Abstract
Bending of large titanium plates for mandibular reconstruction is a tedious task. This is usually done by trial and error over an intraoperatively bent template. By means of rapid prototype technology, accurate three-dimensional models can be obtained. Using these models, it is possible to design, obtain, and adapt custom hardware for individual surgical cases. Reductions of operating room time when using this technology have been reported from 17% to 60%, with an average of 20%. This translates to reduction of cost and risks, improving the overall surgical outcome. The purpose of this article is to establish the indications and contraindication for the use three-dimensional models and prebent plates. We present our experience with five cases in which prebent reconstruction plates were used for mandibular reconstruction. No significant complications occurred, and satisfactory results were achieved in all cases. We found that the models required to obtain the hardware are extremely accurate, have multiple reported applications, and represent a valuable surgical tool in the planning and execution of reconstructive surgery.
Keywords: Prebent plates, stereolithographic models, mandible reconstruction, custom hardware, medical rapid prototyping
The need for reconstruction of defects of the mandible is a challenge commonly faced in head and neck surgery. Benign or malignant tumors, osteomyelitis, trauma, osteoradionecrosis, and most recently bisphosphonate-related osteonecrosis of the jaws are conditions that commonly result in significant continuity defects in the mandible. These types of defects have a dramatic impact in the patients' anatomy, speech, nutrition, self-esteem, and overall quality of life. The use of titanium plates for reconstruction of these defects, whether acquired or congenital, is a common practice in modern head and neck surgery. Proper adaptation of reconstruction plates is critical in the success of the reconstructive procedure. When bone grafts are performed in combination with plate placement, immobility is a determinant factor in graft maturation. A well-adapted plate is essential to achieve stability of the segments, correct spatial position, and occlusion after tumor ablation. All these factors are important for the restoration of form and function.
Adaptation of a reconstruction plate to a resected mandible is usually performed in the operation room. The technique is accomplished by a trial-and-error method intraoperatively using a bent template. Multiple bends may be necessary in the attempt to reproduce the complex architecture of the mandible. Repetitive bending may fatigue the metal in areas of contour, increasing the risk of premature plate fracture. Intraoperative plate bending requires more extensive surgical exposure, increasing surgical time and potentially increasing fluid and blood loss.
The introduction of locking plates has greatly facilitated the hardware adaptation process. Locking the screws in the plate allows the surgeon to achieve stable fixation despite imperfection in adaptation. However, excessive avascular areas of scarring may result from fibrous tissue growth on gaps between the plate and the bone. Scar bands around plates can be up to 440 μm thick, which is more than twice the distance that oxygen and nutrients can diffuse from capillaries.1 This is a disadvantage when performing secondary bone grafting, which depends on plasmatic diffusion during the first 3 to 5 days.2,3
The recent advance of rapid prototype technology in the engineering field has led to the production of prototype parts and models from computerized imaging. Medical rapid prototyping (MRP) is defined as the manufacture of dimensionally accurate physical models of human anatomy derived from medical image data using a variety of rapid prototyping technology.4 MRP was introduced to the medical field in the early 1990s and was first described by Mankovich et al.5 The source of the data for the construction of these models is high-resolution computed tomography (CT) scan. There are several techniques for production of MRP anatomic models. As reported by Winder and Bibb,4 stereolithography (SL) and fused deposition modeling are the most extensively used. However, other techniques like three-dimensional printing, computer controlled milling, and selective laser sintering are available.
The purpose of this article is to discuss the indications, contraindications, advantages, and disadvantages of this technique and our early experience of five cases performed at the Medical College of Georgia Department of Oral and Maxillofacial Surgery.
INDICATIONS
For reconstructions of mandibular continuity defects resulting from trauma and/or pathology, when the extension or location of the defect requires a large plate with a complex bend. This technique is also used for many facial defects requiring alloplastic reconstruction.
CONTRAINDICATIONS
When the margin of the resection cannot be clearly identified in diagnostic imaging. In cases of discrepancy between planned and the definitive resection margins, custom hardware may become useless.
When an accurate scan or model cannot be produced. This may happen when hardware, shrapnel, or any foreign object in the field creates significant artifact in the image.
ADVANTAGES
It decreases surgical time.
It offers improved adaptation of surgical reconstruction plates.
It decreases bone plate gap and thus scar formation.
