The surgical treatment of craniomaxillofacial trauma involves the restoration of both form and function via a complex interplay between the facial bony skeleton and its soft tissue envelope. However, it was not until the introduction of open reduction and internal rigid fixation techniques for the facial skeleton that the basic orthopedic principles of accurate fracture reduction, bone fixation, and healing could be applied. The latter introduced the unprecedented ability to repair unstable and/or displaced bony fractures of the face, providing a stable foundation upon which to reestablish preinjury soft tissue contour.
Advances in the science of internal fixation, improvements in available plating materials and equipment, refinements in exposures to the facial skeleton, and an increase in the volume of facial trauma all fueled the rapid expansion of use of rigid internal fixation for facial fractures in the 1980s.1 With growing experience, surgeons came to appreciate the utility of metallic internal rigid fixation systems, along with the potential pitfalls and complications.2,3,4,5 In addition, the permanence of metallic implants spawned questions of long-term safety,2,5,6,7,8 rates and need for removal,3,9,10 and risks in the growing pediatric skeleton.11,12,13,14,15 Aimed at addressing these concerns, manufacturers began research and development of resorbable rigid fixation systems, which more recently are gathering interest in the management of facial trauma. With this in mind, the authors have attempted to summarize and compare the current data describing use of either metallic or resorbable fixation systems for the treatment of facial fractures in an effort to educate surgeons faced with selecting between these two options. Factors such as complication rates, cost, efficacy, and availability are all considered and summarized in this article.
METALLIC RIGID INTERNAL FIXATION FOR THE TREATMENT OF CRANIOMAXILLOFACIAL TRAUMA
A Historical Primer
Metals have been used to heal wounds since the dawn of medicine. In the first century, Roman writer Aulus Cornelius Celsus described the approximation of a wound's edges with inserted metal pins and thread.16 Metallic fracture fixation, however, required advances in metallurgy many centuries later to permit the production of metallic wire from iron—first used to stabilize a long-bone fracture by Lapeyode and Sicre in 1775.17,18 In 1847, Buck pioneered metallic fixation for the craniomaxillofacial skeleton, using interosseous wiring to fixate a fractured mandible.19
By the early 1800s, Benjamin Bell and others began appreciating difficulties associated with metallic fixation, noting problems of corrosion when different metals were combined for fixation.20 Formal studies of metallic biocompatibility emerged shortly thereafter, such as Levert's descriptions in 1829 of the differences between gold, silver, platinum, and lead sutures in arterial anastomoses in dogs.21 Progress in the field of internal fixation, however, was limited by prohibitive rates of infection, which subsided by the middle of the 19th century due to advances in germ theory, anesthesia, and antisepsis.
Although the first use of rigid internal fixation with a plate and screws is credited to Hansmann in 1858,9 the most significant advances in internal osteosynthesis were contributed by Sir William Arbuthnot Lane and Albin Lambotte. Lane, a Scottish surgeon, noted early on that “no amount of traction upon the ends of the wires could retain the surfaces in accurate apposition after the grip of the lion forceps was relaxed.”22 From 1893 to 1914, he experimented with steel plates and screws to immobilize fractures, but struggled with corrosion.23 In 1912, William Sherman attempted to circumvent problems with corrosion by manufacturing plates from vanadium steel—the first material to be manufactured specifically for the human body.24 Regardless of Lane's tribulations, his introduction of a “no-touch” and strict aseptic technique paved the way for future internal fixation devices.
Albin Lambotte, in turn, was a Belgian surgeon who experimented with several metals including brass, silver, red copper, and aluminum before discovering in 1909 that corrosion could be decreased by plating hardware with gold or nickel.18 Although not intentional, Lambotte also created the first resorbable fixation device when he combined a magnesium plate with gold-plated steel screws, both of which dissolved completely in 8 days, leaving only subcutaneous gas in its place!25
The first application of rigid internal fixation to the facial skeleton is credited to Schede, who described use of steel plates and screws to fixate mandible fractures in 1888.26 It was not until the development of materials that were more resistant to corrosion in the early 20th century, though, that internal fixation for the facial skeleton became more widespread.
Alloys of chromium, nickel, and molybdenum, or “stainless steel,” and later in 1936 Vitallium (an alloy of cobalt, chromium, and molybdenum developed by the Austenal Laboratories, York, PA) paved the way due to their improved corrosion resistance.20 Vitallium found its first use in the face for a mandibular fracture by Bigelow in 1943.27 In an attempt to find a material that had the inertness of Vitallium combined with the usability of stainless steel, Leventhal in 1951 proposed use of titanium for fractures.28 In 1967, Snell described his use of titanium hand fracture plates for the facial skeleton,29 and Luhr introduced one the first dedicated facial plating systems (the Mandibular Compression System) in the late 1960s.30 Rigid fixation of the facial skeleton did not become popular in North America, however, until the 1980s,31,32 and has since remained the gold standard for stabilization of facial fractures and osteotomies.
The Science behind the Metal
The development of rigid fixation systems for the craniomaxillofacial skeleton began by borrowing devices and materials already in use for fracture fixation elsewhere in the body. However, it became apparent that whereas the principles of fracture immobilization were similar in the facial skeleton, specific differences did exist. The “ideal” material required sufficient strength to maintain fracture reduction and resist physiologic stresses until bony healing was complete, yet be sufficiently malleable to allow for in situ plate adaptation. An additional obstacle was the necessity of thin plates (requiring sufficient metal stiffness to resist deformation) to minimize visibility, palpability, and/or discomfort through the often thin soft tissue envelope of the face.
Whereas many metals were tested and abandoned, three materials—stainless steel, titanium, and Vitallium—gained popularity during the evolving era of internal rigid fixation for the facial skeleton.
Stainless steel, popularized by the Champy and AO systems, comprises a mixture of iron (62.5%), chromium (17.6%), nickel (14.5%), molybdenum (2.8%), and smaller amounts of other metals. Stainless steel is strong and extremely rigid, making it difficult to bend and more susceptible to surface damage and resultant corrosion after adaptation.33 Although it was the implant material of choice until the mid-1980s, stainless steel is more corrosive33,34 and produces significantly more radiologic scatter35,36,37 than the other candidate metals and, thus, has largely been replaced from the mainstream of maxillofacial fixation (with the exception of intermaxillary fixation screws, which continue to be made from stainless steel in most fixation systems). For those surgeons who prefer stainless steel due to its strength, options include use of stainless steel miniplate systems available for hand surgery (available from various manufacturers) or by special order (2.4-mm stainless steel screws for mandibular osteotomies or fracture lagging are available from Synthes, West Chester, PA). In addition to being stronger than titanium, stainless steel is also ∼50% less expensive than similar-sized titanium hardware.
