Abstract
Regulation of endocytic transport is controlled by an elaborate network of proteins. Rab GTP-binding proteins and their effectors have well-defined roles in mediating specific endocytic transport steps, but until recently, less was known about the four mammalian dynamin-like C-terminal Eps15 Homology Domain (EHD) proteins that also regulate endocytic events. In recent years, however, great strides have been made in understanding the structure and function of these unique proteins. Indeed, a growing body of literature addresses EHD protein structure, interactions with binding partners, functions in mammalian cells, and the generation of various new model systems. Accordingly, this is now an opportune time to pause and review the function and mechanisms of action of EHD proteins, and to highlight some of the challenges and future directions for the field.
Introduction
Internalisation of receptors and membranes is an essential process required by all mammalian cells, and is vital for multiple cellular processes including nutrient uptake, regulation of surface receptors, adhesion molecules and ion channels, and synaptic vesicle retrieval in neurons [1]. While some internalised receptors are fated for degradation, a subset of receptors is returned to the plasma membrane where these proteins can partake in additional rounds of internalisation. This process, known as endocytic recycling, occurs as receptors are sorted at the early endosome (EE) and transported either directly to the plasma membrane (fast recycling), or through a transitory organelle (slow recycling) known as the endocytic recycling compartment (ERC) (for review, see [2, 3]). Understanding the molecular regulation of these pathways and elucidating the proteins involved has been a challenging process.
Since the early 1990s, the Rab family of small GTP-binding proteins has been characterised as a key group of endocytic regulatory proteins [4, 5]. Over sixty Rabs have been identified, and many of them participate in the regulation of endocytic transport steps. Rabs generally function by cycling from a GDP-bound inactive state in the cytoplasm to a GTP-bound active state on the membrane of a specific organelle. In their GTP-bound state, Rab proteins have a higher affinity for their interaction partners, known as effectors. Interactions between Rabs and their effectors have been implicated in the specificity of SNARE-based fusion between vesicles and target organelles, and in promoting vesicular transport, fission and fusion [6]. Almost a decade after the Rab proteins were discovered, another family of endocytic regulatory proteins was identified. Known as the C-terminal Eps15 Homology Domain (EHD) proteins, all four mammalian family members have been implicated in the regulation of specific endocytic transport steps (reviewed in [7, 8]; see Figure 1). EHDs have been linked to a number of Rab proteins through their association with mutual effectors [9–11; see Table 2], suggesting a coordinate role in endocytic regulation, and highlighting the significance of EHD proteins in these processes (see Figure 2).
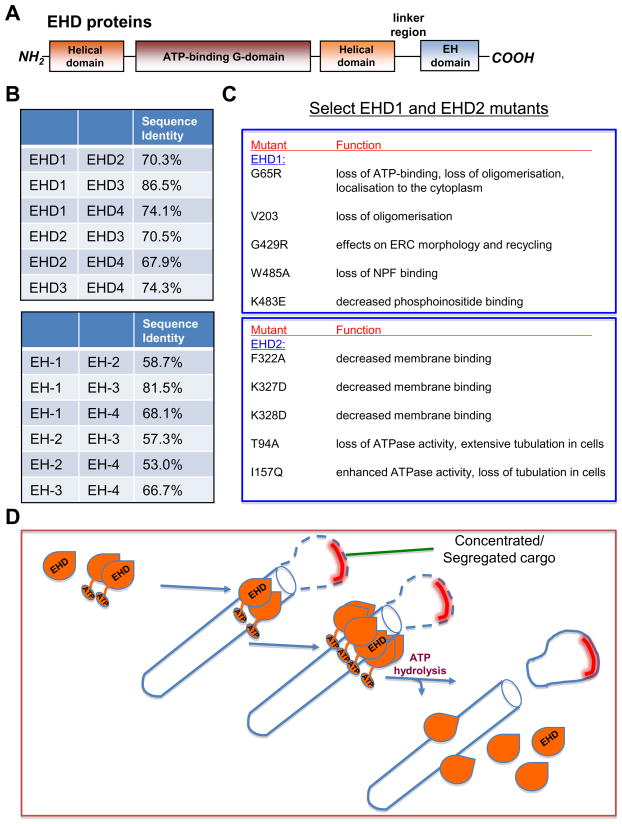
Figure 1.
Domain architecture, conservation and function of C-terminal Eps15 Homology Domain (EHD) proteins. (A) The EHD proteins, comprised of 534–543 amino acids, each contain two helical regions, a conserved ATP-binding domain, a linker region and an EH domain localized to the N-terminus of the protein. (B) Comparison of the amino acid sequence identity of full-length EHD proteins and their individual EH-domains. (C) Partial list of characterized EHD1 and EHD2 mutants and their functions or phenotypes. (D) Proposed model of EHD protein function. Cytoplasmic localized EHD proteins bind ATP and dimerise. EHD dimerisation causes the formation of a membrane binding site and the EHD proteins associate with tubular membranes, where they undergo further oligomerisation. Upon ATP hydrolysis, the membranes are destabilised, leading to scission of vesicles containing concentrated cargo/receptors, thus facilitating vesicular transport.
Table 2.
Interactions between EHD proteins and binding partners.
