Abstract
Background
Sleep disorders are a substantial problem for cancer survivors, with prevalence estimates ranging from 23 to 61%. Although numerous prescription hypnotics are available, few are approved for long-term use or have demonstrated benefit in this circumstance. Hypnotics may have unwanted side effects, are costly, and cancer survivors often wish to avoid prescription drugs. New options with limited side effects are needed. The purpose of this trial was to evaluate the efficacy of a valerian officinalis supplement for sleep in people with cancer who were undergoing cancer treatment.
Methods
Participants were randomized to receive 450 mg of valerian or placebo orally 1 hour before bedtime for 8 weeks. The primary endpoint was area under the curve (AUC) of the overall Pittsburgh Sleep Quality Index. Secondary outcomes included the Functional Outcomes of Sleep Questionnaire, the Brief Fatigue Inventory and the Profile of Mood States. Toxicity was evaluated with both self reported numeric analogue scale questions and the Common Criteria Terminology Criteria (CTCAE) version 3.0. Questionnaires were completed at baseline, 4, and 8 weeks.
Results
A total of 227 patients were randomized onto this study between 3/19/2004 and 3/9/2007, with 119 being evaluable for the primary endpoint. The AUC over the 8 weeks for valerian was 51.4 (SD = 16) while for placebo it was 49.7 (SD = 15) with a p-value of 0.6957. A supplemental, exploratory analysis revealed that several fatigue endpoints, as measured by the BFI and POMS, were significantly better for those taking valerian over placebo. Participants also reported less trouble with sleep and less drowsiness on valerian than placebo. There were no significant differences in toxicities as measured by self report or the CTCAE v3 except for mild alkaline phosphatase increases, which were slightly more common in the placebo group.
Conclusions
This study failed to provide data to support the hypothesis that valerian, 450 mg, at bedtime could improve sleep as measured by the PSQI. However, exploratory analyses revealed improvement in some secondary outcomes, such as fatigue. Further research with valerian exploring physiologic effects in oncology symptom management may be warranted.
Background
Disordered sleep has been found to be common in cancer survivors, and contributes to fatigue and impaired overall functioning. The true prevalence and incidence of sleep disorders in the oncology population is not well documented, though reports range from 23%–61% 1–8. Some research suggests that sleep-wake disturbances are more prevalent in patients with cancer than in other populations 9. In addition, Miller and colleagues 6, suggest that a disturbed sleep-wake cycle may well be a main predictor and contributor of other symptoms such as fatigue, depressed mood and cognitive dysfunction. Evaluating and improving sleep may have broad ramifications for cancer survivors.
Insomnia is present when there is repeated difficulty initiating or maintaining sleep or impairment in sleep quality that occurs despite adequate time and opportunity for sleep, and there is some form of daytime impairment as a result16. Secondary insomnia is denoted when insomnia is prominent and develops in the setting of another primary medical or psychiatric illness, or in the setting of a separate sleep disorder such as sleep apnea 10,11. Sleep disturbance can be associated with poor work performance, increased anxiety and depression, poor cognitive functioning, and impairment of overall QOL 12–15. A recent Institute of Medicine report highlighted the severe costs to individual and society of untreated insomnia.17
Davidson 2 and colleagues conducted a cross-sectional descriptive study in 6 malignant disease clinics from a regional cancer center in Canada. Those surveyed included patients with breast, gastrointestinal, gynecological, genitourinary, lung and non-melanoma skin cancers. Insomnia was defined as a report of trouble sleeping on at least 7 of the previous 28 nights, interfering with daytime functioning. More patients who had treatment within the past 6 months reported insomnia, use of sleeping pills, sleeping more than usual, or fatigue. There were no differences based on type of cancer or treatment. Baker and colleagues 18 surveyed 752 adult patients who had been diagnosed with one of the 10 most commonly occurring cancers to identify which problems cancer survivors experience in dealing with their cancer and its treatment 1 year after diagnosis. Sleep difficulties ranked 5th on the list and were reported by 48% of the sample.
Fatigue is related to sleep disturbance. Although cancer-related fatigue is not necessarily relieved by sleep or rest, insomnia or sleep disturbances clearly contribute to fatigue issues. Fatigue and sleep disturbances are undoubtedly interwoven symptoms and may be difficult to separate. It is not known how much variance in fatigue is explained by sleep problems, nor in what situations sleep is a major contributor.
Pharmacological treatments for insomnia
Because sleep complaints are common, hypnotics are among the most commonly prescribed medications for cancer patients, being prescribed for insomnia in up to 44% of patients.19 Agents most commonly used are benzodiazepine receptor agonists, including true benzodiazepines such as flurazepam, triazolam, quazepam, estazolam, and temazepam, and the non-benzodiazepine agents zolpidem (Ambien®), zaleplon (Sonata®) and eszopiclone (Lunesta®), which decrease subjective time to sleep onset, improve sleep efficiency, decrease the number of awakenings, and increase total sleep duration 20–23 Eszopiclone, extended release formulations of zolpidem (Ambien CR®), and ramelteon (a melatonin receptor agonist) are approved for prolonged use in patient with chronic insomnia24, but other hypnotics lack well-established effectiveness and safety data for use beyond brief intervals in situational insomnia, or as part of a combined approach using cognitive behavioral therapy (CBT) and brief pharmacological therapy.
In general, improvements in various sleep endpoints with pharmacologic therapy have been modest, with mean differences in sleep latency being about 15 minutes, wake after sleep onset improving by about 26 minutes and total sleep time improving by about 40 minutes 22,24,25. Although subjective improvements are often noted, hypnotic medications are associated with a number of risks, including residual next-day hypersomnia, dizziness, lightheadedness, impaired mental status, and increased risk of falls and hip fractures, especially in elderly patients when taking longer-acting hypnotics26–31. Clearly, better options to improve sleep are still needed.
