration of 10nM, significantly inhibited the colony formation of the PCa cells (Fig. 3A). In addition, we also used CytoSelect Transformation Assay that quantitatively measures cell proliferation in a semisolid agar media. As shown in Fig. 3B, we found a significant reduction in the proliferation of cells treated with chronic melatonin (10nM-10μM) in 22Rν1 and PC3 cells (Fig. 3B). Together, these data suggested that melatonin has remarkable anti-proliferative effects in PCa cells, at physiologically achievable low concentration. However, for further studies, we elected to use 1mM and 2mM concentrations of melatonin because of the ease of experimental protocols for acute exposure (versus chronic exposure).
Fig. 3.
Effect of low chronic melatonin treatment on the long-term proliferative capacity of human PCa cells. A) Effect of melatonin on anchorage-independent PCa growth: Cells were treated with melatonin (10nM-10μM; 48 hours) and anchorage-independent growth was assessed as described under ‘Methods’. Colonies were manually counted; B) Effect of melatonin on the transformation of PCa cells: Following melatonin treatment, the effect on cell transformation was assessed by CytoSelect Transformation Assay according to the vendor’s protocol (Cell Biolabs, CA). The data in Fig. 3 are expressed as mean ± SE of three experiments (p-value ≤ 0.01*).
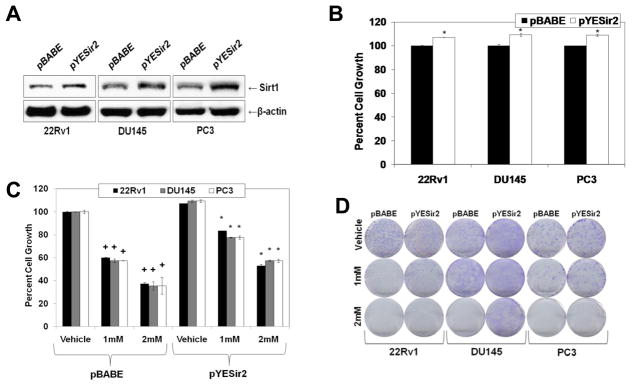
To establish if the anti-proliferative effects of melatonin are mediated via Sirt1 inhibition, we determined if a forced overexpression of Sirt1 rescues PCa cells from the melatonin’s anti-proliferative effects. To achieve this objective, we created stable Sirt1-overexpressing PCa cell lines. Using mammalian retroviral overexpression vectors, pYESir2 (Sirt1 overexpressing) and pBABE (empty vector), we created stable 22Rν1, DU145, and PC3 cell lines that overexpressed Sirt1 protein (Fig. 4A). As expected, we found that overexpression of Sirt1 caused a significant increase in cell growth in all PCa cell lines tested (Fig. 4B). Interestingly, the forced overexpression of Sirt1 was found to result in a significant reversal of melatonin’s anti-proliferative response in PCa cells as melatonin treatment was found to cause significantly less cell growth in Sirt1-transfectant (pYESir2) cells versus control (pBABE) cells (Fig. 4C). This result was further confirmed by colony formation (Fig. 4D) and MTT (data not shown) assays where Sirt1 overexpression was found to have a protective response against the growth inhibitory effects of melatonin. Thus, our data suggested that the anti-proliferative effects of melatonin, at-least partially, are mediated via its Sirt1 inhibitory response. Since we did not observe a complete protection, it is clear that Sirt1 inhibition is not the only mechanism by which melatonin imparts its anti-proliferative effects.
Fig. 4.
