Abstract
The temporal bones are paired structures located on the lateral aspects of the skull and contribute to the skull base. Trauma is usually the result of blunt head injury and can result in damage to the brain and meninges, the middle and internal ear, and the facial nerve. Complications can include intracranial hemorrhage, cerebral contusion, CSF leak and meningitis, hearing loss, vertigo, and facial paralysis. To prevent these complications, diagnosis followed by appropriate medical and surgical management is critical. Diagnosis relies primarily on physical signs and symptoms as well as radiographic imaging. Emergent intervention is required in situations involving herniation of the brain into the middle ear cavity or hemorrhage of the intratemporal carotid artery. Patients with declining facial nerve function are candidates for early surgical intervention. Conductive hearing loss can be corrected surgically as an elective procedure, while sensorineural hearing loss carries a poor prognosis, regardless of management approach. Children generally recover from temporal bone trauma with fewer complications than adults and experience a markedly lower incidence of facial nerve paralysis.
Keywords: Temporal bone, trauma, management
Temporal bone trauma is usually the result of blunt head injury and patients commonly suffer from multiple other body injuries. Motor vehicle accidents are the most common cause, with falls and gunshot wounds contributing to a lesser extent. Because the temporal bone encloses the middle and internal ear, these structures can be damaged by penetrating or concussive trauma to the tympanic membrane through the external acoustic meatus without temporal bone fracture. Iatrogenic trauma to temporal bone structures can also occur, and is usually the result of inadvertent surgical injury to the internal ear or facial nerve.
This article will focus on the diagnosis and treatment of temporal bone trauma, as these injuries can have drastic consequences if they are not recognized and treated promptly and effectively. Complications can include intracranial hemorrhage, cerebral contusion, meningitis, hearing loss, and facial paralysis, all of which may result in death or permanent deficits. A brief description of the anatomy and function of the temporal bone will be presented, followed by a discussion of current guidelines in diagnosing and treating injuries to this structure. Pediatric temporal bone trauma will be addressed separately, as presentation, treatment, and prognosis differ in this population compared with adults.
ANATOMY
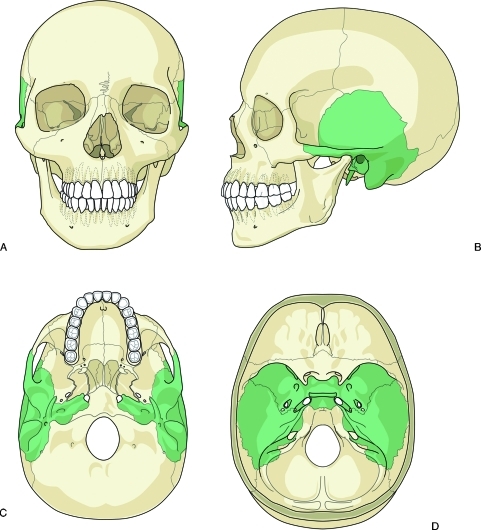
The temporal bones are paired structures located on the lateral aspects of the skull inferior to the parietal bones, posterior to the sphenoid bone, and anterior to the occipital bone (Fig. 1). The temporal bones form parts of the middle and posterior cranial fossae and contribute to the neurocranium or skull base. In addition to protecting the brain, each temporal bone houses several important structures such as the middle and internal ear apparatus including the cochlea, vestibule and the vestibulocochlear nerve (cranial nerve VIII), the facial nerve (cranial nerve VII), the internal carotid artery, and the jugular vein.1 Trauma to the temporal bone can result in injuries to each of these structures.
Figure 1.
Diagram of the paired temporal bones (shaded) in relation to the human skull. Anterior view (A), right lateral view (B), inferior view (C), and interior view of the skull base (D).
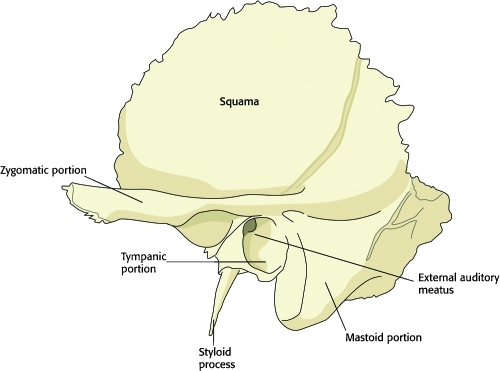
Each temporal bone is divided into five components: squamous, tympanic, styloid, mastoid, and petrous (Fig. 2). The squamous portion lies superior and anterior in relation to the remainder of the temporal bone and comprises much of the lateral wall of the middle cranial fossa. The zygomatic process arises from the squamous part, connecting the temporal bone to the zygomatic bone of the face through the zygomatic arch, as well as providing the superior articulation of the temporomandibular joint (TMJ). The zygomatic arch divides the lateral surface of the head into two anatomic regions, the temporal fossa and the infratemporal fossa, and is also the site of attachment for the masseter muscle, one of the principal muscles of mastication. The squamous part also contributes to the roof of the external acoustic meatus.