The use of valeriana officinalis for sleep
Valeriana officinalis is a perennial herb found in North America, Europe and Asia. In the US, it is primarily sold as a sleeping aid, while in Europe it is used for restlessness, tremors and anxiety. There are three main chemicals that are thought to be the active components of the plant. These are the essential oils, valerenic acid and valenol, valepotriates, and a few alkaloids. Herbal extracts of valeriana officinalis can be aqueous or aqueous-alcoholic extracts using 70% ethanol and herb-to-extract ratios of 4-7:1. Single recommended doses range from 400 mg to 900 mg at bedtime 32. Most sleep studies have used 400 or 450 mg for their trials, with a couple dose finding trials showing that 900 mg was not significantly better than 450 mg33,34. The main impact of valerian from those studies has been on sleep latency (time to fall asleep) and this has improved more in patients who had reported a longer time to fall asleep and who considered themselves poor sleepers 33–37.
Most reviews proclaim valeriana officinalis to be a safe herb with no drug interactions and the only adverse event being daytime sedation at higher doses.38,39 Anecdotal reports of side effects include headaches, nausea, heart palpitations and benzodiazepine-like withdrawal symptoms when stopping the agent40. Some concern has been raised as to whether valerian might interfere with cytochrome P450 metabolism. An article by Budzinski and colleagues reviews numerous herbs and quantitates their interaction with cytochrome P450 41. Out of 21 herbs tested, valeriana officinalis ranked at the bottom of interaction potential, rating a 15 out of a possible 16 (1 being the highest, 16 being the lowest).
The cost of valeriana officinalis, compared to other prescription sleep aids, is less, with a one month supply costing around $10 per month. By contrast, zolpidem, for example, costs over $80 per month.
Therefore, based on the favorable toxicity profile, low cost and promising, but limited, pilot data, this current trial was designed to evaluate 450 mg of valerian at bedtime for sleep disturbance.
Methods
The primary purpose of this trial was to assess the effect of a standardized preparation of valerian in improving sleep in patients undergoing therapy for cancer. Secondary goals were to assess the safety, as well as its effect on anxiety, fatigue and activities of daily living.
Patients eligible for this trial included adults diagnosed with cancer and receiving therapy (radiation, chemotherapy, oral anti-tumor agents, or endocrine therapy). Patients had to report difficulty sleeping of 4 or more on a scale of 0 to 10, had to have a life expectancy ≥ 6 months, and had to have an ECOG Performance Score (PS) of 0 or 1. They could not have an abnormally elevated serum glutamic-oxaloacetic transaminase (SGOT) and/or alkaline phosphatase. Patients were excluded for prior use of valerian for sleep, use of other prescription sleep aids in the past 30 days, a diagnosis of obstructive sleep apnea or primary insomnia per DSM-IV criteria. Pregnant and nursing women were also excluded as well as patients with known sleep disturbance etiologies such as nighttime hot flashes, uncontrolled pain and/or diarrhea.
Participants were randomized to receive 450 mg of oral valerian or placebo, to be taken 1 hour before bedtime for 8 weeks. The valerian used was pure ground, raw root, from one lot and was standardized to contain 0.8% valerenic acid. Valerian capsules and matching placebo, a gelatin capsule, were supplied by Hi-Health from Scottsdale, AZ. Both valerian and placebo were stored in the same containers, in order that the placebo would acquire some of the valerian smell. Self report booklets were completed at baseline, weeks 4 and 8, and contained the Pittsburgh Sleep Quality Index (PSQI)42, the Profile of Moods States (POMS)43, the Functional Outcomes of Sleep Questionnaire (FOSQ) 44 and the Brief Fatigue Inventory (BFI)45. Assessments were scored according to the appropriate algorithms and total and subscale scores were transformed to a 0 to100 scale, with 100 being best. Self-reported symptoms were recorded weekly using a self-report numeric analogue scale, called the Symptom Experience Diary (SED). Toxicity was also assessed every 2 weeks during a CRA/nurse phone call using the Common Terminology Criteria for Adverse Events (CTCAE v 3.0).
The primary endpoint was the normalized (averaged) area under the curve (AUC) of the PSQI between the two arms, compared using the Kruskall Wallis test. Secondary analyses compared AUC scores of other assessments and toxicity incidence. Toxicity comparisons were performed using the Chi-square test or the Kruskall Wallis test, as appropriate. As an intent-to-treat (ITT) analysis, using chi-square tests, patients were categorized as a success if there was a 10 point improvement in the assessment score at week 4 or 8, and a failure if there was no improvement or data were missing.
All hypothesis testing was carried out using a two-sided alternative hypothesis and a 5% Type I error rate. A two-sample t-test with 100 patients per group provided 94% power to detect 50% times the standard deviation of the endpoint under study46. This effect size is considered moderate and has been declared the minimally clinically significant difference for QOL endpoints.47,48
Results
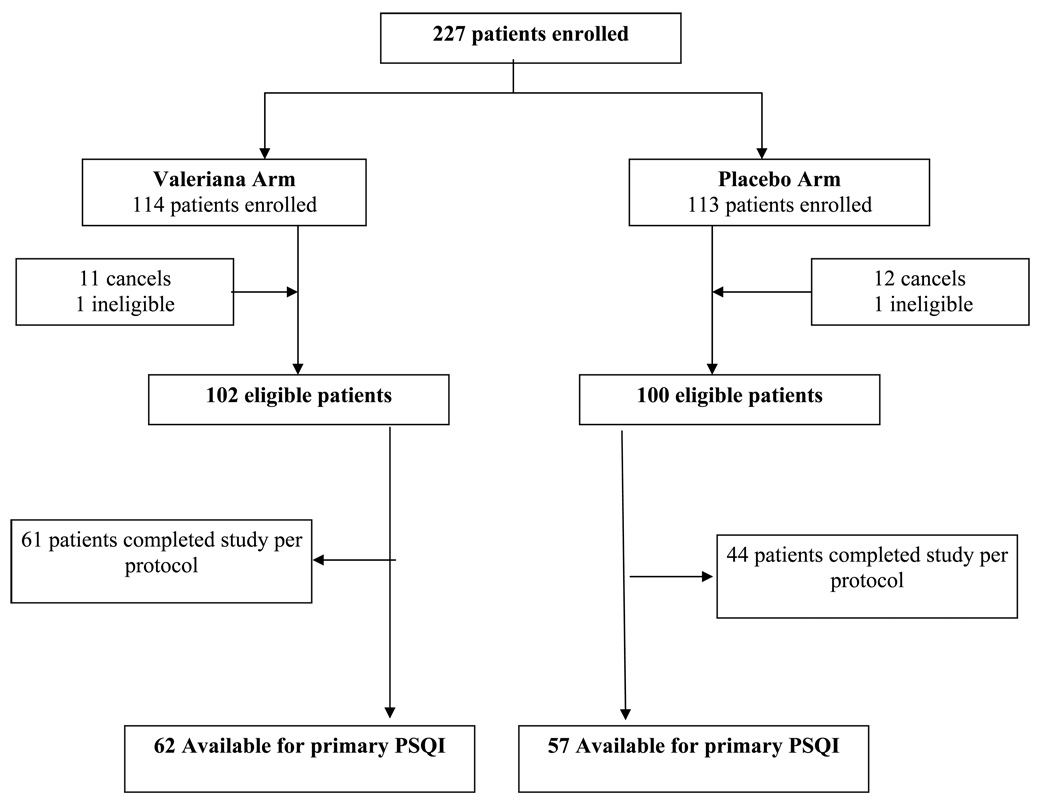
A total of 227 patients were randomized onto this study between 3/19/2004 and 3/9/2007. The consort diagram depicts the flow of data (Figure 1). Twenty-three patients withdrew before starting the study treatment. Primary endpoint data were available on 119 patients (62 receiving valerian and 57 receiving placebo). Baseline characteristics and baseline patient reported outcomes were well balanced between arms with no statistically significant differences (Tables 1 & 2).