Overexpression of Sirt1 in PCa cells partially rescues cells from the anti-proliferative effects of melatonin. A) Sirt1 overexpression: Following retroviral infection (pYESir2 or pBABE) of cells and selection for stable clones with Puromycin, Sirt1 protein levels were detected by Western blot analysis. Equal loading was confirmed by re-probing the blot for β-actin. Data represent three experiments with similar results; B) Effect of Sirt1 overexpression on PCa cell growth: Stable PCa transfected cells were analyzed by Trypan Blue assay to assess cell growth. Cell growth is expressed as percent growth (from total number of cells); C) Effect of melatonin on cell growth in PCa cells overexpressing Sirt1: Stably transfected cells were treated with melatonin (1mM or 2mM; 48 hours) and analyzed by Trypan Blue assay to assess cell growth. Cell growth is expressed as percent growth (from total number of cells); D) Effect of melatonin on the colony formation ability of PCa cells overexpressing Sirt1: Stably transfected cells were treated with melatonin (1mM or 2mM; 14 days), colonies were fixed and stained with 0.5% crystal violet. The data in Fig. 4 are expressed as the mean ± SE of three experiments (+p ≤ 0.01 and *p≤ 0.05).
To establish the in vivo relevance of our in vitro results, we utilized the TRansgenic Adenocarcinoma of the Mouse Prostate (TRAMP) model of prostate cancer. The TRAMP model shows a number of features similar to human PCa [18] and is regarded as an excellent in vivo model of PCa [19–21]. In our experiments, the selected melatonin dose regiment (10mg/L and 20mg/L) was calculated to be physiologically achievable with a human equivalent dose (HED) of 4.86 and 9.72 mg per day (for a 60kg person) (Supplementary Fig. 1) [15]. Our data demonstrated a remarkable prostate cancer inhibitory effect of melatonin in TRAMP mice. As shown in Fig. 5A and 5B, melatonin supplementation resulted in a remarkable decrease in prostate weight in mice. Importantly, melatonin supplementation did not affect the average body weight (Supplementary Fig. 3) or any apparent toxicity in mice. Because PCa in TRAMP model is driven by the expression of SV 40 transgene (T Ag) in the prostatic epithelium, we assessed the effect of melatonin on the expression of the transgene and found that T Ag expression is not affected by melatonin supplementation (Supplementary. Fig. 4). Histological examination of TRAMP mouse prostate tissue at the termination of experiment demonstrated that mice in the control group exhibited mostly poorly differentiated adenocarcinoma (>70% of surface area) composed of scant cytoplasm and marked nuclear pleomorphism. However, the histological findings in the melatonin treated animals were drastically different. In the group that was given 10 mg/L melatonin, the prostate tissues contained of moderately differentiated carcinoma (~60%) and well differentiated carcinoma (~20%). There was ~15% of the area that was observed to be poorly differentiated (Fig. 5C). The group that was provided 20 mg/L of melatonin exhibited even better histopathology with multiple foci of well-differentiated carcinoma (>50%) and moderately differentiated carcinoma (~40%) with only about 5–7% surface area covered by poorly differentiated carcinoma.
Fig. 5.
Effect of melatonin supplementation in TRAMP model. Sixteen-week old TRAMP mice were divided into three treatment groups (0, 10 and 20 mg/L melatonin) of 12 animals each. Blood samples were periodically obtained and at the termination (at 18 weeks of treatment), the mice were euthanized and tissues were collected. A) Effect of melatonin treatment on UGT and prostate size: At the termination of experiment, UGT apparatus and then prostates were removed and photographs were captured. Representative pictures from each group are shown; B) Effect of melatonin on prostate weight: Following dissection, prostates from each mouse were weighed and recorded. The data were obtained from each group (n=12) and are expressed as mean ± SE (*p ≤ 0.01). C) Effect of melatonin treatment on histological morphology of the TRAMP prostate: Histological morphology of TRAMP prostate tissues in control and melatonin treated (10mg/L and 20mg/L) mice at ~34 weeks of age were analyzed by H&E staining. Representative pictures from each treatment taken at 30x magnification are shown.
