Abstract
Background:
Despite the best current treatments, intra-articular fractures commonly cause posttraumatic osteoarthritis. In this disorder, death and dysfunction of chondrocytes associated with acute cartilage injury presumably plays an important role in triggering the pathomechanical cascade that eventually leads to whole-joint degeneration. Information regarding this cell-level cartilage injury, particularly at the whole-organ level in actual human joints, has been lacking. In this study, the distribution and progression of fracture-associated cell-level cartilage damage were assessed using a novel whole-organ model of human ankle intra-articular fracture.
Methods:
Seven normal human ankles harvested immediately following amputation were subjected to a transarticular compressive impaction insult that mimicked an injury mechanism typical of tibial plafond fractures. For each ankle, site-specific, time-dependent changes in chondrocyte viability in the fractured tibial surface were studied by means of live-dead assay, using a confocal laser-scanning microscope. Fractional chondrocyte death was measured at several time points, in the superficial zone of the cartilage in “fracture-edge” regions within 1 mm of the fracture lines, as well as in “non-fracture” regions more than 3 mm centrally away from the fracture lines.
Results:
All seven experimental fractures morphologically replicated tibial plafond fractures. Immediately post-fracture, superficial-zone chondrocyte death was significantly greater (p = 0.001) in fracture-edge regions (fractional cell death = 7.6%) than in non-fracture regions (1.6%). Progression of cell death over the next forty-eight hours was significantly faster in fracture-edge regions (p = 0.007), with the fractional cell death reaching 25.9%, which was again significantly higher (p < 0.001) than in non-fracture regions (8.6%).
Conclusions:
Cell-level cartilage damage in human intra-articular fractures was characterized by acute chondrocyte death that predominated along fracture lines and that spontaneously progressed in the forty-eight hours following injury.
Clinical Relevance:
Progressive chondrocyte damage along fracture lines appears to be a reasonable target of therapeutic treatment to preserve the whole-joint cartilage metabolism in intra-articular fractures, eventually to mitigate the risk of posttraumatic osteoarthritis.
Intra-articular fractures commonly cause posttraumatic osteoarthritis (OA), despite current treatments that effectively reduce and stabilize the fractured surfaces. Clinical series of tibial plafond fractures1-4 have shown that more than half of patients treated by surgical reconstruction of the joint nevertheless develop posttraumatic OA, often within two years of injury. To develop novel treatment strategies that effectively mitigate the risk of posttraumatic OA, there is a clear need to advance the understanding of the pathogenesis of progressive joint degeneration following injury.
There are several factors that presumably contribute to cartilage degeneration in fracture-injured joints5,6. These factors include acute mechanical articular cartilage damage at the instant of the injury event, biological stimuli associated with joint bleeding and/or inflammatory responses, and chronic cartilage overloading resulting from incongruity, instability, and malalignment. Recently, chondrocyte damage involved in human intra-articular fractures has been receiving increasing attention7-9. Death or dysfunction of chondrocytes in fracture-damaged joints necessarily impacts cartilage metabolism, presumably triggering a pathological cascade that eventually leads to whole-joint degeneration. Cell-level cartilage injury appears to be an important pathological process to target for new orthopaedic treatment(s) to forestall the development of OA following intra-articular fractures.
Previous studies documented that chondrocyte damage accompanying fracture-associated articular cartilage injury in humans was distributed near the edges of fracture fragments, particularly in the superficial zone7,8. Unfortunately, in these studies, the use of tissue material obtained at the time of definitive fracture surgery precluded exploration of the characteristics of cell-level cartilage damage occurring in the early acute phase, due to probable post-injury damage progression. In addition, chondrocyte viability in non-fracture regions could not be assessed.
The aim of the present study was to document the distribution and progression of chondrocyte damage in fracture-injured human articular surfaces, at the whole-organ level. The joint of interest was the ankle, in which a relatively high prevalence of intra-articular fractures and predictable OA development had been clinically well documented1-4. A unique model of human distal tibial intra-articular fracture was developed to study site-specific, time-dependent changes in chondrocyte death in fractured cartilage in the early acute phase. It was hypothesized that acute chondrocyte damage in human intra-articular fractures would be concentrated along fracture lines and that this chondrocyte damage would progress in the forty-eight hours after injury.