| EHD protein | Interaction partner | Rab binding by interaction partner? | Mode of EHD interaction | Assay(s) utilized | Reference |
|---|---|---|---|---|---|
| Rme-1 (C. elegans) | Reticulon-C protein | Not demonstrated | C-terminal region | Y2H | [88] |
| Rme-1 (C. elegans) | Alix/ALX-1 | Not demonstrated | 1) Rme-1 EH domain binds ALX-1 NPF motif 2) Rme-1 YPSL motif binds ALX-1 V-domain |
Y2H, PD | [89] |
| EHD1 (D. melanogaster) | Numb | Not demonstrated | EH-domain + NPF | PD, IP | [46] |
| EHD1 | Insulin-like growth factor 1 receptor | Not demonstrated | Not characterized | PD | [27] |
| EHD1 | SNAP29/GS32 | Not demonstrated | EH-domain + NPF | PD | [90] |
| EHD1 | AP-2 α-adaptin | Not demonstrated | Not characterized | PD | [27] |
| EHD1 | Clathrin heavy chain | Not demonstrated | Not characterized | PD | [27] |
| EHD1 | Rabenosyn-5 | Yes | EH-domain + NPF | GST- PD, Y2H | [9] |
| EHD1 | Syndapin I | Not demonstrated | EH-domain + NPF | Y2H, PD, IP | [57, 90] |
| EHD1 | Syndapin II | Not demonstrated | EH-domain + NPF | PD, IP | [57] |
| EHD1 | Rab11-FIP2 | Yes | EH-domain + NPF | Y2H, IP | [10] |
| EHD1 | Vps35/Vps26/ Vps29 complex | Not demonstrated | Not via EH- domain | IP | [28] |
| EHD1 | MICAL-L1 | Yes | EH-domain + NPF | Y2H, PD, IP | [11] |
| EHD1 | KCa2.3 | Not demonstrated | Not characterized | IP | [21] |
| EHD1 | Snapin | Not demonstrated | EH-domain | Y2H, PD, IP | [95] |
| EHD2 | GLUT4 | Not demonstrated | Not characterized | IP | [91] |
| EHD2 | AP-1 μ1 | Not demonstated | Not characterized | PD | [91] |
| EHD2 | AP-2 μ2 | Not demonstrated | Not characterized | PD | [91] |
| EHD2 | CALM | Not demonstrated | Not characterized | PD | [91] |
| EHD2 | EHBP1 | Not demonstrated | EH-domain + NPF | PD | [37] |
| EHD2 | Rabenosyn-5 | Yes | EH-domain + NPF | Y2H | [9] |
| EHD2 | Myoferlin | Not demonstrated | EH-domain + NPF | IP | [39] |
| EHD2 | Prohibitin | Not demonstrated | Not characterized | IP | [92] |
| EHD3 | Syndapin I and II | Not demonstrated | EH-domain + NPF | Y2H, PD, IP | [57] |
| EHD3 | Rabenosyn-5 | Yes | EH-domain + NPF | Y2H | [9] |
| EHD3 | Rab11-FIP2 | Yes | EH-domain + NPF | Y2H, IP | [10] |
| EHD3 | ankyrin-B | Not demonstrated | Coiled-coil region | PD | [41] |
| EHD4 | Numb | Not demonstrated | EH-domain + NPF | PD, IP | [46] |
| EHD4 | Syndapin I and II | Not demonstrated | EH-domain + NPF | Y2H, PD, IP | [57] |
| EHD4 | Type VI collagen | Not demonstrated | Not characterized | Y2H | [93] |
| EHD4 | Cadherin 23 | Not demonstrated | EH-domain (not via NPF) | Y2H, IP | [94] |
IP= immunoprecipitation; Y2H= yeast two-hybrid; PD= pulldown experiment
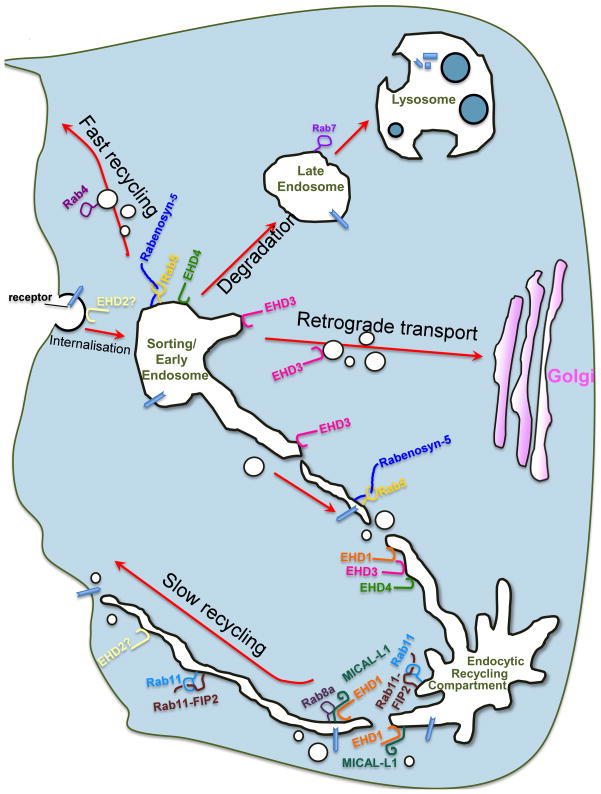
Figure 2.
Regulation of endocytic transport by EHD proteins, Rabs and their effectors. Internalised receptors reach the sorting or early endosome (EE) and are trafficked through one of at least four pathways. Receptors slated for degradation are sorted to EE microdomains containing EHD4, Rab5 and Rabenosyn-5 and transported to late endosomes and lysosomes. Other internalised proteins, such as the Shiga toxin, are transported from EE to the Golgi via an EHD3 and/or EHD1-dependent retrograde pathway. Receptors that recycle back to the plasma membrane can do so directly from EE in a poorly defined manner that requires the function of Rab4 (Fast recycling). Alternatively, many receptors are first directed to the perinuclear endocytic recycling compartment and then shuttled to the plasma membrane (Slow recycling). Slow recycling requires the sequential function of multiple regulators including Rab5, Rab11, Rab8a their effectors (Rabenosyn-5, Rab11-FIP2 and MICAL-L1, respectively), and EHD proteins.
In recent years there has been intense interest in EHD proteins and an exponential number of EHD papers have been added to the literature. Advances have come from multiple directions; for example, EHD proteins have now been studied in a variety of new model systems that include mice, fruit flies, and plants in addition to worms (Box 1). EHD proteins also appear to regulate receptors in a broad range of human organs and tissues, further bolstering their physiological significance. In parallel, great strides have been made in furthering our mechanistic understanding of EHDs at the molecular and atomic levels, spurring the elucidation of new binding partners and shedding light on the mode by which EHD proteins interact with them. It is therefore timely to review these advances and to identify the challenges that lie ahead. In this review, we shall summarize the roles of the four EHD proteins, and tabulate their known interaction partners. While the review will focus primarily on mammalian EHDs, we shall also provide information on the known functions of EHD proteins in model systems (Box 1). Another focus of this review is to assess the significant amount of new structural information that has arisen recently, and to analyse the implications of these findings for interactions between EHD proteins and their interaction partners. Overall, this summary of EHD proteins and their functions will provide the reader with a clearer window into the world of EHD proteins.
Box 1. Model systems for the study of EHD proteins.
Although EHD proteins were initially studied in mammalian cells [60–62], model systems have provided important insight towards understanding the biological functions of these proteins. In parallel with the initial functional studies in mammalian cells [12], the single Caenorhabditis elegans orthologue of human EHD1, known as Rme-1, was demonstrated to control the recycling of internalized receptors from the endocytic recycling compartment to the plasma membrane [63], entirely consistent with subsequent in vitro studies. In Drosophila melanogaster, another invertebrate sytem in which there is also a single EHD orthologue (known as Past1), deletion of the gene leads to the premature death of adult flies and their infertility [64]. In addition, Garland cells derived from flies lacking Past1 display aberrant endocytosis [64]. The original study describing EHD1 knock-down in mice did not discern any phenotype, suggesting a high level of functional redundancy between the EHD paralogues [30]; however mouse embryonic fibroblasts derived from these animals did display delayed receptor recycling [14, 30] and decreased levels of cellular cholesterol [25]. More recently, an additional EHD1 mouse model demonstrated that EHD1 expression is required for normal spermatogenesis and fertility of male mice [32], consistent with the fly phenotype. It is noteworthy that loss of the EHD1 paralogue and oligomerisation partner, EHD4, is not necessary for male mouse fertility, but is needed to allow testis development to normal size [31]. Plants such as Arabidopsis thaliana also have homologues of EHD proteins (AtEHD1 and At EHD2), [65], and studies point to a role for these proteins in regulating endocytosis and signaling [66, 67].
EHD proteins and the regulation of endocytic transport in mammalian cells
While researchers are only at an early stage in understanding the relationship between EHD proteins and human disease (Table 1), marked advances have been made in understanding the functions of these four proteins at the cellular level.
Table 1.