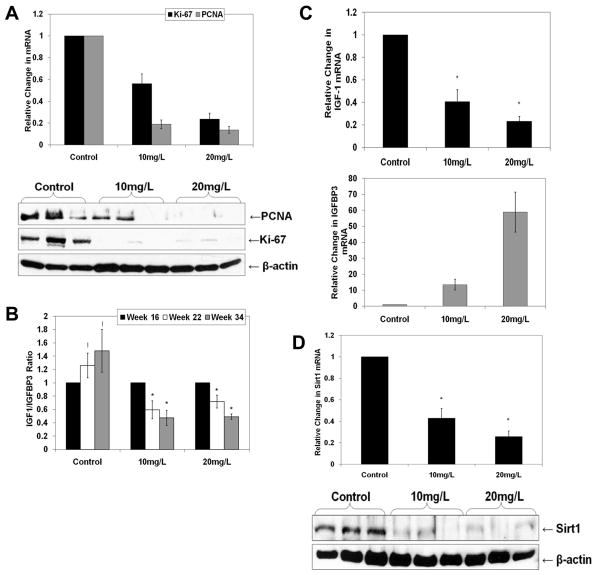
We next assessed the effect of melatonin on proliferation markers in TRAMP prostate. Melatonin supplementation resulted in a significant decrease in Proliferating Cell Nuclear Antigen (PCNA) and Ki-67, both at mRNA and protein levels (Fig. 6A). Additionally, we measured the IGF-1 and IGFBP3 serum levels, as earlier studies have shown IGF-1/IGFBP3 ratio as a marker of cancer progression in TRAMP [19]. We found a progressive increase in the serum IGF-1/IGFBP3 ratio in control mice as a function of age and disease progression (Fig. 6B). Interestingly, melatonin supplementation resulted in a significant decrease in IGF-1/IGFBP3 ratio in serum during disease progression (Fig. 6B). We also determined the levels of IGF-1 and IGFBP3 in the prostate tissues of the TRAMP mice as IGFs can act in an autocrine or a paracrine fashion and are produced locally [22]. Using qRT-PCR analysis, we found melatonin supplementation caused significant decrease in IGF-1 and a concomitant significant increase in IGFBP3 mRNA levels in TRAMP prostate (Fig. 6C). Finally, we determined if the anti-cancer effects of melatonin are mediated via Sirt1 inhibition. As shown in Fig. 6D, melatonin treatment resulted in a significant decrease in Sirt1 expression in the prostate. These data correlate with and support our in vitro finding that the anti-cancer effects of melatonin are mediated via an inhibition of Sirt1.
Fig. 6.
Effect of melatonin treatment on proliferation markers and Sirt1 expression in TRAMP model. Protein and RNA were isolated from prostate tissues of individual mice and then pooled (n=4) into three different samples followed by cDNA synthesis and protein lysates preparation. A) Effect of melatonin on PCNA and Ki-67 mRNA and protein levels: The relative expression levels of PCNA and Ki-67 transcripts were determined by qRT-PCR and the data was normalized to GAPDH. PCNA and Ki-67 protein levels were detected by Western blot analysis. Equal loading was confirmed by re-probing the blot for β-actin. Data represent three experiments with similar results; B) Effect of melatonin treatment on serum IGF-1 and IGFBP3 levels: Serum IGF-1 & IGFBP3 levels were determined by ELISA as described in ‘Methods’; C) Effect of melatonin on IGF-1 and IGFBP3 mRNA levels: The relative expression levels of IGF-1 and IGFBP3 transcripts in TRAMP prostate tissues were determined by qRT-PCR and the data were normalized to GAPDH; D) Effect of melatonin on Sirt1 mRNA and protein levels: The expression level of Sirt1 transcripts in TRAMP prostate tissues was determined by qRT-PCR and the data were normalized to GAPDH. Sirt1 protein levels were detected by Western blot analysis. Equal loading was confirmed by re-probing the blot for β-actin. Data represent three experiments with similar results. The data in Fig. 6 are expressed as mean ± SE of three experiments (*p ≤ 0.01 and !p ≤ 0.05).
