Abstract
Mammalian cells are highly organized to optimize function. For instance, oxidative energy-producing processes in mitochondria are sequestered away from plasma membrane redox signaling complexes and also from nuclear DNA which is subject to oxidant-induced mutation. Proteins are unique among macromolecules in having reversible oxidizable elements, “sulfur switches”, which support dynamic regulation of structure and function. Accumulating evidence shows that redox signaling and control systems are maintained under kinetically limited steady-states which are highly displaced from redox equilibrium and distinct among organelles. Mitochondria are most reducing and susceptible to oxidation under stressed conditions while nuclei are also reducing but relatively resistant to oxidation. Within compartments, the glutathione and thioredoxin systems serve parallel and non-redundant functions to maintain the dynamic redox balance of subsets of protein cysteines which function in redox signaling and control. This organization allows cells to be poised to respond to cell stress but also creates sites of vulnerability. Importantly, disruption of redox organization is a common basis for disease. Research tools are becoming available to elucidate details of subcellular redox organization, and this development highlights an opportunity for a new generation of targeted antioxidants to enhance and restore redox signaling and control in disease prevention.
Translation of basic science knowledge into human research and improved healthcare is a contemporary focus of many funding agencies and research sponsors. In oxidative stress research, however, there is a considerable need for reverse translation, i.e., from clinical observations back to basic science. Such an effort is warranted because large-scale double-blind interventional trials with moderately high doses of free radical scavengers provided little health benefit, even though the basic science research, preclinical studies and observational studies in humans clearly implicate oxidative stress as an underlying mechanism of many disease processes.
The present review addresses one aspect of this reverse translation, investigation of possible cause-effect relationships between oxidative stress, measured in terms of thiol oxidation in human plasma, and its underlying biochemistry at the subcellular level. Most of the information reviewed has not been gained from studies of diabetes or the stressed β-cell, but the mechanistic information is relevant to understanding of β-cell function and dysfunction. Several studies show that plasma thiol/disulphide systems are oxidized in association with diabetes in humans and animal models, and the meaning of changes in oxidation of the thiol systems and subcellular stress signaling is now beginning to emerge.
A seminal observation which led to the current development of redox compartmentalization and cell stress was that two central thiol/disulphide couples in human plasma, the glutathione/glutathione disulphide (GSH/GSSG) and the cysteine/cystine (Cys/CySS) couples, varied little among healthy individuals but were maintained in disequilibrium relative to each other [1]. Each couple was oxidized in association with age, but with different rates [2], thereby showing that the concept of oxidative stress as an imbalance of prooxidants and antioxidants was incorrect [3]. Because two central antioxidants systems were not in balance, there could be no meaning to oxidative stress as a global imbalance of oxidative and reductive processes [3, 4].
Application of quantitative principles to thiol oxidation at the cellular and subcellular levels has now shown that central thiol/disulphide couples are maintained at distinct and stable, non-equilibrium values in different organelles [5, 6]. These central couples, which include Cys/CySS, GSH/GSSG and thioredoxins (Trx), function as reductive counterparts to H2O2 and other oxidants in controlling redox state of oxidizable thiols (“sulfur switches”) in proteins [7, 8]. These sulfur switches are used for cell signaling, protein structure, protein trafficking and regulation of enzyme, transporter, receptor and transcription factor activity. Importantly, these thiol-dependent redox processes typically occur by 2-electron (non-radical) transfers, and usually at rates which are low compared to electron transfer for energy production.
The rates of electron transfer for signaling and control are not known precisely, but probably are in the range of about 1% of the overall O2 consumption by cells [9]. H2O2 is the presumed oxidant in most of these reactions, although O2, hydroperoxides, quinones and other oxidants could be an electron acceptor to support signaling. The rates of peroxide generation and use for signaling and control appear to be considerably greater than the rates of free radical reactions contributing to macromolecular damage [9]. Consequently, the disruption of ongoing thiol-dependent redox signaling and control mechanisms appears to be among the most sensitive and quantitatively important processes in oxidative stress. Because these redox mechanisms control proinflammatory signaling, profibrotic signaling, cell proliferation, apoptosis and a range of other biologic processes without a requirement for macromolecular damage, the results suggest that failure of free radical scavenger trials may have occurred because oxidative stress research over-emphasized the importance of free radical mechanisms and macromolecular damage as an underlying mechanism [9].
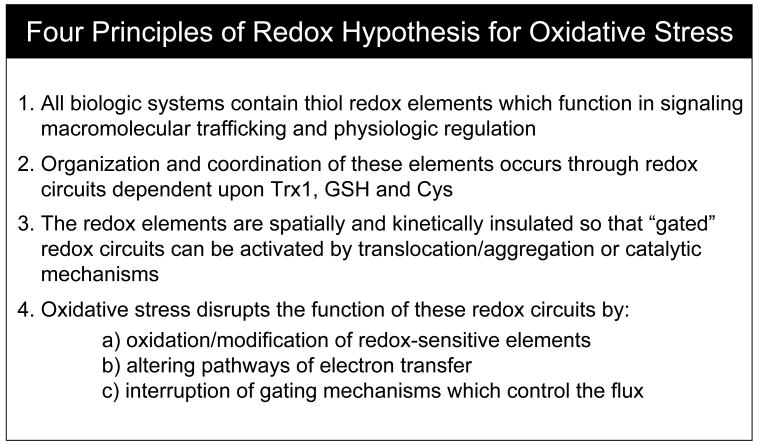
The present consideration of redox compartmentalization and cellular stress is based upon an alternative to the free radical hypothesis which is termed the “redox hypothesis” [9] (Fig. 1). The redox hypothesis postulates that oxidizable thiols are common control elements for biologic processes. These control elements are functionally organized in redox circuits which are controlled by GSH/GSSG, thioredoxins and other control nodes. The redox circuits are kinetically limited and insulated from each other so that they are highly responsive to redox conditions and can function independently in signaling and regulation of different biologic processes. Oxidative stress is a disruption of these circuits due to 1) inhibiting electron transfer and blocking functional redox pathways, 2) short circuiting the otherwise insulated electron transfer pathways or 3) interfering with gating mechanisms which regulate the redox pathways [9].
Fig. 1. The redox hypothesis for oxidative stress.
Oxidative stress research has largely focused on free radical mechanisms as a contributing factor in disease development but large-scale double-blind interventional trials with free radical scavenging antioxidants have been disappointing in providing little evidence of health benefit. The redox hypothesis, consisting of four postulates as indicated, was formulated as an alternative interpretation of experimental data [9].
Redox compartmentalization provides the best evidence to date in support of these concepts. The thiol/disulphide redox couples within organelles are maintained at distinct steady-state redox potentials and respond independently to physiologic signaling. Importantly, the characteristics suggest that the redox signaling and control systems are specialized to organellar function; indeed, optimization of redox environments may have been an important component of the evolution coupling structure and function. Thiols are important elements in structure-function coupling because thiols in proteins can function as transducing elements linking chemistry and biologic structure [9].
Oxidation of Cys residues in proteins to disulphides serves as a common mechanism for stabilizing tertiary structure and is especially important for secreted proteins. Oxidation of protein surface thiols supports intermolecular disulphide formation, well recognized in control of mucus fluidity but also contributing to protein aggregation in cells. At present, the extent to which reversible oxidation of thiols controls dynamic structures in cells is not clear; however, the highly conserved Cys residues in actin, actin-associated proteins (e.g., Keap-1) and other structural proteins (e.g., 14-3-3), raises the possibility that dynamic structure-function relationships occur due to the transduction of redox chemistry to biologic structure through the reversible oxidation of thiols. Such control would necessarily involve local and specific redox reactions [10], and methods are only beginning to be developed which have sufficient resolution and specificity for their study.
