Abstract
Socioemotional abnormalities, including low levels of social interaction and high levels of self-directed activity, were reported when rhesus monkeys with neonatal ablations of either the medial temporal lobe (AH) or the inferior temporal cortex (TE) were paired with unoperated peers at two and six months of age, though these abnormalities were more severe in the AH group (Bachevalier et al., 2001). As they reached adulthood (Experiment 1), the same monkeys were re-evaluated in the same dyads and their reactivity to novel toys, social status, and reactions to separation from age-matched peers were also assessed. Group TE now showed few if any of the abnormal behaviors observed when they were infants. By contrast, Group AH continued to display low levels of social interaction, high levels of self-directed activity and submissive behavior, and reduced responses to separation, although they reacted normally to novel toys. To determine whether this degree of socioemotional impairment was less severe than that produced by the same damage in adulthood, we assessed dyadic social interactions of monkeys raised until adulthood in laboratory conditions similar to those of the earlier groups and then given the AH ablation (Experiment 2). Two months postoperatively these adult-lesioned monkeys showed a small reduction in social interactions that became more pronounced six months postoperatively, yet remained less severe than that seen in the infant-lesioned monkeys. Also, except for an increase in food and water consumption throughout this 6-month period, they showed no other socioemotional effects. The finding that neonatal AH lesions produce more severe socioemotional disturbances than the same lesion in adulthood is the reverse of the effect commonly reported for other cognitive functions after cerebral damage.
Keywords: amygdala, hippocampus, area TE, infants, social interactions
Introduction
Bilateral damage to the medial temporal lobe in adult monkeys yields severe memory loss and profound changes in socioemotional behaviors (see Bachevalier & Meunier, 2005 for review; Klüver & Bucy, 1938, 1939; Mishkin, 1978; Rosvold, Mirsky, & Pribram, 1954). Similar memory loss and behavioral changes, though less severe, are also encountered after bilateral lesions of the inferior temporal area TE, a visual area located on the ventrolateral surface of the temporal neocortex (Gross, 1994). Yet, much less is known on whether these cognitive and behavioral changes also occur when the same damage is incurred in infancy. In a longitudinal study in monkeys, we followed the developmental trajectory of memory and socioemotional behaviors of monkeys that had received neonatal lesions of either the medial temporal lobe or temporal cortical area TE from infancy through adulthood. For memory functions, bilateral medial temporal removals in neonatal monkeys, like those in adult monkeys, produced severe and long-lasting deficits, whereas neonatal lesions of area TE yielded only relatively mild and transient deficits, evident when the monkeys were tested in infancy but not when they were retested as adults (Bachevalier & Mishkin, 1994; Málková, Mishkin, & Bachevalier, 1995). Apparently, the ability of the immature brain to compensate for injury-induced loss of memory function depends critically on the locus of injury. To examine whether a similar conclusion will apply to socioemotional behavior, we assessed the social interactions of the same two groups of neonatally-lesioned monkeys at the ages of 2 months and 6 months (Bachevalier, Malkova, & Mishkin, 2001). Both groups at 2 months displayed socioemotional disturbances and aberrant behaviors that became even more pronounced at 6 months and were particularly severe in the monkeys of the medial temporal group. Their abnormalities consisted of low levels of eye contact and of initiating social interaction, as well as active avoidance when approached by peers. They also showed a progressive increase in behavioral disturbance, such as irritability, locomotor stereotypies, and self-directed activities. The socioemotional disturbances in the inferior temporal group were milder, consisting mainly of initiating fewer social contacts than their controls. To determine whether or not the socioemotional disturbances in the two groups would follow a pattern of differential recovery with maturation similar to that found in memory functions, we reassessed the social interactions of the same monkeys when they reached adulthood (6–8 years of age). In the first experiment, we observed the interactions of each lesioned animal with its age-matched normal control, adding a number of assessments to those used earlier, including reactivity to novel toys, social dominance status, and reactions to separation from peers. Because the results indicated that the neonatally-lesioned medial temporal group had extremely severe and chronic socioemotional abnormality, we conducted a second experiment in which we compared the magnitude of the effects in that group with those in a new group given the same lesion in adulthood. A preliminary report of this work appeared earlier (Málková, Mishkin, Suomi, & Bachevalier, 1997).
Experiment 1
Method
Subjects
The animals were 25 rhesus monkeys (Macaca mulatta) of both sexes, aged 6–8 years and weighing 5.5–7.5 kg at the beginning of the present study. The animals formed four groups, which, for ease of comparison with earlier reports on the same animals when they were infants, are designated as follows: (AH) six monkeys (four males) with bilateral neonatal ablation of the medial temporal lobe, including amygdala, hippocampus, and underlying cortex; (TE) six monkeys (two males) with bilateral neonatal removal of inferior temporal cortex, mainly including von Bonin and Bailey’s area TE (von Bonin & Bailey, 1947); (C) eight normal controls (three males) each reared with one or two lesioned monkeys; and (N) five normal monkeys (four males) each reared with two other normal monkeys (one female out of the original six normal monkeys died before being tested as an adult).
A detailed description of the rearing conditions of these animals when they were infants and juveniles was given in earlier reports (Bachevalier, Brickson, Hagger, & Mishkin, 1990; Bachevalier, Málková, & Mishkin, 2001; Merjanian, Bachevalier, Pettigrew, & Mishkin, 1989). Briefly, all animals were born at the National Institutes of Health Animal Center (Poolesville, MD) and raised in the primate nursery of the Laboratory of Neuropsychology (National Institute of Mental Health, Bethesda, MD). On their arrival at the primate nursery they were grouped in dyads or triads, consisting of either (i) a normal control with one or two lesioned monkeys or (ii) three normal controls. Infant monkeys were handled on many occasions throughout the day and evening by a human caregiver and several experimenters. In addition, each dyad or triad was placed daily in a play pen located in the nursery for 4–6 hours of social interactions, at other times, the animals were kept in individual cages. In their cages, they had continuous access to a surrogate mother, towels, and toys. At the ages of ~2 months and then again at ~6 months, some of the animals participated in social tests (Bachevalier et al., 2001; Merjanian et al., 1989), during which an operated animal and its normal control from the same dyad or triad were observed in a play cage twice a day for six days. In addition, all animals were tested on a series of learning and memory tasks and for emotional reactions, including: (i) preference for novelty at the ages of 5, 15, and 30 days (Bachevalier, Brickson, & Hagger, 1993); (ii) concurrent visual discrimination learning with 24-hour intertrial intervals (24-hr-ITI task) at 3 months (Bachevalier et al., 1990); (iii) emotional reactions towards familiar and novel objects at 9 months (Meunier, Nalwa, & Bachevalier, 2003); and (iv) visual delayed nonmatching-to-sample (DNMS) at 10 months (Bachevalier & Mishkin, 1994). Between 1.5 to 2 years of age, the animals were moved to the NIH Animal Center (Poolesville, MD), where the dyads and triads (AH+C, TE+C, and N+N) were placed in groups of six to eight monkeys in large indoor/outdoor enclosures for 1–2 years. They were then moved to individual cages until they reached 4–5 years of age when they were returned to the Laboratory of Neuropsychology to resume behavioral testing, during which period, the animals were also housed in individual cages. They were retested on two cognitive tasks, i.e. relearning of both the 24-hr-ITI task and visual DNMS at the age of 4–5 years (Málková et al., 1995), and were trained on two new tasks, i.e. tactile and spatial DNMS at the age of 7–8 years (Málková et al., 1995). In the present experiment, conducted when the animals were 6 to 8 years of age, the animals were re-paired exactly as before, but now in a large indoor enclosure, and their behavioral interactions were again recorded. The composition and sex of the monkeys in each dyad are given in Table 1.
Table 1.
Composition of each neonatal dyad
| N | with N | AH | with C | TE | with C |
|---|---|---|---|---|---|
| N 1 (M) | N 2 (M) | AH 2 (M) | C 2 (F) | TE 1 (F) | C 3 (F) |
| N 2 (M) | N 3 (M) | AH 3 (F) | C 8 (M) | TE 3 (F) | C 6 (F) |
| N 1 (M) | N 3 (M) | AH 4 (M) | C 11 (M) | TE 4 (M) | C 6 (F) |
| N 4 (F) | N 5 (M) | AH 6 (F) | C 13 (F) | TE 5 (F) | C 8 (M) |
| AH 7 (M) | C 14 (M) | TE 7 (M) | C 13 (F) | ||
| AH 8 (M) | C 15 (F) | TE 8 (F) | C 14 (F) |
Note: N denotes normal animals paired with other normal animals; AH denotes animals with medial temporal lobe lesions; TE denotes animals with neonatal TE lesions; C denotes normal animals paired with the operated animals; sex of each animal is indicated within the parenthesis. Three of the unoperated controls (C 8, C 13, and C 14) were each paired with one animal with AH lesions and one animal with TE lesions, whereas one unoperated control (C 6) was paired with two animals with TE lesions.
This experiment was conducted under a protocol approved by the Animal Care and Use Committee of the National Institute of Mental Health and in accordance with the Guide for Care and Use of Laboratory Animals adopted by the National Institutes of Health.
Surgery
A detailed description of the neonatal surgical procedures is available in the initial report (Bachevalier et al., 1990). Briefly, the lesions had been made in two stages - in one hemisphere when the animals were about 1 week old, and in the other when they were about 3 weeks old. The surgery was performed using aseptic techniques while the animal was deeply anesthetized, and the aspiration lesions were made under direct visual guidance through a surgical microscope.
The bilateral medial temporal removals (AH Group) included the amygdala, periamygdaloid allocortex, hippocampal formation, and parahippocampal gyrus. Because the lesions extended laterally to the fundus of the rhinal sulcus rostrally and the medial lip of the occipito-temporal sulcus posteriorly, they included all of the entorhinal cortex and most of parahippocampal areas TH and TF, respectively. The bilateral removals of inferior temporal cortex (TE Group) extended ventrally from the fundus of the superior temporal sulcus to the lateral lip of the rhinal sulcus and the fundus of the occipitotemporal sulcus, and anteriorly from a line approximately 9 mm rostral and parallel to the ascending portion of the inferior occipital sulcus to the temporal pole.
Lesion Assessment
The extent of each lesion, evaluated either histologically (AH 6, 7, 8; TE 3, 5, 8) or by magnetic resonance imaging (AH 2, 3, 4; TE 1, 4, 7), has already been reported (Bachevalier, 1994). Coronal sections through two representative cases of each type of lesion (cases AH 6 and TE 5) are presented in Figure 1. All lesions were as intended and damage to adjacent areas was minor.
Figure 1.
Coronal histological sections (thionin stain) through the extent of the neonatal lesions for a representative case with inferior temporal cortical lesion (TE 5) and a representative case with medial temporal lobe lesion (AH 6). The numerals on the left of each coronal sections indicate the distance in millimeters from the interaural plane. The crosses point to sparing of the rostralmost portion of perirhinal areas 35 in case TE 5 and asterisks indicate unintended damage to the caudalmost portion of area TEO in both cases (see level 0). Abbreviations: amt – anterior middle temporal sulcus; ERh – entorhinal cortex; PRh – perirhinal cortex; pmt – posterior middle temporal sulcus; rs – rhinal sulcus; TE, TEO, TF and TH – cytoarchitectonic fields described by von Bonin and Bailey (1947).
In one of the medial temporal cases (AH 7), the caudalmost 1–2.5 mm portion of the hippocampus was spared bilaterally. In all cases, the lesion invaded the fundus of the rhinal sulcus along its entire length, thus encroaching on area 35 of the perirhinal cortex. In addition, at the caudal tip of the rhinal sulcus, the lesions included a small amount of perirhinal cortical area 36. As a result of mechanical and ischemic injury, there was unintended damage to the inferior temporal cortex unilaterally in three cases (AH 2, 3, 7) and bilaterally in three (AH 4, 6–8; see Fig. 1, level 0). This damage was judged to be substantial only in case AH 8, where it averaged 10% bilaterally of areas TE and TEO combined and also extended caudally onto the ventromedial surface of the left hemisphere to include occipital cortex (Bachevalier & Mishkin, 1994).