It decreases fatigue of the metal by significantly reducing the trial-and-error factor.
It protects root tips, the neurovascular bundle, and, when present, tooth buds by identifying them in the model before placement of the screws. This is true only when using SL models.
The required model serves as an aid to diagnosis, patient education, and surgical planning.
It serves as a teaching tool in the academic setting.
DISADVANTAGES
It increases radiation to the patient due to the additional required high-resolution CT scan.
It incurs additional cost of constructing the model and the custom-bent plate.
It increases waiting time from the diagnosis to the actual surgery due to the additional steps in surgical planning.
MATERIALS AND METHODS
We have treated five cases using MRP anatomic models and prebent plates for the reconstruction of mandibular continuity defects. In all cases, we utilized this technique for defects secondary to the treatment of pathology. Diagnoses were obtained in all cases prior to definitive surgical resection and planned reconstructions. Four of the cases were ameloblastoma. The remaining case was a central giant cell granuloma. Patients' ages ranged from 9 to 57 years of age. There were four males and one female. High-resolution CT scans were obtained following the same protocol. The protocol requires helical CT scan with field of view of 25 cm, with gantry tilt of 0 degrees and slice thickness from 0.75 to 1.25 mm. The patient is required to be immobile and the occlusal plane parallel to the image plane. This technique minimized artifact, ensuring an accurate anatomic reproduction and model. At least 2 cm below and beyond the area of interest should be included in the scan. The data were placed in a removable media and sent to Medical Modeling Inc. (Boulder, CO) for fabrication of the model. Models were obtained using either SL or three-dimensional printing. The models were sent back to the Medical College of Georgia for inspection and surgical planning. Resection margins were marked on the surgical model and then sent to Biomet Microfixation (Jacksonville, FL) for preparation of the custom-bent plate. One custom plate was fabricated by Osteomed (Addison, TX). All plates appear to be accurate on preoperative evaluation. Finished plates underwent autoclave sterilization. One case was immediately reconstructed using a free fibula microvascular flap; the remaining four cases were planned for delayed reconstruction with nonvascularized corticocancellous bone from the iliac crest.
CASE REPORTS
Case 1
The first case was a 57-year-old African-American male with a large expansile lesion of the left mandible. Panoramic examination showed a unilocular radiolucent lesion located at the left mandible extending from the left condylar neck to the ipsilateral first molar. Incisional biopsy proved this to be an ameloblastoma. A diagnostic model was obtained using the steps previously described. The resection margin was established distal to the left second bicuspid with planned disarticulation of left condyle. A prebent reconstruction plate with condylar prosthesis was obtained. Arch bars were placed, and the patient was put in maxillomandibular fixation (MMF). The resection was completed using a combined transoral and transcutaneous approach. Minimal adjustment was necessary at the proximal aspect of the plate to achieve ideal position of the condylar prosthesis in the fossa. The articulating disc was sutured around the prosthetic condylar head. Primary closure was achieved at all surgical sites. The total operating time was 6 hours and 55 minutes.
Case 2
The second case was a 14-year-old African-American male with an expansile lesion of the right posterior mandible. Incisional biopsy demonstrated a giant cell tumor. A high-resolution CT scan was obtained followed by construction of the three-dimensional model. The resection margin was defined distal to the right second premolar to include disarticulation of the right mandibular condyle. A prebent reconstruction plate with condylar prosthesis was obtained and sterilized. The patient was placed in MMF. The resection was completed as planned via combined preauricular, submandibular, and transoral approaches. The plate required minimal adjustment at the proximal aspect for ideal condylar positioning. The plate was secured with multiple 2.4-mm screws. The meniscus was secured and sutured around the prosthetic condyle. Total operating time was 5 hours and 7 minutes.