Methods for study of thiol/disulphide couples
Study of the dynamic balance of thiol/disulphide couples in biologic systems has been hampered by the perception that Cys residues present as mixtures of thiol and disulphide forms represent analytic artifacts [11]. Early enzymologists and protein chemists recognized that protein aggregation and loss of enzymatic activity could be prevented by inclusion of thiol reducing agents such as dithiothreitol or GSH. However, protein processing often requires disulphide bond formation [12], so it is clear that the possibility of having artifactual oxidation is not equivalent to oxidation being artifactual. Sies et al [13] addressed this in a discussion of their measurements of GSH/GSSG redox potential (EhGSSG) in rat liver. The steady-state EhGSSG in liver at -255 mV is positive (relatively oxidizing) [14] compared to the redox potential for the donor NADPH/NADP+ couple (EhNADP+, -380 to -405 mV; [15]). Their interpretation was that the GSSG reductase is kinetically limited under physiologic conditions due to a relatively high Km for GSSG. This interpretation was critically reviewed by Gilbert [14], who addressed possible artifacts, and arrived at the same conclusion, i.e., methods are sufficiently efficient to trap the steady-state concentrations and the redox couples are not in redox equilibrium in vivo. Strong evidence in now available to show that thiol/disulfide couples are also not in redox equilibrium in vivo [5, 6].
Several methods are available to measure redox states of thiols and disulphides in body fluids, cells and tissue extracts (Table 1). The methods for plasma Cys/CySS and GSH/GSSG pools were recently reviewed in detail [16] and include discussion of pitfalls in measurement. With appropriate extraction and processing procedures, comparable results are obtained with iodoacetate, iodoacetamide, maleimide, and other thiol reagents [16]. We prefer the expression of redox state as a half-cell redox potential (Eh) value, rather than expression as a simple ratio (e.g., GSH/GSSG) or as fractional oxidation (e.g., % oxidation). Such an expression ranks redox couples according to the reducing or oxidizing force available. With such an expression, one must be aware that the kinetics of electron transfer are often limiting, i.e., the energetics for electron transfer may favor a reaction, but the electron transfer can only occur with an appropriate pathway. As emphasized above, under biologic conditions, electron transfer between thiol/disulphide systems are often kinetically limited.
Table 1. Strategies to measure biologic thiol and disulphide redox states.
| Approach | Analytes | References |
|---|---|---|
| HPLC | Small molecules (GSH, GSSG, Cys, CySS) | [1, 17] |
| Redox western blotting, BIAM | Proteins (Trx, TrxR, Prx…) | [20, 30] |
| Redox ICAT | Global cellular proteins | [25, 26] |
| Genetic manipulation | Subcellular compartment-targeted amino acid sequence (NLS, NES, MLS) incorporation into genes of interest (NLS-Trx1, NES-Trx1, NLS-Prx1, NES-Prx1) | [31] |
| Redox-sensitive GFP | Grx1-roGFP2 | [19] |
We typically use the convention of expressing Eh in mV as oxidation potentials relative to a standard hydrogen electrode, calculated using the molar concentrations of the oxidized and reduced forms, with Eo values at the appropriate pH. Estimates of error due to inaccurate volume and pH estimates have been addressed and usually do not pose major problems for interpretation [17]. For most Cys residues in proteins, however, Eo values are not known so that these must be measured, or expressions can only be made in terms of ratios or fractional oxidation.
Analysis of redox potentials within subcellular compartments poses a much more challenging problem than measurements of cell or tissue averages due to the time and treatments necessary to obtain subcellular fractions. During the process of cell disruption and isolation of organelles, redistribution and artifactual oxidation or reduction can occur. As previously reviewed [5] and summarized below, there is reasonable consensus for major redox couples in most organelles, but some uncertainty remains about the GSH pool in nuclei, where validation of data has been particularly difficult. Indirect evidence for changes in GSH redox state in nuclei has been obtained by measurement of protein S-glutathionylation, which can be expressed as a ratio with total nuclear protein thiol content [18].
Development of assays which depend upon distribution of redox-sensitive proteins has considerably improved the understanding of compartmentalization of redox because these can be measured without fractionation. For instance, a fusion protein of redox-sensitive green fluorescent protein with glutaredoxin allows compartment-specific imaging of GSH redox state [19]. Redox states of Trx-2 in mitochondria [20] and protein disulphide isomerase in the cisternae of the endoplasmic reticulum [21] allow compartment specific values by redox western blot analysis. These redox western blots use conventional western blots in which different redox forms are resolved by pre-treating with thiol reagents which change charge or mass of the protein according to the number of thiols available (disulphides are not reactive). Hwang et al [22] also measured ER redox potential using a small peptide and HPLC. The redox states of Trx1 in the cytoplasmic and nuclear compartments have been measured following fractionation [23]. In addition, a molecular biology technique using epitope-tagged versions of NLS-Trx1 and NES-Trx1, which are specifically localized in nuclei and cytoplasm, respectively, enables measurement without fractionation [24]. A number of variations are available which make these approaches versatile and applicable to a large number of proteins with distinct subcellular distributions [5]. New methods using mass spectrometry [25, 26] create the possibility to map redox-sensitive proteins and pathways in different compartments.
Compartmental redox responses under physiologic stimuli-induced signaling
Early research on the importance of compartmentalization in redox signaling came from research on AP-1 transcriptional regulation. Results showed that oxidative events were involved in activation, but reduction was required for transcription factor binding to the DNA [27]. Evidence for signaling through discrete signaling pathways was obtained with studies of the gp91phox homologue Nox1, which produces H2O2, and induces cell growth, transformation, and tumorigenicity [28]. Investigation of signaling by H2O2 induced by Nox1 overexpression showed that Nox1-derived H2O2 activated a 15-fold increased in an ARE reporter gene expression with no effect on the redox state of the major thiol antioxidant substances, GSH and Trx-1 [29]. H2O2 signaling was mediated by activation of both the c-Jun N-terminal kinase and ERK1/2 pathways modulated by Ras. Thus, the results showed that redox mechanisms which activate kinase signaling pathways occur through a localized redox circuitry without global cellular effects. Compartmentalization in redox signaling was further established through measurement of the redox states of cytosolic and nuclear Trx-1, mitochondrial Trx-2 and cellular GSH during endogenous ROS production induced by EGF (epidermal growth factor) [30]. The results showed that only cytosolic Trx1 was significantly oxidized, providing clear evidence that subcellular compartmental oxidation can occur in the absence of a generalized cellular oxidation.
Compartment-specific signaling by discrete redox pathways was also observed in studies of Nrf-2 signaling [31]. Nrf-2 is a redox-sensitive transcription factor that is activated by an oxidative signal in the cytoplasm but has a critical cysteine that must be reduced to bind to DNA in the nucleus. Both GSH and Trx1 were found to affect Nrf-2 signaling, and studies with selective modification of GSH by metabolic manipulation and Trx-1 by transient transfection showed that these redox systems control different steps in Nrf-2 signaling. Tert-butylhydroquinone (TBHQ) activated Nrf-2 nuclear translocation by a type I (thiylation) redox switch which was regulated by GSH not by Trx-1. In contrast, expression of a Nrf-2-dependent ARE reporter was principally controlled by nuclear-targeted Trx-1 and not by GSH. Thus, GSH and Trx-1 systems have discrete functions in the control of transcriptional regulation by Nrf-2/ARE, and these functions are compartmentalized with oxidative activation regulated by GSH in the cytoplasm and DNA binding regulated by Trx-1 in the nuclei.
Compartmentalization of oxidative signaling has also be demonstrated with tumor necrosis factor-α (TNF-α), a cytokine that can contribute to disease through stimulation of mitochondrial production of ROS. In HeLa cells treated with 5 to 40 ng/ml TNF-α, mitochondrial Trx-2 but not cytoplasmic Trx-1 was oxidized [32] (Table 2). Preferential, significant Trx2 oxidation occurred within 10 min of TNF-α treatment, and overexpression of Trx2, but not Trx1, decreased TNF-α–induced ROS generation. Nuclear translocation of NF-κB was inhibited with Trx2 over-expression but not with the dominant negative active-site mutant C93S Trx2. NF-κB-luciferase reporter expression in response to TNF-α was significantly inhibited by Trx2 overexpression but not with C93S Trx2 expression. Trx2 overexpression, but not C93S Trx2, significantly inhibited TNF-α–induced apoptosis. Thus, in response to TNF-α, compartmentalized generation of ROS occurs in mitochondria and results in Trx2 oxidation, downstream signaling to cytoplasm with NF-κB activation and apoptosis.