As to deviations from the intended inferior temporal lesions, 1–2 mm of tissue lateral to the lateral lip of the rhinal sulcus was spared bilaterally in four cases (TE 1, 3, 7, 8) and unilaterally in one (TE 4), thereby sparing almost all perirhinal cortical areas 36 and 35 in these instances. In one of the TE cases (TE 5) only the rostral portion of the perirhinal cortex was spared bilaterally (Fig. 1, levels +20 and +15. In three cases (TE 3, 5, 8) the lesion encroached unilaterally on the most anterior portion of area TEO, the inferior temporal cortical visual area caudal to TE.
Apparatus and Procedures
The animals’ dyadic interactions were observed in a large, wire-mesh enclosure (3.5 m long, 1.6 m wide, and 2.2 m high) in an otherwise empty room. The enclosure contained perches and toys. The pair of animals remained in the enclosure 24 hrs/day for three consecutive weeks during which their behavioral activities were videorecorded for short periods of time through a one-way vision screen from an adjacent room (this set up prevented audiorecording that could have been systematically analyzed). Throughout the first two weeks, the monkeys were maintained on a diet of ad libitum primate chow (No 5038; PMI Feeds, St. Louis, MO) with fresh fruit supplements (except as indicated below). Feeding times were separated from the videorecording sessions and water was always available. After the third week of observation, the members of the pair were placed in separate home cages out of sight of each other and the videorecording was continued for another week (see below). At the end of the fourth week, another dyad was brought to the enclosure to receive the same schedule of behavioral observation. The four weeks of observation proceeded as follows:
Week 1: Social Interactions
A dyad was placed in the enclosure just before 10 AM on Day 1, and social interactions were recorded for two 5-minute sessions at approximately 10 AM and 4 PM on each day from Days 1 to 5.
Week 2: Novel Objects
A standard set of seven novel objects was placed in the enclosure at 10 AM on Day 1, and the animals were immediately videotaped for 25 minutes. When the normal monkey in the pair served as the control animal in two different dyads, a second set of 7 novels toys was used.
Week 3: Dominance Behavior
Competition for food was induced by providing a single source of food while the monkeys were on a restricted diet (Mirsky, 1960). On Days 1 and 2, after two weeks of ad libitum feeding and two days preceding testing, each animal was fed just eight biscuits/day. On Days 3–5, during the experimental session, 50 large banana pellets (P.J. Noyes Co., 500 mg) were delivered one at a time at 10-sec intervals through a long metal pipe into one of the food wells attached inside the enclosure. After the experimental session on Days 3 and 4, the animals were fed the same amount as on Days 1 and 2, and then were again fed ad libitum after the session on Day 5. The experimental sessions were videorecorded throughout the delivery of the pellets.
Week 4: Social Separation
At the beginning of Week 4, the two animals were separated and placed in the individual home cages, one inside the room with the large enclosure and the other in the room with the videorecorder. Thus, they were in neither tactile nor visual contact but they could hear each other to a limited extent through a closed door between the two rooms. Their behavior was videorecorded for two 5-minute sessions at 10 AM and 4 PM for 4 consecutive days through cameras placed in front of each animal’s cage. During these four days, the animals were fed ad libitum and water was always available.
Data Analysis
For each week, frequency and duration of all behaviors were scored independently by two observers with a Tandy 102 Portable Computer. One of the observers was blind with respect to the animal’s group (lesioned, control, or normal) and rated approximately 80% of the videotapes. The second observer (L.M., not blind with respect to the lesions) rated all the videotapes. Inter-observer correlations (r) for all behavioral categories ranged from 0.90 to 1.00 (all ps < 0.01) except for two. These were Submission and Aggression (r = 0.76 and r = 0.74, respectively), which were observed very rarely, i.e. less then 1% of videorecorded time (Table 3).
Table 3.
Week 1 – Social Interactions
| Behaviors | Sessions | N | C with AH | AH | C with TE | TE |
|---|---|---|---|---|---|---|
| General Activity | ||||||
| Passive behavior | 1st | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 |
| 2–10 | 9 ± 4 | 28 ± 16 | 13 ± 8 | 2 ± 1 | 8 ± 5 | |
| Visual exploration | 1st | 198 ± 24† | 132 ± 30† | 164 ± 12† | 238 ± 19 | 156 ± 33 |
| 2–10 | 262 ± 7 | 182 ± 26 * | 175 ± 8 * | 154 ± 38 | 228 ± 18 | |
| Manipulation | 1st | 2 ±1 | 1 ± 1† | 3 ± 2† | 4 ± 4 | 1 ± 1 |
| 2–10 | 1 ± 0 | 19 ± 9 * | 16 ± 6 * | 10 ± 6 | 8 ± 3 | |
| Locomotion | 1st | 76 ± 19† | 95 ± 33† | 94 ± 18† | 96 ± 32† | 107 ± 28 |
| 2–10 | 6 ± 3 | 16 ± 4 * | 26 ± 5 * | 10 ± 4 | 28 ± 14 | |
| Food and water consumption | 1st | 37 ± 36 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 |
| 2–10 | 21 ± 11 | 18 ± 5 | 16 ± 9 | 0 ± 0 | 8 ± 4 | |
| Social behavior | ||||||
| Approach | 1st | 2 ± 1 | 1 ± 1 | 2 ± 2 | 0 ± 0 | 4 ± 2 |
| 2–10 | 0 ± 0 | 0± 0 | 1 ± 0 | 0 ± 0 | 1 ± 0 | |
| Proximity | 1st | 57 ± 53 | 11 ± 11 | 11 ± 11 | 24 ± 22 | 24 ± 22 |
| 2–10 | 223 ± 28 | 30 ± 17 | 30 ± 17 | 161 ± 32 | 161 ± 32 | |
| Body contacts | 1st | 8 ± 5† | 1 ± 1 | 1 ± 1 | 0 ± 0 | 0 ± 0 |
| 2–10 | 50 ± 29 | 28 ± 27 | 28 ± 27 | 39 ± 13 | 39 ± 13 | |
| Grooming | 1st | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 |
| 2–10 | 0 ± 0 | 0 ± 0 | 1 ± 1 | 17 ± 14 | 2 ± 2 | |
| Withdrawal | 1st | 0 ± 0 | 1 ± 1 | 0 ± 0 | 1 ± 1 | 0± 0 |
| 2–10 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 | |
| Aggression | 1st | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 |
| 2–10 | 0 ± 0 | 0 ± 0 | 1 ± 1 | 0 ± 0 | 0 ± 0 | |
| Submission | 1st | 0 ± 0 | 0 ± 0 | 9 ± 9 | 0 ± 0 | 0 ± 0 |
| 2–10 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 | |
| Total social Contacts (per dyad) | 1st | 63 ± 57† | 13 ± 12 | 13 ± 12 | 24 ± 22† | 24 ± 22† |
| 2–10 | 285 ± 9 | 60 ± 38 * | 60 ± 38 * | 197 ± 35 | 197 ± 35 | |
| Disturbances of behavior | ||||||
| Locomotor stereotypies | 1st | 0 ± 0 | 34 ± 23 | 33 ± 15 * | 42 ± 42 | 31 ± 20 * |
| 2–10 | 6 ± 3 | 28 ± 17 | 12 ± 7 | 15 ± 8 | 4 ± 3 | |
| Self-directed activities | 1st | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 1 ± 1 |
| 2–10 | 7 ± 4 | 13 ± 6 | 36 ± 9 * | 8 ± 3 | 12 ± 2 | |
Means (± SEM) for duration of each behavioral category for the 1st session and the averaged sessions 2 through 10 of recording. Abbreviations: N = normal monkeys paired with other normal monkeys; C with TE = unoperated control monkeys paired with monkeys with neonatal inferior temporal lesions (TE); C with AH = unoperated control monkeys paired with monkeys with neonatal medial temporal lobe lesion.
denotes significant differences between Session 1 and Session 2 and
denotes significant differences from Group N (all ps < .05).
Week 1: Social Interactions
Behavioral categories scored from the videotapes are given in Table 2. Categories followed the descriptions in Bachevalier et al. (2001), although two changes had to be made due to qualitative changes in the animals’ behavior when they reached adulthood. Since the adult animals remained motionless for long periods of time, just passively looking at the surroundings, a behavior labeled Visual exploration was added to the category General activity, and the behavior labeled Passive for the adults included instances where they were sleeping or sitting with eyes closed. Furthermore, in infancy, social interactions consisted mainly of rough-and-tumble play and chasing, which were scored as Approach or Accept approach. In adult monkeys, however, social interactions consisted mainly of keeping in close proximity and/or body contacts, during which the animals usually remained stationary, observing the surroundings or, less frequently, engaging in other activities, such as manipulation or grooming. Thus, for the social interactions in adult monkeys, two behaviors were added: Proximity and Body contact. In addition, the category Total social contacts per dyad for the adult dyads consisted of total amount of time the two animals in a dyad spent in Proximity and Body contact. Behavioral categories were exhaustive but not mutually exclusive, e.g. an animal could manipulate an object while in locomotion.
Table 2.
Behavioral categories.
| Behavior | Description |
|---|---|
| GENERAL ACTIVITY | |
| Passive | Sits with eyes closed or asleep |
| Visual exploration | Visually explores surroundings in a stationary position (sitting or standing) |
| Manipulation | Handles, chews, licks, or smells objects or cage’s parts |
| Locomotion | Walks, runs, climbs, or jumps |
| Food/Water consumption | Eats or drinks |
| SOCIAL BEHAVIOR | |
| Approach | Initiates social contact, i.e. moves within one body length of the other animal |
| Proximity | Remains stationary within one arm’s length of the other animal |
| Body contacts | Touches or holds the other animal |
| Grooming | Spreads, picks or licks the fur of another animal |
| Withdrawal | Moves away or makes an avoidance response after other attempts to initiate social contact |
| Aggression | Makes threatening gestures, i.e. mouth threat, head or body lounge, cage shake, or engages in any active antisocial behavior |
| Submission | Lipsmacks, averts head, presents to other monkey, moves away from the other monkey |
| DISTURBANCES OF BEHAVIOR | |
| Self-directed Behaviors | Engages in self-directed behaviors, i.e. self-grooms, hugs head, self-grabs and bites, presses face with hands, self-holds, closes fists, self-clutches, sexually self-stimulates, prone, or head on chest |
| Locomotor Stereotypies | Paces, somersaults, circles |
For experimental Day 1, behaviors were analyzed separately for each session in order to measure the animals’ reactions when re-introduced to each other in the large enclosure for the first time after 3–4 years of separation. For both frequency and duration of each behavior, including the category Total social contacts, group differences were evaluated by one-way analysis of variance or, for those behavioral categories that occurred infrequently, by nonparametric Kruskal-Wallis analysis of variance. Paired t-tests were then used to compare behavioral changes between the first session, when the animals were just re-introduced, and the second session, when the animals had already adapted to the novel environment for 4–5 hours.
Frequency and duration of each behavior in recording session 2 on Day 1 and in the two recording sessions on each of the four remaining days (i.e. sessions 2 to 10) were analyzed by a repeated-measures analysis of variance (ANOVA) with the factors of Group and Session. Whenever required, Hyunh-Feldt correction was applied to both the degrees of freedom and the p values. The Tukey test was used for paired comparisons. For those behavioral categories that occurred infrequently (i.e. average values of less than 1% percent of the total time per session), the nonparametric Kruskal-Wallis test was used to compare mean values of the behavioral categories across the 9 sessions (i.e. all sessions except the first), followed by the Mann-Whitney test for paired comparisons.