Materials and Methods
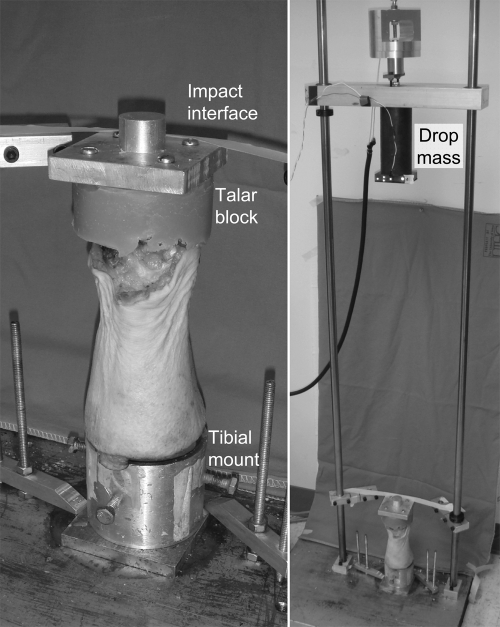
Seven normal human ankles obtained immediately (<4 hours) following surgical amputation, from patients (four men and three women, twenty-nine to seventy-one years old) who had a malignant tumor near or proximal to the knee, were utilized. In every case, the specimen was harvested from discarded tissue after pathological examination, with the only information collected being the age and sex of the patient. The tibial shaft, sectioned at the mid-diaphysis, was potted perpendicularly into a poly(methyl-methacrylate) (PMMA) block, leaving 10 cm of the distal part intact (Fig. 1). The subtalar joint was disarticulated, and the bottom of the talus was potted into another PMMA block. During this potting procedure, the ankle was held in the neutral position. Special attention was paid to align the center of the ankle to both potting blocks. The proximal fibular shaft (sectioned 9 cm above the ankle) and the lateral malleolar tip were kept away from the potting blocks. The skin and subcutaneous soft tissue surrounding the ankle were left intact.
Fig. 1.
A human ankle whole-joint specimen prepared for the fracture-impaction insult (left), and the custom drop-tower system to deliver a fracture-impaction insult (right). An electromagnet holds and releases the drop-mass from a prescribed height.
These ankles were subjected to an impaction insult that mimicked an injury mechanism typical of clinical tibial plafond fractures. Specifically, the ankle was subjected to a high-energy trans-articular compressive force pulse delivered by a custom drop-tower impaction system (Fig. 1). In this device, each specimen was mounted with the tibial side down, and an aluminum impact interface was attached to the upper face of the talar potting block. The specimen was aligned carefully with the axis of mass fall, and secured to the massive base plate, with the talar impact interface held horizontal. A 6.55-kg mass was then dropped from a 78-cm height, delivering approximately 50 J of kinetic energy to the specimen.
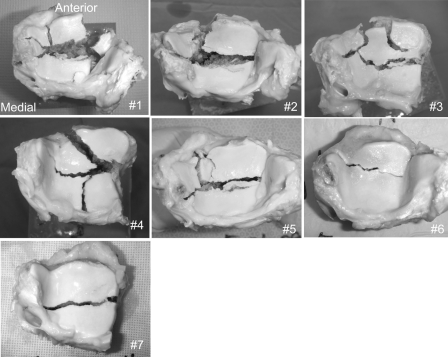
Immediately after impaction, each ankle was disarticulated, and the distal tibial articular surface was digitally photographed (Fig. 2) to record the fracture morphology. Next, under sterile conditions, major osteoarticular fragments were sampled, with juxta-articular bone being trimmed away, while leaving approximately a 5-mm thickness of cancellous bone beneath the cartilage layer (the typical volume per specimen was 1 to 2 cm3). Each osteoarticular fragment was then secured to a specimen holder, such that the articular surface near the fracture edge was held nearly horizontal, by potting in a biocompatible polymer (Polycaprolactone; Sigma-Aldrich, St. Louis, Missouri). The specimens were then immersed in culture medium (approximately 80 mL per specimen), which was comprised of 45% Ham F-12 Nutrient Mixture, 45% high-glucose Dulbecco modified Eagle medium, and 10% fetal bovine serum (all from Invitrogen, Carlsbad, California), with a 1% antibacterial-antifungal mixture of penicillin-streptomycin (Invitrogen) and amphotericin B (Thermo Scientific HyClone, Logan, Utah), in an incubator maintained at 37°C in an atmosphere of 5% CO2 in air.