Table 2. Evidence for compartment-specific redox changes during normal and stressed conditions.
Following treatment with different stimuli as indicated, responses in subcellular compartments were measured by different techniques as discussed in the text. Redox response is indicated by + and no response is indicated by − sign. Compartments not measured are indicated by a blank space. Abbreviations: PM, plasma membrane: Cyto, cytoplasm; ER, endoplasmic reticulum; Mito, mitochondria; Nucl, nuclei. Where different responses occurred for redox components within the same compartment, respective redox sensitive components are given in parentheses. See text for additional details.
| Stimulus | PM | Cyto | ER | Mito | Nucl | Ref | |
|---|---|---|---|---|---|---|---|
| Normal condition | EGF | + | + | - | [30] | ||
| TNF-α | + | + | [32] | ||||
| IGF-1 | + | - | [63] | ||||
| Stressed condition | H2O2 | - | + | - | [20] | ||
| TBH | - | + | - | [20] | |||
| Nuclear H2O2 | - | - | + | [43] | |||
| -Glc & -Gln | + | - | + | [18] | |||
| +Cu; +Fe, or +Ni | + (GSH) - (Trx1) | - (Trx2) | [44] | ||||
| +As; +Cd; or +Hg | - (GSH) + (Trx1) | + (Trx2) | [44] | ||||
| Extracellular EhCySS | + | - | - | + | - | [24] |
The maintenance of thiol/disulphide redox states at different steady-state potentials in different subcellular compartments, the disequilibrium of major thiol/disulphide couples in individual subcellular compartments, and the evidence for redox signaling via discrete mechanisms in the different compartments, supports the interpretation that the maintenance of redox states of thiol/disulphide couples at stable, non-equilibrium conditions in different subcellular compartments represents a vital aspect of biologic organization and function. It follows, then, that specialized redox-dependent functions in subcellular compartments are likely to be differentially sensitive to stressors. Emerging data show compartment-specific dysfunction in response to different cell stresses, underscoring the need for more detailed investigation of compartment specific redox signaling mechanisms.
Redox compartmentalization under normal conditions
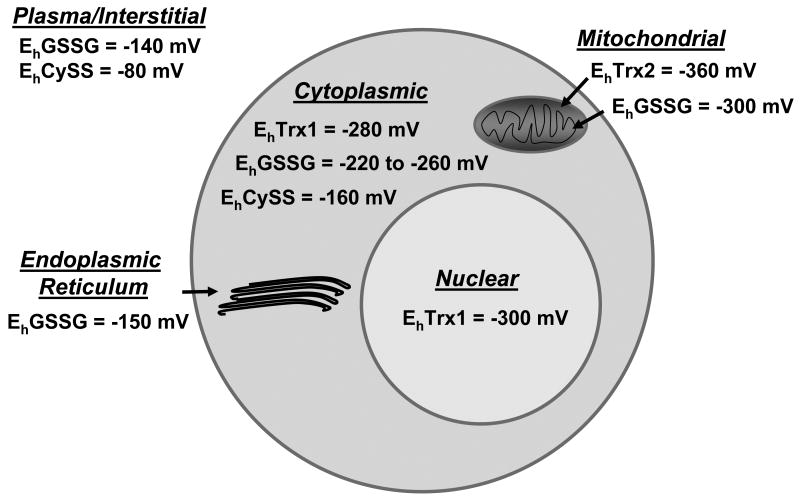
A general picture emerging from these targeted studies is that the compartmental organization of cells is optimized to match oxidative conditions with function ([5]; Fig. 2). For instance, the mitochondria, which are the most active in electron transfer, have Trx2 and GSH maintained at more reducing Eh than other compartments but are the most susceptible to oxidation. Nuclei have Trx1 and GSH maintained under relatively reducing Eh, but these nuclear pools are relatively resistant to oxidation. The lumen of the secretory pathway is relatively oxidizing due to systems which introduce disulphides into proteins during processing for secretion. Endosomes and lysosomes are also relatively oxidizing [33, 34] but have reductive mechanisms to reduce disulphides to facilitate proteolysis [35]. Peroxisomes are specialized compartments with catalase to allow cells to use reactions involving relatively high localized rates of H2O2 production.
Fig 2. Subcellular compartmentalization of thiol/disulfide redox potentials in cultured cells.
Based upon a limited number of cell culture studies, a picture of the normal redox compartmentalization is emerging. The most secure data is for the cytoplasmic GSH redox potential, which varies from about -260 mV in rapidly proliferating cells to about -220 mV in non-dividing cells. A few cell types and metabolic conditions result in more positive (oxidized values), but snap-frozen tissue extracts are typically in this range. Trx-1 redox state has been measured in many cell types and is more reduced than GSH/GSSG. The nuclear Trx1 is more reduced than cytoplasmic Trx1 in monocytic and colonic cell lines. The mitochondrial Trx-2 is more reduced than nuclear and cytoplasmic Trx1, and also more reduced than the mitochondrial GSH/GSSG. The mitochondrial GSH/GSSG is more reduced than cytoplasmic values, and the endoplasmic reticular GSH/GSSG is midway between cytoplasmic and plasma values. Cytoplasmic Cys/CySS redox potential is considerably oxidized relative to the GSH and Trx couples but reduced relative to plasma Cys/CySS.
The information which is currently available only probably gives a superficial picture of the redox circuitry in the different compartments. Cysteine, methionine and selenocysteine are the only amino acids in proteins that undergo reversible oxidation/reduction under biologic conditions, but Cys and Met are present in most proteins. Most redox signaling research has focused on H2O2 and other reactive oxygen species (ROS) as oxidants for signaling proteins. However, oxidation of sulfur-containing side chains of cysteine and methionine by ROS can result in higher oxidation states of sulfur (e.g., sulfinate, sulfonate, sulfone) that have limited or no reversibility under biologic conditions. The steady-state redox potential of Cys/CySS (Eh = −145 mV) in cytoplasm of cells is sufficiently oxidized (>90 mV) relative to the GSH/GSSG (−250 mV) and thioredoxin (Trx1, −280 mV) redox couples for the cysteine/cystine couple to function as an oxidant in sulfur switching [36]. Although speculative, the Cys/CySS couple provides a means to oxidize proteins without direct involvement of more potent oxidants, thereby protecting against over-oxidation. Moreover, inclusion of Cys/CySS as a redox control node which is distinct from GSH and Trx1 significantly expands the redox range over which protein thiol/disulphide couples may operate to control physiologically relevant processes.