To compare the effects of the lesions across ages for all adult animals that had also been tested in infancy, the average behavioral scores in the nine sessions of the present study were compared to the average behavioral scores in the 12 sessions they had received at the age of 6 months (Bachevalier et al., 2001) by a Group × Age ANOVA. The same statistical methods applied to the data for Week 1 were used to analyze the following weeks’ data as well and are designated only by their single letters: F (for ANOVA), t (for t-test), T (for Tukey), H (for Kruskall-Wallis), and U (for Mann-Whitney).
Week 2: Novel Objects
Behavioral categories included Latency to touch first novel object, Number of objects explored, and frequency and duration of Visual exploration of novel objects, Total contact with novel objects (manual and oral), Self-directed activity, Locomotor stereotypies, and Other behavior.
Week 3: Dominance Behavior
The behavioral categories were Number of pellets retrieved from the feeder, as well as frequency of Aggression and Submission, which were summed across the three daily sessions for each animal (for definition of the two latter behaviors, see Table 2).
Week 4: Social Separation
Behavioral categories were the same as those scored during Week 1 (see Table 2), allowing comparison between the effects of separation (eight sessions in Week 4) with those of the social setting (9 sessions in Week 1).
Results
Week 1: Social Interactions
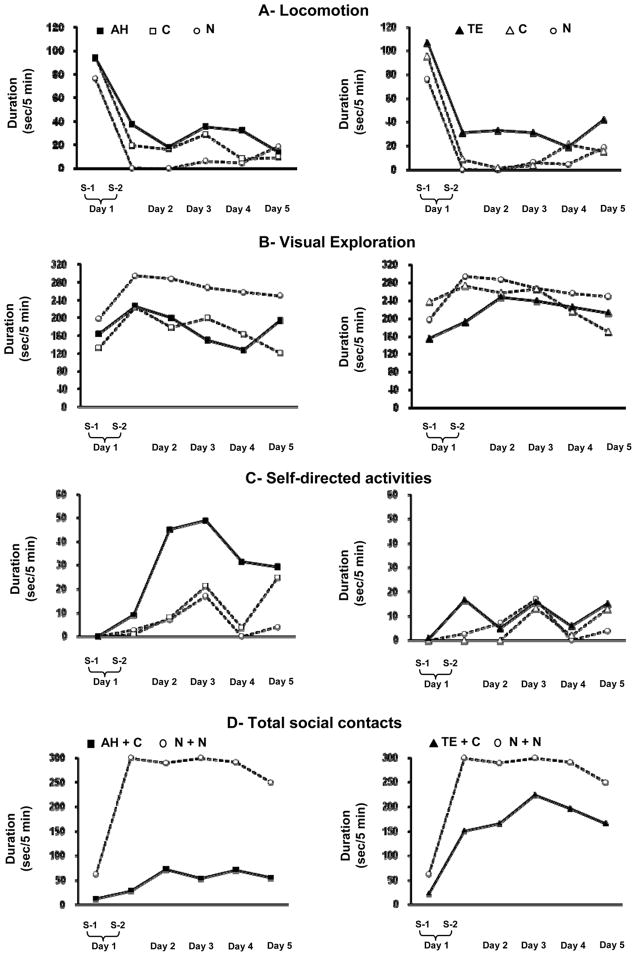
Mean duration scores for the first experimental session as well as mean duration scores averaged over the remaining nine experimental sessions (sessions 2–10) are given for each behavior and each group in Table 3. Figure 2 displays average scores for all groups in behavioral categories for which significant group differences emerged.
Figure 2.
Scores are mean duration of behaviors for the first and second sessions in Day 1 and for the combined AM and PM sessions in Days 2 through 5 for Locomotion, Visual exploration, Self-directed activities, and Total social contacts. Conventions: Group N: animals in the dyads including two normal animals, Group AH: animal with neonatal medial temporal lobe lesion, Group TE: animal with neonatal lateral temporal cortical lesion, and Group C: normal animal paired with an operated monkey. Group comparisons are shown on the left for animal in Groups AH and C with AH and on the right for animals in Groups TE and C with TE. Note that for Total social contacts the mean scores represent the sums of Proximity and Body contacts for the two animals in a dyad (N + N, AH + C, or TE + C).
Normal dyads
Session 1
In the first experimental session, the behavior of the animals in Group N was characterized mainly by exploratory activity, consisting of high frequency and long duration of Locomotion, interrupted by short periods of Visual exploration and Total social contacts.
Session 1 vs. Sessions 2–10
Between the first and the second sessions, Locomotion decreased significantly (frequency, t = 6.50, p < 0.001; duration, t = 4.10, p < 0.005; see Fig. 2A), whereas both Visual exploration and Total social contacts increased (duration, t = 4.40, p < 0.003 and t = 4.19, p < 0.03, respectively; see Figs. 2B and 2D). No other behavioral categories showed any significant changes between the first two sessions. Thus, after the animals had adapted to the experimental situation, they spent most of the time passively exploring the surroundings while remaining in social contact (up to about 90% of time). Across the nine sessions of Week 1, the categories Passive, Locomotion, Manipulation, Self-directed activity, and Locomotor stereotypy, represented only about 10% of behavioral activity. The remaining categories (Approach, Withdrawal, Aggression, Submission, and Grooming) were infrequent and represented less than 1% of each session.
AH+C dyads
Session 1
The animals in both Groups AH and C displayed a pattern of behavior similar to that of Group N: high frequency and duration of Locomotion, high frequency of Visual exploration, and few Total social contacts (Table 3 and Figs. 2A, 2B, and 2D, Session 1). However, as shown in Table 3, both Groups AH and C engaged in Locomotor stereotypy more frequently than Group N (H = 5.91, p < 0.05; U = 32.00, p < 0.05 for AH > N, and U = 36.00, p < 0.05, for C > N) and for longer periods (H = 6.14, p < 0.05; U = 40.00, p < 0.01 for AH > N, although U = 32.00, p = 0.09 for C > N fell short of significance). None of the other behavioral categories showed any group differences in the 1st session.
Session 1 vs. Sessions 2–10
Like Group N, both Groups AH and C engaged in less Locomotion in the second session than in the first (AH and C for frequency, t = 4.48, p < 0.006 and t = 5.03, p < 0.004, respectively; and for duration: t = 3.06, p < 0.03 and t = 2.23, p = 0.076, respectively). –Nevertheless, as shown in Fig. 2A left, both Groups AH and C displayed significantly more Locomotion than group N across Sessions 2–10 [frequency, F(2,17) = 8.33, p < 0.003; duration, F(2/17) = 7.68, p < 0.004; T: AH > N, C > N, ps < 0.01]. Also, both Groups AH and C engaged in more Visual exploration in Session 2 than Session 1, although the magnitude of this increase was less than that observed in Group N as both Groups AH and C engaged in less Visual exploration than Group N in Sessions 2–10 (duration, F[2,17] = 11.80, p < 0.001; T: AH < N and C < N, ps < 0.01; Fig. 2B).
By contrast, whereas Group N did not engage in a significant amount of Manipulation across the 10 sessions (Table 3), Manipulation in Groups AH and C increased significantly from Session 1 to Session 2 and this increase persisted across Sessions 2–10 (frequency, H = 11.18, p < 0.004, AH > N and C > N, U = 47.00, p < 0.002 and U = 41.50, p < 0.02, respectively; duration, H = 10.25, p < 0.006; and for duration, AH > N and C > N, U = 47.00, p < 0.002 and U = 41.5, p < 0.019, respectively; Table 3). More importantly, unlike in the N+N dyads, duration of Total social contacts in the AH+C dyads did not increase from the first to the second session, and remained lower than in the N+N dyads across Sessions 2–10 (F[2,17] = 21.66, p < 0.002; Fig. 2D left). All other social behaviors (Approach, Withdrawal, Aggression, Submission, and Grooming) represented only a minor portion of the total observed time (Table 3) and no group differences were found in any of these categories.
Finally, whereas Self-directed activity were low in all groups in Sessions 1 and 2, they increased significantly in Group AH across sessions 3–10 (Fig. 2C, left) and were significantly higher than in the two control groups (frequency, F[2,17] = 9.76, p < 0.002; duration, F(2,17) = 5.43, p < 0.015).
TE+C dyads
Session 1
Like monkeys in the other groups, those in Groups TE and C engaged in considerable Locomotion, interrupted by short periods of Visual Exploration and Total social contacts (Table 3, and Figs. 2A, B, and D right). In addition, as in the AH+C dyads, both Groups TE and C displayed more Locomotor stereotypy than Group N (Table 3), but significant differences were found only for the comparison between TE and N (frequency, H = 7.34, p < 0.03 and U = 40.00, p < 0.01; duration, H = 6.90, p < 0.04 and U = 40.00, p < 0.01).
Session 1 vs. Sessions 2–10
Also as in the other groups, Locomotion decreased from the first to the second session in both Groups TE and C and remained low for the remaining eight sessions (Fig. 2A right), but this decrease reached significance only for Group C (frequency, t = 10.37, p < 0.001; duration, t = 2.57, p < 0.05). Furthermore, as in the N+N dyads, duration of Total social contacts in the TE+C dyads increased between the first and the second session, and continued to increase gradually across the remaining sessions (Session effect, F[9,72] = 4.78, p < 0.001; Fig. 2D right); as a result, the TE+C dyads did not differ from the N+N dyads on this measure.
Comparison between the effects of AH and TE lesions
The most striking differences in the effects of the two types of lesions were in the small amount of Total social contacts (F[1,10] = 6.92, p < 0.03, Fig. 2D, for duration) and the large amount of Self-directed activity in Group AH (F[1,10] = 7.14, p < 0.03, and F[1,10] = 6.68, p < 0.03, Fig. 2C, for frequency and duration, respectively) compared to the amounts in Group TE. However, in the first session, both lesion groups engaged in more Locomotor stereotypies than did Group N (Table 3).
Summary
With respect to General activity, all three types of dyads adapted equally rapidly to being placed in the novel enclosure, all three displaying equivalent decreases in Locomotion and increases in Visual exploration from the first to the second session, although in the first session, the dyads containing lesioned monkeys exhibited a greater amount of Locomotor stereotypies. In contrast, whereas both the TE+C and N+N dyads increased their social interaction from the first to the second session, as reflected by the large increase in Total social contacts, which then remained at a high level, the social interaction in the AH+C dyads remained dramatically low throughout the entire week. This low level of social interaction coincided with high levels of Self-directed activity and Manipulation (Table 3).
Week 2: Novel Objects
Introduction of novel objects in the play cage did not yield any differences between Groups AH, TE, C, and N (Table 4). The animals with AH and TE lesions approached the novel objects as rapidly as the both types of controls (Groups C and N) and explored the same number of objects.
Table 4.
Week 2 – Novel Objects
| Behaviors | N | C with AH | AH | C with TE | TE |
|---|---|---|---|---|---|
| Latency to touch the first object | 905 ± 210 | 1024 ± 301 | 818 ± 308 | 533 ± 287 | 348 ± 232 |
| Number of objects explored | 1.9 ± 0.8 | 1.7 ± 1.0 | 1.8 ± 1.0 | 2.8 ± 0.8 | 2.3 ± 0.8 |
| Total contact with novel objects | 70 ± 42 | 179 ± 114 | 324 ± 202 | 295 ± 173 | 279 ± 134 |
| Visual exploration of novel objects | 161 ± 18 | 194 ± 32 | 242 ± 81 | 170 ± 44 | 218 ± 65 |
| Disturbances of locomotion | 66 ± 36 | 161 ± 149 | 1.0 ± 0.7 | 230± 173 | 280 ± 127 |
| Self-directed activities | 85 ± 48 | 11 ± 3 | 138 ± 112 | 87 ± 67 | 47 ± 26 |
| Other behaviors | 1109 ± 77 | 941 ± 166 | 780 ± 191 | 705 ± 185 | 676 ± 50 |
Mean latency (± SEM) to touch the first novel object (in sec), number of objects explored, and means (± SEM) for duration of other behavioral categories during the session when novel objects were introduced. Abbreviations see Table 3.