Figure 2.
Diagram of the left temporal bone from a lateral view. The squamous, styloid, and mastoid portions are labeled. The tympanic portion lines the external auditory meatus and the petrous portion is an interior structure and is not visible from a lateral view.
The tympanic portion of the temporal bone provides the remainder of the external acoustic meatus leading to and providing support for the tympanic membrane. The styloid process arises inferior to the tympanic part and gives attachment to the stylohyoid and stylomandibular ligaments, and to the styloglossus and stylopharyngeus muscles. The mastoid portion forms the posterior border of the temporal bone and is highly pneumatized in most individuals, with a communicating honeycomb in its interior known as mastoid cells (Fig. 2). The mastoid process provides the attachment for the sternocleidomastoid (SCM) muscle, which gives the neck much of its mobility.
The petrous portion of the temporal bone is pyramidal in shape and wedged in at the base of the skull between the sphenoid and occipital bones. It is not visible from a lateral view of the temporal bone. This important part of the temporal bone encloses the middle and internal ear structures, along with parts of the facial nerve.
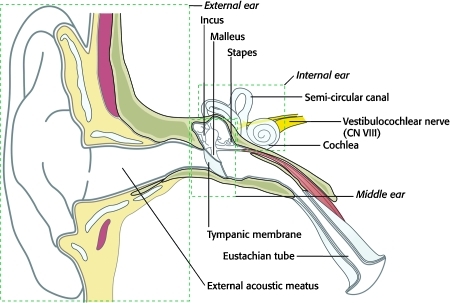
Sound is collected by the auricle (external ear) and then travels through the temporal bone via the external acoustic meatus until it reaches the tympanic membrane (eardrum). The middle ear begins at the internal surface of the tympanic membrane, which is in contact with the chain of ossicles (the malleus, incus, and stapes). Sound is amplified as it travels through these small bony structures until it reaches the oval window, which transmits the sound waves to the internal ear and the cochlea (Fig. 3). At this point, vibrations are translated into neural impulses, which are then conveyed to the brain via cranial nerve (CN) VIII (the vestibulocochlear nerve). Because the middle ear is continuous with the nasopharynx via the pharyngotympanic (eustachian) tube, blood or cerebrospinal fluid (CSF) in the middle ear secondary to trauma may manifest as rhinorrhea, or fluid leaking from the nose.
Figure 3.
Schematic diagram of the right external, middle, and internal ear. Sound travels through the external acoustic meatus causing the tympanic membrane to vibrate. The ossicles of the middle ear (malleus, incus, and stapes) amplify and transmit these vibrations through the oval window to the cochlea, an internal ear structure. Vibrations are then translated into neural impulses and carried to the brain via the vestibulocochlear nerve (CN VIII). CN VIII also transmits information from the vestibular apparatus (balance organ) of the internal ear, which includes the three semicircular canals, and the utricle and saccule (not shown).
The vestibule is also located in the internal ear and is comprised of the utricle and saccule. Three semicircular canals (superior, posterior, and horizontal) communicate with the vestibule and are set at right angles to each other so that they occupy three plains of space. The vestibule, along with the semicircular canals, constitutes an integral part of the body's balancing apparatus, and information regarding the body's acceleration is conveyed from these structures to the brain via CN VIII, along with sound information. Because these important sensory structures are located completely within the temporal bone, trauma to the bone can result in hearing loss and vertigo. Sensorineural hearing loss implies damage to the internal ear structures (e.g., the cochlea) or CN VIII. Conductive hearing loss implies damage distal to the internal ear, including disruption of the ossicle chain within the middle ear and damage to the tympanic membrane (Fig. 3).
The facial nerve (CN VII) has several functions including control of the muscles of facial expression, lacrimation, salivation, general sensation of the external acoustic meatus and scalp, and taste (special sensory) of the anterior two-thirds of the tongue. Each of these functions can be affected secondary to temporal bone trauma. The facial nerve can be divided into intracranial, intratemporal, and extracranial segments.