Figure 1.
Consort Diagram
Table 1.
Demographic Characteristics
| A (N=102) |
B (N=100) |
p value | |
|---|---|---|---|
| Gender | 0.387 | ||
| Female | 82 (80%) | 85 (85%) | |
| Age | 0.546 | ||
| Mean (SD) | 59.5 (11.95) | 58.3 (12.71) | |
| Sleep Scale Group | 0.963 | ||
| Mildly Impaired Sleep Quality | 67 (66%) | 66 (66%) | |
| Mod or Sev Impaired Sleep Quality | 35 (34%) | 34 (34%) | |
| Sleep Scale Score | 0.841 | ||
| Mean (SD) | 6.6 (1.43) | 6.6 (1.69) | |
| Primary Tumor Site | 0.526 | ||
| Breast | 64 (63%) | 66 (67%) | |
| Colon | 9 (9%) | 5 (5%) | |
| Prostate | 3 (3%) | 1 (1%) | |
| Other | 25 (25%) | 27 (27%) | |
| Tumor Status | 0.322 | ||
| Resected with no residual | 64 (64%) | 71 (74%) | |
| Resected with known residual | 17 (17%) | 12 (13%) | |
| Unresected | 19 (19%) | 13 (14%) | |
| Treatment Type | 0.966 | ||
| Radiation therapy | 6 (5.9%) | 6 (6%) | |
| Parenteral chemotherapy | 38 (37%) | 39 (39%) | |
| Oral therapy | 40 (39%) | 40 (40%) | |
| Combined modality | 18 (18%) | 15 (15%) | |
| Concurrent Radiation | 0.926 | ||
| Yes | 23 (23%) | 22 (22%) | |
| Concurrent Cancer Therapy | 0.679 | ||
| Yes | 56 (55%) | 52 (53%) | |
| Planned or Concurrent Hormone | 0.667 | ||
| Yes | 51 (51%) | 53 (54%) |
Table 2.
Distribution of Baseline Assessment Scores
| Valerian (N=101) |
Placebo (N=96) |
p value | |
|---|---|---|---|
| PSQI Total1 | 0.695 | ||
| Mean (SD) | 41.3 (13.92) | 42.4 (14.97) | |
| POMS-SF total | 0.883 | ||
| Mean (SD) | 65.0 (14.28) | 63.9 (16.46) | |
| FOSQ Total Score | 0.927 | ||
| Mean (SD) | 73.7 (16.07) | 72.8 (18.37) | |
| Fatigue NOW | 0.285 | ||
| Mean (SD) | 45.7 (24.41) | 49.4 (25.00) | |
| USUAL Fatigue | 0.216 | ||
| Mean (SD) | 46.8 (23.27) | 51.1 (24.73) | |
| WORST Fatigue | 0.522 | ||
| Mean (SD) | 35.2 (24.67) | 37.9 (26.37) | |
| Total Interference | 0.268 | ||
| Mean (SD) | 61.4 (25.05) | 57.1 (27.37) |
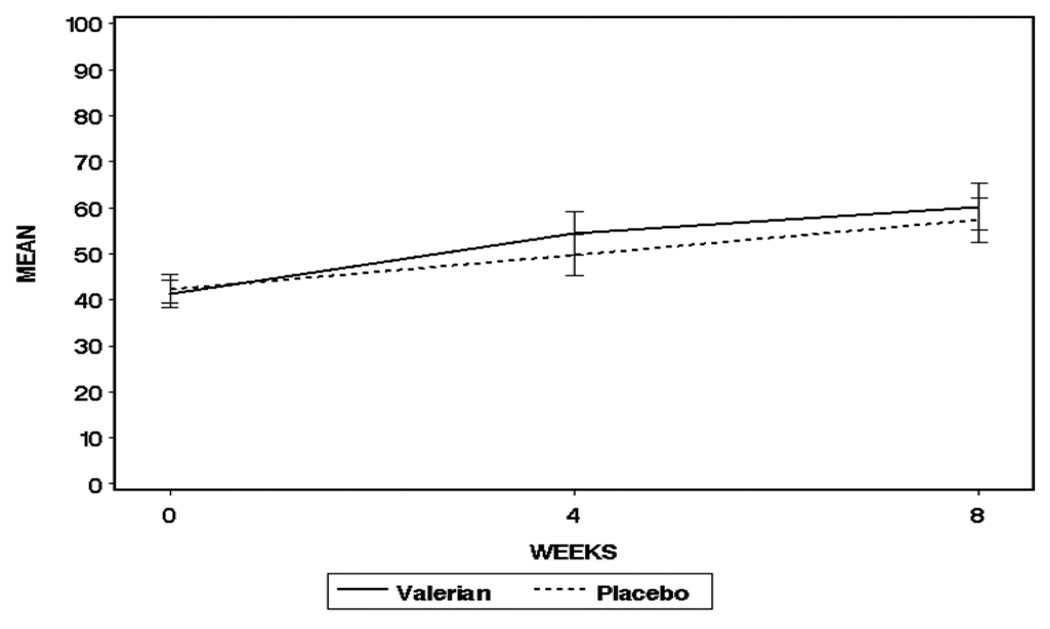
The primary endpoint of treatment effectiveness was measured using the normalized AUC calculated using baseline, week 4 and week 8 PSQI total scores. The Wilcoxon rank-sum test p-value for the total PSQI score was non-significant (valerian AUC = 51.4, SD = 16; placebo AUC=49.7, SD = 15, p=0.696) (Figure 2). Similarly the FOSQ was not significantly different between groups either overall, or on any subscale score.
