Abstract
Controlling the symptoms that are characteristic of patients with pervasive developmental disorders is often challenging. We report on the safety and efficacy of olanzapine in the treatment of 7 patients with pervasive developmental disorders. The patients were all male and ranged in age from 8 to 52 years. They received olanzapine doses of 5–10 mg/d along with their various other drug regimens. Patients were monitored and evaluated for a mean duration of 17.7 (range 12–26) months while on olanzapine therapy. Very few side effects were observed during treatment. All patients showed clinically significant improvement on the Clinical Global Impressions scale, as well as an improved score as measured by the Global Assessment of Functioning scale. Our observations support the use of long-term olanzapine therapy for symptom control in patients with pervasive developmental disorders.
Medical subject headings: antipsychotic agents; child development disorders, pervasive; dopamine; serotonin
Abstract
Il est souvent difficile de soulager les symptômes caractéristiques des patients atteints de troubles de développement profonds. Nous présentons un compte rendu de l'innocuité et l'efficacité de l'olanzapine pour le traitement de sept patients ayant des troubles de développement profonds. Les patients étaient tous de sexe masculin et avaient entre 8 et 52 ans. Ils ont reçu des doses d'olanzapine de 5 à 10 mg/j en plus de leurs divers régimes médicamenteux. Ils ont été surveillés et évalués en moyenne pendant 17,7 (entre 12 et 26) mois pendant leur traitement à l'olanzapine. Très peu d'effets secondaires ont été observés au cours du traitement. Tous les patients ont montré des améliorations importantes sur le plan clinique d'après l'échelle d'impression clinique globale. Leurs résultats se sont aussi améliorés sur l'échelle d'évaluation globale du fonctionnement. Nos observations appuient le recours à un traitement à l'olanzapine à long terme pour soulager les symptômes des patients atteints de troubles de développement profonds.
Introduction
Atypical antipsychotic medications have been well studied for the control of symptoms in schizophrenia, mood disorders and other related psychoses. The use of atypical antipsychotic drugs in other populations, such as children and adults with pervasive developmental disorders (PDDs), has not been studied as extensively. Most drugs found to be consistently effective in the treatment of patients with PDDs target central serotonin (5-HT) or dopamine (DA) pathways.1 For example, a recent study of 101 children with autism revealed risperidone to be an effective and well-tolerated treatment for tantrums, aggression and self-injurious behaviour in a significant proportion of patients.2 However, risperidone was also associated with numerous side effects, such as increased appetite, fatigue, drowsiness, dizziness and drooling.
Although there are significant pharmacologic differences among the atypical antipsychotic agents (olanzapine, clozapine, quetiapine, risperidone and ziprasidone), these agents can generally be said to have an increased affinity for 5-HT2 receptors over D2 receptors compared with traditional antipsychotic drugs such as the phenothiazines. In addition, the blockade of dopaminergic receptors is more pronounced in the limbic system than in the basal ganglia. These characteristics of the atypical neuroleptic drugs result in significantly fewer extrapyramidal effects than with the older, typical neuroleptics.3
Olanzapine, an atypical agent, has been shown to be effective in treating a variety of symptoms in schizophrenia,4 bipolar mania5 and treatment-resistant depression.6 In addition, Keck Jr. et al7 have described the efficacy of olanzapine in treating a broad spectrum of symptoms such as agitation, aggression, depression and suicidality.
To date, there has been limited research published on the use of olanzapine in children and adults with PDDs. Only a small number of case reports and several open-label studies have described symptomatic improvement and drug tolerability. In 1 case, a 17-year-old boy with autism demonstrated stabilization of mood and a significant decrease in pacing and agitation with an olanzapine regimen of 30 mg/d.8 A 10-year-old boy with autistic disorder, severe mental retardation and bipolar disorder showed a reduction in aggression and repetitive behaviours after discontinuation of thioridazine and fenfluramine and initiation of olanzapine (up to 20 mg/d), while continuing with lithium.9 An open-label, prospective pilot study of 8 patients with PDDs also reported that the use of olanzapine monotherapy over a 12-week period was effective and well tolerated in targeting core and related symptoms of PDDs, as measured by standardized rating scales to corroborate clinical impressions.10 Similarly, Kemner et al11 reported on 25 children and adolescents with autism and PDDs who received olanzapine for 12 weeks. Investigations focused on negative symptoms and found that the social interaction and contact with these patients was positively influenced.11 Further, a parallel-group study randomly allocated 12 children with autistic disorder to receive 6 weeks of open-label treatment with olanzapine or haloperidol.12 The results revealed symptom reduction in both groups, as measured by the Clinical Global Impressions (CGI) scale and the Children's Psychiatric Rating Scale (CPRS). Five of 6 participants in the olanzapine group were rated as responders compared with 3 of 6 in the haloperidol group.
These findings notwithstanding, Demb and Roychoudhury13 reported on a chart review of 12 children with developmental disabilities or psychotic disorder who had been treated with olanzapine for an average of 50 days. Of these, only 3 participants responded positively to the drug, and 8 discontinued treatment because of adverse side effects or an exacerbation of target symptoms, or both. Therefore, although some of these findings suggest olanzapine may be a promising treatment for children with autistic disorder, more research is needed in order to demonstrate adequately its clinical efficacy and tolerability.