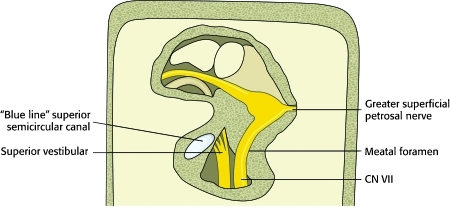
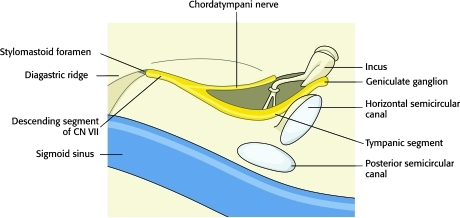
The intratemporal segment is the focus of this discussion and begins as the nerve enters the temporal bone at its internal surface along with CN VIII via the internal acoustic meatus. Shortly after entering the temporal bone, the facial nerve separates from CN VIII in the internal acoustic meatus and enters the facial canal, which can divided into three segments in its course through the temporal bone: labyrinthine, tympanic (horizontal), and mastoid (vertical or descending).2 The labyrinthine portion forms a gradual curve coursing anterolaterally between the vestibule and the cochlea toward the fossa of the geniculate ganglion. The course then takes a sharp turn posterolaterally at the geniculate ganglion, giving off a branch medially (superficial petrosal nerve) to supply the lacrimal gland (Fig. 9).
Figure 9.
Diagram of the facial nerve as exposed in a middle cranial fossa approach. Careful dissection is essential to avoid the superior semicircular canal and the basal turn of the cochlea.
The tympanic portion begins as the nerve leaves the geniculate ganglion and courses posterolaterally, forming a prominence along the medial aspect of the middle ear just above the oval window and the stapes, and below the horizontal semicircular canal. The mastoid segment of the facial nerve extends from the second turn of the facial canal until it reaches the stylomastoid foramen, which transmits the nerve out of the temporal bone between the styloid and mastoid processes.
EPIDEMIOLOGY
Injuries to the temporal bone occur in 30 to 70% of cases involving blunt head trauma.3,4 Although safety measures such as seatbelts, airbags, and bicycle helmets have helped reduce the number of vehicle accidents resulting in head trauma, these types of accidents remain the most common cause of temporal bone injury. Gunshot wounds to the head are an unusual etiology of temporal bone trauma but are increasing in frequency, and more than half of these patients suffer from intracranial trauma as well.5,6 Injuries to the carotid artery and increased mortality are seen more often in these patients than in those suffering from blunt trauma.
Iatrogenic trauma to temporal bone structures most often involves the facial nerve, although severe hearing loss can also occur. Over half of reported cases of surgically induced facial nerve injuries follow mastoid procedures, and 80% of iatrogenic facial nerve injuries are not recognized at the time of initial surgery.7 Direct trauma to the tympanic membrane and middle ear is seen primarily in children. Common instruments for injury include pencils, cotton tipped applicators, and hairpins. Injuries of this type can occasionally occur during removal of cerumen in restless or crying children.8
HISTORY AND PHYSICAL EXAMINATION
Hearing loss is usually immediately apparent to conscious patients and is the most common chief complaint following temporal bone trauma, occurring in as many as 40% of patients with head injury. The hearing loss may or may not be accompanied by tinnitus (ringing in the ears), which has no prognostic significance.9 Loss can be categorized as conductive, due to injury to the conducting system distal to the cochlea, or sensorineural, due to injury of the internal ear including the cochlea and CN VIII. A third category, mixed hearing loss, occurs when the deficit is caused by partial injury to both sets of structures. Conductive and mixed hearing loss is more common in longitudinal fractures of the temporal bone or injuries with no identifiable fracture, while transverse fractures have the highest risk of sensorineural loss. This fracture classification will be described in more detail during the discussion on radiographic evaluation of temporal bone trauma.
Audiometric testing can help differentiate between these types. If necessary, bedside testing is reliable enough for early evaluation and can be confirmed with tuning fork testing. Determining the type of hearing loss is prognostically important but does not influence the timing of surgery, as conductive hearing losses can be repaired at any time and sensorineural loss carries a poor prognosis that is usually not influenced by treatment.