Discussion
The most novel information of this study is the evidence that the pineal hormone melatonin is an inhibitor of Sirt1 in human PCa, which overexpress Sirt1. Sirt1 has been linked to several metabolic processes and longevity [23,24] and its level has been shown to increase in several age-related cancers [4,25]. We have demonstrated that Sirt1 is overexpressed in human PCa and provided evidence that its inhibition could be a viable approach for the management of this age-related malignancy. In this study, we tested our hypothesis that melatonin is an inhibitor of Sirt1 in PCa. The reasoning and rationale for this hypothesis come from several lines of evidence. First, two recent landmark studies have shown an association of Sirt1 with circadian rhythm via regulating PER2 and CLOCK, and melatonin is also known to regulate circadian rhythm [6,26]. Second, the production of melatonin has been shown to decrease with aging and studies have shown that cancer patients (including PCa patients) have lower circulating levels of melatonin [12,27]. Therefore, it is possible that melatonin levels may have an inverse correlation with Sirt1 in PCa. Indeed, melatonin has been reported to possess anti-proliferative effects in several cancers [12,27–30]. Studies have also suggested that melatonin may be useful in cancer management as an adjuvant therapy (reviewed in [31]). We have reviewed this area of research and promoted the idea that melatonin should be explored as an agent for cancer management [12].
We have shown that melatonin strongly inhibits Sirt1 in a wide range of human PCa cells (androgen-sensitive, androgen-insensitive, p53 positive, p53 mutated and p53 null) (Fig. 1). The observed Sirt1 inhibitory response of melatonin was comparable to other known Sirt1 inhibitors, nicotinamide and suramine. This is a novel finding because there is intense research ongoing to discover the non-toxic physiologically feasible inhibitors of Sirt1, which could be evaluated for the management of those cancers where Sirt1 is overexpressed and functionally relevant, PCa is one such cancer type. Our data (Fig. 2) and earlier studies [29,32] have shown that melatonin has anti-proliferative effects against PCa. However, previously published studies have shown effects at a much higher concentration of melatonin that may not be physiologically achievable. We have shown that a low but chronic (physiologically achievable) concentration of melatonin is capable of imparting anti-proliferative effects in human PCa cells (Fig. 3). We reasoned that chronic low melatonin exposure is physiologically more relevant to clinical situations than a single high exposure. However, for mechanistic studies, because of experimental protocol limitations, a single melatonin treatment is a more viable approach. Our data demonstrated that overexpression of Sirt1 partially (but significantly) rescued PCa cells from the anti-proliferative effects of melatonin, suggesting a cause-and-effect association of melatonin-mediated Sirt1 inhibition and its anti-proliferative effects (Fig. 4). We did not see a complete rescue because it is possible that melatonin is affecting other alternative pathways as it has been shown to work through multiple mechanisms (reviewed in [12]).
Employing TRAMP mice, we have also established the in vivo relevance of our in vitro findings and have demonstrated that melatonin, at a physiologically human-achievable dose regimen, significantly inhibits PCa progression (Fig. 5) without any unwanted toxic response. The TRAMP mice on C57BL/6 background appear to be an appropriate model for our study as it has been shown that C57BL/6 mice synthesize melatonin, with low basal levels [33]. We also demonstrated (Fig. 6) that the melatonin inhibits i) the tissue levels of PCNA and Ki-67, which are excellent markers of proliferation, and ii) serum and tissue levels of IGF-1/IGFBP3 ratio, which are believed to be excellent markers of PCa in the human population [34,35] as well as in TRAMP mice [19,36]. Our data also suggest that the anti-cancer effects of melatonin may be mediated via inhibition of Sirt1 (Fig. 6). A recent study has shown that Sirt1ko/ko mice had diminished IGF-1 levels in adipose tissue [37] and we have also found that melatonin-mediated Sirt1 inhibition decreases IGF-1. This is important because IGF-1 has been shown to be involved in PCa progression [19,34–36].
In conclusion, we have shown that melatonin is a novel inhibitor of Sirt1 and Sirt1 inhibition is a viable approach for the management of PCa. Indeed, some studies have suggested a tumor suppressor function of Sirt1 in PCa cells [38,39]. Several other studies [40,41], including our published reports [1,2] and the data presented here clearly demonstrated that Sirt1 inhibition should be thoroughly investigated for the management of PCa as well as other age-related malignancies. Further, alternate mechanism(s) of the anti-cancer effects of melatonin needs to be investigated. Further studies in this direction are ongoing in our laboratory.