Fig. 2.
Distal tibial intra-articular fractures created in the seven human ankle specimens immediately following surgical amputation.
Chondrocyte death in these osteoarticular fragments was assessed with use of calcein AM for labeling live cells and ethidium homodimer-2 for labeling dead cells (Invitrogen). Prior to analysis, the fragments were soaked in culture medium with these fluorescence stains (at a concentration of 1.0 μM each) for approximately one hour at 37°C. After labeling, chondrocytes in the superficial zone, from the cartilage surface to a depth of up to 200 μm (typically 100 to 150 μm), were scanned at 20-μm intervals, using a confocal microscopy system (MRC-1024; Bio-Rad Laboratories, Hercules, California), with the specimen kept immersed in the regular medium during scanning (at room temperature, one to two hours per specimen). After each scanning session, the specimens were washed with Hank's balanced salt solution (#14170; Gibco-Invitrogen, Grand Island, New York) containing 1% of the above-described antibacterial-antifungal mixture and placed into fresh culture media.
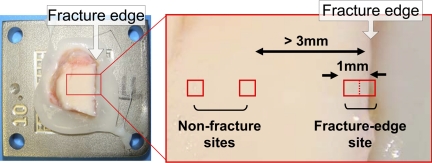
The first two ankles were scanned for cell viability from six to eight hours after the fracture insult. For these ankles, scans were executed at several sites along the primary fracture lines within 1 mm of the fragment edges (fracture-edge regions) and in the regions at least 3 mm centrally (in a direction perpendicular to the fragment edge) away from the fragment edges (non-fracture regions), with use of a ×10 objective lens that allowed scanning of an approximately 1080 × 1080-μm square field per site. The third ankle fracture was scanned after two days of culture. For this ankle, prior to scanning, five representative sites along the primary fracture lines were selected in fracture-edge regions. For each of these sites, two contiguous 540 × 540-μm fields (one including a fracture edge and the next centrally adjacent image field) were scanned with use of a ×20 objective lens (Fig. 3). Scans in non-fracture regions were performed at two nonadjacent sites selected near each fracture-edge scan site but at least 3 mm centrally away from the fragment edges. Scanning at any sites with macroscopically identifiable structural damage (e.g., visible superficial cracks) was avoided. The remaining four ankles were scanned at three time points: immediately (scans completed within six hours), at one day, and at two days post-fracture, in a manner analogous with the third ankle. Prior to the immediate scanning, four to six sets of scanning sites were selected with use of the above-described protocol, and the x-y coordinate information was recorded. Based on this coordinate information, scans at the later time points were repeated at the identical sites, with use of a custom-built programmable microscope x-y axis stage driver (positioning reproducibility, <25 μm).
Fig. 3.
An osteoarticular fragment mounted in the stage-driver system (left), and an example of scan site selection (right).
The scanned images were analyzed with use of ImageJ software (National Institutes of Health, Bethesda, Maryland). Image data for each site and time point consisted of two sets of multi-slice images, one for live cells labeled by green fluorescence and the other for dead cells labeled by red fluorescence. The images, originally in grayscale, were converted to a binary format, allowing fluorescent-labeled cells to be counted with use of the software's particle analysis function. For every image set for live cells, the image intensity threshold for the binary conversion was adjusted such that all plausibly labeled cells were counted, while paying attention to avoid counting the same cells over neighboring multiple slices. The identical binary conversion threshold was utilized for cell counting for the corresponding dead-cell image set. The minimum particle size thresholds for automated cell counting were 50 and 20 μm2 for live and dead cells, respectively. For each site and time point, live and dead cells were counted separately, and the fraction of dead cells among total cells was computed.