Vitallium is a trademarked cobalt-chromium-molybdenum alloy that has twice the tensile strength, 50% more yield strength, and twice the hardness of the other metals.32,38 An added benefit is that the yield strength of Vitallium nearly doubles upon bending, compared with only a mild increase with titanium. The result is that plates manufactured from Vitallium are thinner (0.5-mm-profile microplates) than their steel or titanium counterparts (0.8-mm-profile microplates).32,39 It was additionally touted as having excellent tissue biocompatibility, not unlike titanium.39 However, although experimental in vivo studies confirm that the resistance to corrosion is similarly high for titanium and cobalt-based alloys, they differ in their respective products of corrosion.33 Titanium produces mainly uncharged inorganic compounds as corrosion products, which cause minimal physiologic tissue insult, and, thus, it behaves biologically inert.33 In contrast, alloys such as stainless steel and Vitallium produce charged species (ions), which cause tissue insult and concomitant foreign body reaction or sequestration. Microscopically, this manifests as an absence of vascularized tissue in contact with the implants.40 Although, in theory, Vitallium is sequestered, its foreign body reaction is only mild.41
Currently, Vitallium hardware for maxillofacial surgery is only available from one distributor as the Luhr Modular System (Stryker, Kalamazoo, MI). Vitallium mesh was used successfully by both Sargent42 and Sengezer43 for reconstruction of large orbital and maxillary defects, respectively, noting that its strength permitted the Vitallium mesh and interconnecting bars to be thinner and easier to adapt. Its popularity, however, like stainless steel, waned in spite of its strength due to difficulty adapting the thicker midface/mandible plates as a result of its rigidness, and inferior radiologic scatter properties; the latter having considerable importance due to the frequent reliance on postoperative computed tomography (CT) imaging for verifying fracture reduction and plate positioning. Numerous studies have demonstrated that while stainless steel produces the most scatter, Vitallium produces significantly more scatter than does titanium35,36,37—an effect that can incidentally be significantly reduced by using MediCad software (MediCad Inc, Cedar Knolls, NJ) as demonstrated by Barone et al.35 From a cost perspective, Vitallium hardware is marginally more expensive than titanium components, perhaps due to its smaller production volumes.
Titanium plating systems are made from pure titanium and varying amounts of oxygen or from titanium alloys. Titanium is less rigid and, thus, more easily adaptable than stainless steel while maintaining sufficient strength. It forms a protective oxide that helps it to resist corrosion and achieve good tissue biocompatability.33,34 Titanium also possesses the unique ability to bind to bone, a property known as osseointegration.34 Thus, unlike stainless screws, which typically loosen over time, the release torque of titanium screws ironically exceeds the insertion torque.33 Titanium plating systems for maxillofacial trauma are currently available from all the major manufacturers. Cost-wise, titanium hardware has decreased since its introduction due to widespread use but continues to be higher than that of comparable stainless steel components.
In spite of its popularity as an implant for the maxillofacial skeleton, titanium is not without its own biocompatibility and safety concerns. Although rare, there are reports of toxicity and hypersensitivity to titanium,44,45,46 and studies have documented the presence of titanium within distant organs and lymph nodes.47 In addition, soft tissues removed around explanted anodized titanium craniofacial microplates in human subjects were found to contain trace amounts of titanium due to corrosion, but no evidence of metallosis (accumulations of metal inclusions).8 In contrast, other studies have demonstrated no elevation48 or minimal elevation in local tissue titanium levels, which were further diminished by using anodized titanium.7 At this time, the data are currently inconclusive and, more importantly, do not address the questions that are germane to surgeons—whether these trace amounts of titanium are clinically significant and, more importantly, sufficient to warrant removal after fracture healing?8,49
Although further research is necessary to elucidate these answers, there is a large volume of clinical data that provides information about the safety and complications associated with use of titanium implants. Complications after use of titanium rigid internal fixation for craniomaxillofacial applications are most commonly associated with implant palpability, pain, or thermal sensitivity, especially when used in areas with thin skin cover (e.g., orbital rim).4 Infections, both wound and sinus (due to screws violating the mucosa), hardware loosening, extrusion, and/or migration are all reported.2,3,4,5 Although radiologic scatter is lower for titanium than for other metals used, titanium implants can hinder imaging or cause shielding for radiation therapy. As mentioned earlier, rare reports of sensitization to titanium are also documented.44,45,46
Although percentile complication rates associated with use of titanium rigid internal fixation are reported in the literature,3,4 one must assume that they are influenced by the indication for fixation (elective orthognathic surgery vs. facial trauma) and factors such as bony comminution, tissue devitalization, and contamination. The same variables would presumably affect the percentage of patients requiring a second operation to revise or remove hardware due to these complications (i.e., related to the permanence of titanium hardware). Schmidt reported that 10.6% (20 of 190) of patients who underwent an elective Le Fort I osteotomy required removal of hardware due to complications.10 Interestingly, Francel et al found that a similar proportion (61 of 507, or 12%) of facial trauma patients required removal of hardware for symptoms,3 although presumably the overall complication rate related to the hardware was higher. The most common reasons in their study were location dependent; pain or prominence in the upper face, exposure in the midface, and infection or exposure in the mandible. Thus, in spite of the increased energy of injury and severity associated with facial trauma, titanium hardware appears to be equally well tolerated as in controlled, elective orthognathic surgery. Regardless, when opting to use metallic fixation, one must consider that ∼10% of patients will require a second operation for hardware removal, increasing treatment costs and putting the patient through the risk and morbidity of another surgical procedure, albeit generally a limited one.
For the pediatric patient, the effects of permanent metallic fixation on the growing facial skeleton warrant consideration. Numerous studies to date have demonstrated detrimental effects on craniofacial skeletal growth in animals,12,13,14,15,50,51 although the magnitude of these effects is not likely to be clinically significant in the majority of cases.52 Because of the appositional mechanism of bony growth, screws and plates can be expected to migrate and can translocate intracranially, potentially putting the brain at risk.2,5,53 In spite of these reports, the authors are not aware of any studies that have documented permanent adverse effects on the brain due to hardware translocation. Thus, the choice to use titanium fixation in pediatric craniomaxillofacial surgery or trauma must take these factors into consideration, in addition to the potential need for and timing of removal, as plate extraction can become challenging if deemed necessary once appositional growth has occurred.54,55
RESORBABLE RIGID INTERNAL FIXATION FOR THE TREATMENT OF CRANIOMAXILLOFACIAL TRAUMA
Given the understanding that fracture stabilization by means of rigid internal fixation is necessary only until bone healing is complete, the premise of resorbable rigid fixation was spawned by the desire to reap the benefits of rigid internal fixation without the complications of permanent implants. Further, although orthopedic indications dictated the use of metals as implants to provide the structural rigidity necessary for load-bearing, biomechanical studies of the facial skeleton demonstrated that these bones were not susceptible to the same deforming physiologic forces as those affecting the long bones (with the exception of the mandible).56 The necessity of metallic and permanent rigid fixation for the treatment of facial fractures was thus brought into question. As such, reports of facial fracture fixation using resorbable hardware began appearing in the literature as early as 1971,10,57 gaining acceptance as a viable option for facial fracture treatment only more recently, however, due to necessary advancements in biomaterials, biomechanical research, and clinical experience.
The development of resorbable plates and screws for rigid internal fixation was a natural extension from biodegradeable suture materials already available for many years to assist wound closure. The materials currently used to manufacture resorbable plating systems in common use are polymers of high-molecular-weight α-hydroxy acids including polyglycolic acid (PGA) and polylactic acid (PLA).58 Both materials are initially degraded by hydrolysis into lactic acid, which is then subsequently metabolized by the liver and excreted as carbon dioxide and water.6 However, the rate at which they degrade differs. PGA degradation is rapid and although initially stiff, loses its mechanical strength by 6 weeks and is completely resorbed within a few months.59 Pure PGA hardware is no longer used because its brisk structural degradation provides insufficient structural support for bone healing and is also associated with local inflammatory reaction, osteolysis, and sterile abcesses.54,58
Alternatively, PLA is degraded at a rate far slower than that of PGA, often requiring several years and, thus, increasing the risk of foreign body–type reactions.6,58,60 PLA's stereoisomers, D- and L-lactide, however, have differing degradation characteristics allowing varying combinations of these isomers to confer shorter degradation rates. In a similar manner, mixtures of PGA and PLA, with increasing amounts of the former accelerating the rate of degradation, have been formulated to achieve the same effect. Although the exact formulas vary between manufacturers, the currently available copolymer formulations generally retain structural rigidity for 2 to 3 months and are resorbed completely by 1 to 2 years. Individual variations in resorption times, strength profiles, and available sizes are summarized in Table 1.