Week 3: Dominance Behavior
Two of the AH+N dyads had to be separated during the third week before they completed the experimental sessions due to the excessive aggression of one of the animals. In one of the dyads, the aggression was from a normal male (C-11) towards an lesioned male (AH-4) and in the other, it was from an lesioned male (AH-2) towards a normal female (C-2). Therefore, in Weeks 3 and 4, only data from four of the AH+C dyads were analyzed. As shown in Table 5, animals in Group AH retrieved significantly fewer pellets than their normal controls (U = 0.00, p < 0.02), and displayed significantly more submissive behaviors (U = 16.00, p < 0.02). No group differences were found in the TE+C dyads.
Table 5.
Week 3 – Dominant Behaviors
| Behaviors | C with AH | AH | C with TE | TE |
|---|---|---|---|---|
| Number of pellets retrieved | 99 ± 9 | 47 ± 12 * | 73 ± 28 | 67 ± 30 |
| Submission | 25 ± 7 | 91 ± 14 * | 75 ± 29 | 65 ± 27 |
| Aggression | 10 ± 2 | 4 ± 3 | 8 ± 5 | 6 ± 3 |
Number of counts (± SEM) for each behavior scored during the competition for food. Abbreviations see Table 3.
denotes significant differences from C with AH (p < .05).
Week 4: Social Separation
No behavioral differences were found in Week 4 between Groups N, AH, and C, but Group TE had significantly longer durations of Self-directed activity than Groups N and C (F[2,17] = 9.33, p < 0.01; T: ps < 0.01), though it did not differ from Group AH on this measure (Fig. 3B, Week 4; Table 6).
Figure 3.
Scores are mean duration of behaviors for Locomotor stereotypies (A) and Self-directed activities that each group obtained in Week 1 as compared to Week 4. Conventions as in Fig. 2.
Table 6.
Week 4 – Social Separation
| Behaviors | N | C with AH | AH | C with TE | TE |
|---|---|---|---|---|---|
| General Activity | |||||
| Visual exploration plus Passive | 150 ± 11 | 136 ± 24 | 160 ± 43 | 110 ± 31 | 144 ± 26 |
| Manipulation | 1 ± 1 | 3 ± 2 | 2± 1 | 1 ± 1 | 1 ± 1 |
| Locomotion | 12 ± 4 | 7 ± 3 | 12 ± 5 | 9 ± 3 | 11 ± 9 |
| Disturbances of behavior | |||||
| Locomotor stereotypies | 128 ± 14 | 151 ± 22 | 98 ± 42 | 174 ± 36 | 111 ± 21 |
| Self-directed activities | 2 ± 1 | 1 ± 1 | 17 ± 16 | 3 ± 1 | 23 ± 7 * |
Means (± SEM) for duration of each behavioral category during the separation period. Abbreviations see Table 3.
denotes significant differences from their controls (p < .05).
Confinement of each animal in a separate home cage resulted in a predictable decrease in duration of Locomotion (F[1,23] = 5.05, p < 0.05) across all groups accompanied by a dramatic increase in duration of Locomotor stereotypy (F[1,23] = 52.57, p < 0.001) compared to the levels of these behaviors in Sessions 2–10 of Week 1. Interestingly, the magnitude of increase in Locomotor stereotypies (Fig. 3A) was significant in all groups (ps from < 0.001 to < 0.03) except Group AH. Self-directed activity showed a significant Group by Week interaction (frequency, F[4,23] = 4.33, p < 0.05; but not for duration) due to a significant reduction of this behavior in Group AH (frequency, t = 4.02, p < 0.03; but not for duration, Fig. 3B) combined with a significant increase of this behavior in Group TE. The pattern of results suggests that social separation affected the animals with AH lesions less than it did the others.
Effects of age at testing
In order to better compare the behavioral scores obtained when the animals were adults (6–8 years) with those obtained when they were infants (6 months), some behavioral categories were combined at one or both ages. Adult scores for Visual exploration and Passive were combined to form a new category labeled Inactive/Passive for comparison with the infant category Passive. In addition, the separate infant category Rocking was included in the category Locomotor stereotypy, as it was in the adults. Finally, a new category Social interactions per animal (as compared with Total social contacts measured per dyad) included the adult categories Proximity, Body contacts, and Approach and the infant categories Initiates approach and Accepts approach. All other behavioral catgories remained the same. For each category, the average adult scores for the last nine sessions of Week 1 in the present experiment were compared with the average scores for the 12 sessions of data that were obtained when they were infants.
Considering General activity first, maturation was accompanied by a significant decrease in Locomotion (frequency, F[1,19] = 227.3, p < 0.001; duration, F[1,19] = 33.58, p < .001; Fig. 4A), and Manipulation (frequency, F[1,19] = 165.54, p < 0.001; duration, F[1,19] 104.45, p < 0.001, Fig. 4B]. As a corollary, adult animals showed more Inactivity/Passivity than the infants (frequency, F[1,19] = 9.16, p < 0.007; duration, F[1,19] = 977.25, p < 0.001; Fig. 4C]. In addition, the Group by Age interaction was significant (duration, F[4,19] = 7.76, p < 0.01), indicating that the amount of Inactivity/Passivity, which was similar in all groups in infancy, differed in adulthood, with Group N having the highest amount of this behavior and Group C with TE the lowest (T: N > C, N > AH, ps < 0.05).
Figure 4.
Comparisons of scores obtained 6 months and 6–8 years of age in animals with neonatal lesions and their controls for Locomotion (A), Manipulation (B), Passive (c) and Social interactions per animal (D). Conventions as in Fig. 2.
The average duration of Social interactions per animal increased from infancy to adulthood in both the N+N and TE+C dyads, but not in the AH+C dyads (Group by Age interaction, F[4,19] = 3.14, p < 0.04; paired t-test for each dyad: t = 12.54, p < 0.001, t = 3.28, p < 0.05, for N+N and TE+C dyads, respectively, and t = 0.98, p > 0.05, for AH+C dyads).
Finally, no significant effect of age was found for either Locomotor stereotypies or Self-directed activities.
In summary, in all groups between infancy and adulthood, the level of General activity decreased significantly, reflected in decreased Locomotion and Manipulation, and Passive/Inactive increased, whereas there was no change in either Locomotor stereotypies or Self-directed activity. By contrast, whereas Social interactions per animal increased from infancy to adulthood in both the N+N and TE+N dyads, they did not increase significantly in AH+N dyads.
Experiment 2
The results of Experiment 1 demonstrated that neonatal lesions of the inferior temporal cortex in Group TE resulted in only a transient loss of social interactions, from which the animals recovered by the time they reached adulthood. In contrast, neonatal lesions of the medial temporal lobe in Group AH produced a severe and chronic loss of social interactions. This level of long-lasting socioemotional impairment from neonatal lesions was not predicted, given the special degree of plasticity of the immature brain and the recovery of function that often follows early damage (Goldman, Rosvold, & Mishkin, 1970; Kennard, 1936, 1938; Kolb, 1987; Málková et al., 1995; Malkova et al., 2000; Rushmore, Rigolo, Peer, Afifi, Valero-Cabre, & Payne, 2008; Shupert, Conrwell, & Payne, 1993; Webster, Ungerleider, & Bachevalier, 1995a), raising the question of whether the impairment was nevertheless lower than would be expected from the same damage incurred in adulhood. To address this question, we prepared a new group of monkeys with the same medial temporal ablation as before, but one made when the animals were already fully mature adults. To match the rearing history of the animals in the first experiment, we used a group raised under similar conditions, as described below. We then assessed them for dyadic interactions following the same procedures used in Experiment 1 and compared the results of the socioemotional assessments in the two experiments.
Method
Subjects
The animals were eight rhesus monkeys (Macaca mulatta), five males and three females, aged 7–8 years and weighing 5.5–12.3 kg at the beginning of the experiment. They had been reared under conditions that closely matched those of the animals in Experiment 1. Born at the National Institutes of Health Animal Center (Poolesville, MD), they were separated from their mothers shortly after birth and raised with their peers in the Center’s primate nursery. At the age of two years, they were placed with a larger colony living in an enclosed outdoor area and five years later were brought to the NIMH Laboratory of Neuropsychology, where they were housed in individual cages. Three monkeys received bilateral removals of the medial temporal lobe, including the amygdala, hippocampus, and underlying cortex (Group AHa; ‘a’ for adulthood lesions); two monkeys were unoperated controls paired with the lesioned animals (Group Ca); and three monkeys were normal controls paired with each other (Group Na). The dyads were formed so that the paired animals were familiar with one another before the start of the experiment. Each dyad consisted of either one operated animal and its unoperated control (AHa+Ca) or two normal control animals (Na+Na; Table 7). When paired in the enclosure, one of the lesioned monkeys (AH3a) violently attacked its unoperated control (C3a), necessitating substitution of another normal monkey (N3a) for the original one.
Table 7.
Composition of each adult dyad
| Ad-N with Ad-N | Ad-AH with Ad-C | ||
|---|---|---|---|
| Na 1 (M) | Na 2 (M) | AHa 1 (M) | Ca 1 (F) |
| Na 2 (M) | Na 3 (F) | AHa 2 (M) | Ca 2 (F) |
| Na 3 (F) | Na 1 (M) | AHa 3 (M) | Ca 3 (F) |
Note: Na denotes normal animal paired with another normal animals; AHa denotes animals with medial temporal lobe lesions operated as adults; Ca denotes normal animals paired with the operated animals; Sex of each animal is indicated within the parenthesis. Animal Na 3 is identical with Ca 3.
This experiment was conducted under a protocol approved by the Animal Care and Use Committee of the National Institute of Mental Health and in accordance with the Guide for Care and Use of Laboratory Animals adopted by the National Institutes of Health.
Surgery
The intended medial temporal lobe ablation was the same as that in Experiment 1, except that the bilateral lesions were performed in one stage. Prior to surgery the monkeys were fasted for 12 hours and pretreated with antibiotics. On the day of surgery, each animal was sedated with Ketamine hydrochloride (10 mg/kg, i.m.) and surgical level of anesthesia was attained with gas anesthesia (e.g. isoflurane, 1 to 4%, to effect). The animal was wrapped in a heating blanket with its head secured in a head holder, its vital signs (heart rate, respiration rate, temperature, oxygen saturation, and CO2) monitored continually, and intravenous fluids provided via an iv catheter throughout the surgical procedure. For the lesion in each hemisphere, a part of the temporal bone was removed, the dura mater was opened and reflected, and the intended structures, viewed through an operating microscope, were aspirated using a small-gauge metal sucker. The wound was closed in anatomical layers. All monkeys received a preoperative and postoperative treatment regimen consisting of dexamethasone sodium phosphate (0.4mg/kg) and Di-Trim (24% w/v solution 0.1mg, i.m.; Syntex Animal Health, West Des Moines, IA) for one day before surgery and one week after surgery to minimize trauma and to prevent infection, respectively. They also received postoperative analgesics as determined in consultation with the facility veterinarian.
Histology
At the completion of behavioral testing, the lesioned monkeys were given an overdose of barbiturate (sodium pentobarbital, 100 mg/kg, i.m.) and perfused through the heart with normal saline followed by aldehyde fixatives. The brains were removed and frozen, coronal sections were cut at 50 μ on a freezing microtome, and every fifth section was mounted, stained with thionin, and coverslipped. Histological sections were examined microscopically.
Lesion Assessment
The extent of each lesion was evaluated by histological analysis of the brain sections. Sections through a representative case (AHa 1) are illustrated in Figure 5. All three cases received complete removal of the amygdala, periamygdaloid cortex, hippocampal formation, and parahippocampal gyrus, including the entorhinal cortex and areas TH and TF. There was extensive bilateral encroachment on perirhinal areas 35 and 36 and on area TE in one case (AHa-2).