We report on a series of 7 patients with PDD who were treated with olanzapine to control a variety of symptoms often characteristic of this patient population. In contrast to the studies cited here, these patients received olanzapine for relatively long periods (up to 26 months), thus allowing for the long-term effects of olanzapine on symptoms associated with PDD to be reported, in addition to its initial effects.
Cases
Seven male patients (5 adults and 2 children) are described in this series. The participants ranged in age from 8 to 52 (mean 30.2) years. All had received a primary diagnosis of PDD, according to DSM-IV criteria.14 Five of the participants had been diagnosed with autism, and the remaining 2 with PDD Not Otherwise Specified. Most of the patients had comorbid psychiatric disorders, such as major depressive disorder (4), anxiety disorders (5), obsessive–compulsive disorder (3) and bipolar affective disorder (2). Other medical diagnoses were also represented, such as fragile X syndrome (1), Down's syndrome (1) and microcephaly (1).
Many of these patients presented to our clinic with common symptoms representative of the above diagnoses, such as physical and verbal violence, ritualistic behaviours, vegetative symptoms, intense mood cycling, uncooperativeness and hyperactivity. Most of the patients had received numerous medications in efforts to control these symptoms, all with limited success due to a variety of reasons associated with both efficacy and safety.
Improvement in overall well-being was measured using the Global Assessment of Functioning (GAF) scale14 and the CGI scale.15 These assessments were performed by the principal investigators.
Results
All patients showed clinically significant improvements in behavioural symptoms, measured both clinically using the CGI scale and by the GAF scale. Before olanzapine therapy, mean baseline GAF scores were 37.7 (standard deviation [SD] 8.7), as opposed to mean final scores of 70.7 (SD 4.5). These results were statistically significant in a paired t test for dependent samples (t6 = 13.1, p < 0.001). Meaningful improvements on the CGI scale15 were also noted. After 6 weeks of olanzapine treatment, the condition of 4 participants had improved and 2 showed much improvement (no improvements were observed in 1 participant at this point). After 26 weeks, 2 showed improvement, 4 showed much improvement and the condition of 1 participant had very much improved. By 52 weeks, 1 participant showed improvement, whereas 3 were judged to be much improved and the condition of the remaining 3 participants had very much improved (Fig. 1). Thus, olanzapine was found to be efficacious both in clinical terms and in improved scores on the GAF scale.
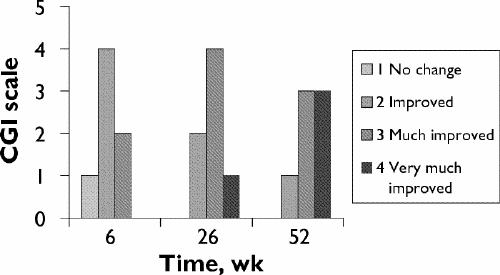
Fig. 1: Study subjects' scores on the Clinical Global Impressions (CGI) scale.
All the patients had been prescribed numerous psychotropic drug regimens in the past to control symptoms. Medications had been stopped for a variety of reasons, ranging from side effects (kidney-related, weight gain, sedation, allergies, extrapyramidal symptoms and seizures) to lack of efficacy and symptom aggravation. Patients received olanzapine doses of between 5 and 10 (mean 7.1 [SD 1.7]) mg/d. Olanzapine was administered to this group of patients for a mean period of 17.7 (SD 5.2, range 12–26) months, with sustained efficacy in all patients. (See Table 1 for information on olanzapine dosages and other drugs administered during the trial.)
Table 1
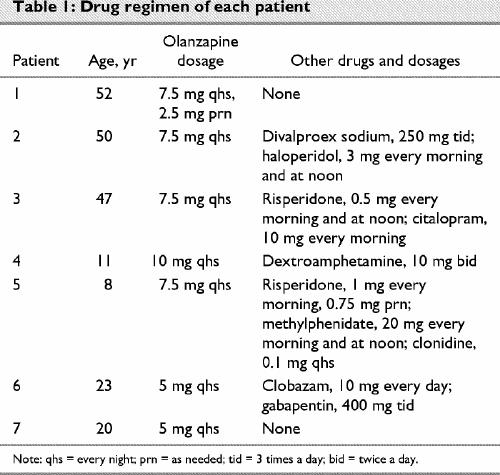
Very few side effects were observed in our group of patients after the addition of olanzapine. The most common issue (4 patients) was sedation, which was managed by administration of olanzapine in the evening. This, along with dose reductions in 3 patients, alleviated the sedation. The only other observed side effect was epilepsy in 1 patient, which was controlled by clobazam, 10 mg once daily, and gabapentin, 400 mg 3 times a day. Epilepsy is not normally associated with olanzapine and was probably not attributable to the drug. There are many reports of patients gaining weight while on olanzapine;11,12 surprisingly, we did not observe this in our patients during the observation period (t6 = 0.04, p = 0.97). Our patients, however, were receiving dietary or behavioural interventions, or both, while on olanzapine; this probably accounts for the lack of weight gain.