Table 1.
Resorbable Rigid Fixation System Product Information Summary
| Stryker Inion CPS* | Synthes Rapid† | Biomet LactoSorb SE‡ | KLS Martin Resorb X and SonicWeld Rx§ | |
|---|---|---|---|---|
| Composition | Varying combinations of L-polylactic acid; D,L-polylactic acid; polyglycolic acid; trimethylene carbonate | 85:15 poly (L-lactide-co-glycolide) | 82:18 poly L-lactic acid: polyglycolic acid | Both systems are 100% poly (D,L-lactic acid) |
| Degradation characteristics | BABY: strength retention 6–9 weeks; resorbed in 1–2 years | 85% strength at 8 weeks; resorbed within 12 months | 70% strength at 8 weeks, resorbed within 12 months | Strength retention to 10 weeks; resorbed in 1–2 years |
| ADULT: strength retention 9–14 weeks; resorbed 2–3 years | ||||
| Available sizes for craniomaxillofacial use | BABY system: 1.5 mm | 1.5-mm system | 1.5-mm system | Both Resorb X (screws) and SonicWeld Rx (pins): 1.6-mm system; 2.1-mm system |
| ADULT system: 1.5, 2.0, 2.5 mm | 2.0-mm system | 2.0-mm system | ||
| 2.8/3.1-mm (screws only) | 2.5/2.8-mm (screws only) | |||
| Plate profile (thickness) | 1.5-mm system: 1.0 mm | 1.5-mm system: 0.8 mm | 1.5-mm system: 1.0 mm | Resorb X and SonicWeld Rx: all plates shapes 1.0 mm |
| 2.0-mm system: 1.3 mm | 2.0-mm system: 1.2 mm | 2.0-mm system: 1.4 mm | ||
| 2.5-mm system: 1.7 mm | ||||
| Screw placement | Self-drilling tap or separate tap | Self-drilling tap or separate tap | Self-drilling tap or separate tap; push screws (no tapping required) | Resorb X: screws with self-drilling tap |
| SonicWeld Rx: drill hole for pins and secure with ultrasonic frequency welder (no tapping required) | ||||
| Indicated for mandible fractures | Yes (with IMF only) | No | No | No |
Inion CPS Biodegradeable Fixation System [product guide]. Kalamazoo, MI: Stryker Craniomaxillofacial; 2008.
Rapid Resorbable Fixation System [product guide]. West Chester, PA: Synthes CMF; 2008.
Lorenz Plating System LactoSorb [product guide]. Jacksonville, FL: Biomet Microfixation; 2008. Note: Biomet was formerly known as Walter Lorenz Surgical (Jacksonville, FL).
SonicWeld Rx [product guide]. Jacksonville, FL: KLS Martin – LP; 2008.
Although inflammatory foreign body reactions and sterile abscesses have been reported for all of these copolymer products, the more gradual degradation characteristics present less risk of adverse reactions or osteolysis.58 That being said, the question of biocompatibility of resorbable hardware, like with titanium, is not entirely agreed upon in spite of manufacturers' claims of complete resorption. At least one study has suggested long-term persistence of crystalline debris from poly-α-hydroxy acid implants (PGA/PLA),6 whereas others have demonstrated an absence of inflammation60,61,62,63,64,65 or residual debris62,63,65 with poly-L-lactic acid/PGA (PLLA/PGA; LactoSorb; Biomet, Jacksonville, FL) implants at long-term follow-up. Whether these findings have any long-term sequelae or clinical consequences remains to be seen. What is known is that successful bony healing using resorbable hardware as fracture fixation has been demonstrated in both animal and clinical studies, regardless of the presence or absence of residual debris or mild inflammation.58,60,61,62,63,64,65,66
From a practical standpoint, there are several important differences between metallic and resorbable rigid fixation materials aside from their physical longevity, including material strength and rigidity, plate adaptation, and screw insertion techniques.
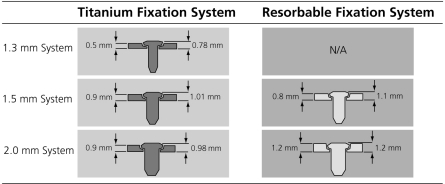
From a strength perspective, the resorbable (co)polymers with useful degradation profiles are relatively weaker and less rigid than metals such as titanium, in addition to being brittle under tensile and bending loads.6 To combat these structural shortcomings, resorbable plates are generally broader and thicker than their metallic counterparts and often employ physical design modifications such as side rails to increase rigidity (Figs. 1 and 2).60 Data from biomechanical studies examining comparative strength of resorbable and titanium systems suggest that additional compensation for material strength differences can be achieved by using a resorbable system that is incrementally larger than that of the metallic system that would have traditionally been selected for a particular indication (e.g., a 2.0-mm system resorbable plate would substitute for a 1.3- to 1.5-mm system titanium plate).67,68,69 Kasrai et al demonstrated that resorbable 1.5-mm or 2.0-mm system plates were similar in efficacy to 1.2-mm system titanium plates in resisting plated zygomaticomaxillary complex fractures in cadavers.69 Both resorbable system sizes, however, were significantly weaker than 1.7-mm titanium plates when performing the same task. Gosain et al compared titanium microplates and larger resorbable plates using both compressive and distractive forces and found that the resorbables performed similarly or even better but were inferior in strength to larger titanium miniplates.68 Although hardware palpability under areas of thin soft tissue cover may be increased due to the bulkier size of resorbable plates (compared with that of metallic plates), this effect is only temporary until resorption takes place.
Figure 1.
Schematic figure demonstrating a cross-sectional comparison of plate profiles (thickness) between varying sizes of rigid titanium or resorbable fixation systems. Note that profiles for both plate alone and plate with a screw in place are demonstrated. (Illustration and plate measurements are courtesy of Synthes, West Chester, PA.)
Figure 2.
Photograph demonstrating varying plate and screw dimensions for resorbable and titanium internal rigid fixation hardware. Plates shown are 1.3-mm titanium, 1.5-mm titanium, 2.0-mm titanium, and 1.5-mm resorbable (from left to right for plates, and bottom to top for screws). Note the relatively broader size of the resorbable plate (compared with the titanium plates) and the addition of reinforcing side rails. (All hardware manufactured by Synthes, West Chester, PA.)
The rigidity of fracture stabilization with resorbable fixation is additionally affected by limitations in compression achievable between the plate and bone surface,55 as resorbable screws are capable of only minor compression due to torsional weakness.70 Biomechanical studies comparing mandible fractures plated with either resorbable or titanium plates have confirmed differing strain patterns neighboring the fracture fixation site depending on the hardware material used.71 However, there is some data to suggest that micromotion, as opposed to absolute rigid fixation, at the fracture site may accelerate bone healing.13 Second, unlike the situation with permanent metallic fixation, as resorbable plates degrade force is gradually transferred to the healing bone, theoretically limiting the risk of disuse bony atrophy related to stress shielding.71
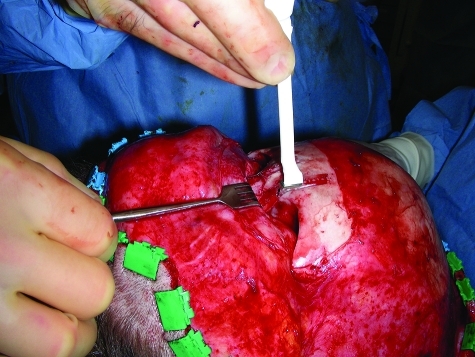
The brittle nature of the resorbable polymers also requires heating for plate adaptation to occur (attempted bending without heating will fracture the plate). Various devices, most commonly a hand-held heating gun (Figs. 3–5) or a warm-water bath are used to achieve this purpose. For this reason, when operating through limited intraoral or orbital incisions, use of a metal template is helpful. The template is bent to the desired contour in situ, then used to mold the resorbable plate using the heat source on a side table.72 Although resorbable plates that can be adapted without heating have been developed by modifying the manufacturing technique (called self-reinforcing), these never became popular in North America due to the tendency to revert back to their preadapted shape.73 In addition, there was a concern that repeated bending during adaptation may weaken the plates. Research is now focusing on the blending of currently available polymers with biodegradeable rubbers to provide the option of room-temperature moldability.6
Figure 3.