Figure 5.
Coronal histological sections (thionin stain) through the extent of the adult medial temporal lobe lesions for a representative case (Ad-AH-1). The numerals on the left of each coronal sections indicate the distance in millimeters from the interaural plane. Abbreviations: amt – anterior middle temporal sulcus; PRh – perirhinal cortex; pmt – posterior middle temporal sulcus; rh – rhinal sulcus; sts – superior temporal sulcus; TE, TEO, TF and TH – cytoarchitectonic fields described by von Bonin and Bailey (1947).
Apparatus and Procedures
The enclosure and videorecording procedures were the same as those in Experiment 1, but the behavioral recording sessions were limited to those described for Week 1: Social Interactions. Also, as with original socioemotional assessments of the neonatally-lesioned animals in the earlier study by Bachevalier and colleagues (2001), recording sessions of the AHa+Ca and Na+Na dyads were carried out twice, two months and six months after surgery.
In both assessment periods, a dyad was released into the enclosure just before 10 AM on the first day and the animals remained together in this cage for 24 hours/day for 5 consecutive days. Their behavior was videorecorded during two 5-minute sessions on each day at approximately 10 AM and 4 PM, although, due to technical difficulties on Day 1, Session 1, some dyads were not videotaped until up to an hour after entering the enclosure. Throughout the week, the monkeys were maintained on a diet of ad libitum primate chow (No 5038; PMI Feeds, St.Louis, MO) and fresh fruit supplements, both provided at times separated from the videorecording sessions. Water was always available.
Data Analysis
Data analysis and statistical procedures were the same as those described in Experiment 1, except that since most behaviors in Sessions 1 and 2 did not change substantially, they are not compared directly.
Results
Two months after surgery
No significant group differences were found for any behavioral category either in Session 1 or between Session 1 and Sessions 2–10 (Table 8; Figure 6). When all 10 sessions were included in the analysis, the only significant Sessions effects were a decrease across all groups in the duration of Locomotion (F[9,81] = 4.85, p < 0.05) and of Total social contacts (F[9,81] = 5.39, p < 0.02]). When Session 1 was removed from the analyses, the significant Sessions effect for duration of Locomotion disappeared. However, the significant Sessions effect for duration of Total social contact persisted, indicating that there were differences (decrease in Day 4 followed by an increase) in this category across the nine sessions. Neither of these two behaviors showed significant Group x Session interaction.
Table 8.
Social Interactions in Monkeys with Adult Lesions
| 2 months postsurgery | 6 months postsurgery | ||||||
|---|---|---|---|---|---|---|---|
| Behavior | Sessions | Na | Ca/AHa | AHa | Na | Ca/AHa | AHa |
| General Activity | |||||||
| Passive behavior | 1st | 0 ± 0 | 0 ± 0 | 0 ± 0 | 1 ± 1 | 0 ± 0 | 0 ± 0 |
| 2–10 | 32 ± 17 | 51 ± 28 | 0.3 ± 0.2 | 36 ± 15 | 11 ± 2 | 4 ± 2 | |
| Visual exploration | 1st | 137 ± 37 | 150 ± 72 | 160 ± 52 | 241 ± 48 | 151 ± 73 | 198 ± 20 |
| 2–10 | 207 ± 32 | 181 ± 13 | 233 ± 11 | 190 ± 29 | 182 ± 43 | 224 ± 7 | |
| Manipulation | 1st | 1 ± 1 | 0 ± 0 | 0 ± 0 | 1 ± 1 | 0 ± 0 | 3 ± 3 |
| 2–10 | 6 ± 4 | 7 ± 5 | 0 ± 0 | 2 ± 1 | 13 ± 7 | 6 ± 3 | |
| Locomotion | 1st | 161 ± 38 | 94 ± 42 | 103 ± 49 | 1 ± 0 | 50 ± 32* | 87 ± 18* |
| 2–10 | 20 ± 9 | 27 ± 11 | 33 ± 7 | 2 ± 1 | 20 ± 7 * | 26 ± 5 * | |
| Food or water consumption | 1st | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 2 ± 2 |
| 2–10 | 4 ± 3 | 16 ± 11 | 41 ± 13 * | 4 ± 3 | 16 ± 11 | 47 ± 23 * | |
| Social behavior | |||||||
| Approach | 1st | 1 ± 0.7 | 0 ± 0 | 18 ± 18 | 0 ± 0 | 0 ± 0 | 8 ± 1 |
| 2–10 | 0 ± 0 | 0.2 ± 0.2 | 2 ± 0 * | 0 ± 0 | 0.1 ± 0.1* | 4 ± 2* | |
| Proximity | 1st | 24 ± 7 | 31 ± 18 | 31 ± 18 | 100 ± 100 | 18 ± 17 | 18 ± 17 |
| 2–10 | 177 ± 73 | 161 ± 37 | 161 ± 37 | 219 ± 30 | 107 ± 23* | 107 ± 23* | |
| Body contacts | 1st | 0 ± 0 | 78 ± 53 | 78 ± 53 | 200 ± 100 | 5 ± 2 | 5 ± 2 |
| 2–10 | 93 ± 70 | 46 ± 31 | 46 ± 31 | 68 ± 34 | 58 ± 17 | 58 ± 17 | |
| Grooming | 1st | 0 ± 0 | 0 ± 0 | 0 ± 0 | 100 ± 100 | 0 ± 0 | 0 ± 0 |
| 2–10 | 64 ± 64 | 6 ± 3 | 10 ± 9 | 58 ± 29 | 6 ± 3 | 10 ± 9 | |
| Total social Contacts (per dyad) | 1st | 24 ± 7 | 109 ± 71 | 109 ± 71 | 300 ± 0 | 23 ±17 | 23 ± 17 |
| 2–10 | 270 ± 15 | 207 ± 23 | 207 ± 23 | 286 ± 4 | 164 ± 38* | 164 ± 38* | |
| Disturbances of behavior | |||||||
| Locomotor stereotypies | 1st | 2 ± 2 | 49 ± 49 | 0 ± 0 | 0 ± 0 | 83 ± 81 | 0 ± 0 |
| 2–10 | 0 ± 0 | 5 ± 4 | 0 ± 0 | 1 ± 1 | 13 ± 13 | 1 ± 1 | |
| Self-directed activities | 1st | 1 ± 1 | 0 ± 0 | 0 ± 0 | 3 ± 2 | 5 ± 5 | 2 ± 2 |
| 2–10 | 8 ± 3 | 16 ± 15 | 1 ± 1 | 28 ± 11 | 35 ± 24 | 6 ± 1 | |
Means (± SEM) for duration (sec) of each behavioral category for the 1st session and the averaged sessions 2 through 10 of recording. Behaviors Withdrawal, Aggression, and Submission had durations less than 1 sec in each group and are not presented in the Table.
Note: Na = normal monkeys paired with other normal monkeys; Ca/AHa = unoperated control monkeys paired with monkeys with adult medial temporal lobe lesions (AHa).
denotes significant difference from Session 1 to sessions 2–10, and
denotes significant differences from Group N.
Figure 6.
Scores are mean duration of behaviors for Approach (A), Proximity (B), Food and water consumption (C), and Total social contacts for animals with medial temporal lobe lesions acquired in adulthood, 2 and 6 months after surgery. Conventions: Na: adult animals in the dyads including two normal animals, AHa: adult animal with medial temporal lobe lesion, and Ca: normal adult animal paired with animals in Group AHa. Note that for Total social contacts the mean scores represent the sum of Proximity and Body contacts for the two animals in the adult dyads (Na + Na and AHa + Ca).
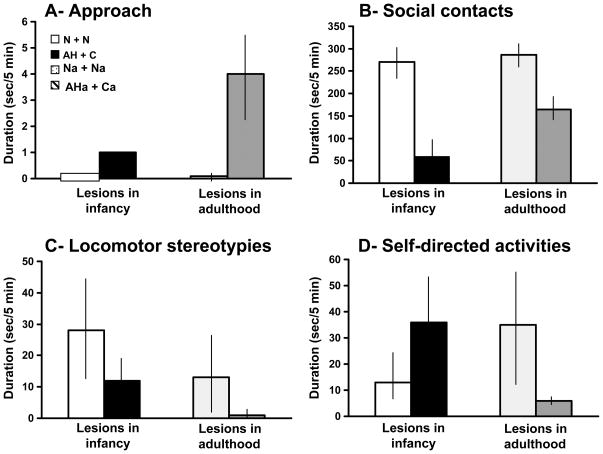
Analysis of the remaining nine sessions revealed that the groups did differ in the frequency of both Locomotion (F[2,9] = 9.44, p < 0.01) and Visual exploration (F[2, 9] = 10.22, p < 0.01), Group AHa displaying both of these behaviors more frequently than Group Na (T: ps < 0.01). In addition, there were significant group differences in Approach due to a greater incidence of this behavior in Group AHa than in either of the other groups (frequency, F[2,9] = 6.27, p<0.01, T: p < 0.01; duration, F = 7.34, p < 0.02, T: p < 0.01; Fig. 6A).
Six months after surgery
The only significant effect in Session 1 of this later assessment was for the Group factor in Locomotion (F[2,9] = 8.49, p < 0.01), with both Groups AHa and Ca displaying a longer duration of this behavior than Group Na (T: ps < 0.01). Duration of Locomotion in Sessions 1–10 showed a main effect of Group (F[2,9] = 13.39, p < 0.01), Session ([(9,18] = 7.47, p < 0.001), and their interaction (F[9,81] = 2.96, p < 0.01). Further analysis indicated that, although Locomotion decreased in both Groups AHa and Ca after the first session, it remained significantly higher than it did in Group Na across all sessions (T: p < 0.01), and this was also the case for Locomotion frequency (F[2,9] = 10.22, p < 0.01; T: ps < 0.01). There was also a significant Group effect for frequency of Food and water consumption (F[2,9] = 10.12, p < 0.01]), with Group AHa engaging in this behavior more frequently than animals in both control groups (ps < 0.05; Table 8 and Fig. 6C).
As was found for the average of Approach across all but the first session at the two-month assessment, the Group effect for the average across all sessions at this six-month follow up was significant (frequency, F[2,9] = 7.53, p < 0.02; duration, F[2,9] = 4.95, p < 0.05), with Group AHa initiating more Approach than either Group Na (T: p < 0.05) or Group Ca (T: p < 0.05, though for frequency only). Conversely, although there were no differences at two months after surgery in either Proximity or Total social contacts, these differences reached significance at six months after surgery, the AHa+Ca dyads spending significantly less time than the Na+Na dyads in Proximity (F[1,4] = 8.74, p < 0.05; Fig. 6B) and Total social contacts (F[1,4] = 14.63, p < 0.02; Fig 6D).
Two months versus six months after surgery
There were no Group effects or Group by Time interactions for any behaviors in the category General activity, except for Food and water consumption, for which there was a significant Group effect (frequency, F(2,9) = 8.74, p < 0.01; duration, F[2,9] = 6.25, p < 0.02; Fig. 6C). Pairwise comparisons indicated that animals in Group AHa consumed more food and water than those in Group Na in both assessment periods (ps < 0.02).
In addition, between the two periods, Total social contacts increased in the Na+Na dyads and decreased in the AHa+Ca dyads, as indicated by both a Group effect (F[1,4] = 8.64, p < 0.05) and a Group × Time Period interaction, which fell just short of significance (F[1,4] = 6.67, p = 0.061). Thus, the loss of social interactions after the AHa lesions became more evident six months after surgery than at two months after (Fig. 6D).
Finally, although the duration of Self-directed activity increased between two and six months after surgery (F[1,9] = 5.22, p < 0.05), there were no significant group differences or Group by Time effects in this category of behavior.