Case 3
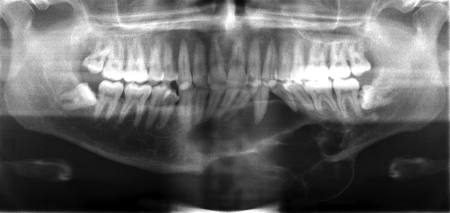
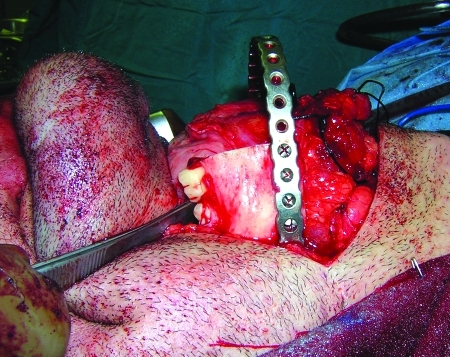
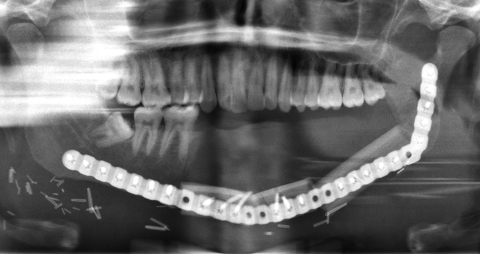
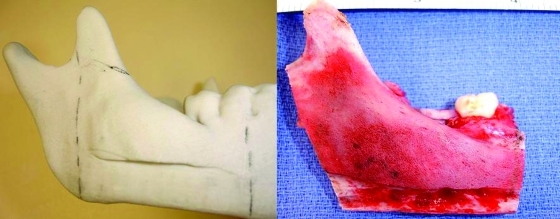
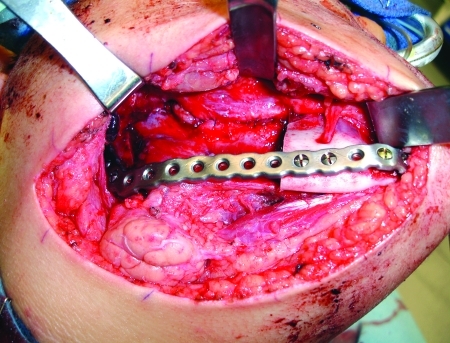
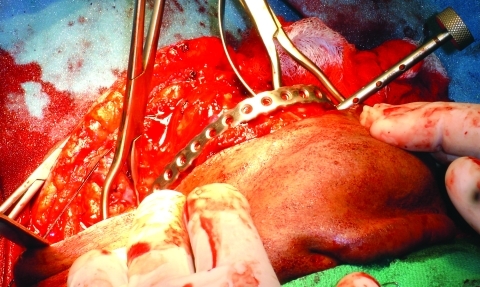
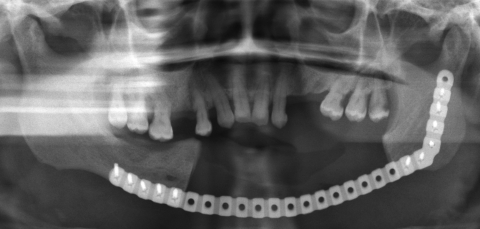
The third case was a 29-year-old white male with an expansile lesion of the anterior mandible. Panoramic radiograph showed a multilocular lesion involving the inferior border of the mandible and extending from the left second molar to the right canine (Fig. 1). Incisional biopsy proved this lesion to be an ameloblastoma. The treatment plan included mandibular resection with 1-cm margin and immediate reconstruction with a free fibula microvascular flap. A tracheostomy for airway protection was also planned. A high-resolution CT scan was obtained followed by construction of a model. A custom prebent plate was obtained using the model as a reference. Using a combined transoral and transcutaneous approach, the mandible was resected from the left angle through the right second bicuspid. The 2.4-mm prebent reconstruction plate was placed (Fig. 2). A free microvascular fibula flap with a skin pedicle was harvested. The fibula was contoured and adapted to the inner aspect of the custom-bent plate and secured with nonlocking screws (Fig. 3). The skin pedicle was used to close the resulting soft tissue defect at the floor of the mouth. A tracheostomy was performed at the end of the case. The total surgical time including all the procedures was 10 hours and 33 minutes.
Figure 1.
Panoramic radiograph showing large expansile multilocular radiolucent lesion. Case 3.
Figure 2.
Reconstruction plate in place after resection. Case 3.
Figure 3.
Postoperative panoramic radiograph. Reconstruction plate with free microvascular fibula graft in place, secured to the plate. Case 3.