In situ adaptation of a resorbable plate using a hand-held, disposable heating gun in the nasofrontal region.
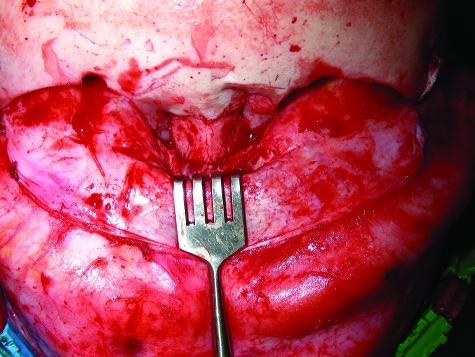
Figure 4.
Intraoperative photo of a pediatric patient with an impacted nasoorbitoethmoidal fracture.
Figure 5.
Reduction and stabilization of the nasoorbitoethmoidal fracture shown in Fig. 4 using 1.5-mm system resorbable fixation (Synthes, West Chester, PA).
Resorbable plates are secured by screw insertion, which requires both predrilling with a traditional drill bit and pretapping with a hand-held tap. Resorbable screws have poor torsional strength compared with that of metallic screws70 and thus require pretapping to prevent screw heads from twisting off during insertion; an additional time-consuming step not necessary with modern metallic screws. More recently, however, self-drilling taps (available for Synthes, Biomet [formerly Walter Lorenz Surgical], Stryker, and KLS Martin resorbable systems) or an ultrasonic device called SonicWeld Rx (KLS Martin, Jacksonville, FL) have been developed to decrease screw insertion times by obviating the need for a separate tapping step. The SonicWeld Rx device uses ultrasound frequency to rapidly “melt” the screw into bone interstices (without tapping) facilitating plate placement over very thin bone (e.g., medial orbital wall or anterior maxillary sinus) or cancellous bone, either of which do not tap readily.
Current indications for resorbable rigid internal fixation vary by manufacturer, with the majority of products having approval for use in fracture fixation and reconstruction of the craniomaxillofacial skeleton in non–load-bearing areas (Table 1). The majority of clinical experience to date has been with its use in elective pediatric cranial vault procedures58,68,74,75 and orthognathic surgery.76,77,78,79,80,81 Whereas their use in load-bearing locations such as the mandible is reported in the literature, use of resorbable hardware alone for mandible fractures is currently off-label (with the exception of the Inion CPS system (Stryker), which is approved for use as an adjunct to metallic fixation or in conjunction with intermaxillary fixation (IMF) for mandible fractures).82 Thus, an appropriate discussion of the current indications for the treatment of maxillofacial fractures with resorbable plating systems requires categorization into load-bearing (i.e., mandible) and non–load bearing (all other facial fractures) applications.
For the latter category, there is a considerable amount of clinical experience supporting the utility of resorbable fixation devices for midfacial and upper facial fractures. In 1997, Eppley and Prevel published their experience with use of resorbable fixation in 30 patients with a variety of zygomaticomaxillary complex, orbit, and Le Fort I or III fractures (mandible fractures were excluded).72 They reported no problems with instability or implant-related complications (i.e., infection, foreign body reaction). Majewski et al had a similarly positive experience with nine facial fractures in which non–load-bearing elements were plated with resorbable hardware and mandibular fractures were stabilized with titanium plates.83 Four maxillofacial fractures treated with resorbable fixation were evaluated by Tatum et al postoperatively at 2 weeks, 3 months, and 6 months with CT scans, demonstrating maintenance of reduction and osseous union with no artifact from the hardware.84 More recently, Bell and Kindsfater published a larger series of 59 pediatric and adult patients with midfacial fractures treated using resorbable hardware.54 They reported two complications including one sterile abscess requiring drainage and a Le Fort I fracture that developed malocclusion requiring reoperation. Although one has to question whether the latter complication was related to use of resorbable fixation from a stability standpoint, the overall complication rate for patients treated with resorbable hardware (3.4%) was less than that for the group treated with titanium hardware (6.3%).
Reports of soft tissue complications related to the degradation process of resorbable hardware are not isolated.80,85 The majority involve self-limited soft tissue inflammatory reactions or sterile abscesses that require simple drainage.54 There are at least two reports of formation of orocutaneous fistulas.80,86 Areas that have thin soft tissue cover such as the orbital area appear to be particularly at risk. Hollier et al reported one patient out of a series of 12 traumatic orbital floor reconstructions with resorbable mesh who developed an inflammatory reaction requiring implant removal.87 Tuncer et al reported similar reconstructions in 17 patients, in which one had migration of the implant causing ectropion and another had a delayed foreign body reaction, both requiring reoperation.88 Although these complications need to be considered when opting to use resorbable fixation, the overall soft tissue complication rate, which is estimated to be ∼6%, with resorbable hardware, compares favorably with the 10% or more chance of reoperation for removal of hardware when using titanium.85 Furthermore, loose or palpable resorbable screws need not be removed, unlike their titanium counterparts, which generally require extraction.54
Although the potential benefits of resorbable fixation for the treatment of mandibular fractures could foreseeably parallel those valued in the upper face, the load-bearing nature and high-functional requirements of the mandible complicate matters. Clear answers are further impeded by our currently inadequate understanding of mandibular fracture dynamics and the great diversity in fracture patterns and locations.89
Nevertheless, numerous experimental studies have attempted to assess the ability of resorbable fixation devices to adequately stabilize mandible fractures, in an attempt to predict their clinical performance. Such studies include that of Tams et al, who demonstrated using a biomechanical model that fracture immobilization with PLA plates was sufficiently stable for angle and parasymphyseal fractures if two plates were used and interfragmentary bone contact was present.90 In spite of these conclusions, Hochuli-Vieira et al found that parasymphyseal fractures in a rabbit model were successfully healed (histologically and clinically) in animals treated with a single resorbable 1.5-mm system plate.63 Of note, there was no period of IMF postoperatively nor any hardware failures.
The published clinical experiences with use of resorbable fixation for mandible fractures have largely purported successful outcomes. In 2002, Kim and Kim published a series of 49 patients with a variety of mandible fractures treated with resorbable fixation and reported satisfactory union in all patients and a complication rate of 12.2% (6 of 49), including four infections and one malocclusion treated with guiding elastics.86 Importantly, one patient developed an orocutaneous fistula requiring reoperation, which was thought to be secondary to degradation-related inflammation. Yerit et al successfully treated 66 patients with mandibular fractures using two 2.0-mm system resorbable plates, only maintaining IMF in patients with concomitant subcondylar fractures.91 They reported no issues with osseous union (except in one noncompliant patient who was reoperated on to convert to titanium fixation), but interestingly reported persistence of mental nerve hypesthesias and/or incision discomfort in 13 of 66 patients at 1-year follow-up. The authors hypothesized that degradation products of the resorbable polymer may be responsible for the persistent nerve irritation in these patients but maintained the opinion that the use of resorbable hardware was stable (without IMF), reliable, and a viable alternative to standard metallic fixation. Favorable results were also reported by Suzuki et al, who successfully treated 14 subcondylar fractures with PLLA resorbable plates, using a variety of techniques (single plate, double plate, and T-plate).92 All patients were treated with 4 weeks of IMF elastic traction.