Supplementary Material
Acknowledgments
This work was partly supported by funding from the National Institutes of Health (T32ES00715-30 and F31 AT005393-01 to BJ-H; R01CA114060 to NA; and T32AR055893 to IAS).
References
- 1.JUNG-HYNES B, NIHAL M, ZHONG W, et al. Role of sirtuin histone deacetylase SIRT1 in prostate cancer. A target for prostate cancer management via its inhibition? J Biol Chem. 2009;284:3823–3832. doi: 10.1074/jbc.M807869200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.JUNG-HYNES B, AHMAD N. Role of p53 in the anti-proliferative effects of Sirt1 inhibition in prostate cancer cells. Cell Cycle. 2009;8:1478–1483. doi: 10.4161/cc.8.10.8408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.LARA E, MAI A, CALVANESE V, et al. Salermide, a Sirtuin inhibitor with a strong cancer-specific proapoptotic effect. Oncogene. 2009;28:781–791. doi: 10.1038/onc.2008.436. [DOI] [PubMed] [Google Scholar]
- 4.OTA H, TOKUNAGA E, CHANG K, et al. Sirt1 inhibitor, Sirtinol, induces senescence-like growth arrest with attenuated Ras-MAPK signaling in human cancer cells. Oncogene. 2006;25:176–185. doi: 10.1038/sj.onc.1209049. [DOI] [PubMed] [Google Scholar]
- 5.ZHAO W, KRUSE JP, TANG Y, et al. Negative regulation of the deacetylase SIRT1 by DBC1. Nature. 2008;451:587–590. doi: 10.1038/nature06515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.ASHER G, GATFIELD D, STRATMANN M, et al. SIRT1 regulates circadian clock gene expression through PER2 deacetylation. Cell. 2008;134:317–328. doi: 10.1016/j.cell.2008.06.050. [DOI] [PubMed] [Google Scholar]
- 7.NAKAHATA Y, KALUZOVA M, GRIMALDI B, et al. The NAD+-dependent deacetylase SIRT1 modulates CLOCK-mediated chromatin remodeling and circadian control. Cell. 2008;134:329–340. doi: 10.1016/j.cell.2008.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.LEE C, ETCHEGARAY JP, CAGAMPANG FR, et al. Posttranslational mechanisms regulate the mammalian circadian clock. Cell. 2001;107:855–867. doi: 10.1016/s0092-8674(01)00610-9. [DOI] [PubMed] [Google Scholar]
- 9.TORRES-FARFAN C, SERON-FERRE M, DINET V, et al. Immunocytochemical demonstration of day/night changes of clock gene protein levels in the murine adrenal gland: differences between melatonin-proficient (C3H) and melatonin-deficient (C57BL) mice. J Pineal Res. 2006;40:64–70. doi: 10.1111/j.1600-079X.2005.00279.x. [DOI] [PubMed] [Google Scholar]
- 10.IMBESI M, ARSLAN AD, YILDIZ S, et al. The melatonin receptor MT1 is required for the differential regulatory actions of melatonin on neuronal ‘clock’ gene expression in striatal neurons in vitro. J Pineal Res. 2008;46:87–94. doi: 10.1111/j.1600-079X.2008.00634.x. [DOI] [PubMed] [Google Scholar]
- 11.KARASEK M. Does melatonin play a role in aging processes? J Physiol Pharmacol. 2007;58 (Suppl 6):105–113. [PubMed] [Google Scholar]
- 12.JUNG B, AHMAD N. Melatonin in cancer management: progress and promise. Cancer Res. 2006;66:9789–9793. doi: 10.1158/0008-5472.CAN-06-1776. [DOI] [PubMed] [Google Scholar]
- 13.REAGAN-SHAW S, NIHAL M, AHSAN H, et al. Combination of vitamin E and selenium causes an induction of apoptosis of human prostate cancer cells by enhancing Bax/Bcl-2 ratio. Prostate. 2008;68:1624–1634. doi: 10.1002/pros.20824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.RASMUSSEN DD, BOLDT BM, WILKINSON CW, et al. Daily melatonin administration at middle age suppresses male rat visceral fat, plasma leptin, and plasma insulin to youthful levels. Endocrinology. 1999;140:1009–1012. doi: 10.1210/endo.140.2.6674. [DOI] [PubMed] [Google Scholar]