Neonatal lesions versus lesions in adulthood
The socioemotional assessments of the monkeys in Group AH with neonatal lesions (Experiment 1, Week 1, Sessions 2–10) were compared with those of the monkeys in Group AHa with lesions imposed when they were adults (Experiment 2, six months after surgery, Sessions 2–10). Nonparametric analyses revealed significant differences in the durations, but not in frequencies, of several behaviors. Compared to the animals with neonatal lesions, those with lesions in adulthood exhibited more Approach behavior (U = 1.00, p < 0.05; Fig. 7A) and engaged in less Self-directed activity (U = 18.00, p < 0.02; Fig. 7D). In addition, Total social contacts were at a higher level in AHa than in the now mature AH (Fig. 7B), although this difference did not reached significance. Because Group AHa consisted of three male monkeys, whereas the neonatally lesioned Group AH included two female as well as four males, we reanalyzed the behavioral scores limiting the comparison to male monkeys. The two all-male groups differed, as before, in Approach (U=0.00, p<0.05) and Self-directed activity (U=12.00, p<0.05), reported above, but Total social contact was now also significant (F[1,5] = 9.51, p < 0.03) and two other behaviors approached significance, with the males in Group AHa tending to show more Grooming (U = 10.00, p = 0.078) and less Locomotor stereotypy (U = 11.00, p = 0.074) than those in Group AH.
Figure 7.
Comparisons of the scores obtained for the animals with neonatal lesions, the animals that acquired the same lesions in adulthood, and their age-matched controls for Approach (A), Total social contacts (B), Locomotor stereotypies (C), and Self-directed activities (D). Conventions as in Figs. 2 and 6.
Discussion
The effects of medial temporal lesions in adult monkeys
Medial temporal lobectomies (including the amygdala, the hippocampus, and the underlying and surrounding cortical areas) in adult monkeys resulted in a set of symptoms first described by Kluver and Bucy (1938, 1939) and later named Klüver-Bucy syndrome. These symptoms included visual agnosia (“psychic blindness”), the inability to recognize the meaning of objects by vision only, hyperorality, a tendency to explore all stimuli in the animal’s environment orally, and “hypermetamorphosis”, a tendency to attend and react to every stimulus. Other symptoms included loss of fear and anger, change in dietary habits, and hypersexuality. Later studies demonstrated that ablations restricted to the anterior temporal lobes, including the amygdala, produced only some of the symptoms, namely tameness and tendency to explore objects and the environment indiscriminately (Weiskrantz, 1956). The original studies, however, did not test the effects of these lesions on social interactions. The first systematic study of the consequences of medial temporal lesions, that included the amygdala and the underlying cortical areas but spared most of the hippocampus, on social behavior was done by Rosvold, Mirsky, and Pribram (1954). This study reported social disturbances including loss of dominance in two previously dominant monkeys that were reintroduced in the same group of male monkeys after the surgery. Abnormalities in social interactions after the removals of the amygdala were later extensively studied and confirmed by Kling and colleagues (Kling & Brothers, 1992; Kling & Steklis, 1976) in various laboratory and natural settings.
Monkeys with medial temporal lesions acquired in adulthood (Group AHa) showed some but not all of the symptoms described by Kluver and Bucy (1938, 1939). Thus, they did not exhibit an increased amount of manual and oral manipulation, compulsive exploration or hypersexuality, but they displayed greater consumption of food and water and reduced social interactions. These differences could be due to the differences in the lesion extent as well as environmental factors. First, the extent of medial temporal lesions in the present study, like that of the medial temporal lobectomies performed by Kluver and Bucy study (1938 (1939), included both the amygdala and the hippocampus, but were substantially more restricted with respect to damage to the surrounding cortical areas. Thus, lesions in the present study spared the inferior temporal cortex and the temporal pole and the cortical damage was largely limited to most medial cortical areas, i.e. the entorhinal and perirhinal cortices anteriorly and the parahippocampal cortex posteriorly. Thus, because of the sparing of most of the temporal cortex, we predicted a much milder effects of the lesions on social behaviors. This prediction was also substantiated by Kling and Mass (Kling & Mass, 1974) observations that some of the Klüver-Bucy symptoms, such as hyperorality and hypersexuality, occur only in an impoverished laboratory environment, such as laboratory cages, and may not be observed in more natural conditions. Indeed, the monkeys with adult medial temporal lesions in the present experiment did not exhibit increased manipulation (manual and oral) or compulsive exploration, or hypersexuality. The lack of these tendencies can be explained both by the more restricted extent of the lesions and the more enriched laboratory environment since these monkeys were tested in dyads, i.e. in the presence of another conspecific, and in the large enclosure with various stimuli present.
Interestingly, the monkeys in Group AHa showed significantly increased Food and water consumption compared with normal controls. This finding is consistent with previous findings of hyperfagia and, in some cases, obesity in monkeys (Brown & Schafer, 1888; Bucy & Klüver, 1955; Pribram & Bagshaw, 1953; Schwartzbaum, 1961), humans (Marlowe, Mancall, & Thomas, 1975; Terzian & Ore, 1955), dogs (Fonberg, 1971) and rats (Rollins & Kling, 2000) following bilateral temporal lobectomies or bilateral amygdala resections as well as more circumscribed lesions or some of its nuclei.
Monkeys with adult medial temporal lesions in the present study showed a decrease amount of social interactions (total social contact), which became even more pronounced at 6 months after surgery. These findings are consistent with a host of previous findings that demonstrated a loss of social interactions after ablations of the amygdala (Kling & Brothers, 1992). This decrease in social contacts was present even though Group AHa initiated approach significantly more often than the normal controls. This latter finding is consistent with that obtained after either large (Kling & Brothers, 1992; Klüver & Bucy, 1939) or more circumscribed excitotoxic (Emery et al., 2001; Machado et al., 2008) amygdala lesions.
The effects of medial temporal lesions in infant monkeys
As published previously (Bachevalier et al., 2001), the monkeys from the present study tested in infancy showed a lack of social interactions and increased behavioral disturbances. However, when tested systematically for their reactions towards novel and familiar objects they did not display the compulsive exploration evidenced after similar lesions in adulthood (Klüver & Bucy, 1939) and retained some ability to detect novelty (Meunier et al., 2003) despite their known memory impairments (Bachevalier & Mishkin, 1994; Málková et al., 1995). This lack of compulsive exploration and lack of hyperorality were still present when the monkeys were retested as adults in the present study. Although they showed significantly higher manipulation (manual and oral combined) during the first week of testing as compared with the monkeys in the normal dyads (N + N), the same increase in manipulation was also observed in their normal controls. A possible interpretation of this increase in both the operated monkeys and their controls is that they substituted these behaviors (as well as more locomotion) for the impoverished social interactions they displayed. When presented with a set of novel objects, the operated monkeys showed normal exploratory activities and no abnormal behaviors directed towards the objects. These results indicate that similarly to the adult lesions (see above), neonatal medial temporal lesions sustained in infancy did not yield all the components of the Klüver-Bucy syndrome, namely hyperorality and hypermetamorphosis.
When retested as adults for social interactions, the monkeys with neonatal medial temporal lesions maintained a severe lack of social interactions and increased amount of self-directed activities. Whereas the amount of social interactions increased from infancy to adulthood in the normal dyads, it remained at the same low level in the dyads including monkeys with neonatal medial temporal lesions. The operated animals also displayed a lack of dominance and increase in submissive behaviors when observed during the food competition. These findings are consistent with those after both large amygdala lesions in adulthood (Kling & Brothers, 1992; Mirsky, 1960; Rosvold et al., 1954) and bilateral excitotoxic amygdala lesions done in infancy (Bauman, Toscano, Mason, Lavenex, & Amaral, 2006). It is interesting to note that the observation of two dyads containing the neonatally operated animals had to be terminated during this part of the experiment because of aggressive attacks. Although in one case the aggressive attack was from the normal control towards the operated monkey and in the other case from the operated monkey towards the control, these findings suggest that the operated monkeys engaged in inappropriate behaviors that led to these attacks since no excessive aggression was observed in the other two dyads, i.e. the normal dyads or those including animals with TE lesions and their controls.
Finally, in the separation period, the monkeys with neonatal medial temporal lesions seemed to be less disturbed by the social separation as reflected by the lowest increase of Locomotor streotypies they displayed as compared to all other groups and by their decrease in Self-directed activities as compared to their normal controls and those with TE lesions who by contrast showed an increase in these abnormal behaviors after social separation.
The dramatic lack of social interactions in monkeys with neonatal medial temporal lesions was even more severe than that observed in the monkeys that received the same lesions in adulthood (see above). In addition, although medial temporal lesions in adult monkeys resulted in reduced social ineraction, they did not yield behavioral abnormalities, e.g. Self-directed activities, observed in those operated on in infancy. The finding of more profound socioemotional disturbances after neonatal lesions suggests that the monkeys given lesions in adulthood retained at least some aspects of the socioemotional repertoire they had acquired during maturation, whereas the neonatally operated animals never succeeded in acquiring them. This finding also represents a reverse pattern from that commonly reported for other types of behavioral effects after early versus late cerebral damage (Goldman et al., 1970; Kennard, 1936, 1938; Kolb, 1987; Málková et al., 1995; Malkova et al., 2000; Rushmore et al., 2008; Shupert et al., 1993; Webster et al., 1995a) indicating greater deficits after the adult than the infant lesions. Thus, the present findings have important implications for socioemotional as distinct from other types of behavioral and cognitive development after medial temporal damage in children.
The question then becomes which structures within the large medial temporal lobe lesions are responsible for the behavioral changes observed. It has already been shown that many of the emotional and behavioral abnormalities following large medial temporal lesions (Klüver & Bucy, 1938, 1939) can be found after ablations restricted to the amygdala and underlying cortical areas while largely sparing the hippocampus (Kling & Brothers, 1992). This finding pointed to the critical role of the amygdala in emotional reactivity and social interactions. Similarly, behavioral changes qualitatively similar to those observed in the monkeys with neonatal medial temporal lesions had been previously reported after early ablations restricted to the amygdala and adjacent cortex (Bachevalier, 1994; Thompson, 1981). The amygdalectomized monkeys displayed transient increase in passivity and a decrease in social interactions, which became more severe when the animals matured. These findings suggest that the severe impairment observed in our study could predominantly be due to the effect of the amygdala ablations. However, recent findings in adult monkeys also point to the role of the perirhinal cortex in the control of emotional reactivity (Meunier & Bachevalier, 2002; Meunier et al., 2006). In addition, hippocampus and adjacent parahippocampal cortices when ablated in infancy yielded socioemotional abnormalities when the monkeys were retested as adults (Beauregard, Málková, & Bachevalier, 1995). These results indicate, that the cortical areas surrounding the amygdala may also contribute to the socioemotional behavior and/or exacerbate the effects of amygdala lesions on social interactions.