Case 4
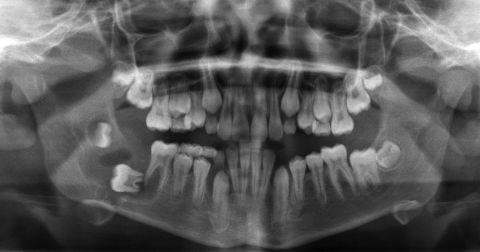
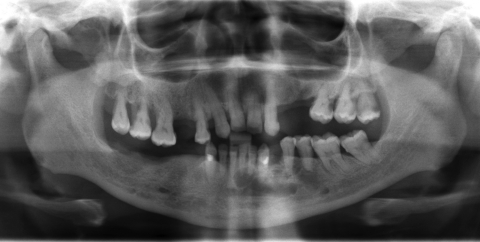
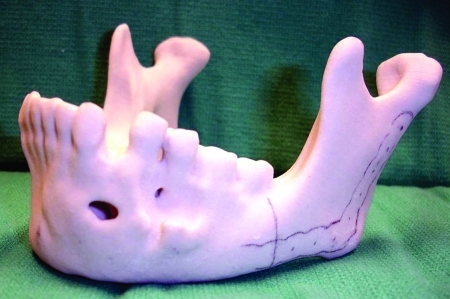
The fourth case was a 9-year-old white male referred to our service with a biopsy-proven ameloblastoma of the right mandible. Panoramic radiographic examination showed a unilocular radiolucent lesion extending from the right first mandibular molar up into the ascending ramus (Fig. 4). A high-resolution CT scan was followed by the construction of a three-dimensional model (Fig. 5). The resection with 1-cm margins was planned over the model extending vertically from the right sigmoid notch to the gonial angle and anteriorly through to the ipsilateral first premolar. Because of the marked lateral expansile nature of the lesion, the model and the specimen (prior to resection) were altered at the area of the inferior border to facilitate hardware adaptation (Fig. 6). A prebent reconstruction plate was obtained and sterilized preoperatively. The resection was performed via a combined transoral and transcutaneous approach. Screw holes were drilled using the prebent plate as a template prior to completing the osteotomies. This was done to ensure correct position of the segments. After the resection, the plate was repositioned without modification utilizing the previous predrilled holes (Fig. 7). The occlusion was assessed intraoperatively and was found to be unchanged from preoperatively. The total surgical time was 3 hours and 34 minutes.
Figure 4.
Preoperative panoramic radiograph showing unilocular radiolucent lesion at the left mandible. Significant tooth displacement is noted. Case 4.
Figure 5.
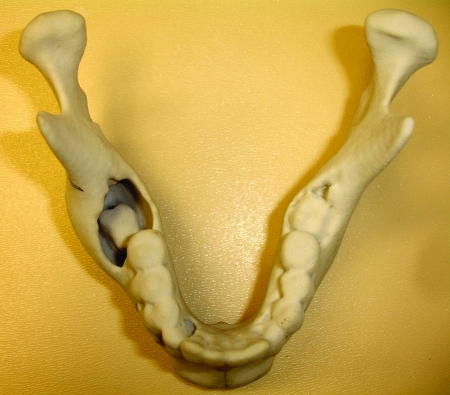
Anatomic three-dimensional model of the mandible. Noted lateral expansion and displaced second molar. Case 4.
Figure 6.
The altered model and specimen to facilitate adaptation of the plate prior to resection. This step will ensure correct contour and position of the segments after resection. Case 4.
Figure 7.
Custom prebent plate in position after resection. Case 4.
Case 5
The fifth case was a 52-year-old African-American female referred for evaluation of a recurrent expansile multilocular lesion involving the symphysis and left body of the mandible (Fig. 8). Incisional biopsy proved this to be an ameloblastoma. A high-resolution CT scan was obtained for construction of a three-dimensional model. The model resection margins incorporated 1 cm of healthy bone (Fig. 9). A custom prebent plate was obtained and sterilized. Combined transoral and transcutaneous approach was used. Prior to the resection, the custom plate was placed to record the preresected proximal and distal segments position and then removed (Fig. 10). The resection was performed as planned from the left mandibular angle to the right parasymphysis just anterior to the mental foramen. The custom-bent plate was resecured without difficulty (Fig. 11). The total surgical time was 4 hours and 17 minutes.
Figure 8.
Preoperative panoramic radiograph showing multilocular radiolucent lesion at the left mandible. Case 5.
Figure 9.
Three-dimensional model showing the lesion at the left mandible and planned posterior resection margin. Case 5.