In their 2006, 30-patient series, Landes and Ballon caution the optimism of other authors, reporting two patients with angle fractures treated using a resorbable two-plate technique that fractured at 6 weeks postoperatively—one while eating hard food (against recommendations) and the other while having a dental impression made.93 The authors concluded that resorbable fixation of mandibular angle fractures may be unreliable due to inadequate loading tolerance and stress the importance of strict patient compliance.
DECIDING BETWEEN METALLIC OR RESORBABLE RIGID INTERNAL FIXATION FOR THE TREATMENT OF CRANIOMAXILLOFACIAL TRAUMA
The choice to use metallic versus resorbable rigid fixation materials is based on several variables—not the least of which is the comfort level and experience of the treating surgeon with the particular plating system. Although many surgeons were trained during a time when metallic fixation was the sole option, as summarized in this article, there is a significant amount of published clinical experience providing support for resorbable fixation in elective orthognathic and pediatric craniofacial surgery and, to a lesser extent, in the management of acute facial fractures. Although some of the results from elective maxillofacial and cranial vault surgery can be extrapolated, facial trauma introduces variables including limited exposures, increased fracture comminution, tissue devitalization, and contamination that may render such analogies moot.72 The decision process must therefore be individualized to fracture location and severity and must take into account other variables such as patient age, comparative complication rates, and hardware costs of resorbable versus metallic fixation. The salient points are summarized below.
For the pediatric facial fracture patient, the benefits of resorbable fixation seem obvious. These include avoidance of potential problems with growth restriction, decreased risk of injury to tooth buds, decreased functional demands on mandibular fixation, and the obviated necessity of potentially challenging metallic device removal.54,55,94,95 Although titanium fixation can be safely used, a second operation for hardware removal is the rule rather than the exception, making resorbable fixation a more cost-effective strategy also.
In the adult facial trauma patient, the benefit of resorbable fixation is less clear-cut. For uncomplicated fractures of the maxilla or zygomaticomaxillary complex (including the orbit floor), the published data suggest that fixation is equally effective with resorbable or titanium fixation.54,60,72,84 One consideration in the treatment of orbit floor fractures using resorbable mesh implants lies in the difficulty in assessing the position of the device on postoperative imaging, which can be important in fractures that extend posteriorly toward the orbital apex near the optic nerve. In addition, there is a learning curve associated with the different methods of plate adaptation required when using resorbable fixation and, thus, potentially slightly longer operative times initially. The risk of complications, however, appear to be lower with use of resorbable fixation for uncomplicated non–load-bearing fractures, with rates published between 3.4%54 and 9%.80 The majority of the latter complications do not require reoperation compared with those associated with use of titanium hardware, which require a second surgery in 10 to 18%.3,4
The costs associated with repeat operative procedures would also likely outweigh the relative cost difference between resorbable and titanium fixation. Although prices vary slightly between manufacturers, at our institution resorbable plates are ∼20% more expensive (with the exception of orbit floor implants, which are more expensive in titanium), whereas the screws are virtually the same cost. As an example, the hardware costs associated with a standard 4-point fixation of an uncomplicated zygomaticomaxillary fracture (2 L-plates, 1 orbit rim plate, 1 orbit floor implant, 16 screws, and 1 disposable drill bit/tap) with the resorbable system are $2433.00 versus $2381.00 if the titanium system is used—a difference of only 2.2%. The titanium systems do, however, have an additional cost benefit from the ability to resterilize hardware.
Thus, whereas resorbable hardware appears to be a viable option for uncomplicated maxillofacial fractures, those involving complex panfacial fractures, high-energy injuries with tissue devitalization and loss, large orbit floor defects, and the potential need for bone grafting are indications for the proven reliability of metallic fixation.54,60,72,87 The thin-boned and often severely comminuted fractures of the nasoethmoidal area and anterior maxillary sinus wall are not well-engaged with currently available resorbable fixation and are better treated with fine titanium microplates.60,72
For the treatment of mandibular fractures, the selection of rigid fixation material is best individualized. Laughlin prospectively analyzed 50 mandible fractures treated with either resorbable fixation (two-plate technique with 2 weeks of IMF postoperatively and a liquid diet for 8 weeks) and compared those with historical controls treated with titanium fixation. Nonunion, infection, and hardware removal rates were 0.0%, 6.0%, and 4.0%, respectively, for the resorbable group and 3.9%, 13.0%, and 18.9%, respectively, for the historical titanium group. Acknowledging the lack of a true control group and any selection bias of patients chosen to receive resorbable fixation, these results nonetheless suggest that an argument can be made for the safety and efficacy of resorbable fixation for the treatment of mandibular fractures. The importance of careful case selection cannot be overstressed, with avoidance of cases involving fracture comminution, infection, and patient noncompliance.86
The merit of titanium fixation in the treatment of mandible fractures cannot, however, be overlooked. Metallic fixation is stable, reliable, and generally avoids the necessity of IMF, which is most often considered a requirement when resorbable fixation of mandible fractures is selected. Resorbable systems also do not permit use of lag screw techniques and are unsuitable for complex, comminuted mandibular fractures where large, spanning and locking reconstruction plates are invaluable.
CONCLUSION
The option of resorbable rigid internal fixation material for the treatment of craniomaxillofacial trauma is now a reality. Advances in available resorbable plating materials, expansion of published clinical experience, and decreased product costs have all contributed to this rise in popularity. The utility of titanium rigid internal fixation devices, however, will not be replaced entirely by the resorbables, as metallic fixation continues to maintain its superiority for specific tasks. Instead, the two modalities will likely reach a new equilibrium in which they will be used in concert to maximize and balance the benefits of stability and bioresorption for each individual patient.