Relationship of EHD proteins to human disease.
| EHD Protein | Disease or condition | Aberrancy phenotype | transcript or protein | Fold increase or decrease | Reference |
|---|---|---|---|---|---|
| EHD1 | Pseudoexfoliation glaucoma | enhanced levels of serum antibodies | N/A | N/A | [73] |
| EHD1 | Apergillus fumigatus infection | enhanced EHD1 expression in monocytes | transcript | 1.5–2 fold increase | [74] |
| EHD1 | Metastatic ability of well-differentiated pancreatic endocrine neoplasms | decreased expression | transcript | 3.1 fold decrease | [75] |
| EHD1 | Sickle cell disease | decreased expression | protein | not given | [76] |
| EHD1 | Aeromonas hydrophila cytotoxic enterotoxin induced-genes | increased expression in macrophages | transcript | 3–4 fold increase | [77] |
| EHD1 | Plasmodium falciparum infected erythrocytes | increased expression in erythrocytes | transcript | 4.9 fold increase | [78] |
| EHD1 | Prostate cancer | secreted from exosomes of prostate cancer cells | protein | N/A | [79] |
| EHD1 | Cutaneous T cell lymphoma | increased expression in lesion | transcript | 1.5 fold increase | [80] |
| EHD1–4 | Glioma | gene loss proposed | N/A | N/A | [81] |
| EHD2 | Diabetes mellitus-associated bladder dysfunction | decreased EHD2 expression | protein | 1.5 fold decrease | [82] |
| EHD2 | Primary pigmented nodular adrenocortical disease | increased EHD2 expression | transcript | 0.19% expression compared to 0% in control | [83] |
| EHD3 | Oral squamous cell carcinoma | decreased EHD3 expression | transcript | 11.9 fold decrease | [84] |
| EHD3 | Small-cell lung cancer | increased EHD3 expression | transcript | abundant increase (value not given) | [85] |
| EHD3 | Acute myeloid leukemias | gene methylation | N/A | N/A | [86] |
| EHD4 | Systemic onset juvenile idiopathic arthritis | increased EHD4 expression | transcript | 0.8 fold increase | [87] |
EHD1
Perhaps because it generally exhibits the highest level of sequence homology to the single EHD orthologue expressed in invertebrate organisms, EHD1 is the best characterized of the four mammalian EHD proteins. Initial functional studies utilizing a dominant-negative EHD1 in Chinese Hamster Ovary cells elucidated a role for EHD1 in regulating the recycling of the transferrin receptor (TfR) from the endocytic recycling compartment to the plasma membrane [12]. Subsequently, the role of EHD1 was broadened to include regulating the recycling of receptors that, unlike TfR, are internalized through clathrin-independent mechanisms, such as major histocompatibility complex (MHC) class I proteins [13], and β1 integrins [14]. Since these studies, it has been reported that EHD1 regulates the recycling of a wide array of receptors, including the cystic fibrosis transmembrane conductance regulator [15], the insulin-regulated GLUT4 glucose transporter [16], AMPA type glutamate receptors [17], MHC class II molecules [18], the hyperpolarization-activated cyclic nucleotide-gated (HCN) ion channel family members HCN1, HCN2 and HCN4 [19], G protein-activated inwardly rectifying potassium channels [20], and the calcium-activated potassium channel KCa2.3 [21]. The interaction of EHD1 with Rab11-FIP2 [10], and its localization to peripheral endosomes and functional relationship with Rab35 and connecdenn [22, 23] suggest that EHD1 also plays a role in the transport of receptors from EE to the ERC. Indeed, a new study has established that EHD1 is linked to dynein motors that drive EE to ERC transport via a complex including MICAL-L1 and the collapsin response mediator protein-2 (Crmp2) [24].
It is noteworthy that in addition to its well documented role in the control of receptor recycling, EHD1 also regulates the internalisation of the low density lipoprotein (LDL) receptor [25] and the L1/neuron glia cell adhesion molecule (NgCAM) [26], and has been proposed as a modulator of insulin-like growth factor 1 receptor signaling [27]. Moreover, EHD1 also interacts with members of the Vps35-Vps26-Vps29 complex involved in retrograde transport from endosomes to the Golgi, and stabilizes tubules containing sorting nexin 1 [28], a role consistent with that described for its closest paralogue, EHD3 [29]. Thus while EHD1 acts as a gatekeeper to promote ERC-to-plasma membrane recycling of multiple receptors, it clearly has evolved to carry out additional endocytic functions for select receptors and in specialized cells such as neurons.
EHD2
Although the absence of EHD1 and EHD4 in mouse knock-down models leads to relatively minor phenotypes in vivo [30–32], suggesting compensation by the other EHD paralogs, EHD2 displays only 70% sequence identity with EHD1 (compared to 86% and 74% by EHD3 and EHD4, respectively; see Fig. 1B). EHD2 also appears to be unique among the EHDs; while there is strong evidence for hetero-oligomerisation by other EHDs [33–35], EHD2 forms homo-oligomers [36]. Although EHD2 is the first and only EHD protein whose complete structure has been solved [36], and despite evidence clearly implicating EHD2 in the regulation of endocytic transport, there is not yet a consensus on its precise function.
For example, EHD2 interacts with EH domain binding protein 1 (EHBP1) [37], a binding partner that contains a cytoskeleton-interacting Calponin Homology (CH) domain, as well as five asparagine-proline-phenylalanine (NPF) motifs that typically interact with EH domains (Box 2). Functionally, both EHBP1 and EHD2 have been linked to internalisation of receptors such as transferrin and GLUT4, potentially by linking clathrin-dependent endocytosis to filamentous actin via the EHBP1 CH domain [37]. On the other hand, a subsequent study provides evidence that EHD2 may function redundantly with EHD1, because its depletion leads to delayed transferrin recycling from the ERC [38]. Moreover, in myoblasts derived from mice lacking the EHD2 binding partner, myoferlin, a delay in transferrin recycling was also observed [39]. In addition, EHD2 itself has been implicated in myoblast fusion [39]. It is possible that EHD2 might display partial functional redundancy with EHD1 in some tissues, but in others, such as adipocytes, EHD2 may play a more central role in receptor internalisation.
BOX 2. The EH domain.
Initially, studies identified three homologous ~100 residue regions at the N-terminus of the epidermal growth factor receptor tyrosine kinase substrate Eps15 [68, 69]. Since then, numerous proteins have been identified that contain EH domains in all eukaryotic cells (reviewed in [70]). Multiple EH domain solution structures have been solved (reviewed in [53]), revealing a high level of structural homology with each domain containing two calcium-binding helix-loop-helix motifs (EF-hands) joined by a short antiparallel β-sheet. A breakthrough in understanding the function of EH domains came from studies showing that these domains are specialized protein interaction modules that bind tightly to the tripeptide asparagine-proline-phenylalanine (NPF) [47, 48]. Nuclear magnetic resonance (NMR) studies demonstrated that the NPF residues, in the conformation of a type I βturn, access a conserved hydrophobic pocket between the αB and αC helices within the EH domain, allowing close contact between the asparagine and a conserved tryptophan in the pocket [49, 50]. More recently, an additional mode of NPF binding has been described for the second EH domain of Eps15, utilizing another region containing a hydrophobic pocket [71]. EH domains are also capable of direct interactions with phosphorylated phosphoinositides [72].