Redox compartmentalization under stressed conditions
Oxidative stress in cytoplasm and mitochondria in response to exogenous peroxides
Oxidative stress induced by added peroxides is among the most extensively studied types of cell stress. Although early evidence supported a selective vulnerability of mitochondria, perhaps exemplified most clearly by GSH depletion in hepatic mitochondria during chronic alcohol exposure [37], the capability to directly demonstrate redox effects was limited to measurement of decreases in the thiol because of poor detection of the disulphide. However, following discovery of Trx-2 [38] and demonstration that overexpression of Trx2 protected against oxidant-induced apoptosis [39], several studies have shown that Trx2 is selectively vulnerable to oxidative stress. These studies have mostly relied upon redox western blot analysis [30] using the thiol reagent AMS [40] to quantify the EhTrx-2. Results with an osteosarcoma cell line show that Trx-2 is selectively oxidized relative to cytoplasmic Trx-1, nuclear Trx-1 and cellular GSH when cells are treated with H2O2 or tert-butylhydroperoxide (tBH) [20]. Trx-2 was also oxidized by the thiol oxidant, diamide, and overexpression of Trx-2 protected against diamide-induced oxidation and cytotoxicity. Oxidation of Trx-2 occurred by knocking down its reductase, and this also caused increased susceptibility to cell death. Similarly, in cultured SH-SY5Y human neuroblastoma cells, overexpression of Trx-2 inhibited apoptosis and loss of mitochondrial membrane potential induced by tBH. Responses to the calcium ionophore (Br-A23187) were not affected by Trx-2, suggesting the protection was specific against oxidative injury. The mitochondrial GSH pool was oxidized by tBH, and this oxidation was not inhibited by increased Trx-2. Consequently, the antioxidant function of Trx-2 is not redundant to that of GSH. The catalytically inactive C90,93S Trx-2 was ineffective in protecting against tBH-induced cytoxicity. Together, these studies show that the redox status of Trx-2 is a regulatory mechanism underlying the vulnerability of mitochondria to oxidative stress.
Oxidative stress in cytoplasm and nuclei in response to exogenous peroxides
Trx-1 is a key redox control system within the nucleus, which is enhanced by translocation of cytoplasmic Trx-1 to nuclei during periods of stress [41]. Studies of oxidant-induced changes in the redox states of nuclear Trx1, cytoplasmic Trx1, and cellular GSH in THP1 cells showed that nuclear Trx1 was more reducing than cytoplasmic Trx1 and cellular GSH. tBH caused an increase in the total amount of nuclear Trx1, and a 60 mV oxidation. However, the extent of oxidation was less than that of the cytoplasm, showing that the nuclear pool responds to stress with improved capability to maintain redox state.
A more specific function of Trx1 in the nucleus was demonstrated by studies of peroxiredoxins (Prx) in HeLa cells. Prx are widely distributed and abundant proteins, which function as Trx-dependent peroxidases [42]. Accumulating evidence indicates that Prx have an important role in redox signaling [42]. Prx1 is found in the cytoplasm and nucleus, and studies with targeted expression to increase Prx1 in nuclei (NLS-Prx1) and cytoplasm (NES-Prx1) showed that cytoplasmic Prx1 inhibited NF-κB activation and nuclear translocation while nuclear Prx1 did not affect NF-κB nuclear translocation but increased activity of an NF-κB reporter. Both nuclear and cytoplasmic Prx1 inhibited NF-κB p50 oxidation, suggesting that oxidation of the redox-sensitive cysteine in p50's DNA-binding domain is regulated via peroxide metabolism in both compartments. Nuclear Trx-1 oxidation by added H2O2 was protected by NLS-Prx1, and cytoplasmic Trx-1 was protected by NES-Prx1. These compartmental differences due to Prx1 show that the redox poise of cytoplasmic and nuclear thiol systems can be dynamically controlled through changing peroxide elimination. These data provide strong support for the concept that the balance of peroxide generation/metabolism in subcellular compartments is a specific component of redox signaling and that perturbation at the level of subcellular compartments controls cell responses to stress.
Oxidative stress in cytoplasm and nuclei in response to endogenous peroxide
To examine stress induced by H2O2 in nuclei, a fusion protein (NLS-DAAO) of an H2O2-producing enzyme, D-amino acid oxidase (DAAO) and a nuclear localization sequence (NLS) was used to generate H2O2 in the nuclei of HeLa cells [43]. Addition of N-acetyl-D-alanine (NADA), a substrate of DAAO, caused a twofold increase in ROS production, and staining of cellular thiols confirmed that NLS-DAAO–induced ROS selectively modified the nuclear thiol pool, whereas the cytoplasmic pool remained unchanged. NLS-DAAO/NADA–induced ROS caused significant oxidation of the nuclear GSH pool as measured by nuclear protein S-glutathionylation (Pr-SSG), but did not cause significant oxidation of nuclear Trx1. NF-κB reporter activity was diminished by nuclear H2O2 generation. The results show that in HeLa cells, nuclear GSH is more susceptible to localized oxidation than is nuclear Trx1. In addition to other studies showing that Trx1 is important in regulating NF-κB activity in nuclei, these results show that critical nuclear proteins are also subject to control by S-glutathionylation during nuclear oxidative stress.
Oxidative stress in cytoplasm, nuclei, and mitochondria to energy precursor deficiency
The relative sensitivities of thiol antioxidant systems in nuclei, mitochondria, and cytoplasm to conditions of energy limitation were studied in human colonic epithelial (HT29) cells by depletion of glucose (Glc) and glutamine (Gln) from the culture medium [18]. Increased oxidation of dichlorofluoroscein (DCF) indicated an increased level of ROS, and redox western blot analysis showed oxidation of cytosolic Trx1 and mitochondrial Trx2, but little oxidation of nuclear Trx1. The Trx1 substrate, redox factor-1 (Ref-1), was also oxidized in cytosol but was reduced in nuclei. Protein S-glutathionylation (PrSSG), expressed as a ratio of protein thiol (PrSH), was increased in the cytosol, while nuclear PrSSG/PrSH was not. These data show that oxidative stress induced by depletion of Glc and Gln affects the redox states of proteins in the cytoplasm and mitochondria more than those in the nucleus. The results show that nuclei have better protection against oxidative stress than cytoplasm or mitochondria and suggest that energy and/or substrate supply may contribute to sensitivity of mitochondrial and cytoplasmic systems to oxidative stress.
Oxidative stress in cytoplasm and mitochondria in response to metals
Excess exposure to transition metals is a common component of disease which can occur due to direct metal ion interactions with macromolecules and also by generation of ROS and subsequent oxidative stress. The effects of arsenic, cadmium, cesium, copper, iron, mercury, nickel, and zinc on cellular GSH, cytoplasmic Trx1 and mitochondrial Trx2 redox states were studied in HeLa cells [44]. Copper, iron, and nickel showed significant oxidation of GSH but relatively little oxidation of either Trx1 or Trx2. In contrast, arsenic, cadmium, and mercury showed little oxidation of GSH but significantly oxidized both Trx1 and Trx2. The magnitude of effects of arsenic, cadmium, and mercury was greater for the mitochondrial Trx2 (>60 mV) compared to the cytoplasmic Trx1 (20 to 40 mV). Apoptosis signal-regulating kinase 1 (ASK1) activation and cell death were observed with metals that oxidized thioredoxins but not with metals that oxidized GSH. Although oxidation was greater in mitochondria, ASK1 activation occurs in both the mitochondria and cytoplasm [45]. Consequently, the differential oxidation of the major thiol antioxidant systems by metal ions suggest that the GSH/GSSG and Trx systems convey different levels of control in apoptotic and toxic signaling pathways, but do not clearly show whether this is predominantly activated in mitochondria or shared between the mitochondrial and cytoplasmic systems.
Phenotypic alterations in response to extracellular EhCySS oxidation
As indicated in the introduction, many disease states, including diabetes, are associated with systemic oxidative stress as measured by thiol/disulphide couples in the plasma [46]. The cause-effect relationship of oxidation of the plasma Cys/CySS couple has been studied in many cell models using a redox clamp model [47]. In this model, extracellular Cys/CySS redox potential is systematically varied for short-term studies by changing the Cys and CySS concentrations with constant total Cys equivalents. Using this model, endothelial cells exposed to a more oxidized (0 mV) potential had stimulated H2O2 production but did not have increased nitric oxide production [48]. Exposure of cells to the oxidized EhCySS activated NF-κB, increased expression of adhesion molecules (intercellular adhesion molecule-1, platelet endothelial cell adhesion molecule-1, P-selectin), and stimulated monocytes binding to endothelial cells. Extracellular Eh regulated thiol/disulphide redox states of cell surface membrane proteins, and cell impermeant alkylating agents blocked H2O2 production, indicating that variation in extracellular Eh is detected and signaled at the cell surface.