References
- Rahn B A. Theoretical considerations in rigid fixation of facial bones. Clin Plast Surg. 1989;16:21–27. [PubMed] [Google Scholar]
- Fearon J A, Munro I R, Bruce D A. Observations on the use of rigid fixation for craniofacial deformities in infants and young children. Plast Reconstr Surg. 1995;95:634–637. [PubMed] [Google Scholar]
- Francel T J, Birely B C, Ringelman P R, Manson P N. The fate of plates and screws after facial fracture reconstruction. Plast Reconstr Surg. 1992;90:568–573. doi: 10.1097/00006534-199210000-00004. [DOI] [PubMed] [Google Scholar]
- Orringer J S, Barcelona V, Buchman S R. Reasons for removal of rigid internal fixation devices in craniofacial surgery. J Craniofac Surg. 1998;9:40–44. doi: 10.1097/00001665-199801000-00009. [DOI] [PubMed] [Google Scholar]
- Yu J C, Bartlett S P, Goldberg D S, et al. An experimental study of the effects of craniofacial growth on the long-term positional stability of microfixation. J Craniofac Surg. 1996;7:64–68. doi: 10.1097/00001665-199601000-00014. [DOI] [PubMed] [Google Scholar]
- Cordewener F W, Schmitz J P. The future of biodegradable osteosyntheses. Tissue Eng. 2000;6:413–424. doi: 10.1089/107632700418119. [DOI] [PubMed] [Google Scholar]
- Jorgenson D S, Centeno J A, Mayer M H, et al. Biologic response to passive dissolution of titanium craniofacial microplates. Biomaterials. 1999;20:675–682. doi: 10.1016/s0142-9612(98)00225-7. [DOI] [PubMed] [Google Scholar]
- Jorgenson D S, Mayer M H, Ellenbogen R G, et al. Detection of titanium in human tissues after craniofacial surgery. Plast Reconstr Surg. 1997;99:976–979. doi: 10.1097/00006534-199704000-00006. [DOI] [PubMed] [Google Scholar]
- Beals S P, Munro I R. The use of miniplates in craniomaxillofacial surgery. Plast Reconstr Surg. 1987;79:33–38. doi: 10.1097/00006534-198701000-00005. [DOI] [PubMed] [Google Scholar]
- Schmidt B L, Perrott D H, Mahan D, Kearns G. The removal of plates and screws after Le Fort I osteotomy. J Oral Maxillofac Surg. 1998;56:184–188. doi: 10.1016/s0278-2391(98)90865-5. [DOI] [PubMed] [Google Scholar]
- Berryhill W E, Rimell F L, Ness J, Marentette L, Haines S J. Fate of rigid fixation in pediatric craniofacial surgery. Otolaryngol Head Neck Surg. 1999;121:269–273. doi: 10.1016/S0194-5998(99)70183-X. [DOI] [PubMed] [Google Scholar]
- Eppley B L, Platis J M, Sadove A M. Experimental effects of bone plating in infancy on craniomaxillofacial skeletal growth. Cleft Palate Craniofac J. 1993;30:164–169. doi: 10.1597/1545-1569_1993_030_0164_eeobpi_2.3.co_2. [DOI] [PubMed] [Google Scholar]
- Lin K Y, Bartlett S P, Yaremchuk M J, et al. An experimental study on the effect of rigid fixation on the developing craniofacial skeleton. Plast Reconstr Surg. 1991;87:229–235. doi: 10.1097/00006534-199102000-00003. [DOI] [PubMed] [Google Scholar]
- Resnick J I, Kinney B M, Kawamoto H K., Jr The effect of rigid internal fixation on cranial growth. Ann Plast Surg. 1990;25:372–374. doi: 10.1097/00000637-199011000-00005. [DOI] [PubMed] [Google Scholar]
- Wong L, Richtsmeier J T, Manson P N. Craniofacial growth following rigid fixation: suture excision, miniplating, and microplating. J Craniofac Surg. 1993;4:234–244. [PubMed] [Google Scholar]
- Majno G. The Healing Hand: Man and Wound in the Ancient World. Cambridge, MA: Harvard University Press; 1991.
- Conn H. The internal fixation of fractures. J Bone Joint Surg. 1931;13:261–268. [Google Scholar]
- Ferguson A. Historical use of metals in the human body. In: In: Bechtol C, Ferguson ALP, editor. Metals and Engineering in Bone and Joint Surgery. Baltimore, MD: Williams and Wilkins Company; 1959. pp. 1–18. [Google Scholar]
- Mukerji R, Mukerji G, McGurk M. Mandibular fractures: historical perspective. Br J Oral Maxillofac Surg. 2006;44:222–228. doi: 10.1016/j.bjoms.2005.06.023. [DOI] [PubMed] [Google Scholar]
- Rowe N, Kiley H. Fractures of the Facial Skeleton. London: E & S Livingstone Limited; 1955.
- Rushton N. Biomaterials. In: In: Klenerman L, editor. Evolution of Orthopedic Surgery. London: RSM Press; 2002. pp. 83–89. [Google Scholar]
- Jones A R. Sir William Arbuthnot Lane. J Bone Joint Surg Br. 1952;34:478–482. doi: 10.1302/0301-620X.34B3.478. [DOI] [PubMed] [Google Scholar]
- Peltier L. Fractures: A History and Iconography of Their Treatment. Novato, CA: Norman Publishing; 1990.
- Sherman W. Vanadium steel plates and screws. Surg Gynecol Obstet. 1912;14:629–634. [Google Scholar]
- Staiger M P, Pietak A M, Huadmai J, Dias G. Magnesium and its alloys as orthopedic biomaterials: a review. Biomaterials. 2006;27:1728–1734. doi: 10.1016/j.biomaterials.2005.10.003. [DOI] [PubMed] [Google Scholar]
- Dorrance G, Bransfield J, Mann J. The History of Treatment of Fractured Jaws. Philadelphia, PA: Privately published; 1941.
- Bigelow H. Vitallium bone screws and appliances for treatment of fracture of mandible. J Oral Surg (Chic) 1943;1:131. [Google Scholar]
- Leventhal G S. Titanium, a metal for surgery. J Bone Joint Surg Am. 1951;33:473–474. [PubMed] [Google Scholar]
- Snell J, Dott W. The use of metallic plates in surgery of the facial skeleton. Rome: Presented at: the 4th International Congress of Plastic and Reconstructive Surgery; 1967.
- Luhr H G. [On the stable osteosynthesis in mandibular fractures] Dtsch Zahnarztl Z. 1968;23:754. [PubMed] [Google Scholar]
- Luhr H G. Philadelphia, PA: Presented at: Symposium on Methods of Facial Skeletal Fixation; December 3, 1983.
- Munro I R. The Luhr fixation system for the craniofacial skeleton. Clin Plast Surg. 1989;16:41–48. [PubMed] [Google Scholar]
- Steinemann S. Metal for craniomaxillofacial internal fixation implants and its physiologic implications. In: In: Greenberg A, Prein J, editor. Craniomaxillofacial Reconstructive and Corrective Bone Surgery. New York, NY: Springer; 2006. pp. 107–112. [Google Scholar]
- Steinmann S G, Eulenberger J, Mausli P A, Schroeder A. Biological and Biomechanical Performance of Biomaterials. Amsterdam: Elsevier Science; 1986.
- Barone C M, Eisig S, Wallach S, Mitnick R, Mednick R. Effects of rigid fixation device composition on three-dimensional computed axial tomography imaging: direct measurements on a pig model. J Oral Maxillofac Surg. 1994;52:737–740. doi: 10.1016/0278-2391(94)90490-1. [DOI] [PubMed] [Google Scholar]
- Fiala T G, Novelline R A, Yaremchuk M J. Comparison of CT imaging artifacts from craniomaxillofacial internal fixation devices. Plast Reconstr Surg. 1993;92:1227–1232. [PubMed] [Google Scholar]
- Sullivan P K, Smith J F, Rozzelle A A. Cranio-orbital reconstruction: safety and image quality of metallic implants on CT and MRI scanning. Plast Reconstr Surg. 1994;94:589–596. [PubMed] [Google Scholar]
- Hobar P C. Methods of rigid fixation. Clin Plast Surg. 1992;19:31–39. [PubMed] [Google Scholar]
- Luhr H G. A micro-system for cranio-maxillofacial skeletal fixation: preliminary report. J Craniomaxillofac Surg. 1988;16:312–314. doi: 10.1016/s1010-5182(88)80069-6. [DOI] [PubMed] [Google Scholar]
- Simpson J P, Geret V, Brown S A, Merrit K. Implant retrieval: material and biologic analysis. 1981. pp. 395–422. NBS Spec Publ 601.