EHD3
EHD3 shares the highest level of sequence identity with EHD1, but unlike EHD1 and EHD2 it appears to be more variable in its expression, with high levels of protein expressed in brain, liver and kidney [40]. A recent study indicates that EHD3 and the other EHDs are also expressed in mouse myocytes, and demonstrates that EHD3 depletion affects the expression and function of the sodium/calcium exchanger through its interaction with ankyrin-B [41].
Based on yeast two hybrid analysis and immunoprecipitations of over-expressed EHDs, it was suggested that EHD3 interacts with EHD1 [10, 33, 38, 40]. Indeed, EHD3 may cooperate with EHD1 and Rab8a to interact with Myosin Vb motors [42]. However, unlike EHD1, when the latter is expressed EHD3 does not appear to play a primary role in regulating exit from the ERC to the plasma membrane. It is of interest that whereas endogenous EHD3 is expressed at low levels in many tissues as well as HeLa cells, its depletion nevertheless results in impaired transport, with receptor cargo accumulating in peripheral EE and failing to reach the perinuclear ERC region [10]. Consistent with these findings, a recent study demonstrated involvement of EHD3 in EE-to-Golgi retrograde transport aberrant Golgi morphology, function, and delayed biosynthetic transport of lysosomal enzymes [29]. On the other hand, the modest phenotypes described for EHD1 knock-down mice [30, 31] suggest that with 86% identity to EHD1, EHD3 may be highly functionally redundant with EHD1 in most tissues. Ultimately, the generation of double knock-out mice will be key to determining the degree of EHD1/EHD3 functional redundancy.
EHD4
Although both EHD3 and EHD4 are expressed in the brain, EHD4 exhibits a more consistent pattern of tissue expression [40]. EHD4 localizes to a subset of EE and has been implicated in the regulation of receptor transport from EE to the ERC, as well as from EE to the lysosomal degradation pathway [34, 38]. EHD4 depletion leads to the enlargement of EE, likely resulting from activation of Rab5, recruitment of phosphoinositide-3-kinase and generation of phosphatidylinositol-3-phosphate [34, 38].
Although EHD4 hetero-oligomerises with EHD1 [34, 38], experiments in cell lines suggest a function for EHD4 at the EE, upstream of EHD1. Despite exhibiting only modest phenotypes (likely resulting from functional overlap), EHD1 and EHD4 knockout animals display some striking similarities, with each knock-out animal exhibiting defects in either prepubertal testis size (Ehd4; [31]) or in spermatogenesis and male fertility (Ehd1; [32]).
In neuronal cells EHD4 (also known as "pincher") functions in the internalisation of the TrkA and TrkB nerve growth factor receptors [43, 44], and an active fragment of Nogo-A, a highly potent inhibitor of axonal growth [45]. By oligomerising with EHD1, EHD4 also cooperates in the control of NgCAM endocytosis [26]. EHD4 also interacts with the adaptor protein, NUMB, which is responsible for cell fate specification of Drosophila cells in the nervous system [46]. Studies thus far suggest a tissue specific role for EHD4; however as no severe neurological phenotypes have been reported in Ehd4 knock-out mice, there is likely a high level of functional redundancy with other EHDs in living organisms.
One of the best ways to elucidate EHD function is to identify interaction partners and determine how the binding pair cooperates in the regulation of endocytic transport. As noted, the tripeptide asparagine-proline-phenylalanine (NPF) is a well-characterized motif for interacting with EH domains [47–50] (Box 2). In the C. elegans proteome alone there are 839 predicted proteins that contain at least a single NPF motif [51], and mammalian cells appear to have even greater diversity. This highlights the importance of understanding the mechanism by which EHDs selectively interact with a distinct subset of NPF-containing proteins to regulate endocytic trafficking.
Choices, choices: Selectivity of EHD proteins for specific NPF-containing partners
The four C-terminal EHD proteins have evolutionarily divergent EH domain sequences from the many EH domain-containing proteins (previously reviewed in [7, 8]), and the vast majority of EHD interactions occur via EH domain-NPF motif binding (Table 2). This section will review what determines the selectivity of EHD proteins for specific NPF-containing interaction partners.
Mechanism of EHD1-EH domain structure in selectivity
A clue to the mechanism of EHD selectivity for NPF motifs came from the recent NMR solution structure of the EHD1 EH domain. While the three dimensional structure of this domain showed a high level of similarity to previously solved N-terminal EH domains [52, 53], a striking difference between the EHD EH domains and other EH domains is the presence of a highly positively charged surface [52]. N-terminal EH domains, such as those of Eps15 and intersectin, generally have a negatively charged surface area and elegant studies have demonstrated that the first and third EH domains of the Eps15 related (Eps15R) protein prefer binding to NPF motifs that are immediately followed by a positively charged arginine [47].
Owing to the positively charged surface of EHD EH domains observed in all orthologues examined, it seems unlikely that this family of EH domains would prefer binding to an NPF motif followed by a positively charged residue. Indeed, the vast majority of EHD interaction partners contain NPF residues followed by at least two acidic residues, thus conforming to the consensus: N-P-F-[D,E]-[D,E] [54]. These findings led to the hypothesis that positively charged (and evolutionarily divergent) C-terminal EH domains selectively bind to a subset of NPF-containing proteins that contain acidic residues following the NPF tripeptide [54, 55]. Indeed, two recent studies utilizing a variety of biophysical and biochemical approaches strongly support this notion [54, 55] and the NMR solution structure of the EHD1 EH domain with the bound NPFEEEEED peptide (of MICAL-L1) provided a mechanistic explanation for these findings and demonstrated that the first two glutamate residues flanking the NPF are situated in a position favorable for the formation of salt bridges with lysine residues in the EH domain [54]. These findings shed new light on how EHD proteins selectively interact with specific NPF-containing partners that are involved in endocytic transport, and provide a means to identify potential new binding partners.
Interactions with XPF motifs
The first crystal structure of a full-length EHD protein, that of mouse EHD2, has provided much new insight toward understanding EHDs and their function, as well as eliciting some surprising new findings regarding EH domains [36]. Based on their level of sequence identity, all four EHD proteins are comprised of an N-terminal helical domain, an ATP-binding dynamin-like G-domain, a central helical domain followed by a 'linker-region' and the EH domain at the C-terminus [36] (see Figure 1). This study showed that EHD2 homo-oligomerises and identified a GPF motif localised to the linker region that is predicted to bind to the EH domain of the opposing dimeric EHD2 [36]. Although the affinity of GPF motifs for EH domains in solution is several orders of magnitude lower than that of the NPF motifs of interaction partners [56], the one-to-one stoichiometry and proximity of these GPF motifs to the EH domains in vivo may compensate for the relative low affinity of binding. Thus oligomerisation might regulate EHD interactions with NPF-containing proteins. In addition, it has been proposed that ATP hydrolysis of oligomeric EHD structures leads to membrane scission, promoting the transport/recycling of endocytic vesicles [36] (see Figure 1D).
Around the bend: EHD proteins as dynamin-like ATPases and 'benders of membranes'
Based on sequence homology, EHD proteins were originally predicted to contain a nucleotide-binding site similar to those found in Ras and Dynamin-family GTPases [13], and this notion has received strong support from several studies demonstrating nucleotide binding [10, 35, 36], and culminating in the crystal structure of EHD2 in the presence of a non-hydrolysable ATP analog [36]. Because of its similarity to GTP-binding proteins, it was surprising that EHD1 and its paralogs display a much higher affinity for ATP than GTP [35]. To explain the role of EHDs in endocytic transport, a model has been proposed whereby EHD dimers utilize a highly curved interface to insert themselves into membranes resulting in the bending of the membrane towards the dimer, and the hydrolysis of ATP would induce conformational changes leading to membrane destabilization and fission of transport vesicles [36] (see Figure 1D).