The EhCySS dependence of monocyte adhesion to endothelial cells was found to involve responses in both monocytes and endothelial cells. In vitro exposure of monocytes to oxidized EhCySS increased expression of the proinflammatory cytokine, interleukin-1β (IL-1β), suggesting that plasma EhCySS could be a systemic indicator of stress which provides a mechanistic link between oxidative stress and chronic inflammation [49]. In experiments to determine whether EhCySS specifically affects proinflammatory signaling or has other effects on monocytes, gene expression arrays and mass spectrometry–based proteomics were used to evaluate global changes in protein redox state, gene expression, and protein abundance in monocytes in response to EhCySS. Pathway analysis revealed that in addition to IL-1β-related pathways, components of stress/detoxification and cell death pathways were increased by oxidized EhCySS, while components of cell growth and proliferation pathways were increased by a reduced potential. Although no specific compartmental analysis was performed, results included membranal, cytoplasmic, nuclear and mitochondrial proteins. Phenotypic studies confirmed that a cell stress response occurred with oxidized Eh and that cell proliferation was stimulated with reduced Eh [49].
More detailed studies in U937 monocytes showed that oxidized Eh Cys/CySS is a determinant of IL-1β levels [50]. A 1.7-fold increase in secreted pro-IL-1β levels was observed in cells exposed to oxidized Eh Cys/CySS (-46 mV), compared to controls exposed to a normal physiological Eh of -80 mV. In LPS-challenged mice, preservation of plasma EhCySS from oxidation by dietary sulfur amino acid (SAA) supplementation, was associated with a 1.6-fold decrease in plasma IL-1β compared to control mice fed an isonitrogenous SAA-adequate diet. In humans, analysis of EhCySS and IL-1β in plasma revealed a significant positive association between oxidized EhCySS and IL-1β after controlling for age, gender, and BMI. These data show that oxidation of Cys/CySS in the plasma compartment represents an important stress which is linked to proinflammatory signaling.
Additional studies of the stress of oxidized EhCySS in endothelial cells showed that mitochondria are a major source of ROS in this response [24]. The increase in ROS was blocked by overexpression of mitochondrial Trx-2 in endothelial cells from Trx2-transgenic mice. Mass spectrometry-based redox proteomics showed that several classes of plasma membrane and cytoskeletal proteins involved in inflammation responded to this redox switch, including vascular cell adhesion molecule, integrins, actin, and several Ras family GTPases [24]. Together, the data show that the proinflammatory effects of oxidized plasma EhCySS are due to a mitochondrial signaling pathway that is mediated through redox control of downstream effector proteins.
The redox clamp model has also been used to study the subcellular responses to extracellular EhCySS in a model of oxidant-induced apoptosis in cultured human retinal pigment epithelial (hRPE) cells [51]. The hRPE cells were incubated in culture medium with Eh from -16 mV (most oxidized) to -158 mV (most reduced) and apoptosis was induced with tBH. The hRPE cells were sensitized to tBH-induced apoptosis in the more oxidized extracellular conditions (Eh>-55 mV) compared with the reduced conditions (Eh <-89 mV). Loss of mitochondrial membrane potential (mtΔψ), release of cytochrome c, and activation of caspase 3 after tBH treatments increased under the more oxidized conditions. However, the extracellular redox state did not affect expression of Fas or FasL in hRPE cells. The results show that hRPE cells exposed to a more oxidized Eh have increased susceptibility to oxidant-induced apoptosis through the mitochondrial compartment but not through the cytoplasmic/nuclear signaling of the Fas system, suggesting the same compartmentalization of responses as in endothelial cells described above.
Studies using the redox clamp model have also linked systemic oxidant stress to fibrogenesis in a model of profibrotic signaling [52]. Primary murine lung fibroblasts exposed to an oxidized EhCySS (-46 mV) had stimulated proliferation and enhanced expression of fibronectin, a matrix glycoprotein highly expressed in fibrotic lung diseases. This stimulation was dependent on protein kinase C activation and increased the phosphorylation of cAMP response element binding protein, a transcription factor known for its ability to stimulate fibronectin expression. Oxidized EhCySS increased the expression of mRNAs and proteins coding for the transcription factors nuclear factors NF-κB and mothers against decapentaplegic homolog 3, SMAD3. Fibronectin expression in response to an oxidized EhCySS was associated with expression of transforming growth factor-β1 (TGF-β1) and was inhibited by an anti-TGF-β1 antibody and SB-431542, a TGF-β1 receptor inhibitor. The details of the compartmentalization of signaling from the extracellular Eh to the nuclei which control lung fibroblast proliferation and matrix expression have not been studied.
Reductive stress through extracellular EhCySS
The possibility that disease could be caused by excessive reduction has recently been discussed [53]. Reductive stress resulting from increased GSH level has been linked to disruption of antioxidative pathways in mice [54, 55]. In addition, deleterious effects of reductive stress were shown in different organisms, such as reduced life span of yeast and C. elegans and age-related protein aggregation disease in mice [55, 56]. Although this is included in the redox hypothesis as a disruption of redox signaling and control, excessively reducing conditions are distinct from more common conditions of excessive oxidants which have been frequently linked to disease. Reductive stress was considered by Chance [57]as a possible mechanism of anoxic injury, and subsequently as a component of hypoxic potentiation of halothane-induced liver toxicity [58], reoxygenation injury [59] and enhanced killing of hypoxic tumor cells [60].
More recent data show that human cells in culture require controlled extracellular Eh for optimal growth [61] and that EhCySS is sufficient to control this response [62, 63]. A series of studies show that reductive stress created by -150 mV EhCySS stimulates growth-factor insensitive growth in a human colon carcinoma cell line (Caco2). Eh effects on DNA synthesis and increase in cell number were equivalent to those attained with insulin-like growth factor-1 (IGF-1). epidermal growth factor (EGF), keratinocyte growth factor or glutamine. The signaling was independent of cellular GSH and occurred through compartmentalized signaling at the plasma membrane [64]. Growth signaling through EGF receptor was mediated at least in part by metalloproteinase release of TGF-α. A similar growth response was observed in normal hRPE cells [51] and endothelial cells (observation from our unpublished data), but was not as robust as in cancer cell lines. Thus, reductive stress may be important in signaling excessive growth in cancer cells, and additional investigation is warranted.
Although not studied extensively, redox state of cytoplasmic GSH/GSSG and Cys/CySS varies as a function of proliferation, differentiation and apoposis [36, 65]. The detoxification enzyme inducer, benzyl isothiocyanate caused a 16-mV oxidation in proliferating cells (from -260 to -244 mV) but a 40-mV oxidation (from -200 to -160 mV) in differentiated cells. Glutathione S-transferase (GST) and nicotinamide adenine dinucleotide phosphate (NADPH):quinone reductase activities (N:QR) changed in association with the Eh, showing a sufficient magnitude of change to control the activity of redox-sensitive proteins. Induction of detoxification enzymes is usually associated with a protective response, but in colonic epithelial cells this appears to be a stress signal which activates a delayed apoptotic signaling [66].
Future research needs
Additional research is needed to obtain a more systematic understanding of signaling with the life cycle of cells. The studies summarized in the current review include a number of cell lines, but no studies are available to address the variation in redox characteristics of the subcellular compartments in different organ systems in vivo. Consequently, there is an important need to apply available methods to understand variations which occur in animal models. As indicated above, differences are expected to occur due to cell cycle and differentiation programs; it is currently unknown whether redox stress at specific times of development trigger epigenetic changes with long-term health consequences.