Discussion
Switching to olanzapine, in conjunction with other drug regimens, was associated with improvements in overall behaviour problems and negative symptoms in the 7 cases described here. We observed a maintenance of response in patients who received olanzapine for up to 26 months. These findings are more positive than those reported by Demb and Roychoudhury,13 who found that only one-quarter of participants responded favourably to olanzapine treatment. Malone et al12 and Potenza et al10 have suggested that adequate doses of olanzapine may be useful in treating symptoms for the short term (6 and 12 weeks, respectively), but that longer durations of therapy should be studied. In our research, the therapeutic effectiveness and positive long-term symptom improvement observed at dosages similar to acute dosages suggest that long-term olanzapine therapy for patients with PDD may be a valid treatment option. This will need to be investigated using double-blind, placebo-controlled trials.
Acknowledgments
We gratefully acknowledge the nonremunerated help of Shannon Muldrew, MA, C. Psych. Assoc., Neuroscience Specialty Representative, Eli Lilly Canada, and Sherilyn G. Walker, BPHE (Hons), Regional Research Associate, Neuroscience Eli Lilly Canada.
Footnotes
Competing interests: None declared.
Correspondence to: Dr. Chrissoula Stavrakaki, Child and Family Psychiatric Unit, Children's Hospital of Eastern Ontario, 311 McArthur Ave., Suite 200, Ottawa ON K1L 8M3; fax 613 738-4891; csgm91@rogers.com
Submitted Sept. 11, 2002; Revised Dec. 18, 2002; Accepted May 20, 2003.
References
- 1.Cook EH. Autism: review of neurochemical investigation. Synapse 1990;6:292-308. [DOI] [PubMed]
- 2.Research Units on Pediatric Psychopharmacology Autism Network. Risperidone treatment in children with autism and serious behavioral problems. N Engl J Med 2002;347:14-21.
- 3.Burns MJ. The pharmacology and toxicology of atypical antipsychotic agents. Clin Toxicol 2001;39(1):1-14. [DOI] [PubMed]
- 4.Tran P, Hamilton SH, Kuntz AJ, Potvin JH, Andersen SW, Beasley C, et al. Double-blind comparison of olanzapine versus risperidone in the treatment of schizophrenia and other psychotic disorders. J Clin Psychopharmacol 1997;17:407-18. [DOI] [PubMed]
- 5.Tohen M, Jacobs TG, Grundy SL, McElroy SL, Banov MC, Janicak PG, et al. Efficacy of olanzapine in acute bipolar mania: a double-blind, placebo-controlled study. Arch Gen Psychiatry 2000;57:841-9. [DOI] [PubMed]
- 6.Shelton RC, Tollefson GD, Tohen M, Stahl S, Gannon KS, Jacobs TG, et al. A novel augmentation strategy for treating resistant major depression. Am J Psychiatry 2001;158:131-4. [DOI] [PubMed]
- 7.Keck PE Jr, Strakowski SM, McElroy SL. The efficacy of atypical antipsychotics in the treatment of depressive symptoms, hostility, and suicidality in patients with schizophrenia. J Clin Psychiatry 2000;61(Suppl 3):4-9. [PubMed]
- 8.Rubin M. Use of atypical antipsychotics in children with mental retardation, autism, and other developmental disabilities. Psychiatr Ann 1997;27:219-21.
- 9.Horrigan JP, Barnhill LJ, Courvoisie HE. Olanzapine in PDD. J Am Acad Child Adolesc Psychiatry 1997;36:1166-7. [DOI] [PubMed]
- 10.Potenza MN, Holes JP, Kanes SJ, McDougle CJ. Olanzapine treatment of children, adolescents, and adults with pervasive developmental disorder: an open-label pilot study. J Clin Psychopharmacol 1999;19:37-44. [DOI] [PubMed]
- 11.Kemner C, Willemsen-Swinkels SH, de Jonge M, Tuynman-Qua H, van Engeland H. Open-label study of olanzapine in children with pervasive development disorder. J Clin Psychopharmacol 2002;22:455-60. [DOI] [PubMed]
- 12.Malone RP, Cater J, Sheikh RM, Choudhury MS, Delaney MA. Olanzapine vs. haloperidol in children with autistic disorder: an open pilot study. J Am Acad Child Adolesc Psychiatry 2001; 40:887-94. [DOI] [PubMed]
- 13.Demb HD, Roychoudhury K. Comments on “Olanzapine treatment of children, adolescents, and adults with pervasive developmental disorders: an open-label pilot study.” J Clin Psychopharmacol 2000;20:580-1. [DOI] [PubMed]
- 14.American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4th ed. Washington: The Association; 1994.
- 15.Guy W. Clinical global impressions. In: EDCEU assessment manual for psychopharmacology. Rev. ed. US Department of Health, Education, and Welfare: Rockville (MD); 1976. p. 217-22.