EHDs and tubulation: a binder or a bender be?
Since 2002, a hallmark of EHD proteins has been their unique subcellular localization to an array of tubulo-vesicular membranes, often appearing to spiral out of the perinuclear region like spokes from a bicycle wheel [9, 13, 38, 40, 51, 57]. Despite their intriguing appearance, the physiological significance of these unusual membrane structures has remained difficult to decipher. Recently, it was shown that a non-tubular EHD1 mutant displayed an impaired ability to recycle receptors, suggesting that the tubules are required for efficient recycling [58]. Although all four EHD proteins can be observed to varying degrees on tubular membranes [10, 13, 33, 34, 36, 38, 40, 57], EHD1 and EHD4 are the only endogenous EHDs that have been observed on tubules [13, 34].
Since the identification of EHD1-decorated tubules, one of the most intriguing and difficult questions that has remained incompletely answered is whether EHD1 binds to pre-existing tubular membranes, or whether it actually tubulates membranes and/or stabilizes them. Because the other endogenous EHD paralogs display significantly less tubule localization, one possibility (awaiting experimentation) is that their rates of ATP hydrolysis are faster than that of EHD1, resulting in more rapid fission that induces membrane scission and supports vesicular transport.
EHD proteins as membrane benders
The crystal structure of mouse EHD2, which displays >70% sequence identity to EHD1, has provided key new evidence in support of EHD proteins as membrane benders [36]. In this study, it was demonstrated that, similar to dynamin superfamily GTPases, purified EHD2 tubulates liposomes in vitro, forms ring-like oligomers around lipid tubules, and induces ATP hydrolysis upon lipid binding [36]. Indeed, EHD2 has been characterized as a protein that bends membranes in a 'scaffolding mechanism' resembling that of BAR domain-containing proteins [59]. This is supported by mathematical modeling predicting that EHD2 rings are key elements of the membrane fission machinery, a process that may require the participation of additional proteins [59].
EHD proteins as membrane binders
While it has been demonstrated that EHD proteins clearly have the ability to induce tubulation of liposomes in vitro, whether they do so in cells is not certain. EHD1-decorated tubules have been characterized as being enriched in phosphatidylinositol-4-phosphate and phosphatidylinositol-(4,5)-bisphosphate. Additional markers of these structures (Rab8a and the double palmitoylated and farnesylated carboxy-terminus of H-Ras) continued to localise to these tubules in the absence of EHD1 expression [58]. Recently, a novel EHD1 interaction partner was identified, MICAL-L1, which displays nearly 100% co-localisation with EHD1 to tubular membranes and now serves as the best-known marker of EHD1 tubules [11]. However, EHD1 depletion had no discernable effect on the localisation of MICAL-L1 to these tubular membranes. While it cannot be entirely ruled out that the functional redundancy of other EHDs contributes to the localization of MICAL-L1 to tubules, taken together, these two studies suggest that in vivo the activity of EHD1 is not required for the generation and maintenance of these tubular membranes.
A recent study may help reconcile the role of EHD1 in the tubulation process. In C. elegans and in mammalian cells, the BAR domain-containing protein AMPH-1/Amphiphysin/Bin1 interacts with EHD1, suggesting a cooperative role in tubule generation [51]. In vitro, complexes of purified AMPH-1-EHD1 induced the tubulation of liposomes; however, these short membrane tubules were qualitatively distinct from those generated by either protein individually [51]. Thus, in vivo, an elaborate cooperative enterprise between BAR and EHD proteins may regulate the generation and maintenance of tubular endosomal membranes.
Concluding remarks
Over the past decade, the C-terminal EHD proteins have become known as integral regulators of endocytic trafficking. Each of the four EHD proteins regulates selected transport steps, and the EHDs coordinate their activities with members of the Rab family of GTP-binding proteins through common interactions with Rab effectors. Now that these relationships have been defined, while links between EHD proteins and motor proteins have recently been described [24, 42], an important next step will be to better understand how EHD proteins connect with motor proteins to regulate microtubule-based transport. Another avenue that shows great promise of advancing the current understanding of EHD function comes from the recently described mouse model systems. To date, knock-downs of protein function in cell culture and non-mammalian systems such as C. elegans have provided the most significant evidence for EHD function in endocytic pathways. It is possible that yeast manage without EHDs because they rely more heavily on actin-based transport than microtuble-based transport. On the other hand, only a single EHD orthologue has been identified in D. melanogaster and C. elegans. The complexity of mammalian organisms, and the multitude of organs and tissues, may have driven the evolution of the three EHD1 paralogs. However, thus far functional redundancy and compensation in mice hinder the ability to discern the true functions of individual EHDs in vivo. Obtaining double, triple and quadruple EHD knock-out mice will likely lead to a better understanding of the role of these proteins in mammals. In vitro, future studies will need to elucidate how each of the four EHD proteins discern between different NPF motifs. For example, EHD1 binds to MICAL-L1, whereas EHD4 does not exhibit detectable binding. One possibility is that residues upstream to the NPF might allow 'fine-tuning' of the NPF motif to individual EHDs. An additional question regarding the function of the four EHDs is what regulates their differential subcellular localisation. Since these proteins display such a high level of amino acid identity, it is likely that short, specific sequences may control EHD localisation patterns. Overall, it is clear that the EHD proteins are key conductors of endocytic trafficking, and improved knowledge of their functions both mechanistically in vitro, and in living organisms is central to advancing our understanding of the mode by which receptors and lipids are transported through the cell.