A second needed area of research concerns discrimination of beneficial and adverse effects. Stress responses do not necessarily result in adverse effects so that translation of in vitro results into in vivo studies is needed to evaluate the biologic importance of subcellular redox responses. For instance, sensing by Nrf-2 in the cytoplasm is among the most sensitive of redox responses. Oxidants are detected by Keap-1, an actin associated protein with 26 thiols. Oxidant or electrophile reaction with Keap-1 causes release of Nrf-2 and translocation of Nrf-2 into the nucleus. The regulatory process is integrated with kinase/phosphatase and ubiquitin/proteosome degradation control mechanisms and both of these are sensitive to redox control. In the nucleus, Nrf-2 binding to DNA is also redox dependent. Consequently, the integration of redox control with other cell regulatory processes is complex, requires advanced system biology models for prediction of responses, and does not allow simple interpretation concerning whether responses are beneficial or adverse.
A third area requiring additional investigation includes the mechanisms controlling transmembranal redox gradients. Transport systems for GSH occur in the plasma membrane [67], mitochondria [68] and endoplasmic reticulum [69]. ATP-dependent GSSG efflux occurs in most, in not all cells [70]. Earlier studies showed that regulation of GSH transport is sensitive to adrenergic agonists [71], and GSH efflux occurs during activation of apoptosis [72], but additional research is needed to understand physiologic as opposed to toxicologic responses. Numerous Cys and CySS transport systems are known [73], but there is little understanding of how these systems are integrated to control transmembranal redox gradients. The quantitative contribution of transmembranal electron transfer from NADPH to O2 by NADPH oxidases and from NADH to other electron acceptors also needs further investigation.
A rapidly developing opportunity exists for application of mass spectrometry-based redox proteomics methods [25, 26, 74] which can be expected to help elucidate the critical redox pathways functioning in compartmental control. Development of a consortium for this could allow proteomics databases to be expanded to contain systematic data on redox sensitivity of specific Cys residues in proteins.
Finally, development of an understanding of redox compartmentalization associated with membrane-delineated structures may be only an approximation of a spatially limited microcompartmentalization. D'Autreaux and Toledano [10] described the concept of specificity in redox signaling due to spatial distribution of signaling components. Actin and actin-associated proteins have highly conserved Cys residues which are susceptible to oxidation, glutathionylation and other covalent modifications. Evidence is available to show functional changes by these redox mechanisms [75]. Consequently, standing redox gradients within cells could direct macromolecular trafficking or protein distribution. Spatial differences in redox state could also result in localized effects on translation, proteolysis and protein processing. Processes such as protein import into mitochondria, protein import into nuclei (e,g., glucocorticoid receptor) and protein export by nuclei, are redox-sensitive and could be locally affected. The development of selective redox-sensitive probes such as the Grx-GFP fusion, will be needed to test such possibilities in live cells.
Summary and Conclusions
Considerable advances in understanding redox compartmentalization and responses to stress have been obtained using techniques to examine steady-state oxidation/reduction balance of proteins with specific subcellular distribution. The results of these studies provide a general picture that mitochondria and nuclei are relatively reducing compartments which differ in that mitochondria are highly sensitive to oxidation while nuclei are relatively resistant. The mitochondrial compartment is relatively susceptible to deficiency in metabolic substrates but the nuclear compartment is not. The cytoplasmic compartment undergoes oxidation in the normal life cycle of cells, with the GSH system being more responsive to the signaling in differentiated cells than in proliferating cells. The secretory pathway and endosome/lysosome compartments are relatively oxidized compared to the cytoplasm, apparently reflecting the transition between the cytoplasm and the more oxidized extracellular compartment.
Acknowledgments
The research of the authors is supported by NIH grants, ES009047, ES011195 and ES016731.
References
- 1.Jones DP, Carlson JL, Mody VC, Cai J, Lynn MJ, Sternberg P. Redox state of glutathione in human plasma. Free Radic Biol Med. 2000;28:625–635. doi: 10.1016/s0891-5849(99)00275-0. [DOI] [PubMed] [Google Scholar]
- 2.Jones DP, Mody VC, Jr, Carlson JL, Lynn MJ, Sternberg P., Jr Redox analysis of human plasma allows separation of pro-oxidant events of aging from decline in antioxidant defenses. Free Radic Biol Med. 2002;33:1290–1300. doi: 10.1016/s0891-5849(02)01040-7. [DOI] [PubMed] [Google Scholar]
- 3.Jones DP. Redefining oxidative stress. Antioxid Redox Signal. 2006;8:1865–1879. doi: 10.1089/ars.2006.8.1865. [DOI] [PubMed] [Google Scholar]
- 4.Sies H, Jones D. Oxidative stress. 2nd. Elsevier; 2007. [Google Scholar]
- 5.Go YM, Jones DP. Redox compartmentalization in eukaryotic cells. Biochim Biophys Acta. 2008;1780:1273–1290. doi: 10.1016/j.bbagen.2008.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kemp M, Go YM, Jones DP. Nonequilibrium thermodynamics of thiol/disulfide redox systems: a perspective on redox systems biology. Free Radic Biol Med. 2008;44:921–937. doi: 10.1016/j.freeradbiomed.2007.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Forman HJ, Maiorino M, Ursini F. Signaling functions of reactive oxygen species. Biochemistry. 2010;49:835–842. doi: 10.1021/bi9020378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schafer FQ, Buettner GR. Redox environment of the cell as viewed through the redox state of the glutathione disulfide/glutathione couple. Free Radic Biol Med. 2001;30:1191–1212. doi: 10.1016/s0891-5849(01)00480-4. [DOI] [PubMed] [Google Scholar]
- 9.Jones DP. Radical-free biology of oxidative stress. Am J Physiol Cell Physiol. 2008;295:C849–868. doi: 10.1152/ajpcell.00283.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.D'Autreaux B, Toledano MB. ROS as signalling molecules: mechanisms that generate specificity in ROS homeostasis. Nat Rev Mol Cell Biol. 2007;8:813–824. doi: 10.1038/nrm2256. [DOI] [PubMed] [Google Scholar]
- 11.Ziegler DM. Role of reversible oxidation-reduction of enzyme thiols-disulfides in metabolic regulation. Annu Rev Biochem. 1985;54:305–329. doi: 10.1146/annurev.bi.54.070185.001513. [DOI] [PubMed] [Google Scholar]
- 12.Sitia R, Braakman I. Quality control in the endoplasmic reticulum protein factory. Nature. 2003;426:891–894. doi: 10.1038/nature02262. [DOI] [PubMed] [Google Scholar]
- 13.Sies H, Summer KH. Hydroperoxide-metabolizing systems in rat liver. Eur J Biochem. 1975;57:503–512. doi: 10.1111/j.1432-1033.1975.tb02325.x. [DOI] [PubMed] [Google Scholar]
- 14.Gilbert HF. Molecular and cellular aspects of thiol-disulfide exchange. Adv Enzymol Relat Areas Mol Biol. 1990;63:69–172. doi: 10.1002/9780470123096.ch2. [DOI] [PubMed] [Google Scholar]