- 15.REAGAN-SHAW S, NIHAL M, AHMAD N. Dose translation from animal to human studies revisited. FASEB J. 2008;22:659–661. doi: 10.1096/fj.07-9574LSF. [DOI] [PubMed] [Google Scholar]
- 16.ZHIGANG Z, JIEMING L, SU L, et al. Serum insulin-like growth factor I/free prostate specific antigen (IGF-I/fPSA) ratio enhances prostate cancer detection in men with total PSA 4.0–10.0 ng/ml. J Surg Oncol. 2007;96:54–61. doi: 10.1002/jso.20784. [DOI] [PubMed] [Google Scholar]
- 17.GUPTA S, HASTAK K, AHMAD N, et al. Inhibition of prostate carcinogenesis in TRAMP mice by oral infusion of green tea polyphenols. Proc Natl Acad Sci U S A. 2001;98:10350–10355. doi: 10.1073/pnas.171326098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.GREENBERG NM, DEMAYO F, FINEGOLD MJ, et al. Prostate cancer in a transgenic mouse. Proc Natl Acad Sci U S A. 1995;92:3439–3443. doi: 10.1073/pnas.92.8.3439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.ADHAMI VM, SIDDIQUI IA, AHMAD N, et al. Oral consumption of green tea polyphenols inhibits insulin-like growth factor-I-induced signaling in an autochthonous mouse model of prostate cancer. Cancer Res. 2004;64:8715–8722. doi: 10.1158/0008-5472.CAN-04-2840. [DOI] [PubMed] [Google Scholar]
- 20.GUPTA S, ADHAMI VM, SUBBARAYAN M, et al. Suppression of prostate carcinogenesis by dietary supplementation of celecoxib in transgenic adenocarcinoma of the mouse prostate model. Cancer Res. 2004;64:3334–3343. doi: 10.1158/0008-5472.can-03-2422. [DOI] [PubMed] [Google Scholar]
- 21.RAINA K, RAJAMANICKAM S, SINGH RP, et al. Chemopreventive efficacy of inositol hexaphosphate against prostate tumor growth and progression in TRAMP mice. Clin Cancer Res. 2008;14:3177–3184. doi: 10.1158/1078-0432.CCR-07-5275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.SHI R, BERKEL HJ, YU H. Insulin-like growth factor-I and prostate cancer: a meta-analysis. Br J Cancer. 2001;85:991–996. doi: 10.1054/bjoc.2001.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.LONGO VD, KENNEDY BK. Sirtuins in aging and age-related disease. Cell. 2006;126:257–268. doi: 10.1016/j.cell.2006.07.002. [DOI] [PubMed] [Google Scholar]
- 24.MARMORSTEIN R. Structure and chemistry of the Sir2 family of NAD+-dependent histone/protein deactylases. Biochem Soc Trans. 2004;32:904–909. doi: 10.1042/BST0320904. [DOI] [PubMed] [Google Scholar]
- 25.HIDA Y, KUBO Y, MURAO K, et al. Strong expression of a longevity-related protein, SIRT1, in Bowen’s disease. Arch Dermatol Res. 2007;299:103–106. doi: 10.1007/s00403-006-0725-6. [DOI] [PubMed] [Google Scholar]
- 26.NAKAHATA Y, SAHAR S, ASTARITA G, et al. Circadian control of the NAD+ salvage pathway by CLOCK-SIRT1. Science. 2009;324:654–657. doi: 10.1126/science.1170803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.BLASK DE, SAUER LA, DAUCHY RT. Melatonin as a chronobiotic/anticancer agent: cellular, biochemical, and molecular mechanisms of action and their implications for circadian-based cancer therapy. Curr Top Med Chem. 2002;2:113–132. doi: 10.2174/1568026023394407. [DOI] [PubMed] [Google Scholar]