Figure 2.
Pittsburgh Sleep Quality Index Overall Score, AUC
Supplemental and exploratory analyses using changes from baseline, however, showed a significant difference in the change from baseline in the amount of sleep at night at week 4 (p=0.008), favoring the valerian group. Change from baseline in the categorical value for sleep latency was also significantly different at week 4 where 10% of valerian patients indicated longer time to fall asleep compared to 28% on placebo, and 43% of valerian patients reported less time to fall asleep compared to 32% on placebo (p=0.03) (Table 3). The intent to treat analysis indicated that about 9% more patients experienced a success on valerian relative to placebo, but this was not statistically significant. When scores on the PSQI were divided into less than or equal to 5 and over 5 (this latter group representing sleep problems), there were fewer patients in the valerian group to have sleep problems by week 8 (64% vs 80%, p=0.56).
Table 3.
Percent of patients reporting categorical changes from baseline on the Pittsburgh Sleep Quality Index Subscales
| Valerian | Placebo | P-value | |||
|---|---|---|---|---|---|
| Sleep Quality | Week 4 | 0.199 | |||
| Worse | 2(3%) | 5(8%) | |||
| Same | 33(49%) | 37(57%) | |||
| Better | 33(49%) | 23(35%) | |||
| Week 8 | 0.927 | ||||
| Worse | 3(5%) | 2(3%) | |||
| Same | 26(41%) | 25(42%) | |||
| Better | 35(55%) | 32(54%) | |||
| Sleep Latency | Week 4 | 0.030 | |||
| Worse | 6(10%) | 18(28%) | |||
| Same | 30(48%) | 26(40%) | |||
| Better | 27(43%) | 21(32%) | |||
| Week 8 | 0.072 | ||||
| Worse | 3(5%) | 11(18%) | |||
| Same | 28(47%) | 29(48%) | |||
| Better | 27(47%) | 21(34%) | |||
| Sleep Duration | Week 4 | 0.244 | |||
| Worse | 6(9%) | 10(16%) | |||
| Same | 26(39%) | 29(46%) | |||
| Better | 34(52%) | 24(38%) | |||
| Week 8 | 0.148 | ||||
| Worse | 8(13%) | 4(7%) | |||
| Same | 19(31%) | 28(48%) | |||
| Better | 34(56%) | 27(46%) | |||
| Sleep Efficiency | Week 4 | 0.295 | |||
| Worse | 7 (12%) | 13 (22%) | |||
| Same | 26 (43%) | 23 (39%) | |||
| Better | 28 (46%) | 23 (39%) | |||
| Week 8 | 0.758 | ||||
| Worse | 11 (19%) | 9 (16%) | |||
| Same | 19 (33%) | 22 (39%) | |||
| Better | 28 (48%) | 25 (45%) | |||
|
Sleep Disturbance |
Week 4 | 0.738 | |||
| Worse | 9 (15%) | 11 (18%) | |||
| Same | 41 (66%) | 40 (67%) | |||
| Better | 12 (19%) | 9 (15%) | |||
| Week 8 | 0.177 | ||||
| Worse | 10 (16%) | 7 (13%) | |||
| Same | 35 (57%) | 41 (73%) | |||
| Better | 16 (26%) | 8 (14%) | |||
|
Daytime Dysfunction |
Week 4 | 0.114 | |||
| Worse | 6 (9%) | 13 (19%) | |||
| Same | 42 (60%) | 40 (60%) | |||
| Better | 22 (31%) | 14 (21%) | |||
| Week 8 | 0.478 | ||||
| Worse | 6 (10%) | 8 (13%) | |||
| Same | 27 (43%) | 31 (50%) | |||
| Better | 30 (48%) | 23 (37%) |
While the POMS AUC scores indicated no difference between treatment arms, the mean change from baseline at weeks 4 and 8 was significantly different for the fatigue-inertia subscale at weeks 4 (p=0.004) and 8 (p=0.02), with the valerian arm reporting better scores (Table 4). On the BFI, the valerian arm scored significantly better than the placebo arm in the mean change from baseline at weeks 4 and 8 on the “fatigue now” (p=0.003 and p=0.01, respectively) and “usual fatigue” items (p=0.02 and p=0.046, respectively) (Table 4).
Table 4.