In statistical analysis, to account for the repeated-measures and unbalanced-design characteristics of the data, a repeated-measures analysis of variance was performed by fitting a general linear mixed-effects model with use of the SAS PROC MIXED procedure (SAS 9.1.3; SAS Institute, Cary, North Carolina). This estimation approach allowed for unbalanced data. Specifically, each specimen could have a different number of observations at each time point and/or could have no observations at one or more time points10. The MIXED approach also afforded flexibility in the specification of the variance-covariance structure. Variances for each location/day combination were allowed to differ. Covariance within specimens (i.e., for a pair of observations from the same specimen, but from different location/day combinations) was modeled by including a random subject effect, which resulted in the same within-specimen covariance between two location/day combinations. Since the variances were allowed to differ, the within-specimen correlations differed although the within-specimen covariance was constant. (Note: the [adjusted] means and 95% confidence intervals reported in the Results section are based on this model; these adjusted means are not conventional arithmetic means of the subject-level observations, but rather are weighted optimally by the MIXED procedure to take into account correlations, variance differences, and sample-size differences.) All tests were performed with a significance level of alpha set at 0.05. For descriptive purposes, the interquartile ranges (IQRs) in the form (25th percentile, 75th percentile) based on the raw data (i.e., the percentiles are computed as though the data values are independent) were also reported.
Source of Funding
This research was supported by the University of Iowa Biological Science Funding Program, by an Orthopaedic Trauma Association Research Grant, and by NIH Grant P50 AR055533.
Results
In all seven of these human ankles, comminuted fractures at the distal tibial surface occurred with the single impaction insult, with the fracture morphology (Fig. 2) being consistent with clinical tibial plafond fractures. Prior examination of these joints revealed no appreciable cartilage degeneration or synovitis in any of the specimens.
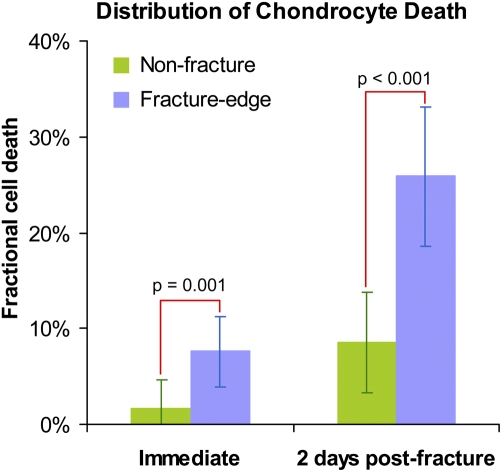
Fractional chondrocyte death immediately post-fracture impaction was measured at twenty-four fracture-edge sites and at forty-five non-fracture sites across six ankles. Cell death ranged from 0.0% to 29.6% (IQR: 2.9% to 12.4%) in fracture-edge regions and from 0.0% to 34.2% (IQR: 0.0% to 2.8%) in non-fracture regions, variable across both sites and ankles (Table I). However, the mean value in fracture-edge regions (7.6%, confidence interval [CI]: 4.0% to 11.3%, Fig. 4) was significantly higher (p = 0.001) than in non-fracture regions (1.6%, CI: 0.0% to 4.7%). Cell death at two days post-fracture was assessed at twenty-five fracture-edge sites and at forty-seven non-fracture sites, across five ankles. Cell death ranged from 2.8% to 62.4% (IQR: 12.4% to 38.6%) in fracture-edge regions and from 0.0% to 68.8% (IQR: 0.8% to 11.5%) in non-fracture regions. Again, the mean value in fracture-edge regions (25.9%, CI: 18.7% to 33.1%) was significantly higher (p < 0.001) than it was in non-fracture regions (8.6%, CI: 3.4% to 13.8%).
TABLE I.