- 15.Sies H. Nicotinamide nucleotide compartmentation. London: Academic Press; 1982. [Google Scholar]
- 16.Jones DP, Liang Y. Measuring the poise of thiol/disulfide couples in vivo. Free Radic Biol Med. 2009;47:1329–1338. doi: 10.1016/j.freeradbiomed.2009.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jones DP. Redox potential of GSH/GSSG couple: assay and biological significance. Methods Enzymol. 2002;348:93–112. doi: 10.1016/s0076-6879(02)48630-2. [DOI] [PubMed] [Google Scholar]
- 18.Go YM, Ziegler TR, Johnson JM, Gu L, Hansen JM, Jones DP. Selective protection of nuclear thioredoxin-1 and glutathione redox systems against oxidation during glucose and glutamine deficiency in human colonic epithelial cells. Free Radic Biol Med. 2007;42:363–370. doi: 10.1016/j.freeradbiomed.2006.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gutscher M, Pauleau AL, Marty L, et al. Real-time imaging of the intracellular glutathione redox potential. Nat Methods. 2008;5:553–559. doi: 10.1038/nmeth.1212. [DOI] [PubMed] [Google Scholar]
- 20.Chen Y, Cai J, Jones DP. Mitochondrial thioredoxin in regulation of oxidant-induced cell death. FEBS Lett. 2006;580:6596–6602. doi: 10.1016/j.febslet.2006.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mezghrani A, Fassio A, Benham A, Simmen T, Braakman I, Sitia R. Manipulation of oxidative protein folding and PDI redox state in mammalian cells. The EMBO journal. 2001;20:6288–6296. doi: 10.1093/emboj/20.22.6288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hwang C, Lodish HF, Sinskey AJ. Measurement of glutathione redox state in cytosol and secretory pathway of cultured cells. Methods Enzymol. 1995;251:212–221. doi: 10.1016/0076-6879(95)51123-7. [DOI] [PubMed] [Google Scholar]
- 23.Watson WH, Jones DP. Oxidation of nuclear thioredoxin during oxidative stress. FEBS Lett. 2003;543:144–147. doi: 10.1016/s0014-5793(03)00430-7. [DOI] [PubMed] [Google Scholar]
- 24.Go YM, Park H, Koval M, et al. A key role for mitochondria in endothelial signaling by plasma cysteine/cystine redox potential. Free Radic Biol Med. 2010;48:275–283. doi: 10.1016/j.freeradbiomed.2009.10.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Go YM, Pohl J, Jones DP. Quantification of redox conditions in the nucleus. Methods in molecular biology (Clifton, NJ. 2009;464:303–317. doi: 10.1007/978-1-60327-461-6_17. [DOI] [PubMed] [Google Scholar]
- 26.Leichert LI, Gehrke F, Gudiseva HV, et al. Quantifying changes in the thiol redox proteome upon oxidative stress in vivo. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:8197–8202. doi: 10.1073/pnas.0707723105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xanthoudakis S, Curran T. Identification and characterization of Ref-1, a nuclear protein that facilitates AP-1 DNA-binding activity. The EMBO journal. 1992;11:653–665. doi: 10.1002/j.1460-2075.1992.tb05097.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lambeth JD. NOX enzymes and the biology of reactive oxygen. Nature reviews. 2004;4:181–189. doi: 10.1038/nri1312. [DOI] [PubMed] [Google Scholar]
- 29.Go YM, Gipp JJ, Mulcahy RT, Jones DP. H2O2-dependent activation of GCLC-ARE4 reporter occurs by mitogen-activated protein kinase pathways without oxidation of cellular glutathione or thioredoxin-1. J Biol Chem. 2004;279:5837–5845. doi: 10.1074/jbc.M307547200. [DOI] [PubMed] [Google Scholar]
- 30.Halvey PJ, Watson WH, Hansen JM, Go YM, Samali A, Jones DP. Compartmental oxidation of thiol-disulphide redox couples during epidermal growth factor signalling. Biochem J. 2005;386:215–219. doi: 10.1042/BJ20041829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hansen JM, Watson WH, Jones DP. Compartmentation of Nrf-2 redox control: regulation of cytoplasmic activation by glutathione and DNA binding by thioredoxin-1. Toxicol Sci. 2004;82:308–317. doi: 10.1093/toxsci/kfh231. [DOI] [PubMed] [Google Scholar]
- 32.Hansen JM, Zhang H, Jones DP. Mitochondrial thioredoxin-2 has a key role in determining tumor necrosis factor-alpha-induced reactive oxygen species generation, NF-kappaB activation, and apoptosis. Toxicol Sci. 2006;91:643–650. doi: 10.1093/toxsci/kfj175. [DOI] [PubMed] [Google Scholar]
- 33.Austin CD, Wen X, Gazzard L, Nelson C, Scheller RH, Scales SJ. Oxidizing potential of endosomes and lysosomes limits intracellular cleavage of disulfide-based antibody-drug conjugates. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:17987–17992. doi: 10.1073/pnas.0509035102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hwang C, Sinskey AJ, Lodish HF. Oxidized redox state of glutathione in the endoplasmic reticulum. Science (New York, NY. 1992;257:1496–1502. doi: 10.1126/science.1523409. [DOI] [PubMed] [Google Scholar]
- 35.Chakravarthi S, Jessop CE, Bulleid NJ. The role of glutathione in disulphide bond formation and endoplasmic-reticulum-generated oxidative stress. EMBO reports. 2006;7:271–275. doi: 10.1038/sj.embor.7400645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jones DP, Go YM, Anderson CL, Ziegler TR, Kinkade JM, Jr, Kirlin WG. Cysteine/cystine couple is a newly recognized node in the circuitry for biologic redox signaling and control. FASEB J. 2004;18:1246–1248. doi: 10.1096/fj.03-0971fje. [DOI] [PubMed] [Google Scholar]
- 37.Fernandez-Checa JC, Ookhtens M, Kaplowitz N. Effect of chronic ethanol feeding on rat hepatocytic glutathione. Compartmentation, efflux, and response to incubation with ethanol. The Journal of clinical investigation. 1987;80:57–62. doi: 10.1172/JCI113063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Spyrou G, Enmark E, Miranda-Vizuete A, Gustafsson J. Cloning and expression of a novel mammalian thioredoxin. J Biol Chem. 1997;272:2936–2941. doi: 10.1074/jbc.272.5.2936. [DOI] [PubMed] [Google Scholar]
- 39.Chen Y, Cai J, Murphy TJ, Jones DP. Overexpressed human mitochondrial thioredoxin confers resistance to oxidant-induced apoptosis in human osteosarcoma cells. J Biol Chem. 2002;277:33242–33248. doi: 10.1074/jbc.M202026200. [DOI] [PubMed] [Google Scholar]
- 40.Damdimopoulos AE, Miranda-Vizuete A, Pelto-Huikko M, Gustafsson JA, Spyrou G. Human mitochondrial thioredoxin. Involvement in mitochondrial membrane potential and cell death. J Biol Chem. 2002;277:33249–33257. doi: 10.1074/jbc.M203036200. [DOI] [PubMed] [Google Scholar]
- 41.Wei SJ, Botero A, Hirota K, et al. Thioredoxin nuclear translocation and interaction with redox factor-1 activates the activator protein-1 transcription factor in response to ionizing radiation. Cancer research. 2000;60:6688–6695. [PubMed] [Google Scholar]
- 42.Rhee SG, Chae HZ, Kim K. Peroxiredoxins: a historical overview and speculative preview of novel mechanisms and emerging concepts in cell signaling. Free Radic Biol Med. 2005;38:1543–1552. doi: 10.1016/j.freeradbiomed.2005.02.026. [DOI] [PubMed] [Google Scholar]
- 43.Halvey PJ, Hansen JM, Johnson JM, Go YM, Samali A, Jones DP. Selective oxidative stress in cell nuclei by nuclear-targeted D-amino acid oxidase. Antioxid Redox Signal. 2007;9:807–816. doi: 10.1089/ars.2007.1526. [DOI] [PubMed] [Google Scholar]
- 44.Hansen JM, Zhang H, Jones DP. Differential oxidation of thioredoxin-1, thioredoxin-2, and glutathione by metal ions. Free Radic Biol Med. 2006;40:138–145. doi: 10.1016/j.freeradbiomed.2005.09.023. [DOI] [PubMed] [Google Scholar]