Acknowledgments
We thank Dr. Keith Johnson for critical reading of this manuscript. Work in the laboratory of SC and NN is supported by National Institutes of Health grants R01GM074876, R01GM087455, P20 RR018759 from the National Center for Research Resources, and the Nebraska Department of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Conner SD, Schmid SL. Regulated portals of entry into the cell. Nature. 2003;422:37–44. doi: 10.1038/nature01451. [DOI] [PubMed] [Google Scholar]
- 2.Maxfield FR, McGraw TE. Endocytic recycling. Nat Rev Mol Cell Biol. 2004;5:121–132. doi: 10.1038/nrm1315. [DOI] [PubMed] [Google Scholar]
- 3.Grant BD, Donaldson JG. Pathways and mechanisms of endocytic recycling. Nat Rev Mol Cell Biol. 2009;10:597–608. doi: 10.1038/nrm2755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pfeffer S, Aivazian D. Targeting Rab GTPases to distinct membrane compartments. Nat Rev Mol Cell Biol. 2004;5:886–896. doi: 10.1038/nrm1500. [DOI] [PubMed] [Google Scholar]
- 5.Grosshans BL, et al. Rabs and their effectors: achieving specificity in membrane traffic. Proc Natl Acad Sci U S A. 2006;103:11821–11827. doi: 10.1073/pnas.0601617103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Novick P, et al. Interactions between Rabs, tethers, SNAREs and their regulators in exocytosis. Biochem Soc Trans. 2006;34:683–686. doi: 10.1042/BST0340683. [DOI] [PubMed] [Google Scholar]
- 7.Naslavsky N, Caplan S. C-terminal EH-domain-containing proteins: consensus for a role in endocytic trafficking, EH? J Cell Sci. 2005;118:4093–4101. doi: 10.1242/jcs.02595. [DOI] [PubMed] [Google Scholar]
- 8.Grant BD, Caplan S. Mechanisms of EHD/RME-1 Protein Function in Endocytic Transport. Traffic. 2008;9:2043–52. doi: 10.1111/j.1600-0854.2008.00834.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Naslavsky N, et al. Rabenosyn-5 and EHD1 Interact and Sequentially Regulate Protein Recycling to the Plasma Membrane. Mol Biol Cell. 2004;15:2410–2422. doi: 10.1091/mbc.E03-10-0733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Naslavsky N, et al. Interactions between EHD Proteins and Rab11-FIP2: A Role for EHD3 in Early Endosomal Transport. Mol Biol Cell. 2006;17:163–177. doi: 10.1091/mbc.E05-05-0466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sharma M, et al. MICAL-L1 links EHD1 to tubular recycling endosomes and regulates receptor recycling. Mol Biol Cell. 2009;20:5181–5194. doi: 10.1091/mbc.E09-06-0535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lin SX, et al. Rme-1 regulates the distribution and function of the endocytic recycling compartment in mammalian cells. Nat Cell Biol. 2001;3:567–572. doi: 10.1038/35078543. [DOI] [PubMed] [Google Scholar]
- 13.Caplan S, et al. A tubular EHD1-containing compartment involved in the recycling of major histocompatibility complex class I molecules to the plasma membrane. EMBO J. 2002;21:2557–2567. doi: 10.1093/emboj/21.11.2557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jovic M, et al. EHD1 regulates beta1 integrin endosomal transport: effects on focal adhesions, cell spreading and migration. J Cell Sci. 2007;120:802–814. doi: 10.1242/jcs.03383. [DOI] [PubMed] [Google Scholar]
- 15.Picciano JA, et al. Rme-1 regulates the recycling of the cystic fibrosis transmembrane conductance regulator. Am J Physiol Cell Physiol. 2003;285:C1009–1018. doi: 10.1152/ajpcell.00140.2003. [DOI] [PubMed] [Google Scholar]
- 16.Guilherme A, et al. Role of EHD1 and EHBP1 in perinuclear sorting and insulin-regulated GLUT4 recycling in 3T3-L1 adipocytes. J Biol Chem. 2004;279:40062–40075. doi: 10.1074/jbc.M401918200. [DOI] [PubMed] [Google Scholar]
- 17.Park M, et al. Recycling endosomes supply AMPA receptors for LTP. Science. 2004;305:1972–1975. doi: 10.1126/science.1102026. [DOI] [PubMed] [Google Scholar]
- 18.Walseng E, et al. Major histocompatibility complex class II-peptide complexes internalize using a clathrin- and dynamin-independent endocytosis pathway. J Biol Chem. 2008;283:14717–14727. doi: 10.1074/jbc.M801070200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hardel N, et al. Recycling endosomes supply cardiac pacemaker channels for regulated surface expression. Cardiovasc Res. 2008;79:52–60. doi: 10.1093/cvr/cvn062. [DOI] [PubMed] [Google Scholar]
- 20.Chung HJ, et al. Neuronal activity regulates phosphorylation-dependent surface delivery of G protein-activated inwardly rectifying potassium channels. Proc Natl Acad Sci U S A. 2009;106:629–634. doi: 10.1073/pnas.0811615106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gao Y, et al. Recycling of the Ca2+-activated K+ channel, KCa2.3 is dependent upon RME-1, Rab35/EPI64C and an N-terminal domain. J Biol Chem. 2010;285:17938–17953. doi: 10.1074/jbc.M109.086553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Allaire PD, et al. The Connecdenn DENN domain: a GEF for Rab35 mediating cargo-specific exit from early endosomes. Mol Cell. 2010;37:370–382. doi: 10.1016/j.molcel.2009.12.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sato M, et al. Regulation of endocytic recycling by C. elegans Rab35 and its regulator RME-4, a coated-pit protein. EMBO J. 2008;27:1183–1196. doi: 10.1038/emboj.2008.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rahajeng J, et al. Collapsin response mediator protein-2 (Crmp2) regulates trafficking by linking endocytic regulatory proteins to dynein motors. J Biol Chem. 2010 doi: 10.1074/jbc.C110.166066. published online Aug. 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Naslavsky N, et al. EHD1 regulates cholesterol homeostasis and lipid droplet storage. Biochem Biophys Res Commun. 2007;357:792–799. doi: 10.1016/j.bbrc.2007.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yap CC, et al. Alterations of EHD1/EHD4 protein levels interfere with L1/NgCAM endocytosis in neurons and disrupt axonal targeting. J Neurosci. 2010;30:6646–6657. doi: 10.1523/JNEUROSCI.5428-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rotem-Yehudar R, et al. Association of insulin-like growth factor 1 receptor with EHD1 and SNAP29. J Biol Chem. 2001;276:33054–33060. doi: 10.1074/jbc.M009913200. [DOI] [PubMed] [Google Scholar]
- 28.Gokool S, et al. EHD1 interacts with retromer to stabilize SNX1 tubules and facilitate endosome-to-Golgi retrieval. Traffic. 2007;8:1873–1886. doi: 10.1111/j.1600-0854.2007.00652.x. [DOI] [PubMed] [Google Scholar]
- 29.Naslavsky N, et al. EHD3 regulates early-endosome-to-Golgi transport and preserves Golgi morphology. J Cell Sci. 2009;122:389–400. doi: 10.1242/jcs.037051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rapaport D, et al. Recycling to the plasma membrane is delayed in EHD1 knockout mice. Traffic. 2006;7:52–60. doi: 10.1111/j.1600-0854.2005.00359.x. [DOI] [PubMed] [Google Scholar]