- Gross P P, Gold L. The compatibility of Vitallium and Austanium in completely buried implants in dogs. Oral Surg Oral Med Oral Pathol. 1957;10:769–780. doi: 10.1016/s0030-4220(57)80073-5. [DOI] [PubMed] [Google Scholar]
- Sargent L A, Fulks K D. Reconstruction of internal orbital fractures with Vitallium mesh. Plast Reconstr Surg. 1991;88:31–38. doi: 10.1097/00006534-199107000-00006. [DOI] [PubMed] [Google Scholar]
- Sengezer M, Sadove R C. Reconstruction of midface bone defects with vitallium micromesh. J Craniofac Surg. 1992;3:125–133. doi: 10.1097/00001665-199211000-00003. [DOI] [PubMed] [Google Scholar]
- Lalor P A, Revell P A, Gray A B, et al. Sensitivity to titanium: a cause of implant failure? J Bone Joint Surg Br. 1991;73:25–28. doi: 10.1302/0301-620X.73B1.1991768. [DOI] [PubMed] [Google Scholar]
- Moran C A, Mullick F G, Ishak K G, Johnson F B, Hummer W B. Identification of titanium in human tissues: probable role in pathologic processes. Hum Pathol. 1991;22:450–454. doi: 10.1016/0046-8177(91)90130-h. [DOI] [PubMed] [Google Scholar]
- Redline S, Barna B P, Tomashefski J F, Jr, Abraham J L. Granulomatous disease associated with pulmonary deposition of titanium. Br J Ind Med. 1986;43:652–656. doi: 10.1136/oem.43.10.652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weingart D, Steinemann S, Schilli W, et al. Titanium deposition in regional lymph nodes after insertion of titanium screw implants in maxillofacial region. Int J Oral Maxillofac Surg. 1994;23:450–452. doi: 10.1016/s0901-5027(05)80045-1. [DOI] [PubMed] [Google Scholar]
- Moberg L E, Nordenram A, Kjellman O. Metal release from plates used in jaw fracture treatment: a pilot study. Int J Oral Maxillofac Surg. 1989;18:311–314. doi: 10.1016/s0901-5027(89)80102-x. [DOI] [PubMed] [Google Scholar]
- Rosenberg A, Gratz K W, Sailer H F. Should titanium miniplates be removed after bone healing is complete? Int J Oral Maxillofac Surg. 1993;22:185–188. doi: 10.1016/s0901-5027(05)80249-8. [DOI] [PubMed] [Google Scholar]
- Marschall M A, Chidyllo S A, Figueroa A A, Cohen M. Long-term effects of rigid fixation on the growing craniomaxillofacial skeleton. J Craniofac Surg. 1991;2:63–68. doi: 10.1097/00001665-199102020-00005. [DOI] [PubMed] [Google Scholar]
- Yaremchuk M J, Fiala T G, Barker F, Ragland R. The effects of rigid fixation on craniofacial growth of rhesus monkeys. Plast Reconstr Surg. 1994;93:1–10. [PubMed] [Google Scholar]
- Manson P N. Discussion on the long-term effects of rigid fixation on the growing craniomaxillofacial skeleton. J Craniofac Surg. 1991;2:69–70. doi: 10.1097/00001665-199102020-00005. [DOI] [PubMed] [Google Scholar]
- Goldberg D S, Bartlett S, Yu J C, Hunter J V, Whitaker L A. Critical review of microfixation in pediatric craniofacial surgery. J Craniofac Surg. 1995;6:301–307. doi: 10.1097/00001665-199507000-00008. [DOI] [PubMed] [Google Scholar]
- Bell R B, Kindsfater C S. The use of biodegradable plates and screws to stabilize facial fractures. J Oral Maxillofac Surg. 2006;64:31–39. doi: 10.1016/j.joms.2005.09.010. [DOI] [PubMed] [Google Scholar]
- Bos R R. Treatment of pediatric facial fractures: the case for metallic fixation. J Oral Maxillofac Surg. 2005;63:382–384. doi: 10.1016/j.joms.2004.11.010. [DOI] [PubMed] [Google Scholar]
- Rudderman R H, Mullen R L. Biomechanics of the facial skeleton. Clin Plast Surg. 1992;19:11–29. [PubMed] [Google Scholar]
- Cutright D E, Hunsuck E E, Beasley J D. Fracture reduction using a biodegradable material, polylactic acid. J Oral Surg. 1971;29:393–397. [PubMed] [Google Scholar]
- Suuronen R, Lindqvist C. Bioresorbable materials for bone fixation: review of biological concepts and mechanical aspects. In: In: Greenberg A, Prein J, editor. Craniomaxillofacial Reconstructive and Corrective Bone Surgery. New York, NY: Springer; 2006. [Google Scholar]
- Gerlach K L, Eitenmuller J. Biomaterials and Clinical Applications. Amsterdam: Elsevier Science; 1987. pp. 439–445.
- Eppley B L, Prevel C D, Sadove A M, Sarver D. Resorbable bone fixation: its potential role in cranio-maxillofacial trauma. J Craniomaxillofac Trauma. 1996;2:56–60. [PubMed] [Google Scholar]
- Edwards R C, Kiely K D, Eppley B L. The fate of resorbable poly-L-lactic/polyglycolic acid (LactoSorb) bone fixation devices in orthognathic surgery. J Oral Maxillofac Surg. 2001;59:19–25. doi: 10.1053/joms.2001.19267. [DOI] [PubMed] [Google Scholar]
- Eppley B L, Reilly M. Degradation characteristics of PLLA-PGA bone fixation devices. J Craniofac Surg. 1997;8:116–120. doi: 10.1097/00001665-199703000-00010. [DOI] [PubMed] [Google Scholar]
- Hochuli-Vieira E, Cabrini Gabrielli M A, Pereira-Filho V A, Gabrielli M F, Padilha J G. Rigid internal fixation with titanium versus bioresorbable miniplates in the repair of mandibular fractures in rabbits. Int J Oral Maxillofac Surg. 2005;34:167–173. doi: 10.1016/j.ijom.2004.03.016. [DOI] [PubMed] [Google Scholar]
- Quereshy F A, Goldstein J A, Goldberg J S, Beg Z. The efficacy of bioresorbable fixation in the repair of mandibular fractures: an animal study. J Oral Maxillofac Surg. 2000;58:1263–1269. doi: 10.1053/joms.2000.16627. [DOI] [PubMed] [Google Scholar]
- Wiltfang J, Merten H A, Becker H J, Luhr H G. The resorbable miniplate system Lactosorb in a growing cranio-osteoplasty animal model. J Craniomaxillofac Surg. 1999;27:207–210. doi: 10.1016/s1010-5182(99)80030-4. [DOI] [PubMed] [Google Scholar]
- Eppley B L, Sadove A M. A comparison of resorbable and metallic fixation in healing of calvarial bone grafts. Plast Reconstr Surg. 1995;96:316–322. doi: 10.1097/00006534-199508000-00009. [DOI] [PubMed] [Google Scholar]
- Synthes CMF. Mechanical Test Data for Synthes Resorbable vs. Titanium Implants. West Chester, PA: Synthes CMF; 2000.