Dizziness and dysequilibrium are often noticed later after temporal bone trauma unless severe labyrinthine injury has occurred. Many patients notice imbalance only after becoming ambulatory. Nystagmus (rapid, involuntary movements of the eyes) is often a sign of vestibular injury and the direction of nystagmus is usually away from the affected ear. Extensive physical assessment of vestibular complaints immediately after temporal bone trauma is unnecessary, as complete recovery from imbalance and nystagmus is to be expected.
Complaints of facial paresis (weakness) or paralysis and facial asymmetry are indicative of injury to the facial nerve (CN VII) and are most likely to dictate the need for early surgical intervention of all other complications of temporal bone trauma. The timing of onset of these symptoms is an important factor in planning treatment. Weakness is considered to be immediate if the symptoms begin in the first few hours after injury; however, early onset can easily go unnoticed by patients due to facial swelling, lacerations, and abrasions. Late-onset paresis or paralysis may be delayed for days or weeks and is common after temporal bone trauma.
Mapping facial nerve function is of little or no value in determining the location of the nerve injury with the exception that if not all the branches of the facial nerve are injured, trauma to the nerve is likely to have occurred outside the temporal bone (extracranial).10 Electroneuronography (ENOG) is the most effective method for testing facial nerve function and can be performed by comparing the summated action potential of the affected side with that of the uninjured side.11 Any observation of facial paralysis should be followed with ENOG to determine improvement or worsening of function. ENOG is generally performed 2 to 3 days after facial nerve injury but within 2 to 3 weeks.
Patients with facial nerve paresis who benefit from early surgical intervention include those with immediate paralysis and no evidence of return of function after 1 week, and patients with immediate paralysis and progressive decline in ENOG functioning to less than 10% of the normal side. Patients with immediate paralysis with computed tomography (CT) evidence of significant temporal bone disruption, indicating severe nerve laceration or sectioning also benefit from early surgery.9 If eye closure is compromised following facial nerve injury, prophylactic corneal care (i.e., artificial tears and lubricant) should be administered to prevent drying out. Failure to detect electromyographic (EMG) or clinical evidence of return of function after 1 year indicates complete severing of the facial nerve and precludes the spontaneous return of function in the future.
Because the temporal bone provides part of the inferior wall of the cranial cavity, trauma may result in leakage of cerebrospinal fluid (CSF) into the middle ear cavity. Provided the tympanic membrane is intact, this fluid may travel through the eustachian tube, resulting in rhinorrhea (nasal drainage). If the tympanic membrane has also been damaged, otorrhea (drainage through the external acoustic meatus) may result (Fig. 4). Bleeding from these orifices early after injury can mask the presence of CSF. A circular ring of lighter color surrounding blood stains on dressings covering the ear can provide a clue for the presence of CSF. Evidence of CSF leakage should prompt a thorough evaluation, as its presence implies a tear in the dura, which can provide a route of infection to the brain. Herniation of brain tissue into the middle ear cavity may also occur with CSF leakage after severe temporal bone trauma.
Figure 4.
Cerebrospinal fluid (CSF) leaking from the ear (otorrhea) indicates that a temporal bone fracture has occurred and that the tympanic membrane has been disrupted. Leakage from the nose (rhinorrhea) is also indicative of a basilar skull fracture.
Facial hypesthesia (decreased or absent touch sensation) and diplopia (double vision) are indicative of CN V (trigeminal nerve) and CN VI (abducens nerve) injury respectively and are uncommon complaints after temporal bone trauma. Neither deficit is usually noticed immediately after injury leading to speculation that edema and not direct trauma is responsible for the damage. Spontaneous recovery of both facial hypesthesia and diplopia is the general rule.
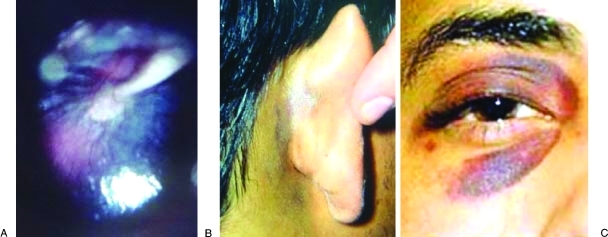
In addition to the above signs and symptoms commonly seen after temporal bone trauma, a presumptive diagnosis of fracture can be made based on three physical findings (Fig. 5): hemotympanum (blood observed behind the tympanic membrane), postauricular ecchymosis or Battle's sign (arch-shaped bruising behind the auricle), and periorbital ecchymosis or raccoon sign (circular bruising around the eye). These signs along with a history of head trauma are sufficient for the diagnosis of temporal bone fracture, even in the absence of radiographic evidence. Concussive blows to the external acoustic meatus or penetration of foreign objects into the meatus can result in severe trauma to the tympanic membrane, ossicular chain, facial nerve, and labyrinth without fracture of the temporal bone.