- 31.George M, et al. Ehd4 is required to attain normal pre-pubertal testis size but dispensable for fertility in male mice. Genesis. 2010;48:328–342. doi: 10.1002/dvg.20620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rainey MA, et al. The endocytic recycling regulator EHD1 is essential for spermatogenesis and male fertility in mice. BMC Dev Biol. 2010;10:37. doi: 10.1186/1471-213X-10-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Galperin E, et al. EHD3: a protein that resides in recycling tubular and vesicular membrane structures and interacts with EHD1. Traffic. 2002;3:575–589. doi: 10.1034/j.1600-0854.2002.30807.x. [DOI] [PubMed] [Google Scholar]
- 34.Sharma M, et al. A role for EHD4 in the regulation of early endosomal transport. Traffic. 2008;9:995–1018. doi: 10.1111/j.1600-0854.2008.00732.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lee DW, et al. ATP Binding regulates oligomerization and endosome association of RME-1 family proteins. J Biol Chem. 2005;280:280–290. doi: 10.1074/jbc.M412751200. [DOI] [PubMed] [Google Scholar]
- 36.Daumke O, et al. Architectural and mechanistic insights into an EHD ATPase involved in membrane remodelling. Nature. 2007;449:923–927. doi: 10.1038/nature06173. [DOI] [PubMed] [Google Scholar]
- 37.Guilherme A, et al. EHD2 and the Novel EH Domain Binding Protein EHBP1 Couple Endocytosis to the Actin Cytoskeleton. J Biol Chem. 2004;279:10593–10605. doi: 10.1074/jbc.M307702200. [DOI] [PubMed] [Google Scholar]
- 38.George M, et al. Shared as well as distinct roles of EHD proteins revealed by biochemical and functional comparisons in mammalian cells and C. elegans. BMC Cell Biol. 2007;8:3. doi: 10.1186/1471-2121-8-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Doherty KR, et al. The endocytic recycling protein EHD2 interacts with myoferlin to regulate myoblast fusion. J Biol Chem. 2008 doi: 10.1074/jbc.M802306200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Blume JJ, et al. EHD proteins are associated with tubular and vesicular compartments and interact with specific phospholipids. Exp Cell Res. 2007;313:219–231. doi: 10.1016/j.yexcr.2006.10.006. [DOI] [PubMed] [Google Scholar]
- 41.Gudmundsson H, et al. EH Domain Proteins Regulate Cardiac Membrane Protein Targeting. Circ Res. 2010;107:84–95. doi: 10.1161/CIRCRESAHA.110.216713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Roland JT, et al. Myosin Vb interacts with Rab8a on a tubular network containing EHD1 and EHD3. Mol Biol Cell. 2007;18:2828–2837. doi: 10.1091/mbc.E07-02-0169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shao Y, et al. Pincher, a pinocytic chaperone for nerve growth factor/TrkA signaling endosomes. J Cell Biol. 2002;157:679–691. doi: 10.1083/jcb.200201063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Valdez G, et al. Pincher-mediated macroendocytosis underlies retrograde signaling by neurotrophin receptors. J Neurosci. 2005;25:5236–5247. doi: 10.1523/JNEUROSCI.5104-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Joset A, et al. Pincher-generated Nogo-A endosomes mediate growth cone collapse and retrograde signaling. J Cell Biol. 2010;188:271–285. doi: 10.1083/jcb.200906089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Smith CA, et al. The Cell Fate Determinant Numb Interacts with EHD/Rme-1 Family Proteins and Has a Role in Endocytic Recycling. Mol Biol Cell. 2004;15:3698–3708. doi: 10.1091/mbc.E04-01-0026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Paoluzi S, et al. Recognition specificity of individual EH domains of mammals and yeast. Embo J. 1998;17:6541–6550. doi: 10.1093/emboj/17.22.6541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Salcini AE, et al. Binding specificity and in vivo targets of the EH domain, a novel protein-protein interaction module. Genes Dev. 1997;11:2239–2249. doi: 10.1101/gad.11.17.2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.de Beer T, et al. Structure and Asn-Pro-Phe binding pocket of the Eps15 homology domain. Science. 1998;281:1357–1360. doi: 10.1126/science.281.5381.1357. [DOI] [PubMed] [Google Scholar]
- 50.de Beer T, et al. Molecular mechanism of NPF recognition by EH domains. Nat Struct Biol. 2000;7:1018–1022. doi: 10.1038/80924. [DOI] [PubMed] [Google Scholar]
- 51.Pant S, et al. AMPH-1/Amphiphysin/Bin1 functions with RME-1/Ehd1 in endocytic recycling. Nat Cell Biol. 2009;11:1399–1410. doi: 10.1038/ncb1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kieken F, et al. EH domain of EHD1. J Biomol NMR. 2007;39:323–329. doi: 10.1007/s10858-007-9196-0. [DOI] [PubMed] [Google Scholar]
- 53.Confalonieri S, Di Fiore PP. The Eps15 homology (EH) domain. FEBS Lett. 2002;513:24–29. doi: 10.1016/s0014-5793(01)03241-0. [DOI] [PubMed] [Google Scholar]
- 54.Kieken F, et al. Mechanism for the selective interaction of C-terminal Eps15 homology domain proteins with specific Asn-Pro-Phe-containing partners. J Biol Chem. 2010;285:8687–8694. doi: 10.1074/jbc.M109.045666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Henry GD, et al. Charge effects in the selection of NPF motifs by the EH domain of EHD1. Biochemistry. 2010;49:3381–3392. doi: 10.1021/bi100065r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kieken F, et al. Structural insight into the interaction of proteins containing NPF, DPF, and GPF motifs with the C-terminal EH-domain of EHD1. Protein Sci. 2009;18:2471–2479. doi: 10.1002/pro.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Braun A, et al. EHD proteins associate with syndapin I and II and such interactions play a crucial role in endosomal recycling. Mol Biol Cell. 2005;16:3642–3658. doi: 10.1091/mbc.E05-01-0076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Jovic M, et al. Eps15 homology domain 1-associated tubules contain phosphatidylinositol-4-phosphate and phosphatidylinositol-(4,5)-bisphosphate and are required for efficient recycling. Mol Biol Cell. 2009;20:2731–2743. doi: 10.1091/mbc.E08-11-1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Campelo F, et al. Modeling membrane shaping by proteins: focus on EHD2 and N-BAR domains. FEBS Lett. 2010;584:1830–1839. doi: 10.1016/j.febslet.2009.10.023. [DOI] [PubMed] [Google Scholar]
- 60.Haider NB, et al. Evaluation and molecular characterization of EHD1, a candidate gene for Bardet-Biedl syndrome 1 (BBS1) Gene. 1999;240:227–232. doi: 10.1016/s0378-1119(99)00395-9. [DOI] [PubMed] [Google Scholar]
- 61.Mintz L, et al. EHD1--an EH-domain-containing protein with a specific expression pattern. Genomics. 1999;59:66–76. doi: 10.1006/geno.1999.5800. [DOI] [PubMed] [Google Scholar]
- 62.Pohl U, et al. EHD2, EHD3, and EHD4 encode novel members of a highly conserved family of EH domain-containing proteins. Genomics. 2000;63:255–262. doi: 10.1006/geno.1999.6087. [DOI] [PubMed] [Google Scholar]
- 63.Grant B, et al. Evidence that RME-1, a conserved C. elegans EH-domain protein, functions in endocytic recycling. Nat Cell Biol. 2001;3:573–579. doi: 10.1038/35078549. [DOI] [PubMed] [Google Scholar]
- 64.Olswang-Kutz Y, et al. Drosophila Past1 is involved in endocytosis and is required for germline development and survival of the adult fly. J Cell Sci. 2009;122:471–480. doi: 10.1242/jcs.038521. [DOI] [PubMed] [Google Scholar]