More recently, Amaral and colleagues (Bauman, Lavenex, Mason, Capitanio, & Amaral, 2004; Bauman, Toscano, Mason, Lavenex, & Amaral, 2006; Bauman, Toscano, Babineau, Mason, & Amaral, 2008; Prather et al., 2001; Toscano, Bauman, Mason, & Amaral, 2009) investigated the effects of excitotoxic amygdala lesions sparing the underlying cortices on emotional and social behavior in infant monkeys. Despite the emergence of decreased fear of objects but increased social fear, submissivity, and lower rank in social hierarchy (Bauman et al., 2004; Bauman et al., 2006; Bauman et al., 2008; Prather et al., 2001), these operated monkeys developed a species-typical repertoire of social behaviors. They displayed interest in conspecifics during social encounters, and did not show any of the behavioral abnormalitites, e.g. self directed activities, found in the monkeys with large medial temporal ablation from the present experiment. Nevertheless, abnormal behaviors in the operated animals did emerge when the animals were juveniles (Bauman et al., 2008). When placed in larger social groups, they displayed lower frequency of positive social behaviors (approach, grooming, physical contact) and decreased social interactions with conspecifics (Bauman, Toscano, Mason, & Amaral, 2007). Furthermore, the lesioned female monkeys showed a significantly decreased interest in infants of other monkeys and displayed fewer affiliative vocalizations and facial expressions towards them (Toscano et al., 2009). In addition to differences in extent of lesion, which can be an obvious explanation for the behavioral differences in the two studies, the monkeys with the excitotoxic amygdala lesions in those studies were raised in more natural conditions, i.e. they were returned to their mothers after the surgery and stayed with their mothers in small social groups until the age of 6 months, as compared to monkeys in the present experiments which were laboratory raised since their birth. The environmental rearing conditions could also play a significant role in the time course and severity of symptoms following neonatal amygdala lesions. This conclusion has received additional support from a recent pilot study (Raper, Stephens, Goursaud, Wallen, & Bachevalier, 2008; Wallen, Raper, Stephens, Goursaud, & Bachevalier, 2006) demonstrating that monkeys with neonatal excitotoxic amygdala lesions, which were reintroduced with their mother in a large semi-naturalistic social group of 165 animals, displayed more social play, reduced fearfulness, increased aggression, and more sexual displays. They were also more independent of their mother, spending more time in contact with other female monkeys as compared to sham-operated controls. Yet, in contrast to the monkeys in Bauman et al. study (2004), they did not show increased social fear. These studies indicate that the amygdala is involved in regulation and/or modulation of emotional behavioral and social interactions and is critical for normal development of socioemotional behavior. Importantly, these studies also stressed the role of the social environment complexity in the behavioral outcomes observed after neonatal amygdala lesions in monkeys.
The effects of inferior temporal cortical lesions in infant monkeys
Extensive ablations or disconnections of the inferior temporal cortex in adult monkeys also result in behavioral and emotional abnormalities, such as hyperorality, hypermetamorphosis, or decrease aggressivity (Horel, Keating, & Misantone, 1975; Horel & Misantone, 1974; Iwai, Nishio, & Yamaguchi, 1986). Inferior temporal lesions that included the temporal pole also yielded social deficits (Akert, Gruesen, Woolsey, & Myers, 1961; Myers, 1958, 1975; Myers & Swett, 1970). In contrast, the impairment in socioemotional behavior observed in monkeys with neonatal inferior temporal lesions was only transient. Thus, although these operated animals displayed less social interactions with their controls when they were tested at 6 months of age (Bachevalier et al., 2001) and abnormal patterns of vocalizations when tested as juveniles (Newman & Bachevalier, 1997), they had normal amount of social contacts when re-tested as adults and demonstrated greater emotional reactivity to separation from their peers. The significant sparing of socio-emotional behaviors is consistent with the sparing found in the same animals in visual recognition (Málková et al., 1995). Thus, as compared to the neonatal medial temporal lobe lesions (see above), the findings on neonatal inferior temporal cortical lesions demonstrate that damage to the temporal cortical areas results in a transient impairment in both socio-emotional behaviors and memory.
Conclusion
Neonatal medial temporal lesions resulted in long-lasting socioemotional disturbances that were still present when the animals were retested as adults and that were even more severe than the social deficits found after the same lesions in adult monkeys that were raised in similar conditions as infants and juveniles. This outcome after neonatal versus adult medial temporal lesions is in contrast with the finding that the same monkeys with neonatal lesions exhibited a long-lasting and severe global recognition memory loss, which was, nevertheless, slightly less severe than the memory loss after the same lesions in adulthood (Málková et al., 1995). The possible explanation of the slight sparing of the memory function after the early lesions was that the medial cortical tissue in and around the rhinal sulcus, region which is now known to be critical for visual recognition memory (Meunier, Bachevalier, Mishkin, & Murray, 1993), was partially spared in the present neonatal medial temporal lobe lesions. Partial sparing of this cortical tissue could have more beneficial consequences during the period of development because of the connectional plasticity of the cortical tissue (see below). In contrast, subcortical structures such as the amygdala, which seems to be critical for the development of normal social interactions, may not possess the same magnitude of plasticity. This could possibly explain the more severe impairment in socioemotional behavior than in visual memory after early versus late medial temporal lobe lesions. Unlike adult lesions, neonatal lesions of the inferior temporal cortex (TE) resulted in sparing of both visual recognition (Málková et al., 1995) and socioemotional behavior when the animals were reassessed as adults. This sparing of functions indicate that compensatory mechanisms could operate early in infancy and yield to behavioral effects that are only transient. Such compensatory mechanisms have already been studied after neonatal area TE lesions and include the maintenance of exuberant projections that would have normally disappeared during maturation and sprouting of new projections (Webster, Bachevalier, & Ungerleider, 1994, 1995b; Webster, Ungerleider, & Bachevalier, 1991a,b).
Summary
When retested as adults, monkeys with neonatal medial temporal lesions (Group AH) maintained a severe lack of Social interaction and low levels of Self-directed activity, Manipulation, Locomotion, and Submissive behaviors. Their dramatic lack of Social interaction was even more severe than that observed in the monkeys that received the same lesions in adulthood (Group AHa). In contrast, the impairment in adult socioemotional behavior observed in monkeys with neonatal inferior temporal lesions was only transient since when these animals reached adulthood, they no longer showed the lack of social interactions or any abnormal behaviors observed when they were infants. Thus, the pattern of abnormalities found in social behavior after the two types of lesions seems to be similar to that described earlier for memory functions, that is, whereas medial temporal lobe lesions yielded a permanent memory loss, the inferior temporal lesions had only a transient effect (Málková et al., 1995).
Acknowledgments
We thank Bryan Kirkpatrick for behavioral analyses of the videotapes as the second observer. We also thank Norma Minters for post-operative care of the animals, John Sewell III for histological processing of the brains, and Richard C. Saunders for MRI scans of the animals.
This work was supported by Intramural Research Program of the National Institute of Mental Health and Division of Intramural Research, Eunice Kennedy Shriver National Institute of Child Health and Human Development, the work on the manuscript was supported in part by KO2HD42269 and R01MH084069 to LM and R01MH58846 and P01HD35471 to JB.
Footnotes
Publisher's Disclaimer: The following manuscript is the final accepted manuscript. It has not been subjected to the final copyediting, fact-checking, and proofreading required for formal publication. It is not the definitive, publisher-authenticated version. The American Psychological Association and its Council of Editors disclaim any responsibility or liabilities for errors or omissions of this manuscript version, any version derived from this manuscript by NIH, or other third parties. The published version is available at www.apa.org/pubs/journals/BNE
References
- Akert K, Gruesen RA, Woolsey CN, Meyer DR. Klüver-Bucy syndrome in monkeys with neocortical ablations of temporal lobe. Brain. 1961;84:480–498. doi: 10.1093/brain/84.3.480. [DOI] [PubMed] [Google Scholar]
- Bachevalier J. Medial temporal lobe structures and autism: A review of clinical and experimental findings. Neuropsychologia. 1994;32:627–648. doi: 10.1016/0028-3932(94)90025-6. [DOI] [PubMed] [Google Scholar]
- Bachevalier J, Mishkin M. Effects of selective neonatal temporal lobe lesions on visual recognition memory in rhesus monkeys. Journal of Neuroscience. 1994;14:2128–2139. doi: 10.1523/JNEUROSCI.14-04-02128.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachevalier J, Meunier M. The neurobiology of social-emotional cognition in nonhuman primates. In: Easton A, Emery NJ, editors. The cognitive neuroscience of social behaviour. London: Psychology Press; 2005. pp. 19–58. [Google Scholar]
- Bachevalier J, Brickson M, Hagger C. Limbic-dependent recognition memory in monkeys develops early in infancy. Neuroreport. 1993;4:77–80. doi: 10.1097/00001756-199301000-00020. [DOI] [PubMed] [Google Scholar]
- Bachevalier J, Malkova L, Mishkin M. Effects of selective neonatal temporal lobe lesions on socioemotional behavior in infant rhesus monkeys (Macaca mulatta) Behavioral Neuroscience. 2001;115:545–559. doi: 10.1037//0735-7044.115.3.545. [DOI] [PubMed] [Google Scholar]
- Bachevalier J, Brickson M, Hagger C, Mishkin M. Age and sex differences in the effects of selective temporal lobe lesion on the formation of visual discrimination habits in rhesus monkeys (Macaca mulatta) Behavioral Neuroscience. 1990;104:885–899. doi: 10.1037//0735-7044.104.6.885. [DOI] [PubMed] [Google Scholar]
- Bauman MD, Toscano JE, Mason WA, Amaral DG. Continued analysis of social development in rhesus monkeys with neonatal amygdala lesions. Society for Neuroscience Abstracts. 2007;748:2/EEE19. [Google Scholar]
- Bauman MD, Lavenex P, Mason WA, Capitanio JP, Amaral DG. The development of social behavior following neonatal amygdala lesions in rhesus monkeys. Journal of Cognitive Neuroscience. 2004;16:1388–1411. doi: 10.1162/0898929042304741. [DOI] [PubMed] [Google Scholar]
- Bauman MD, Toscano JE, Mason WA, Lavenex P, Amaral DG. The expression of social dominance following neonatal lesions of the amygdala or hippocampus in rhesus monkeys (Macaca mulatta) Behavioral Neuroscience. 2006;120:749–760. doi: 10.1037/0735-7044.120.4.749. [DOI] [PubMed] [Google Scholar]
- Bauman MD, Toscano JE, Babineau BA, Mason WA, Amaral DG. Emergence of stereotypies in juvenile monkeys (Macaca mulatta) with neonatal amygdala or hippocampus lesions. Behavioral Neuroscience. 2008;122:1005–1015. doi: 10.1037/a0012600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beauregard M, Malkova L, Bachevalier J. Stereotypies and loss of social affiliation after early hippocampectomy in primates. Neuroreport. 1995;6:2521–2526. doi: 10.1097/00001756-199512150-00018. [DOI] [PubMed] [Google Scholar]