- 28.MARTIN-RENEDO J, MAURIZ JL, JORQUERA F, et al. Melatonin induces cell cycle arrest and apoptosis in hepatocarcinoma HepG2 cell line. J Pineal Res. 2008;45:532–540. doi: 10.1111/j.1600-079X.2008.00641.x. [DOI] [PubMed] [Google Scholar]
- 29.TAM CW, CHAN KW, LIU VW, et al. Melatonin as a negative mitogenic hormonal regulator of human prostate epithelial cell growth: potential mechanisms and clinical significance. J Pineal Res. 2008;45:403–412. doi: 10.1111/j.1600-079X.2008.00608.x. [DOI] [PubMed] [Google Scholar]
- 30.JOO SS, YOO YM. Melatonin induces apoptotic death in LNCaP cells via p38 and JNK pathways: therapeutic implications for prostate cancer. J Pineal Res. 2009;47:8–14. doi: 10.1111/j.1600-079X.2009.00682.x. [DOI] [PubMed] [Google Scholar]
- 31.DZIEGIEL P, PODHORSKA-OKOLOW M, ZABEL M. Melatonin: adjuvant therapy of malignant tumors. Med Sci Monit. 2008;14:RA64–RA70. [PubMed] [Google Scholar]
- 32.JOO SS, YOO YM. Melatonin induces apoptotic death in LNCaP cells via p38 and JNK pathways: therapeutic implications for prostate cancer. J Pineal Res. 2009;47:8–14. doi: 10.1111/j.1600-079X.2009.00682.x. [DOI] [PubMed] [Google Scholar]
- 33.VIVIEN-ROELS B, MALAN A, RETTORI MC, et al. Daily variations in pineal melatonin concentrations in inbred and outbred mice. J Biol Rhythms. 1998;13:403–409. doi: 10.1177/074873098129000228. [DOI] [PubMed] [Google Scholar]
- 34.CHOKKALINGAM AP, POLLAK M, FILLMORE CM, et al. Insulin-like growth factors and prostate cancer: a population-based case-control study in China. Cancer Epidemiol Biomarkers Prev. 2001;10:421–427. [PubMed] [Google Scholar]
- 35.PLATZ EA, POLLAK MN, LEITZMANN MF, et al. Plasma insulin-like growth factor-1 and binding protein-3 and subsequent risk of prostate cancer in the PSA era. Cancer Causes Control. 2005;16:255–262. doi: 10.1007/s10552-004-3484-8. [DOI] [PubMed] [Google Scholar]
- 36.KAPLAN PJ, MOHAN S, COHEN P, et al. The insulin-like growth factor axis and prostate cancer: lessons from the transgenic adenocarcinoma of mouse prostate (TRAMP) model. Cancer Res. 1999;59:2203–2209. [PubMed] [Google Scholar]
- 37.LI H, RAJENDRAN GK, LIU N, et al. SirT1 modulates the estrogen-insulin-like growth factor-1 signaling for postnatal development of mammary gland in mice. Breast Cancer Res. 2007;9:R1. doi: 10.1186/bcr1632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.DAI Y, NGO D, FORMAN LW, et al. Sirtuin 1 is required for antagonist-induced transcriptional repression of androgen-responsive genes by the androgen receptor. Mol Endocrinol. 2007;21:1807–1821. doi: 10.1210/me.2006-0467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.SOLOMON JM, PASUPULETI R, XU L, et al. Inhibition of SIRT1 catalytic activity increases p53 acetylation but does not alter cell survival following DNA damage. Mol Cell Biol. 2006;26:28–38. doi: 10.1128/MCB.26.1.28-38.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.HUFFMAN DM, GRIZZLE WE, BAMMAN MM, et al. SIRT1 is significantly elevated in mouse and human prostate cancer. Cancer Res. 2007;67:6612–6618. doi: 10.1158/0008-5472.CAN-07-0085. [DOI] [PubMed] [Google Scholar]
- 41.YANG Y, HOU H, HALLER EM, et al. Suppression of FOXO1 activity by FHL2 through SIRT1-mediated deacetylation. EMBO J. 2005;24:1021–1032. doi: 10.1038/sj.emboj.7600570. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.