- 65.Bar M, et al. AtEHDs, novel Arabidopsis EH-domain-containing proteins involved in endocytosis. Plant J. 2008;55:1025–1038. doi: 10.1111/j.1365-313X.2008.03571.x. [DOI] [PubMed] [Google Scholar]
- 66.Bar M, Avni A. EHD2 inhibits ligand-induced endocytosis and signaling of the leucine-rich repeat receptor-like protein LeEix2. Plant J. 2009;59:600–611. doi: 10.1111/j.1365-313X.2009.03897.x. [DOI] [PubMed] [Google Scholar]
- 67.Bar M, et al. The coiled-coil domain of EHD2 mediates inhibition of LeEix2 endocytosis and signaling. PLoS One. 2009;4:e7973. doi: 10.1371/journal.pone.0007973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Fazioli F, et al. eps15, a novel tyrosine kinase substrate, exhibits transforming activity. Mol Cell Biol. 1993;13:5814–5828. doi: 10.1128/mcb.13.9.5814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wong WT, et al. A protein-binding domain, EH, identified in the receptor tyrosine kinase substrate Eps15 and conserved in evolution. Proc Natl Acad Sci U S A. 1995;92:9530–9534. doi: 10.1073/pnas.92.21.9530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Miliaras NB, Wendland B. EH Proteins: Multivalent Regulators of Endocytosis (and Other Pathways) Cell Biochem Biophys. 2004;41:295–318. doi: 10.1385/CBB:41:2:295. [DOI] [PubMed] [Google Scholar]
- 71.Rumpf J, et al. Structure of the Eps15-stonin2 complex provides a molecular explanation for EH-domain ligand specificity. EMBO J. 2008;27:558–569. doi: 10.1038/sj.emboj.7601980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Naslavsky N, et al. EHD1 and Eps15 Interact with Phosphatidylinositols via Their Eps15 Homology Domains. J Biol Chem. 2007;282:16612–16622. doi: 10.1074/jbc.M609493200. [DOI] [PubMed] [Google Scholar]
- 73.Dervan EW, et al. Protein macroarray profiling of serum autoantibodies in pseudoexfoliation glaucoma. Invest Ophthalmol Vis Sci. 2010;51:2968–2975. doi: 10.1167/iovs.09-4898. [DOI] [PubMed] [Google Scholar]
- 74.Cortez KJ, et al. Functional genomics of innate host defense molecules in normal human monocytes in response to Aspergillus fumigatus. Infect Immun. 2006;74:2353–2365. doi: 10.1128/IAI.74.4.2353-2365.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hansel DE, et al. Met proto-oncogene and insulin-like growth factor binding protein 3 overexpression correlates with metastatic ability in well-differentiated pancreatic endocrine neoplasms. Clin Cancer Res. 2004;10:6152–6158. doi: 10.1158/1078-0432.CCR-04-0285. [DOI] [PubMed] [Google Scholar]
- 76.Ammann LP, Goodman SR. Cluster analysis for the impact of sickle cell disease on the human erythrocyte protein interactome. Exp Biol Med (Maywood) 2009;234:703–711. doi: 10.3181/0806-RM-211. [DOI] [PubMed] [Google Scholar]
- 77.Galindo CL, et al. Identification of Aeromonas hydrophila cytotoxic enterotoxin-induced genes in macrophages using microarrays. J Biol Chem. 2003;278:40198–40212. doi: 10.1074/jbc.M305788200. [DOI] [PubMed] [Google Scholar]
- 78.Tripathi AK, et al. Plasmodium falciparum-infected erythrocytes induce NF-kappaB regulated inflammatory pathways in human cerebral endothelium. Blood. 2009;114:4243–4252. doi: 10.1182/blood-2009-06-226415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Jansen FH, et al. Exosomal secretion of cytoplasmic prostate cancer xenograft-derived proteins. Mol Cell Proteomics. 2009;8:1192–1205. doi: 10.1074/mcp.M800443-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Shin J, et al. Lesional gene expression profiling in cutaneous T-cell lymphoma reveals natural clusters associated with disease outcome. Blood. 2007;110:3015–3027. doi: 10.1182/blood-2006-12-061507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Maher EA, et al. Malignant glioma: genetics and biology of a grave matter. Genes Dev. 2001;15:1311–1333. doi: 10.1101/gad.891601. [DOI] [PubMed] [Google Scholar]
- 82.Yohannes E, et al. Proteomics analysis identifies molecular targets related to diabetes mellitus-associated bladder dysfunction. Mol Cell Proteomics. 2008;7:1270–1285. doi: 10.1074/mcp.M700563-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Horvath A, et al. Serial analysis of gene expression in adrenocortical hyperplasia caused by a germline PRKAR1A mutation. J Clin Endocrinol Metab. 2006;91:584–596. doi: 10.1210/jc.2005-1301. [DOI] [PubMed] [Google Scholar]
- 84.Chen C, et al. Gene expression profiling identifies genes predictive of oral squamous cell carcinoma. Cancer Epidemiol Biomarkers Prev. 2008;17:2152–2162. doi: 10.1158/1055-9965.EPI-07-2893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Taniwaki M, et al. Gene expression profiles of small-cell lung cancers: molecular signatures of lung cancer. Int J Oncol. 2006;29:567–575. [PubMed] [Google Scholar]
- 86.Desmond JC, et al. Discovery of epigenetically silenced genes in acute myeloid leukemias. Leukemia. 2007;21:1026–1034. doi: 10.1038/sj.leu.2404611. [DOI] [PubMed] [Google Scholar]
- 87.Allantaz F, et al. Blood leukocyte microarrays to diagnose systemic onset juvenile idiopathic arthritis and follow the response to IL-1 blockade. J Exp Med. 2007;204:2131–2144. doi: 10.1084/jem.20070070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Iwahashi J, et al. Caenorhabditis elegans reticulon interacts with RME-1 during embryogenesis. Biochem Biophys Res Commun. 2002;293:698–704. doi: 10.1016/S0006-291X(02)00282-6. [DOI] [PubMed] [Google Scholar]
- 89.Shi A, et al. A novel requirement for C. elegans Alix/ALX-1 in RME-1-mediated membrane transport. Curr Biol. 2007;17:1913–1924. doi: 10.1016/j.cub.2007.10.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Xu Y, et al. Mutually exclusive interactions of EHD1 with GS32 and Syndapin II. Molecular Membrane Biology. 2004;21:269–277. doi: 10.1080/09687680410001716871. [DOI] [PubMed] [Google Scholar]
- 91.Park SY, et al. EHD2 interacts with the insulin-responsive glucose transporter (GLUT4) in rat adipocytes and may participate in insulin-induced GLUT4 recruitment. Biochemistry. 2004;43:7552–7562. doi: 10.1021/bi049970f. [DOI] [PubMed] [Google Scholar]
- 92.Ande SR, Mishra S. Palmitoylation of prohibitin at cysteine 69 facilitates its membrane translocation and interaction with Eps 15 homology domain protein 2 (EHD2) Biochem Cell Biol. 2010;88:553–558. doi: 10.1139/o09-177. [DOI] [PubMed] [Google Scholar]
- 93.Kuo HJ, et al. Characterization of EHD4, an EH domain-containing protein expressed in the extracellular matrix. J Biol Chem. 2001;276:43103–43110. doi: 10.1074/jbc.M106128200. [DOI] [PubMed] [Google Scholar]
- 94.Sengupta S, et al. EHD4 and CDH23 are interacting partners in cochlear hair cells. J Biol Chem. 2009;284:20121–20129. doi: 10.1074/jbc.M109.025668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Wei S, et al. EHD1 is a synaptic protein that modulates exocytosis through binding to snapin. Mol Cell Neurosci. 2010 doi: 10.1016/j.mcn.2010.07.014. published online Aug. 6. [DOI] [PubMed] [Google Scholar]