Figure 5.
A presumptive diagnosis of temporal bone fracture can be made with the presence of three physical findings. Hemotympanum (A) is a collection of blood in the middle ear and gives the tympanic membrane and reddish-blue hue when visualized externally. Postauricular ecchymosis, or Battle's sign (B), is an arch-shaped bruise behind the external ear. Periorbital ecchymosis, or Raccoon sign (C), is circular bruising around the eye.
RADIOGRAPHIC EVALUATION
High-resolution CT scans with bone algorithms are the standard in diagnosis of temporal bone trauma. Axial and coronal thin-section CT scans can establish fracture sites in most cases, and more than one-third of fractures detected by CT are missed by clinical diagnosis alone.12 In general, the fracture lines run parallel to the line of the blow delivered and extend through foramina, which weaken the bone. Fractures may be single or multiple and are classically referred to as longitudinal or transverse (Figs. 6, 7), although careful observation demonstrates that most are actually oblique. The original terminology persists as it predicts well-known sequelae.
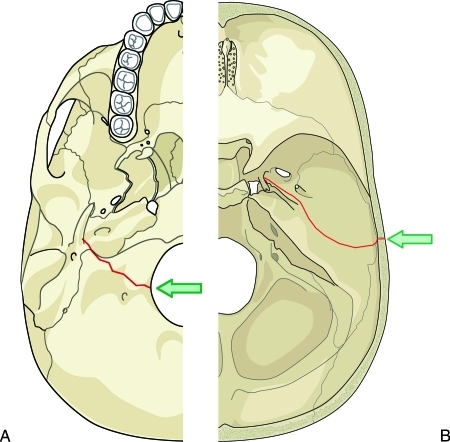
Figure 6.
Diagram of common fracture patterns of the temporal bone. Transverse fractures (A) result most often from a blow to the back of the head. The fracture extends from the jugular foramen through the petrous pyramid to the foramen spinosum and foramen lacerum. Longitudinal fractures (B) result most often from a blow to the side of the head. The fracture extends from the squamous portion of the temporal bone to the carotid and jugular foramina.
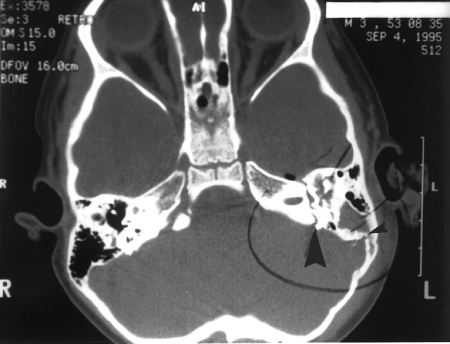
Figure 7.
Axial computed tomography (CT) scan of the showing both a longitudinal fracture (small arrowhead) and a transverse fracture (large arrowhead) of the left temporal bone.
Approximately 70 to 80% of temporal bone fractures are longitudinal, resulting from a blow to the temporal or parietal region of the skull.13 These fractures follow the path of least resistance, which usually leads through the petrosquamous suture line and continues anterior to the otic capsule. Involvement of the middle ear causes frequent hemotympanum and ossicular disruption, resulting in conductive hearing loss. The facial nerve is involved in 15 to 20% of these fractures.
Transverse fractures are less common and result from forces generated along the anterior-posterior axis, usually blows to the back of the head. Fractures begin at the jugular foramen and extend across the petrous pyramid to the area of the foramen spinosum and foramen lacerum. Sensorineural hearing loss and vertigo are secondary to direct injury of the inner ear, and occur in up to 50% of these fractures. The facial nerve is involved in nearly half of these fractures as well.
In addition to determining fracture type and location, CT scans are invaluable in assessing the location of facial nerve injury, as well as for planning surgical approaches. Magnetic resonance imaging (MRI) can also be useful in corroborating cranial nerve injury. In the case of gunshot wounds or other penetrating trauma, angiography or MRA (magnetic resonance angiography) is always indicated because of the greater possibility of injury to the internal carotid artery.