Individual Specimen Information and Fractional Cell-Death Data
| Case (Age, Sex) | Number of Fragments Harvested | Fracture-Edge Region |
Non-Fracture Region |
||||||
| Number of Scan Sites | Mean Fractional Cell Death (Range) (%) |
Number of Scan Sites | Mean Fractional Cell Death (Range) (%) |
||||||
| Day 0 | Day 1 | Day 2 | Day 0 | Day 1 | Day 2 | ||||
| #1 (in 40s, M) | 2 | 3 | 4.9 (0.0 to 14.5) | — | — | 2 | 0.1 (0.1 to 0.2) | — | — |
| #2 (in 50s, M) | 2 | 2 | 1.2 (0.1 to 2.2) | — | — | 2 | 1.3 (0.0 to 2.6) | — | — |
| #3 (57, F) | 5 | 5 | — | — | 13.6 (2.8 to 28.9) | 10 | — | — | 7.5 (0.2 to 32.8) |
| #4 (29, F) | 4 | 5 | 11.5 (4.7 to 29.6) | 20.3 (0.8 to 41.8) | 25.5 (4.6 to 58.1) | 8 | 0.8 (0.0 to 5.6) | 0.5 (0.0 to 1.2) | 4.8 (0.0 to 25.0) |
| #5 (71, F) | 3 | 5 | 6.1 (1.3 to 15.8) | 9.4 (3.5 to 15.6) | 40.7 (30.8 to 62.4) | 15 | 0.9 (0.0 to 3.1) | 2.4 (0.0 to 11.8) | 11.3 (0.0 to 37.6) |
| #6 (57, M) | 3 | 6 | 12.2 (3.7 to 18.4) | 26.7 (10.4 to 47.9) | 34.0 (14.0 to 58.7) | 12 | 5.4 (0.2 to 34.2) | 13.6 (0.6 to 55.6) | 14.9 (1.1 to 68.8) |
| #7 (68, M) | 2 | 4 | 6.2 (3.5 to 10.0) | 7.4 (3.1 to 11.5) | 14.0 (10.0 to 18.8) | 8 | 2.0 (0.0 to 4.4) | 1.7 (0.0 to 6.2) | 1.2 (0.0 to 5.9) |
Fig. 4.
Chondrocyte viability in fracture-edge regions vs. non-fracture regions, immediately and at two days post-fracture. The values of percent fractional cell death are given as (adjusted) means calculated in the MIXED procedure. The dispersion bars indicate 95% confidence intervals.
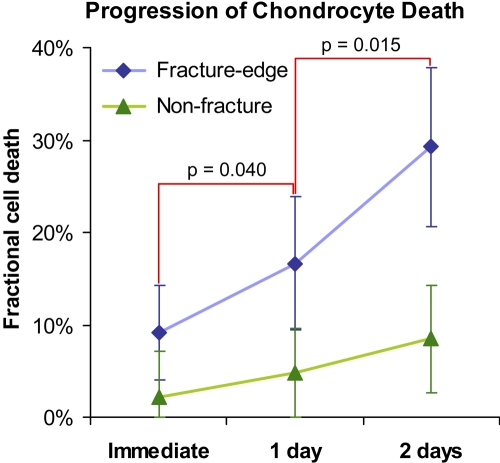
The three-time-point chondrocyte death analysis was performed at twenty fracture-edge sites and at forty-one non-fracture sites across the last four of the tested seven ankles. Cell death in fracture-edge regions, whose mean was 9.1% (CI: 4.0% to 14.2%) immediately post-fracture, increased to 16.6% (CI: 9.4% to 23.8%) at one day post-fracture (p = 0.040, vs. immediately post-fracture), and further increased to 29.2% (CI: 20.6% to 37.9%) at two days post-fracture (p = 0.015, vs. one day post-fracture) as shown in Figures 5 and 6. Although cell death in non-fracture regions also increased from immediately to two days post-fracture (p = 0.014), the fractions remained relatively low, below 10% throughout the test period in the majority (76%) of the non-fracture scan sites, and neither of the one-day comparisons (one day vs. immediately post-fracture and two days vs. one day) attained significance. The result of repeated-measures two-way analysis of variance revealed that progression of chondrocyte death from immediate to two days post-fracture in fracture-edge regions was significantly faster than in non-fracture regions (p = 0.007), with the near-fracture mean change at 20.1% (CI: 11.6% to 28.7%) and the non-fracture change at 6.3% (CI: 1.3% to 11.3%).
Fig. 5.
Time-dependent changes in human chondrocyte viability at fracture-edge regions and non-fracture regions. The values of percent fractional cell death are given as (adjusted) means calculated in the MIXED procedure. The dispersion bars indicate 95% confidence intervals.