Brief Fatigue Inventory (BFI) and Profile of Mood States (POMS) Change from baseline – higher numbers are better
| Side Effect | Week | Valerian | Placebo | P-value |
|---|---|---|---|---|
| BFI | ||||
| Fatigue Now | Week 4 | 13.2 | 1.5 | <0.01 |
| Week 8 | 22.1 | 10.5 | <0.01 | |
| Usual Fatigue | Week 4 | 12.8 | 4.2 | 0.02 |
| Week 8 | 19.4 | 10.0 | 0.05 | |
| Worst Fatigue | Week 4 | 11.2 | 3.2 | 0.03 |
| Week 8 | 14.8 | 12.4 | 0.65 | |
| Activity Interference | Week 4 | 6.2 | 4.1 | 0.75 |
| Week 8 | 12.3 | 10.8 | 0.75 | |
| POMS | ||||
| Anger-Hostility | Week 4 | 3.5 | 2.0 | 0.53 |
| Week 8 | 3.9 | 4.2 | 0.89 | |
| Vigor-Activity | Week 4 | 2.0 | −0.4 | 0.43 |
| Week 8 | 2.0 | 4.7 | 0.34 | |
| Depression-Dejection | Week 4 | 3.7 | 5.5 | 0.21 |
| Week 8 | 3.7 | 5.4 | 0.25 | |
| Confusion-Bewilderment | Week 4 | 4.8 | 2.6 | 0.26 |
| Week 8 | 5.3 | 3.4 | 0.79 | |
| Fatigue-Inertia | Week 4 | 13.9 | 2.8 | <0.01 |
| Week 8 | 17.5 | 9.2 | 0.02 | |
| Tension-Anxiety | Week 4 | 6.3 | 5.6 | 0.85 |
| Week 8 | 9.2 | 8.9 | 0.54 | |
| Total Score | Week 4 | 5.7 | 3.0 | 0.19 |
| Week 8 | 6.9 | 6.0 | 0.90 |
In terms of toxicity, there were no significant differences between arms for the self reported side effect items (headache, trouble waking, nausea) at baseline, week 4 or week 8 (Table 5). The valerian arm change from baseline at both weeks 4 and 8 showed significant improvement in drowsiness (p=0.04 and p=0.03, respectively) and sleep problems (p=0.005 and p=0.03, respectively) compared to placebo (Table 5). The maximum severity over time for each self reported toxicity resulted in no significant differences between arms. There was a significant difference in the CTCAE reporting of alkaline phosphatase, with the placebo arm having a higher incidence of grade 1 toxicity (p=0.049).
Table 5.
Self Reported Side Effects Change from baseline – higher numbers are better
| Side Effect | Week | Valerian | Placebo | P-value |
|---|---|---|---|---|
| Nausea | Week 4 | 3.0 | −2.1 | .07 |
| Week 8 | 3.4 | 0.0 | .06 | |
| Headache | Week 4 | 4.8 | 1.5 | .09 |
| Week 8 | 6.7 | 4.6 | .27 | |
| Trouble waking | Week 4 | 8.8 | 4.3 | .42 |
| Week 8 | 9.5 | 5.7 | .36 | |
| Drowsiness | Week 4 | 21.0 | 9.7 | .04 |
| Week 8 | 24.0 | 14.0 | .03 | |
| Sleep problems | Week 4 | 18.7 | 4.3 | <.01 |
| Week 8 | 24.0 | 13.0 | .03 |
Discussion
This study failed to identify any significant improvements in sleep as measured by the overall PSQI or the FOSQ in this population. This corroborates data from a recent study by Taibi and colleagues 49 who evaluated 300 mg of valerian, taken ½ hour before bed. Taibi reports that valerian did not improve any self reported or polysomnographic sleep outcomes significantly more than placebo. The Taibi study has several possible limitations including the small sample size (n=16), a dose lower than that used in the majority of pilot trials with promising results, and a duration of only 15 days on the study agent.
The current study is one of the few randomized placebo-controlled trials evaluating pharmacological treatment of insomnia complaints among cancer patients. Most randomized trials of treatments directed at insomnia in cancer patients compare cognitive-behavioral therapy with usual care or wait-list care and find it of substantial benefit.50–59 One prior trial in terminal cancer patients evaluated intravenous agents for effectiveness, and another controlled trial found mirtazapine effective in improving sleep complaints in cancer patients with depression.51,60 Otherwise, there are no apparent other controlled trials assessing pharmacologic agents to primarily address sleep-related complaints in cancer patients.
While there was no significant improvement in sleep quality as assessed by the PSQI, there were consistent improvements in the secondary fatigue outcomes as measured by both the Brief Fatigue Inventory and the Profile of Mood States fatigue-inertia subscale. Although caution is required in interpreting these secondary results, the raw differences in change scores between the two arms are fairly large, often over 10 points (on a 100 point scale). In addition, several other secondary endpoints; change from baseline related to sleep latency, amount of sleep per night, improvement in sleep problems, and less drowsiness, all support the valerian arm outperforming placebo.
There are several hypotheses related to the inconsistencies in the results. The PSQI may measure different dimensions of well-being than the BFI or POMS, the former concentrating on sleep quality measures, while the latter two measures concentrate on daytime symptoms. The correlation between sleep quality and daytime symptoms may not be very strong in this study’s population. Another possibility is that there was a beta-error. Some of the data were incomplete due to the patient’s inability to complete the questionnaires appropriately. The power analysis suggested 100 patients per arm were required, and only about 60 per group provided data for analysis. Another hypothesis is that the effects of valerian were too modest and limited to one aspect, perhaps sleep latency, that were not detectable with multidimensional scales such as the PSQI or the FOSQ that look at impact on activity.
There were more patients who withdrew from the placebo arm early, compared to the valerian arm. The reasons for this are not known. However, patients on this trial were getting active treatment for cancer, so numerous and varied reasons could explain early withdrawals including complications from treatment, increased fatigue and worsening sleep problems.
In summary, this trial did not provide data to support that valerian is helpful in improving sleep during cancer treatment in this population. It is not clear whether valerian may have helpful physiologic activity supporting research in oncology symptom management related to fatigue. Perhaps further exploration is warranted.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
This study was conducted as a collaborative trial of the North Central Cancer Treatment Group and Mayo Clinic and was supported in part by Public Health Service grants CA-25224, CA-37404, CA-124477 (Mentorship Grant) CA-35431, CA-63848, CA-35195, CA-35133, CA-35267, CA-35269, CA-35103, CA-35101, CA-63849, CA-35119, CA-52352, CA-35448, CA-35103, CA-03011, CA-107586, CA-35261, CA-67575, CA-95968, CA-67753 and CA-35415. The content is solely the responsibility of the authors and does not necessarily represent the views of the National Cancer Institute or the National Institute of Health.