MANAGEMENT
Emergent intervention is necessary in two situations following temporal bone trauma. Obvious brain herniation (encephalocele) into the middle ear, mastoid, or external acoustic meatus requires immediate neurologic and medical stabilization, and CT scanning to allow planning for emergent surgical correction. The second situation is massive bleeding from intratemporal carotid artery laceration and is an uncommon complication of temporal bone trauma. Balloon occlusion of the vessel by an interventional radiologist is generally faster than surgical ligation and repair in this situation.
As mentioned previously, the natural history of temporal bone fractures is closely related to the initial evaluation of cranial nerve function. Patients with initially good facial nerve function generally do well without surgery, even though late onset paralysis can occur. Surgical management is dictated if facial nerve function is determined to have a poor prognosis via testing results or if there is CT evidence of severe disruption or displacement of the facial nerve. Careful review of CT scans should be performed to determine if the nerve injury is located proximal or distal to the geniculate ganglion.
The transmastoid approach is suitable only for lesions determined to be distal to the geniculate ganglion (Fig. 8). In patients with postauricular ecchymosis (Battle's sign), the fracture defect usually involves the mastoid cortex or squamous portion. The fracture line can be followed medially to the point of facial nerve injury. If the fracture is not identified laterally, the nerve should be exposed in the facial recess and traced along its path until the area of injury can be determined. The incus can be temporarily removed to facilitate this exposure.
Figure 8.
Diagram of the facial nerve and other middle ear structures as exposed in a transmastoid approach. This approach is suitable for patients whose nerve injury lies distal to the geniculate ganglion. The incus can be temporarily removed to facilitate this approach.
Once the facial nerve injury is located, any bone chips should be removed and the area should be examined for stretching, compression, laceration, or transection. If the nerve is largely intact, decompression of the epineural sheath is performed in proximal to distal. Partial transection can be repaired with suture, but separation of more than 50% of the axons usually requires an interpositional nerve graft.9 The greater auricular nerve, which normally provides sensory information from the skin overlying the parotid gland, mastoid process, and both surfaces of the outer ear, is commonly used as a source of graft tissue because of its size and proximity. One or two epineural sutures are recommended but topical thrombin or “tissue glue” is usually sufficient. Adequate length should be used for shrinkage of nerve interposition grafts, and the approximate diameter of the facial nerve should be reestablished. Standard mastoidectomy closure and postoperative care are used.
For patients who have facial nerve injury proximal to the geniculate ganglion and no sensorineural hearing deficits, the middle cranial fossa approach is appropriate (Fig. 9). A temporal bone flap is created with its base parallel to the middle cranial fossa floor with two-thirds of the square anterior and one-third posterior to a line drawn vertically through the external acoustic meatus. Great care must be exercised when elevating the flap because branches of the middle meningeal artery often are embedded in the inner table. If encountered, these are controlled with bipolar cauterization or suture ligation. Dural elevation from the floor of the middle cranial fossa proceeds from posterior to anterior to avoid avulsion injury to the geniculate ganglion. Elevation continues until the petrous ridge is reached and the arcuate eminence and greater superficial petrosal nerve are identified. Bone dissection begins with exposure of the greater petrosal nerve and geniculate ganglion until connection is made with a previous tympanic dissection. Careful dissection is essential to avoid the superior semicircular canal and basal turn of the cochlea. Once entirely exposed, the nerve can be examined and tested for integrity or injury and subsequently repaired via interpositional nerve grafting if necessary. Previously harvested temporalis fascia is used to seal the dural defect at the internal acoustic meatus, preventing temporal lobe herniation into the middle ear. Reposition of the bone flap and layered closure is then accomplished.
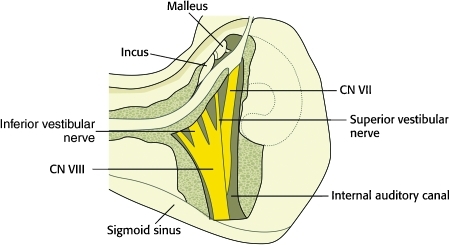
For patients with sensorineural hearing loss that is unlikely to improve, the transmastoid-translabyrinthine approach can be employed, which has less associated morbidity than the middle cranial fossa approach. Patients suitable for this procedure generally have transverse fractures, and the translabyrinthine exposure allows visibility of the entire intratemporal course of the facial nerve, including the brain stem origin (Fig. 10). Suturing of interposition grafts is more difficult in the posterior fossa, and additional support from collagen or silastic sleeves may be needed. Closure is performed with abdominal fat grafts to maintain a CSF tight closure.
Figure 10.