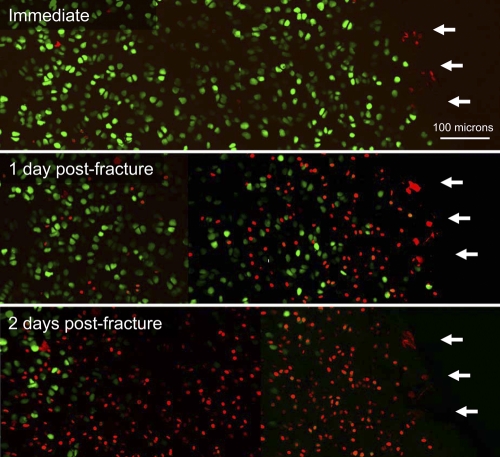
Fig. 6.
Confocal microscope images of human superficial chondrocyte viability at a representative fracture-edge scan site, at the immediate, one day, and two days post-fracture time points. Live cells are labeled by green fluorescence, while dead cells are labeled by red fluorescence. White arrows indicate the edge of cartilage on the fracture line.
Discussion
To the best of our knowledge, this is the first study of the distribution and progression of whole-joint chondrocyte death in human intra-articular fractures, in which both specimens and an insult method have closely replicated conditions in human clinical intra-articular fractures. Specifically, fracture-associated articular cartilage pathology was modeled in fresh human cartilage in an anatomical location that clinically frequently sustains intra-articular fracture. For every ankle tested, the fracture insult was delivered within four hours after surgical amputation, and with the joint capsule having been kept closed. Given that articular cartilage is avascular, the consequence of this relatively short duration of blood supply disruption on chondrocyte biological activities was minimal, as confirmed by the immediate low fractional chondrocyte death in the non-fracture regions. It therefore seems reasonable to regard these experimental conditions, under which articular cartilage in these joints was fractured, as approximating those in closed human intra-articular fractures in vivo. Nevertheless, a limitation of this study is the post-fracture conditions, including the lack of blood supply and synovial and cellular reaction the injury. These otherwise may contribute to subsequent progression of chondrocyte death. However, an advantage of the lack of these “extrinsic” biological influences following injury is that it was possible to study the isolated effect of mechanical insult to the articular surface. Another potential limitation is that, since the ankles utilized were from patients who had malignant tumor, these joints may have been load-protected for a certain period prior to amputation. Systemic chemotherapy before surgery also might have influenced chondrocyte activity. However, cells in non-fracture regions retained high fractional viability at most sites even at two days post-fracture, suggesting that the articular cartilage in these joints was initially reasonably healthy. Similarly, the potential adverse effects of ex vivo experimentation on chondrocyte viability were also presumed to be noncritical. A strength of this study was that the insult methodology utilized to produce the fractures in these joints mimicked an injury mechanism typical of clinical tibial plafond fractures. Given that the fractures introduced were morphologically consistent with this type of fracture as seen clinically, the physiologic clinical injury mechanism arguably was appropriately replicated in this model.
The appreciable chondrocyte death identified along fracture lines in the present study is consistent with observations in previous studies that explored fracture-associated cartilage pathology in small osteoarticular fragments during surgery for intra-articular fracture fixation7-9. The low fractions of cell death across non-fracture regions, documented in our study, indicate that chondrocyte death in fractured human joints occurs predominantly near fracture lines. Chondrocyte damage in intra-articular fractures appears to be closely associated with structural damage of the articular surface, just as suggested by cartilage blunt-impact experiments11,12 and by blunt-wounding experiments13,14 (in which blunt wounding introduced more severe chondrocyte damage than a sharp scalpel cut). Assuming that the majority of chondrocyte damage occurs along fracture lines, the degree of whole-joint chondrocyte dysfunction would presumably be correlated with the total length of fracture lines, which in turn correlates with mechanical energy absorption during the fracture event15,16. This observation supports the concept that joints with more severely comminuted articular surfaces sustain a greater insult to cartilage metabolism, and therefore are at a greater risk of posttraumatic OA.