Additional participating institutions include: Duluth Clinic CCOP, Duluth, MN 55905 (Daniel A. Nikcevich, M.D., Ph.D.); CCOP Sioux Community Cancer Consortium, Sioux Falls, SD (Loren K. Tschetter, M.D.); Iowa Oncology Research Association CCOP, Des Moines, IA 50309-1016 (Robert J. Behrens, M.D.); Mayo Clinic Arizona, Fitch, Scottsdale AZ 85259 (Tom R. Fitch, M.D.) Missouri Valley Cancer Consortium CCOP, Omaha, NE 68106 (Gamini S. Soori, M.D.); Medical College of Georgia Minority-Based CCOP, Augusta, GA 30912 (Anand P. Jillella, M.D.); Columbus CCOP, Columbus, OH 43215 (J. Philip Kuebler, M.D., Ph.D.); Upstate Carolina CCOP, Spartanburg, SC 29303 (James D. Bearden, M.D.) Cedar Rapids Oncology Project CCOP, Cedar Rapids, IA 52403-1206 (Martin Wiesenfeld, M.D. Altru Cancer Center, Grand Forks, ND 53201-4030 (Todor Dentchev, M.D.) Montana Cancer Consortium CCOP, Billings, MT 591010 (Benjamin T. Marchello, M.D.); Saint Vincent Hospital CCOP, Green Bay, WI 54307 (Anthony J. Jaslowski, M.D.); Colorado Cancer Research Program CCOP, Denver, CO 80224 (Eduardo R. Pajon, Jr., M.D.);Geisinger Medical Center CCOP, Danville, PA 17822 (Albert M. Bernath, Jr, M.D.); Rapid City Regional Hospital, Rapid City, SD 57701 (Richard C. Tenglin, M.D.); Siouxland Hematology Oncology Associates, Sioux City, IA 51101-1733 (Donald B. Wender, M.D., Ph.D.); Toledo Community Hospital Oncology Program CCOP, Toledo, OH 43623 (Paul L. Schaefer, M.D.);
References
- 1.Savard J, Morin CM. Insomnia in the context of cancer: a review of a neglected problem. J Clin Oncol. 2001;19:895–908. doi: 10.1200/JCO.2001.19.3.895. [DOI] [PubMed] [Google Scholar]
- 2.Davidson JR, MacLean AW, Brundage MD, et al. Sleep disturbance in cancer patients. Soc Sci Med. 2002;54:1309–1321. doi: 10.1016/s0277-9536(01)00043-0. [DOI] [PubMed] [Google Scholar]
- 3.Hu DS, Silberfarb PM. Management of sleep problems in cancer patients. Oncology (Williston Park) 1991;5:23–27. discussion 28. [PubMed] [Google Scholar]
- 4.Fiorentino L, Ancoli-Israel S. Insomnia and its treatment in women with breast cancer. Sleep Med Rev. 2006;10:419–429. doi: 10.1016/j.smrv.2006.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mao JJ, Armstrong K, Bowman MA, et al. Symptom burden among cancer survivors: impact of age and comorbidity. J Am Board Fam Med. 2007;20:434–443. doi: 10.3122/jabfm.2007.05.060225. [DOI] [PubMed] [Google Scholar]
- 6.Miller AH, Ancoli-Israel S, Bower JE, et al. Neuroendocrine-immune mechanisms of behavioral comorbidities in patients with cancer. J Clin Oncol. 2008;26:971–982. doi: 10.1200/JCO.2007.10.7805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Savard J, Villa J, Ivers H, et al. Prevalence, Natural Course, and Risk Factors of Insomnia Comorbid With Cancer Over a 2-Month Period. J Clin Oncol. 2009;27(31):5233–5239. doi: 10.1200/JCO.2008.21.6333. [DOI] [PubMed] [Google Scholar]
- 8.Oxana G, Palesh J, Roscoe K, et al. Prevalence, Demographics, and Psychological Associations of Sleep Disruption in Patients with Cancer: University of Rochester Cancer Center – Community Clinical Oncology Program. J Clin Oncol. 2010;8(2):292–298. doi: 10.1200/JCO.2009.22.5011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Owen DC, Parker KP, McGuire DB. Comparison of subjective sleep quality in patients with cancer and healthy subjects. Oncol Nurs Forum. 1999;26:1649–1651. [PubMed] [Google Scholar]
- 10.Morin AK, Jarvis CI, Lynch AM. Therapeutic options for sleep-maintenance and sleep-onset insomnia. Pharmacotherapy. 2007;27:89–110. doi: 10.1592/phco.27.1.89. [DOI] [PubMed] [Google Scholar]
- 11.Morgenthaler T, Kramer M, Alessi C, et al. Practice parameters for the psychological and behavioral treatment of insomnia: an update. An American Academy of Sleep Medicine report. Sleep. 2006;29:1415–1419. [PubMed] [Google Scholar]
- 12.Roehrs T, Roth T. Sleep-wake state and memory function. Sleep. 2000;23 Suppl 3:S64–S68. [PubMed] [Google Scholar]
- 13.Payne J, Piper B, Rabinowitz I, et al. Biomarkers, fatigue, sleep, and depressive symptoms in women with breast cancer: a pilot study. Oncol Nurs Forum. 2006;33:775–783. doi: 10.1188/06.ONF.775-783. [DOI] [PubMed] [Google Scholar]
- 14.Benca RM, Ancoli-Israel S, Moldofsky H. Special considerations in insomnia diagnosis and management: depressed, elderly, and chronic pain populations. J Clin Psychiatry. 2004;65 Suppl 8:26–35. [PubMed] [Google Scholar]
- 15.Katz DA, McHorney CA. The relationship between insomnia and health-related quality of life in patients with chronic illness. J Fam Pract. 2002;51:229–235. [PubMed] [Google Scholar]
- 16.American Academy of Sleep Medicine. Diagnostic and Coding Manual. 2nd ed. Westchester, IL: American Academy of Sleep Medicine; 2005. International Classification of Sleep Disorders. [Google Scholar]
- 17.Colten HR, Altevogt BM. Institute of Medicine (U.S.) Sleep disorders and sleep deprivation : an unmet public health problem. Washington, DC: National Academies Press; 2006. Committee on Sleep Medicine and Research. [PubMed] [Google Scholar]