Diagram of the facial nerve as exposed in a translabyrinthine approach. When combined with a transmastoid approach, the entire intratemporal course of the facial nerve can be visualized.
CSF otorrhea induced by temporal bone trauma usually resolves spontaneously within 2 weeks without intervention. Antibiotics are not routinely prescribed in cases with CSF leakage, for fear of masking early infection. Patients should be questioned frequently about meningeal symptoms (headaches with nuchal rigidity, photophobia, etc) and a lumbar puncture should be performed if meningitis is suspected, before beginning antibiotic therapy. Surgery is indicated for continuous CSF otorrhea or rhinorrhea persisting longer than 14 days. If lumbar drainage for 72 hours fails, surgical exploration is recommended for closure of the dural tear and prevention of meningitis. Careful review of CT scans is necessary to locate the source of the dural tear. Tears can be repaired with suturing and dural grafting, and dehiscent brain tissue extending into the temporal bone is nonfunctional and can be removed by electrocautery.14
Generally, conductive hearing loss secondary to hemotympanum resolves without intervention, and remaining conductive losses due to ossicular disruption can be repaired electively. Surgery is not recommended earlier than 3 months after trauma because of postinjury edema, bleeding, and friability of healing tissues. Sensorineural hearing loss may show improvement over time but tends to persist and is refractory to treatment.
Intravenous corticosteroids are sometimes used for both sensorineural hearing loss and facial nerve injury following temporal bone trauma. While there is little data examining the efficacy of such an approach, the therapy is relatively inexpensive, and a short course of steroids presents minimal risk of complications.15 These agents inhibit inflammation and thus reduce edema in and around the nerve. The decreased compression to the nerve is expected to improve functioning.
Dysequilibrium usually responds to activity and should resolve without additional intervention. Benign paroxysmal positional vertigo can follow head injury after a period of days to weeks but also resolves spontaneously. Vestibular suppressants can be used briefly. Intravenous droperidol provides the most effective immediate relief, and intramuscular promethazine is usually sufficient for subsequent treatment. These medications should only be used briefly and should be tapered rapidly to allow for central nervous system (CNS) compensation to the injury. Early ambulation also stimulates CNS compensation.
PEDIATRIC TEMPORAL BONE TRAUMA
Temporal bone fractures in children are three times more common in males than in females and occur in a bimodal distribution, with peaks at 3 years and 12 years of age.16,17 The greatest percentage of these injuries in the younger age group are due to falls and motor vehicle accidents. In older children, motor vehicle accidents are the most common cause of temporal bone fracture. Biking accidents and blows to the head are minor contributors, but more common in older children. Child abuse must be considered as a possible etiology in children presenting with injuries inconsistent with the reported history or in children with a prior history of traumatic injuries.
As in adults, the majority of temporal bone fractures in children are longitudinal, with only 4 to13% classified as transverse.17,18 CSF fistula also occurs at similar rates in children with temporal bone trauma, with a reported incidence of 20 to 26%. Management of CSF leakage is conservative unless there is evidence of brain herniation, recurrent episodes of meningitis, or the leakage persists for 14 days. Prophylactic antibiotics are usually not given, and may actually result in a higher incidence of meningitis.19
Hearing loss is more common after temporal bone trauma in children than adults and occurs in more than 80% of cases, 75% of which resolve without invasive treatment. In those with persistent hearing loss, ossicular disruption, especially incudostapedial joint dislocation, is the most common abnormality. For persistent hearing loss, a repeat audiogram at 3 to 4 weeks, with exploration of the middle ear if the patient is found to have continued conductive hearing loss greater than 30 dB, is recommended. High-frequency sensorineural hearing loss is less common in children than adults following temporal bone trauma, occurring in less than 20% of pediatric patients. Improvement of sensorineural loss also appears to be greater in children than adults, although studies to date are highly variable and may be plagued by poor long-term follow-up.17
The most significant difference between children and adults with regard to complications from temporal bone trauma is a markedly lower incidence of facial nerve paralysis in children compared with adults (3% in children compared with 30 to 70% in adults). Liu-Shindo hypothesized that decreased ossification and resultant flexibility of children's skulls may contribute to this striking difference.20 Management of children with facial nerve paresis or paralysis is similar to the management of adults.