The measured time-dependent increase of cell death in fracture-edge regions indicates that chondrocyte damage in these fractures propagates spontaneously with time. The chondrocyte death identified immediately post-fracture was presumably cell necrosis, due to excessive acute mechanical stresses from the fracture. These physical stresses might have caused delayed apoptosis, which would have contributed to the acute-phase propagation of chondrocyte damage identified at the later observation times. Another possible contributing factor is the cytotoxic effect of biochemical mediators released from damaged chondrocytes and/or from the disrupted extracellular matrix, similar to that observed in cartilage-explant blunt-impact studies17-19. In intra-articular fractures in vivo, acutely damaged cartilage is also subsequently subjected to biological stimuli associated with joint bleeding and/or inflammatory responses, including factors known to accelerate cartilage degeneration, such as reactive oxygen species, matrix-damaging enzymes, inflammatory cells, and inflammatory cytokines20-25. Arguably, chondrocyte damage in fractured joints is therefore not fully established at the instant of injury event. There may be a certain window of time during which biological treatment may be able to inhibit progression of chondrocyte damage in the early acute phase, potentially moderating the pathological cascade that leads to posttraumatic OA.
Chondrocyte death that predominated along fracture lines in this study suggests that whole-organ cell-level cartilage damage in a fractured human ankle joint is dependent on the total fracture line length and is therefore closely associated with the initial injury severity. Progression of chondrocyte death during the two days of cartilage culture suggests that the fracture-associated cell-level cartilage damage propagates spontaneously with time during the early acute phase. In the somewhat analogous situations of acute myocardial infarction and ischemic stroke, early thrombolytic therapy to minimize progressive cell death has led to dramatic improvement of clinical outcomes26,27. A similar concept may apply to treatment of intra-articular fracture. Arguably, the most effective treatment to mitigate the risk of posttraumatic OA would not only treat macroscopic damage with anatomical fracture reduction and fixation but would also seek to preserve viable, metabolically effective chondrocytes.
Footnotes
Disclosure: In support of their research for or preparation of this work, one or more of the authors received, in any one year, outside funding or grants in excess of $10,000 from the University of Iowa Biological Science Funding Program, the Orthopaedic Trauma Association (a research grant), and the National Institutes of Health (Grant P50 AR055533). Neither they nor a member of their immediate families received payments or other benefits or a commitment or agreement to provide such benefits from a commercial entity.
References
- 1.Etter C, Ganz R. Long-term results of tibial plafond fractures treated with open reduction and internal fixation. Arch Orthop Trauma Surg. 1991;110:277-83 [DOI] [PubMed] [Google Scholar]
- 2.Marsh JL, Weigel DP, Dirschl DR. Tibial plafond fractures. How do these ankles function over time? J Bone Joint Surg Am. 2003;85:287-95 [PubMed] [Google Scholar]
- 3.Ovadia DN, Beals RK. Fractures of the tibial plafond. J Bone Joint Surg Am. 1986;68:543-51 [PubMed] [Google Scholar]
- 4.Teeny SM, Wiss DA. Open reduction and internal fixation of tibial plafond fractures. Variables contributing to poor results and complications. Clin Orthop Relat Res. 1993;292:108-17 [PubMed] [Google Scholar]
- 5.Buckwalter JA, Brown TD. Joint injury, repair, and remodeling: roles in post-traumatic osteoarthritis. Clin Orthop Relat Res. 2004;423:7-16 [PubMed] [Google Scholar]
- 6.Furman BD, Olson SA, Guilak F. The development of posttraumatic arthritis after articular fracture. J Orthop Trauma. 2006;20:719-25 [DOI] [PubMed] [Google Scholar]
- 7.Hembree WC, Ward BD, Furman BD, Zura RD, Nichols LA, Guilak F, Olson SA. Viability and apoptosis of human chondrocytes in osteochondral fragments following joint trauma. J Bone Joint Surg Br. 2007;89:1388-95 [DOI] [PubMed] [Google Scholar]