- Gosain A K, Song L, Corrao M A, Pintar F A. Biomechanical evaluation of titanium, biodegradable plate and screw, and cyanoacrylate glue fixation systems in craniofacial surgery. Plast Reconstr Surg. 1998;101:582–591. doi: 10.1097/00006534-199803000-00004. [DOI] [PubMed] [Google Scholar]
- Kasrai L, Hearn T, Gur E, Forrest C R. A biomechanical analysis of the orbitozygomatic complex in human cadavers: examination of load sharing and failure patterns following fixation with titanium and bioresorbable plating systems. J Craniofac Surg. 1999;10:237–243. doi: 10.1097/00001665-199905000-00012. [DOI] [PubMed] [Google Scholar]
- Shetty V, Caputo A A, Kelso I. Torsion-axial force characteristics of SR-PLLA screws. J Craniomaxillofac Surg. 1997;25:19–23. doi: 10.1016/s1010-5182(97)80020-0. [DOI] [PubMed] [Google Scholar]
- Chacon G E, Dillard F M, Clelland N, Rashid R. Comparison of strains produced by titanium and poly D, L-lactide acid plating systems to in vitro forces. J Oral Maxillofac Surg. 2005;63:968–972. doi: 10.1016/j.joms.2005.03.013. [DOI] [PubMed] [Google Scholar]
- Eppley B L, Prevel C D. Nonmetallic fixation in traumatic midfacial fractures. J Craniofac Surg. 1997;8:103–109. doi: 10.1097/00001665-199703000-00008. [DOI] [PubMed] [Google Scholar]
- Tormala P, Vasenius J, Vainionpaa S, et al. Ultra-high-strength absorbable self-reinforced polyglycolide (SR-PGA) composite rods for internal fixation of bone fractures: in vitro and in vivo study. J Biomed Mater Res. 1991;25:1–22. doi: 10.1002/jbm.820250102. [DOI] [PubMed] [Google Scholar]
- Eppley B L. Resorbable biotechnology for craniomaxillofacial surgery. J Craniofac Surg. 1997;8:85–86. [PubMed] [Google Scholar]
- Kumar A V, Staffenberg D A, Petronio J A, Wood R J. Bioabsorbable plates and screws in pediatric craniofacial surgery: a review of 22 cases. J Craniofac Surg. 1997;8:97–99. doi: 10.1097/00001665-199703000-00006. [DOI] [PubMed] [Google Scholar]
- Edwards R C, Kiely K D. Resorbable fixation of Le Fort I osteotomies. J Craniofac Surg. 1998;9:210–214. doi: 10.1097/00001665-199805000-00005. [DOI] [PubMed] [Google Scholar]
- Edwards R C, Kiely K D, Eppley B L. Resorbable PLLA-PGA screw fixation of mandibular sagittal split osteotomies. J Craniofac Surg. 1999;10:230–236. doi: 10.1097/00001665-199905000-00011. [DOI] [PubMed] [Google Scholar]
- Edwards R C, Kiely K D, Eppley B L. Resorbable fixation techniques for genioplasty. J Oral Maxillofac Surg. 2000;58:269–272. doi: 10.1016/s0278-2391(00)90049-1. [DOI] [PubMed] [Google Scholar]
- Haers P E, Sailer H F. Biodegradable self-reinforced poly-L/DL-lactide plates and screws in bimaxillary orthognathic surgery: short term skeletal stability and material related failures. J Craniomaxillofac Surg. 1998;26:363–372. doi: 10.1016/s1010-5182(98)80069-3. [DOI] [PubMed] [Google Scholar]
- Landes C A, Ballon A. Five-year experience comparing resorbable to titanium miniplate osteosynthesis in cleft lip and palate orthognathic surgery. Cleft Palate Craniofac J. 2006;43:67–74. doi: 10.1597/04-167r1.1. [DOI] [PubMed] [Google Scholar]
- Shand J M, Heggie A A. Use of a resorbable fixation system in orthognathic surgery. Br J Oral Maxillofac Surg. 2000;38:335–337. doi: 10.1054/bjom.1999.0287. [DOI] [PubMed] [Google Scholar]
- Stryker Craniomaxillofacial. Inion CPS Biodegradeable Fixation System [product guide] Kalamazoo, MI: Stryker Craniomaxillofacial; 2008.
- Majewski W T, Yu J C, Ewart C, Aguillon A. Posttraumatic craniofacial reconstruction using combined resorbable and nonresorbable fixation systems. Ann Plast Surg. 2002;48:471–476. doi: 10.1097/00000637-200205000-00004. [DOI] [PubMed] [Google Scholar]
- Tatum S A, Kellman R M, Freije J E. Maxillofacial fixation with absorbable miniplates: computed tomographic follow-up. J Craniofac Surg. 1997;8:135–140. doi: 10.1097/00001665-199703000-00014. [DOI] [PubMed] [Google Scholar]
- Landes C A, Ballon A, Roth C. Maxillary and mandibular osteosyntheses with PLGA and P(L/DL)LA implants: a 5-year inpatient biocompatibility and degradation experience. Plast Reconstr Surg. 2006;117:2347–2360. doi: 10.1097/01.prs.0000218787.49887.73. [DOI] [PubMed] [Google Scholar]
- Kim Y K, Kim S G. Treatment of mandible fractures using bioabsorbable plates. Plast Reconstr Surg. 2002;110:25–31. doi: 10.1097/00006534-200207000-00006. [DOI] [PubMed] [Google Scholar]
- Hollier L H, Rogers N, Berzin E, Stal S. Resorbable mesh in the treatment of orbital floor fractures. J Craniofac Surg. 2001;12:242–246. doi: 10.1097/00001665-200105000-00009. [DOI] [PubMed] [Google Scholar]
- Tuncer S, Yavuzer R, Kandal S, et al. Reconstruction of traumatic orbital floor fractures with resorbable mesh plate. J Craniofac Surg. 2007;18:598–605. doi: 10.1097/01.scs.0000246735.92095.ef. [DOI] [PubMed] [Google Scholar]
- Rudderman R H, Mullen R L, Phillips J H. The biophysics of mandibular fractures: an evolution toward understanding. Plast Reconstr Surg. 2008;121:596–607. doi: 10.1097/01.prs.0000297646.86919.b7. [DOI] [PubMed] [Google Scholar]
- Tams J, Otten B, Loon J P Van, Bos R R. A computer study of fracture mobility and strain on biodegradable plates used for fixation of mandibular fractures. J Oral Maxillofac Surg. 1999;57:973–981. doi: 10.1016/s0278-2391(99)90020-4. [DOI] [PubMed] [Google Scholar]
- Yerit K C, Hainich S, Turhani D, et al. Stability of biodegradable implants in treatment of mandibular fractures. Plast Reconstr Surg. 2005;115:1863–1870. doi: 10.1097/01.prs.0000165075.51898.6f. [DOI] [PubMed] [Google Scholar]
- Suzuki T, Kawamura H, Kasahara T, Nagasaka H. Resorbable poly-L-lactide plates and screws for the treatment of mandibular condylar process fractures: a clinical and radiologic follow-up study. J Oral Maxillofac Surg. 2004;62:919–924. doi: 10.1016/j.joms.2004.01.016. [DOI] [PubMed] [Google Scholar]
- Landes C A, Ballon A. Indications and limitations in resorbable P(L70/30DL)LA osteosyntheses of displaced mandibular fractures in 4.5-year follow-up. Plast Reconstr Surg. 2006;117:577–587. doi: 10.1097/01.prs.0000200915.65693.29. [DOI] [PubMed] [Google Scholar]
- Senel F C, Tekin U S, Imamoglu M. Treatment of a mandibular fracture with biodegradable plate in an infant: report of a case. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006;101:448–450. doi: 10.1016/j.tripleo.2005.07.020. [DOI] [PubMed] [Google Scholar]
- Yerit K C, Hainich S, Enislidis G, et al. Biodegradable fixation of mandibular fractures in children: stability and early results. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2005;100:17–24. doi: 10.1016/j.tripleo.2004.11.013. [DOI] [PubMed] [Google Scholar]