- 18.Baker F, Denniston M, Smith T, et al. Adult cancer survivors: how are they faring? Cancer. 2005;104:2565–2576. doi: 10.1002/cncr.21488. [DOI] [PubMed] [Google Scholar]
- 19.Stiefel FC, Kornblith AB, Holland JC. Changes in the prescription patterns of psychotropic drugs for cancer patients during a 10-year period. Cancer. 1990;65(4):1048–1053. doi: 10.1002/1097-0142(19900215)65:4<1048::aid-cncr2820650434>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 20.Ebert B, Wafford KA, Deacon S. Treating insomnia: Current and investigational pharmacological approaches. Pharmacol Ther. 2006;112:612–629. doi: 10.1016/j.pharmthera.2005.04.014. [DOI] [PubMed] [Google Scholar]
- 21.Moller HJ. Effectiveness and safety of benzodiazepines. J Clin Psychopharmacol. 1999;19:2S–11S. doi: 10.1097/00004714-199912002-00002. [DOI] [PubMed] [Google Scholar]
- 22.Barbera J, Shapiro C. Benefit-risk assessment of zaleplon in the treatment of insomnia. Drug Saf. 2005;28:301–318. doi: 10.2165/00002018-200528040-00003. [DOI] [PubMed] [Google Scholar]
- 23.Bellon A. Searching for new options for treating insomnia: are melatonin and ramelteon beneficial? J Psychiatr Pract. 2006;12:229–243. doi: 10.1097/00131746-200607000-00005. [DOI] [PubMed] [Google Scholar]
- 24.Roth T, Seiden D, Sainati S, et al. Effects of ramelteon on patient-reported sleep latency in older adults with chronic insomnia. Sleep Med. 2006;7:312–318. doi: 10.1016/j.sleep.2006.01.003. [DOI] [PubMed] [Google Scholar]; 22 Morin CM, Bootzin RR, Buysse DJ, et al. Psychological and behavioral treatment of insomnia:update of the recent evidence (1998–2004) Sleep. 2006;29:1398–1414. doi: 10.1093/sleep/29.11.1398. [DOI] [PubMed] [Google Scholar]; 23 Morin CM, Culbert JP, Schwartz SM. Nonpharmacological interventions for insomnia: a meta-analysis of treatment efficacy. Am J Psychiatry. 1994;151:1172–1180. doi: 10.1176/ajp.151.8.1172. [DOI] [PubMed] [Google Scholar]; 24 Smith MT, Perlis ML, Park A, et al. Comparative meta-analysis of pharmacotherapy and behavior therapy for persistent insomnia. Am J Psychiatry. 2002;159:5–11. doi: 10.1176/appi.ajp.159.1.5. [DOI] [PubMed] [Google Scholar]
- 25.Morgenthaler Timothy I, Holbrook AM, Crowther R, Lotter A, et al. Meta-analysis of benzodiazepine use in the treatment of insomnia. Can Med Assoc. 2000;J162:225–233. Jan 8, '10, 4:06 PM. [PMC free article] [PubMed] [Google Scholar]
- 26.Hall N. Taking policy action to reduce benzodiazepine use and promote self-care among seniors. J Appl Gerontol. 1998;17:318–351. [Google Scholar]
- 27.Johnson LC, Chernik DA. Sedative-hypnotics and human performance. psychopharmacology (Berl) 1982;76:101–113. doi: 10.1007/BF00435262. [DOI] [PubMed] [Google Scholar]
- 28.Ray WA, Griffin MR, Downey W. Benzodiazepines of long and short elimination half-life and the risk of hip fracture. JAMA. 1989;262:3303–3306. [PubMed] [Google Scholar]
- 29.Foy A, O’Connell D, Henry D, et al. Benzodiazepine use as a cause of cognitive impairment in elderly hospital inpatients. J Gerontol A Biol Sci Med Sci. 1995;50:M99–M106. doi: 10.1093/gerona/50a.2.m99. [DOI] [PubMed] [Google Scholar]
- 30.Gray SL, Lai KV, Larson EB. Drug-induced cognition disorders in the elderly: Incidence prevention and management. Drug Saf. 1999;21:101–122. doi: 10.2165/00002018-199921020-00004. [DOI] [PubMed] [Google Scholar]
- 31.Tune LE, Bylsma FW. Benzodiazepine-induced and anticholinergic-induced delirium in the elderly. Int Psychogeriatr. 1991;3:397–408. doi: 10.1017/s1041610291000832. [DOI] [PubMed] [Google Scholar]
- 32.Houghton PJ. The scientific basis for the reputed activity of Valerian. J Pharm Pharmacol. 1999;51:505–512. doi: 10.1211/0022357991772772. [DOI] [PubMed] [Google Scholar]
- 33.Balderer G, Borbely AA. Effect of valerian on human sleep. Psychopharmacology (Berl) 1982;87:406–409. doi: 10.1007/BF00432503. [DOI] [PubMed] [Google Scholar]
- 34.Leathwood PD, Chauffard F. Aqueous extract of valerian reduces latency to fall asleep in man. Planta Med. 1985:144–148. doi: 10.1055/s-2007-969430. [DOI] [PubMed] [Google Scholar]
- 35.Leathwood PD, Chauffard F, Heck E, et al. Aqueous extract of valerian root (Valeriana officinalis L.) improves sleep quality in man. Pharmacol Biochem Behav. 1982;17:65–71. doi: 10.1016/0091-3057(82)90264-7. [DOI] [PubMed] [Google Scholar]
- 36.Schulz H, Stolz C, Muller J. The effect of valerian extract on sleep polygraphy in poor sleepers: a pilot study. Pharmacopsychiatry. 1994;27:147–151. doi: 10.1055/s-2007-1014295. [DOI] [PubMed] [Google Scholar]
- 37.Lindahl O, Lindwall L. Double blind study of a valerian preparation. Pharmacol Biochem Behav. 1989;32:1065–1066. doi: 10.1016/0091-3057(89)90082-8. [DOI] [PubMed] [Google Scholar]
- 38.Hodgson B, Kizior R. Nursing Drug Handbook. Philadelphia, PA: Saunders; 2000. [Google Scholar]
- 39.Thompson CA. USP moves forward in providing information on botanical products. Am J Health Syst Pharm. 1998;55:527–530. doi: 10.1093/ajhp/55.6.527. [DOI] [PubMed] [Google Scholar]