- 8.Kim HT, Lo MY, Pillarisetty R. Chondrocyte apoptosis following intraarticular fracture in humans. Osteoarthritis Cartilage. 2002;10:747-9 [DOI] [PubMed] [Google Scholar]
- 9.Murray MM, Zurakowski D, Vrahas MS. The death of articular chondrocytes after intra-articular fracture in humans. J Trauma. 2004;56:128-31 [DOI] [PubMed] [Google Scholar]
- 10.Verbeke G, Molenberghs G. Linear mixed models for longitudinal data. New York: Springer; 2000. p 41-99 [Google Scholar]
- 11.Ewers BJ, Dvoracek-Driksna D, Orth MW, Haut RC. The extent of matrix damage and chondrocyte death in mechanically traumatized articular cartilage explants depends on rate of loading. J Orthop Res. 2001;19:779-84 [DOI] [PubMed] [Google Scholar]
- 12.Lewis JL, Deloria LB, Oyen-Tiesma M, Thompson RC, Jr, Ericson M, Oegema TR., Jr Cell death after cartilage impact occurs around matrix cracks. J Orthop Res. 2003;21:881-7 [DOI] [PubMed] [Google Scholar]
- 13.Tew SR, Kwan AP, Hann A, Thomson BM, Archer CW. The reactions of articular cartilage to experimental wounding: role of apoptosis. Arthritis Rheum. 2000;43:215-25 [DOI] [PubMed] [Google Scholar]
- 14.Redman SN, Dowthwaite GP, Thomson BM, Archer CW. The cellular responses of articular cartilage to sharp and blunt trauma. Osteoarthritis Cartilage. 2004;12:106-16 [DOI] [PubMed] [Google Scholar]
- 15.Beardsley CL, Anderson DD, Marsh JL, Brown TD. Interfragmentary surface area as an index of comminution severity in cortical bone impact. J Orthop Res. 2005;23:686-90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Anderson DD, Mosqueda T, Thomas T, Hermanson EL, Brown TD, Marsh JL. Quantifying tibial plafond fracture severity: absorbed energy and fragment displacement agree with clinical rank ordering. J Orthop Res. 2008;26:1046-52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Clements KM, Burton-Wurster N, Lust G. The spread of cell death from impact damaged cartilage: lack of evidence for the role of nitric oxide and caspases. Osteoarthritis Cartilage. 2004;12:577-85 [DOI] [PubMed] [Google Scholar]
- 18.Levin A, Burton-Wurster N, Chen CT, Lust G. Intercellular signaling as a cause of cell death in cyclically impacted cartilage explants. Osteoarthritis Cartilage. 2001;9:702-11 [DOI] [PubMed] [Google Scholar]
- 19.Goodwin W, McCabe D, Sauter E, Reese E, Walter M, Buckwalter JA, Martin JA. Rotenone prevents impact-induced chondrocyte death. J Orthop Res. 2010;28:1057-63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Goldring SR, Goldring MB. The role of cytokines in cartilage matrix degeneration in osteoarthritis. Clin Orthop Relat Res. 2004;427 Suppl:S27-36 [DOI] [PubMed] [Google Scholar]
- 21.Hedbom E, Häuselmann HJ. Molecular aspects of pathogenesis in osteoarthritis: the role of inflammation. Cell Mol Life Sc. 2002;59:45-53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Henrotin Y, Kurz B, Aigner T. Oxygen and reactive oxygen species in cartilage degradation: friends or foes? Osteoarthritis Cartilage. 2005;13:643-54 [DOI] [PubMed] [Google Scholar]
- 23.Hooiveld M, Roosendaal G, Wenting M, van den Berg M, Bijlsma J, Lafeber F. Short-term exposure of cartilage to blood results in chondrocyte apoptosis. Am J Pathol. 2003;162:943-51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.John T, Stahel PF, Morgan SJ, Schulze-Tanzil G. Impact of the complement cascade on posttraumatic cartilage inflammation and degradation. Histol Histopathol. 2007;22:781-90 [DOI] [PubMed] [Google Scholar]
- 25.Martin JA, McCabe D, Walter M, Buckwalter JA, McKinley TO. N-acetylcysteine inhibits post-impact chondrocyte death in osteochondral explants. J Bone Joint Surg Am. 2009;91:1890-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Indications for fibrinolytic therapy in suspected acute myocardial infarction: collaborative overview of early mortality and major morbidity results from all randomised trials of more than 1000 patients Fibrinolytic Therapy Trialists’ (FTT) Collaborative Group. Lancet. 1994;343:311-22 [PubMed] [Google Scholar]
- 27.Wardlaw JM, Zoppo G, Yamaguchi T, Berge E. Thrombolysis for acute ischaemic stroke. Cochrane Database Syst Rev. 2003;3:CD000213. [DOI] [PubMed] [Google Scholar]