- Brown S, Schafer A. An investigation into the fundtions of the occipital and temporal lobes of the monkey’s brain. Philosophical Transactions of the Royal Society fo London (Biology) 1888;179:303–327. [Google Scholar]
- Bucy PC, Klüver H. An anatomical investigation of the temporal lobe in the monkey (Macaca mulatta) Journal of Comparative Neurology. 1955;103:151–251. doi: 10.1002/cne.901030202. [DOI] [PubMed] [Google Scholar]
- Emery NJ, Capitanio JP, Mason WA, Machado CJ, Mendoza SP, Amaral DG. The effects of bilateral lesions of the amygdala on dyadic social interactions in rhesus monkeys (Macaca mulatta) Behavioral Neuroscience. 2001;115:515–544. [PubMed] [Google Scholar]
- Fonberg E. Hyperphagia produced by lateral amygdalar lesions in dogs. Acta Neurobiologica Experiemntalis (Warsaw) 1971;31:19–32. [PubMed] [Google Scholar]
- Goldman PS, Rosvold HE, Mishkin M. Selective sparing of function following prefrontal lobectomy in infant monkeys. Experimental Neurology. 1970;29:221–226. doi: 10.1016/0014-4886(70)90053-1. [DOI] [PubMed] [Google Scholar]
- Gross CG. How inferior temporal cortex became visual area. Cerebral Cortex. 1994;4:455–469. doi: 10.1093/cercor/4.5.455. [DOI] [PubMed] [Google Scholar]
- Horel JA, Misantone LJ. The Klüver-Bucy syndrome produced by partial isolation of the temporal lobe. Experimental Neurology. 1974;42:101–112. doi: 10.1016/0014-4886(74)90010-7. [DOI] [PubMed] [Google Scholar]
- Horel JA, Keating EG, Misantone LJ. Partial-Bucy syndrome produced by destroying temporal neocortex or amygdala. Brain Research. 1975;94:347–359. doi: 10.1016/0006-8993(75)90067-0. [DOI] [PubMed] [Google Scholar]
- Iwai E, Nishio T, Yamaguchi K. Neuropsychological basis of K-B sign in Klüver-Bucy syndrome produced following total removal of inferotemporal cortex of macaque monkeys. In: Oomura Y, editor. Emotions: Neural and chemical control. Tokyo: Japan: Scientific Societies Press; 1986. pp. 299–311. [Google Scholar]
- Kennard ME. Age and other factrs in motor recovery from precentral lesions in monkeys. American Journal of Physiology. 1936;115:138–146. [Google Scholar]
- Kennard ME. Reorganization of motor function in the cerebral cortex in monkeys deprived of motor and premotor areas in infancy. Journal of Neurophysiology. 1938;1:477–496. [Google Scholar]
- Kling AS, Mass R. Alterations of social behavior with neural lesions in nohuman primates. In: Holloway RL, editor. Primate Aggression, Territoriality, and Zenophobia. New York: Academy Press; 1974. pp. 361–386. [Google Scholar]
- Kling AS, Steklis HD. A neural substrate for affiliative behavior in nonhuman primates. Brain, Behavior, & Evolution. 1976;13:216–238. doi: 10.1159/000123811. [DOI] [PubMed] [Google Scholar]
- Kling AS, Brothers LA. The amygdala and social behavior. In: Aggleton JP, editor. The amygdala: Neurobiological aspects of emotion, memory, and mental dysfunction. New York: Wiley-Liss; 1992. pp. 353–377. [Google Scholar]
- Klüver H, Bucy PC. An analysis of certain effects of bilateral lobectomy in the rhesus monkey with special reference to “psychic blindness”. Journal of Psychology. 1938;5:33–54. [Google Scholar]
- Klüver H, Bucy PC. Preliminary analysis of functioning of the temporal lobes in monkeys. Archives of Neurology and Psychiatry. 1939;42:979–1000. [Google Scholar]
- Kolb B. Recovery from early cortical damage in rats. I. Differential behavioral and anatomical effects of frontal lesions at different ages of neural maturation. Behavioral Brain Research. 1987;25:205–220. doi: 10.1016/0166-4328(87)90069-6. [DOI] [PubMed] [Google Scholar]
- Machado CJ, Emery NJ, Capitanio JP, Mason WA, Mendoza SP, Amaral DG. Bilateral neurotoxic amygdala lesions in rhesus monkeys (Macaca mulatta): consistent pattern of behavior across different social contexts. Behavioral Neuroscience. 2008;122:251–266. doi: 10.1037/0735-7044.122.2.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malkova L, Mishkin M, Bachevalier J. Long-term effects of selective neonatal temporal lobe lesions on learning and memory in monkeys. Behavioral Neuroscience. 1995;109:212–226. doi: 10.1037//0735-7044.109.2.212. [DOI] [PubMed] [Google Scholar]
- Malkova L, Mishkin M, Suomi SJ, Bachevalier J. Socioemotional behavior in adult rhesus monkeys after early versus late lesions of the medial temporal lobe. Annals of the New York Academy of Sciences. 1997;807:538–540. doi: 10.1111/j.1749-6632.1997.tb51961.x. [DOI] [PubMed] [Google Scholar]
- Malkova L, Bachevalier J, Webster M, Mishkin M. Effects of neonatal inferior prefrontal and medial temporal lesions on learning the rule for delayed nonmatching-to-sample. Developmental Neuropsychology. 2000;18:399–421. doi: 10.1207/S1532694207Malkova. [DOI] [PubMed] [Google Scholar]
- Marlowe WB, Mancall EL, Thomas JJ. Complete Klüver-Bucy syndrome in man. Cortex. 1975;11:53–59. doi: 10.1016/s0010-9452(75)80020-7. [DOI] [PubMed] [Google Scholar]
- Merjanian P, Bachevalier J, Pettigrew KD, Mishkin M. Behavioral disturbances in the developing rhesus monkeys following neonatal lesions of inferior temporal cortex (Area TE) resemble those of attention-deficit hyperactivity disorder. Society for Neuroscience Abstracts. 1989;15:302. [Google Scholar]
- Meunier M, Bachevalier J. Comparison of emotional responses in monkeys with rhinal cortex or amygdala lesions. Emotion. 2002;2:147–161. doi: 10.1037/1528-3542.2.2.147. [DOI] [PubMed] [Google Scholar]
- Meunier M, Nalwa V, Bachevalier J. Reactions to familiar and novel objects in infant monkeys with neonatal temporal lesions. Hippocampus. 2003;13:489–493. doi: 10.1002/hipo.10143. [DOI] [PubMed] [Google Scholar]
- Meunier M, Cirilli L, Bachevalier J. Responses to affective stimuli in monkeys with entorhinal or perirhinal cortex lesions. Journal of Neuroscience. 2006;26:7718–7722. doi: 10.1523/JNEUROSCI.1949-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meunier M, Bachevalier J, Mishkin M, Murray EA. Effects on visual recognition of combined and separate ablations of the entorhinal and perirhinal cortex in rhesus monkeys. Journal of Neuroscience. 1993;13:5418–5432. doi: 10.1523/JNEUROSCI.13-12-05418.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirsky AF. Studies of the effects of brain lesions on social behavior in Macaca mulatta: methodological and theoretical considerations. Annals of the New York Academy of Sciences. 1960;85:785–794. doi: 10.1111/j.1749-6632.1960.tb50000.x. [DOI] [PubMed] [Google Scholar]
- Mishkin M. Memory in monkeys severely impaired by combined but not separate removal of amygdala and hippocampus. Nature. 1978;273:297–298. doi: 10.1038/273297a0. [DOI] [PubMed] [Google Scholar]
- Myers DP. Some psychological determinants of sparing and loss following damage to the brain. In: Harlow HF, Woolsey CN, editors. Biological & Biochemical Bases of Behavior. Madison, WI: University of Wisconsin Press; 1958. pp. 173–192. [Google Scholar]
- Myers RE. Neurology of social behavior and affect in primates: a study of prefrontal and anterior temporal cprtex. In: Zulch KE, editor. Cerebral Localization. New York: Springer-Verlag; 1975. pp. 161–170. [Google Scholar]
- Myers RE, Swett C., Jr Social behavior deficits of free-ranging monkeys after anterior temporal cortex removal: a preliminary report. Brain Research. 1970;18:551–556. doi: 10.1016/0006-8993(70)90140-x. [DOI] [PubMed] [Google Scholar]
- Newman JD, Bachevalier J. Neonatal ablations of the amygdala and inferior temporal cortex alter the vocal response to social separation in rhesus macaques. Brain Research. 1997;758:180–186. doi: 10.1016/s0006-8993(97)00212-6. [DOI] [PubMed] [Google Scholar]
- Prather MD, Lavenex P, Mauldin-Jourdain ML, Mason WA, Capitanio JP, Mendoza SP, Amaral DG. Increased social fear and decreased fear of objects in monkeys with neonatal amygdala lesions. Neuroscience. 2001;106:653–658. doi: 10.1016/s0306-4522(01)00445-6. [DOI] [PubMed] [Google Scholar]
- Pribram KH, Bagshaw M. Further analysis of the temporal lobe syndrome utilizing fronto-temporal ablations. Journal of Comparative Neurology. 1953;99:347–375. doi: 10.1002/cne.900990208. [DOI] [PubMed] [Google Scholar]
- Raper JR, Stephens S, Goursaud AP, Wallen K, Bachevalier J. Society for Neuroscience Abstracts. Vol. 34. 2008. Neonatal lesions of the amygdala result in reduced fear behavior in male rhesus monkeys (Macaca mulatta) reared in a semi-naturalistic environment. online. [Google Scholar]
- Rollins BL, Kling BM. Amygdala-lesion obesity: what is the role of the various amygdaloid nuclei? American Journal of Physiology Regulatory, Comparative, and Physiological Psycholoy. 2000;279:R1348–1356. doi: 10.1152/ajpregu.2000.279.4.R1348. [DOI] [PubMed] [Google Scholar]
- Rosvold HE, Mirsky AF, Pribram KH. Influence of amygdalectomy on social behavior in monkeys. Journal of Comparative and Physiological Psychology. 1954;47:173–178. doi: 10.1037/h0058870. [DOI] [PubMed] [Google Scholar]
- Rushmore RJ, Rigolo L, Peer AK, Afifi LM, Valero-Cabre A, Payne BR. Age-dependent sparing of visual function after bilateral lesions of primary visual cortex. Behavioral Neuroscience. 2008;122:1274–1283. doi: 10.1037/a0013586. [DOI] [PubMed] [Google Scholar]
- Schwartzbaum JS. Some characteristics of ‘amygdaloid hyperphagia’ in monkeys. American Journal of Psychology. 1961;74:252–259. [PubMed] [Google Scholar]
- Shupert C, Cornwell P, Payne B. Differential sparing of depth perception, orienting, and optokinetic nystagmus after neonatal versus adult lesions of cortical areas 17, 18, and 19 in the cat. Behavioral Neuroscience. 1993;107:633–650. doi: 10.1037//0735-7044.107.4.633. [DOI] [PubMed] [Google Scholar]
- Terzian H, Ore GD. Syndrome of Klüver and Bucy; reproduced in man by bilateral removal of the temporal lobes. Neurology. 1955;5:373–380. doi: 10.1212/wnl.5.6.373. [DOI] [PubMed] [Google Scholar]
- Thompson CI. Long-term behavioral development of rhesus monkeys after amygdalectomy in infancy. In: Ben Ari Y, editor. The amygdala complex. Amsterdam: Elsevier North-Holland Biomedical Press; 1981. pp. 259–270. [Google Scholar]
- Toscano JE, Bauman MD, Mason WA, Amaral DG. Interest in infants by female rhesus monkeys with neonatal lesions of the amygdala or hippocampus. Neuroscience. 2009;162:881–891. doi: 10.1016/j.neuroscience.2009.05.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Bonin G, Bailey P. The neocortex of macaca mulatta. Urbana, Il: University of Illinois; 1947. [Google Scholar]
- Wallen K, Raper J, Stephens S, Goursaud AP, Bachevalier J. Behavioral effects of neonatal amygdala lesions in male monkeys living in a semi-naturalistic environment. Society for Neuroscience Abstracts. 2006;578:520/MM103. [Google Scholar]
- Webster MJ, Ungerleider LG, Bachevalier J. Lesions of inferior temporal area TE in infant monkeys alter cortico-amygdalar projections. Neuroreport. 1991a;2:769–772. doi: 10.1097/00001756-199112000-00010. [DOI] [PubMed] [Google Scholar]
- Webster MJ, Ungerleider LG, Bachevalier J. Connections of inferior temporal areas TE and TEO with medial temporal-lobe structures in infant and adult monkeys. Journal of Neuroscience. 1991b;11:1095–1116. doi: 10.1523/JNEUROSCI.11-04-01095.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webster MJ, Bachevalier J, Ungerleider LG. Connections of inferior temporal areas TEO and TE with parietal and frontal cortex in macaque monkeys. Cereb Cortex. 1994;4:470–483. doi: 10.1093/cercor/4.5.470. [DOI] [PubMed] [Google Scholar]
- Webster MJ, Ungerleider LG, Bachevalier J. Development and plasticity of the neural circuitry underlying visual recognition memory. Canadian Journal of Physiology and Pharmacol. 1995a;73:1364–1371. doi: 10.1139/y95-191. [DOI] [PubMed] [Google Scholar]
- Webster MJ, Bachevalier J, Ungerleider LG. Transient subcortical connections of inferior temporal areas TE and TEO in infant macaque monkeys. Journal of Comparative Neurolology. 1995b;352:213–226. doi: 10.1002/cne.903520205. [DOI] [PubMed] [Google Scholar]
- Weiskrantz L. Behavioral changes associated with ablation of the amygdaloid complex in monkeys. Journal of Comparative and Physiological Psychology. 1956;49:381–391. doi: 10.1037/h0088009. [DOI] [PubMed] [Google Scholar]