References
- Gunlock M G, Gentry L R. Anatomy of the temporal bone. Neuroimaging Clin N Am. 1998;8(1):195–209. [PubMed] [Google Scholar]
- Proctor B. The anatomy of the facial nerve. Otolaryngol Clin North Am. 1991;24(3):479–504. [PubMed] [Google Scholar]
- Hasso A N, Ledington J A. Traumatic injuries of the temporal bone. Otolaryngol Clin North Am. 1988;21(2):295–316. [PubMed] [Google Scholar]
- Wiet R J, Valvassori G E, Kotsanis C A, et al. Temporal bone fractures: state of the art review. Am J Otol. 1985;6(3):207–215. [PubMed] [Google Scholar]
- Shindo M L, Fetterman B L, Shih L, Maceri D R, Rice D H. Gunshot wounds of the temporal bone: a rational approach to evaluation and management. Otolaryngol Head Neck Surg. 1995;112(4):533–539. doi: 10.1177/019459989511200405. [DOI] [PubMed] [Google Scholar]
- Moore P L, Selby G, Irving R M. Gunshot injuries to the temporal bone. J Laryngol Otol. 2003;117(1):71–74. doi: 10.1258/002221503321046702. [DOI] [PubMed] [Google Scholar]
- Green J D, Jr, Shelton C, Brackmann D E. Iatrogenic facial nerve injury during otologic surgery. Laryngoscope. 1994;104(8 Pt 1):922–926. doi: 10.1288/00005537-199408000-00002. [DOI] [PubMed] [Google Scholar]
- Bellucci R J. Traumatic injuries of the middle ear. Otolaryngol Clin North Am. 1983;16(3):633–650. [PubMed] [Google Scholar]
- Kamerer D B, Thompson S W. Middle ear and temporal bone trauma. In: In: Bailey BJ, editor. Head and Neck Surgery—Otolaryngology. 3rd ed. Philadelphia: Lippincott Williams & Wilkins; 2001. [Google Scholar]
- Grobman L R, Pollak A, Fisch U. Entrapment injury of the facial nerve resulting from longitudinal fracture of the temporal bone. Otolaryngol Head Neck Surg. 1989;101(3):404–408. doi: 10.1177/019459988910100318. [DOI] [PubMed] [Google Scholar]
- Fisch V. Management of intratemporal facial palsy of traumatic origin. In: In: Fisch V, editor. Facial Nerve Surgery. Birmingham: Kugler/Aesculapius; 1977. [Google Scholar]
- Exadaktylos A K, Sclabas G M, Nuyens M, et al. The clinical correlation of temporal bone fractures and spiral computed tomographic scan: a prospective and consecutive study at a level I trauma center. J Trauma. 2003;55(4):704–706. doi: 10.1097/01.TA.0000038550.11890.A5. [DOI] [PubMed] [Google Scholar]
- Goodwin W J., Jr Temporal bone fractures. Otolaryngol Clin North Am. 1983;16(3):651–659. [PubMed] [Google Scholar]
- Kamerer D B, Caparosa R J. Temporal bone encephalocele—diagnosis and treatment. Laryngoscope. 1982;92(8 Pt 1):878–882. [PubMed] [Google Scholar]
- Chang C YJ, Cass S P. Management of facial nerve injury due to temporal bone trauma. Am J Otol. 1999;20(1):96–114. [PubMed] [Google Scholar]
- McGuirt W F, Stool S E. Temporal bone fractures in children: a review with emphasis on long-term sequelae. Clin Pediatr (Phila) 1992;31(1):12–18. doi: 10.1177/000992289203100103. [DOI] [PubMed] [Google Scholar]
- Lee D, Honrado C, Har-El G, Goldsmith A. Pediatric temporal bone fractures. Laryngoscope. 1998;108(6):816–821. doi: 10.1097/00005537-199806000-00008. [DOI] [PubMed] [Google Scholar]
- Harwood-Nash D C. Fractures of the petrous and tympanic parts of the temporal bone in children: a tomographic study of 35 cases. AJR. 1970;110(3):598–607. doi: 10.2214/ajr.110.3.598. [DOI] [PubMed] [Google Scholar]
- Rathore M H. Do prophylactic antibiotics prevent meningitis after basilar skull fracture? Pediatr Infect Dis J. 1991;10(2):87–88. doi: 10.1097/00006454-199102000-00001. [DOI] [PubMed] [Google Scholar]
- Liu-Shindo M, Hawkins D B. Basilar skull fractures in children. Int J Pediatr Otorhinolaryngol. 1989;17(2):109–117. doi: 10.1016/0165-5876(89)90086-4. [DOI] [PubMed] [Google Scholar]