- 45.Zhang R, Al-Lamki R, Bai L, et al. Thioredoxin-2 inhibits mitochondria-located ASK1-mediated apoptosis in a JNK-independent manner. Circulation research. 2004;94:1483–1491. doi: 10.1161/01.RES.0000130525.37646.a7. [DOI] [PubMed] [Google Scholar]
- 46.Samiec PS, Drews-Botsch C, Flagg EW, et al. Glutathione in human plasma: decline in association with aging, age-related macular degeneration, and diabetes. Free Radic Biol Med. 1998;24:699–704. doi: 10.1016/s0891-5849(97)00286-4. [DOI] [PubMed] [Google Scholar]
- 47.Go YM, Jones DP. Redox clamp model for study of extracellular thiols and disulfides in redox signaling. Methods Enzymol. 2010 doi: 10.1016/S0076-6879(10)74010-6. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Go YM, Jones DP. Intracellular proatherogenic events and cell adhesion modulated by extracellular thiol/disulfide redox state. Circulation. 2005;111:2973–2980. doi: 10.1161/CIRCULATIONAHA.104.515155. [DOI] [PubMed] [Google Scholar]
- 49.Go YM, Craige SE, Orr M, Gernert KM, Jones DP. Gene and protein responses of human monocytes to extracellular cysteine redox potential. Toxicol Sci. 2009;112:354–362. doi: 10.1093/toxsci/kfp205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Iyer SS, Accardi CJ, Ziegler TR, et al. Cysteine redox potential determines pro-inflammatory IL-1beta levels. PLoS One. 2009;4:e5017. doi: 10.1371/journal.pone.0005017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jiang S, Moriarty-Craige SE, Orr M, Cai J, Sternberg P, Jr, Jones DP. Oxidant-induced apoptosis in human retinal pigment epithelial cells: dependence on extracellular redox state. Invest Ophthalmol Vis Sci. 2005;46:1054–1061. doi: 10.1167/iovs.04-0949. [DOI] [PubMed] [Google Scholar]
- 52.Ramirez A, Ramadan B, Ritzenthaler JD, Rivera HN, Jones DP, Roman J. Extracellular cysteine/cystine redox potential controls lung fibroblast proliferation and matrix expression through upregulation of transforming growth factor-beta. Am J Physiol Lung Cell Mol Physiol. 2007;293:L972–981. doi: 10.1152/ajplung.00010.2007. [DOI] [PubMed] [Google Scholar]
- 53.Ralser M, Benjamin IJ. Reductive stress on life span extension in C. elegans. BMC research notes. 2008;1:19. doi: 10.1186/1756-0500-1-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ghyczy M, Boros M. Electrophilic methyl groups present in the diet ameliorate pathological states induced by reductive and oxidative stress: a hypothesis. The British journal of nutrition. 2001;85:409–414. doi: 10.1079/bjn2000274. [DOI] [PubMed] [Google Scholar]
- 55.Rajasekaran NS, Connell P, Christians ES, et al. Human alpha B-crystallin mutation causes oxido-reductive stress and protein aggregation cardiomyopathy in mice. Cell. 2007;130:427–439. doi: 10.1016/j.cell.2007.06.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ralser M, Wamelink MM, Kowald A, et al. Dynamic rerouting of the carbohydrate flux is key to counteracting oxidative stress. Journal of biology. 2007;6:10. doi: 10.1186/jbiol61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chance B. Cellular oxygen requirements. Federation proceedings. 1957;16:671–680. [PubMed] [Google Scholar]
- 58.de Groot H, Noll T. The crucial role of hypoxia in halothane-induced lipid peroxidation. Biochem Biophys Res Commun. 1984;119:139–143. doi: 10.1016/0006-291x(84)91629-2. [DOI] [PubMed] [Google Scholar]
- 59.Granger DN, Rutili G, McCord JM. Superoxide radicals in feline intestinal ischemia. Gastroenterology. 1981;81:22–29. [PubMed] [Google Scholar]
- 60.Clarke ED, Goulding KH, Wardman P. Nitroimidazoles as anaerobic electron acceptors for xanthine oxidase. Biochem Pharmacol. 1982;31:3237–3242. doi: 10.1016/0006-2952(82)90556-1. [DOI] [PubMed] [Google Scholar]
- 61.Hwang C, Sinskey A. The role of oxidation-reduction potential in monitoring growth of cultured mammalian cells. Oxford; Halley Court: 1991. [Google Scholar]
- 62.Jonas CR, Gu LH, Nkabyo YS, et al. Glutamine and KGF each regulate extracellular thiol/disulfide redox and enhance proliferation in Caco-2 cells. American journal of physiology. 2003;285:R1421–1429. doi: 10.1152/ajpregu.00702.2002. [DOI] [PubMed] [Google Scholar]
- 63.Jonas CR, Ziegler TR, Gu LH, Jones DP. Extracellular thiol/disulfide redox state affects proliferation rate in a human colon carcinoma (Caco2) cell line. Free Radic Biol Med. 2002;33:1499–1506. doi: 10.1016/s0891-5849(02)01081-x. [DOI] [PubMed] [Google Scholar]
- 64.Nkabyo YS, Go YM, Ziegler TR, Jones DP. Extracellular cysteine/cystine redox regulates the p44/p42 MAPK pathway by metalloproteinase-dependent epidermal growth factor receptor signaling. Am J Physiol Gastrointest Liver Physiol. 2005;289:G70–78. doi: 10.1152/ajpgi.00280.2004. [DOI] [PubMed] [Google Scholar]
- 65.Kirlin WG, Cai J, Thompson SA, Diaz D, Kavanagh TJ, Jones DP. Glutathione redox potential in response to differentiation and enzyme inducers. Free Radic Biol Med. 1999;27:1208–1218. doi: 10.1016/s0891-5849(99)00145-8. [DOI] [PubMed] [Google Scholar]
- 66.Kirlin WG, Cai J, DeLong MJ, Patten EJ, Jones DP. Dietary compounds that induce cancer preventive phase 2 enzymes activate apoptosis at comparable doses in HT29 colon carcinoma cells. J Nutr. 1999;129:1827–1835. doi: 10.1093/jn/129.10.1827. [DOI] [PubMed] [Google Scholar]
- 67.Garcia-Ruiz C, Fernandez-Checa JC, Kaplowitz N. Bidirectional mechanism of plasma membrane transport of reduced glutathione in intact rat hepatocytes and membrane vesicles. J Biol Chem. 1992;267:22256–22264. [PubMed] [Google Scholar]
- 68.Lash LH. Mitochondrial glutathione transport: physiological, pathological and toxicological implications. Chemico-biological interactions. 2006;163:54–67. doi: 10.1016/j.cbi.2006.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Csala M, Fulceri R, Mandl J, Benedetti A, Banhegyi G. Glutathione transport in the endo/sarcoplasmic reticulum. BioFactors (Oxford, England) 2003;17:27–35. doi: 10.1002/biof.5520170104. [DOI] [PubMed] [Google Scholar]
- 70.Leier I, Jedlitschky G, Buchholz U, et al. ATP-dependent glutathione disulphide transport mediated by the MRP gene-encoded conjugate export pump. Biochem J. 1996;314(Pt 2):433–437. doi: 10.1042/bj3140433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sies H, Akerboom TP. Glutathione disulfide (GSSG) efflux from cells and tissues. Methods Enzymol. 1984;105:445–451. doi: 10.1016/s0076-6879(84)05062-x. [DOI] [PubMed] [Google Scholar]
- 72.van den Dobbelsteen DJ, Nobel CS, Schlegel J, Cotgreave IA, Orrenius S, Slater AF. Rapid and specific efflux of reduced glutathione during apoptosis induced by anti-Fas/APO-1 antibody. J Biol Chem. 1996;271:15420–15427. doi: 10.1074/jbc.271.26.15420. [DOI] [PubMed] [Google Scholar]
- 73.Bannai S. Transport of cystine and cysteine in mammalian cells. Biochim Biophys Acta. 1984;779:289–306. doi: 10.1016/0304-4157(84)90014-5. [DOI] [PubMed] [Google Scholar]
- 74.Butterfield DA, Sultana R. Redox proteomics: understanding oxidative stress in the progression of age-related neurodegenerative disorders. Expert review of proteomics. 2008;5:157–160. doi: 10.1586/14789450.5.2.157. [DOI] [PubMed] [Google Scholar]
- 75.Mieyal JJ, Gallogly MM, Qanungo S, Sabens EA, Shelton MD. Molecular mechanisms and clinical implications of reversible protein S-glutathionylation. Antioxid Redox Signal. 2008;10:1941–1988. doi: 10.1089/ars.2008.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]