- 40.Garges H, Varia I, Doraiswamy P, et al. Cardiac Complications and Delirium Associated with Valerian Root Withdrawl. JAMA. 1998;280:1566–1567. doi: 10.1001/jama.280.18.1566-a. [DOI] [PubMed] [Google Scholar]
- 41.Budzinski JW, Foster BC, Vandenhoek S, et al. An in vitro evaluation of human cytochrome P450 3A4 inhibition by selected commercial herbal extracts and tinctures. Phytomedicine. 2000;7:273–282. doi: 10.1016/S0944-7113(00)80044-6. [DOI] [PubMed] [Google Scholar]
- 42.Buysse DJ, Reynolds CF, 3rd, Monk TH, et al. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28:193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 43.Curran S, Andrykowsky M, Studts J. Short Form of the Profile of Mood States (POMS-SF): Psychometric Information. Psychological Assessment. 1995;7:80–83. [Google Scholar]
- 44.Weaver TE, Laizner AM, Evans LK, et al. An instrument to measure functional status outcomes for disorders of excessive sleepiness. Sleep. 1997;20:835–843. [PubMed] [Google Scholar]
- 45.Mendoza TR, Wang XS, Cleeland CS, et al. The rapid assessment of fatigue severity in cancer patients: use of the Brief Fatigue Inventory. Cancer. 1999;85:1186–1196. doi: 10.1002/(sici)1097-0142(19990301)85:5<1186::aid-cncr24>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 46.Lipsitz SR, Fitzmaurice GM, Orav EJ, et al. Performance of generalized estimating equations in practical situations. Biometrics. 1994;50:270–278. [PubMed] [Google Scholar]
- 47.Cohen J. Statistical Power Analysis for the Behavioral Sciences. Hillsdale, NJ: Lawrence Erlbaum Associates; 1988. [Google Scholar]
- 48.Sloan J, Symonds T, Vargas-Chanes D, et al. Practical guidelines for assessing the clinical significance of health-related quality of life changes within clinical trials. Drug Information Journal. 2003;37:23–31. [Google Scholar]
- 49.Taibi DM, Vitiello MV, Barsness S, et al. A randomized clinical trial of valerian fails to improve self-reported, polysomnographic, and actigraphic sleep in older women with insomnia. Sleep Med. 2009;10:319–328. doi: 10.1016/j.sleep.2008.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Berger A, Kuhn B, Farr J, et al. Behavioral therapy intervention trial to improve sleep quality and cancer-related fatigue. Psycho-Oncology. 2009;18:634–646. doi: 10.1002/pon.1438. [DOI] [PubMed] [Google Scholar]
- 51.Cankurtaran E, Ozalp E, Soygur H, et al. Mirtazapine improves sleep and lowers anxiety and depression in cancer patients: superiority over imipramine. Support Care Cancer. 2008;16:1291–1298. doi: 10.1007/s00520-008-0425-1. [DOI] [PubMed] [Google Scholar]
- 52.Espie C, Fleming L, Cassidy J, et al. Randomized Controlled Clinical Effectiveness Trial of Cognitive Behavior Therapy Compared With Treatment As Usual for Persistent Insomnia in Patients With Cancer. J Clin Oncol. 2008;26(28):4651–4658. doi: 10.1200/JCO.2007.13.9006. [DOI] [PubMed] [Google Scholar]
- 53.Epstein D, Dirksen S. Randomized Trial of a Cognitive-Behavioral Intervention for Insomnia in Breast Cancer Survivors Oncology Nursing Forum. 2007;34(5):E51–E59. doi: 10.1188/07.ONF.E51-E59. [DOI] [PubMed] [Google Scholar]
- 54.Savard J, Simard S, Giguère I, et al. Randomized clinical trial on cognitive therapy for depression in women with metastatic breast cancer: psychological and immunological effects. Palliat Support Care. 2006;4(3):219–237. doi: 10.1017/s1478951506060305. [DOI] [PubMed] [Google Scholar]
- 55.Savard J, Simard S, Ivers H, et al. Randomized Study on the Efficacy of Cognitive-Behavioral Therapy for Insomnia Secondary to Breast Cancer, Part I: Sleep and Psychological Effects. J Clin Oncol. 2005;23(25):6083–6096. doi: 10.1200/JCO.2005.09.548. [DOI] [PubMed] [Google Scholar]
- 56.Savard J, Simard S, Ivers H. Randomized study on the efficacy of cognitive behavioral therapy for insomnia secondary to breast cancer, part II: Immunologic effects. J Clin Oncol. 2005;23(25):6097–6106. doi: 10.1200/JCO.2005.12.513. [DOI] [PubMed] [Google Scholar]
- 57.Sherwood P, Given B, Given C, et al. A Cognitive Behavioral Intervention for Symptom Management in Patients with Advanced Cancer. Oncol Nrsg Forum. 2005;32(6):1190–1198. doi: 10.1188/05.ONF.1190-1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Quesnel C, Savard J, Simard S, et al. Efficacy of Cognitive-Behavioral Therapy for Insomnia in Women Treated for Nonmetastatic Breast Cancer. J Consulting and Clinical Psychology. 2003;71(1):189–200. [PubMed] [Google Scholar]
- 59.Davidson J, Waisberg J, Brundage M, et al. Nonpharmacologic Group Treatment of Insomnia: A Preliminary Study with Cancer Survivors. Psycho-Oncology. 2001;10:389–397. doi: 10.1002/pon.525. [DOI] [PubMed] [Google Scholar]
- 60.Matsuo N, Morita T. Efficacy, Safety, and Cost Effectiveness of Intravenous Midazolam and Flunitrazepam for Primary Insomnia in Terminally Ill Patients with Cancer: A Retrospective Multicenter Audit Study. J Palliative Medicine. 2007;10(5):1054–1062. doi: 10.1089/jpm.2007.0016. [DOI] [PubMed] [Google Scholar]