Figure 10.
Placement of the prebent reconstruction plate prior to performing the osteotomies to record the preresection position of the proximal and distal segments. Case 5.
Figure 11.
Postoperative panoramic radiograph showing the 14-cm mandibular defect with reconstruction plate in place. Case 5.
RESULTS
All the anatomic models used as a reference were found to be extremely accurate. All reconstruction plates were placed without difficulty and required minimal or no adjustments at all. MMF was required in cases where the resection included disarticulation. In the case where immediate reconstruction was performed, the free fibula flap was secured to the plate and satisfactory stability was achieved. All cases demonstrated excellent symmetry and esthetics. The preoperative occlusion and vertical dimension were maintained, with the exception of one patient in whom the specimen included all the remaining mandibular teeth. The mean total surgical time was 6 hours and 5 minutes, ranging from 3.5 to 10.5 hours. No significant complications occurred during surgery.
DISCUSSION
In our experience, three-dimensional models are an effective surgical tool when planning resections in the mandible. They serve not only in treatment planning and construction of custom-made hardware but also as an aid in patient education and consultation. There are several other applications and benefits from the use of MRP as reported by Erben et al.6 Results were collected from questionnaires sent out to partners of the Phidias Network in regards to the use of SL models. Over 170 responses indicated the following range of applications:
To aid production of a surgical implant
To improve surgical planning
To act as an orienting aid during surgery
To enhance diagnostic quality
To be useful in preoperative simulation
To achieve patient's agreement prior to surgery
To prepare a template for resection
Erickson et al collected data from 38 surveys and reported that 69% of the responding surgeons used the models for diagnosis; 73% used the model for patient education; 38% believed that the use of models minimized the wound size and exposure; 65% found that exposure to MRP changed the way they handled the patient's surgery; and 96% though that SL models were a useful adjunct to patient care.7
One of the difficulties we encountered was the bending of the plate over a deformed model due to an expansile lesion. We solved this issue by modifying the model using the opposite site as reference (Fig. 6). Coward et al8 described a technique to create a mirror image wax model for reconstruction of an ear using the contralateral unaffected side. Using the same principle, Hannen9 reported two cases in which, with the help of software, the corresponding unaffected area from the contralateral side was digitally separated, mirrored, and fitted into the resection defect, obtaining a symmetric model used as a template for custom-bent plates. Another common complication is placement of the plate before performing the osteotomies. This was done on cases 4 and 5 with the objective of recording the position of the segments prior to resection. Modifying the mandible at expense of the tumor violates the oncological surgical principles and is not viable on the deformed morbid anatomy secondary to malignancy. An external pin fixation device could be placed to accomplish this goal when the screw holes cannot be drilled before the resection.
We found that saving time in the operating room is one of the major advantages of the use of prebent plates, not only for financial reasons but also by decreasing the exposure of the patient to general anesthesia time; the possible complications and the recovery period are also decreased. Time savings from 17 to 60%, with median of 20%, have been reported7; in long surgeries, this may represent 1 or 2 hours.7,10 Toro et al11 reported reduction of the operating time of 1 to 1.5 hours when using CT-guided SL and virtual reality surgical planning. Savings in operative time is important because operating room cost average 30 to 40% of hospital expenses.12 This plus the savings in comparison with the cost of secondary corrections13 justifies the use of the SL modeling and custom-bent plates. Improvement in technology and increased clinical applications for MRP should reduce the price of three-dimensional model production.11
Models represent an invaluable tool to help patients better understand their condition and subsequent proposed surgical treatment. Reconstructed models can also be used as a didactic instrument in academic settings.
CONCLUSION
MRP models and custom prebent hardware are valuable tools in reconstructive surgery. In our experience, they have proven to be beneficial for the surgeon and patient alike. The reduction in overall surgical time is significant and represents a great advance in oncological surgery. Additional costs of obtaining models and custom preadapted hardware are justified and ultimately rewarded by time savings, lowered risks, and overall patient satisfaction.
References
- Marx R E, Stern D. Oral and Maxillofacial Pathology: A Rationale for Diagnosis and Treatment. Carol Stream, IL: Quintessence Publishing Co., Inc.; 2003. pp. 635–703.
- Mark R E, Stevens M R. Atlas of Oral and Extraoral Bone Harvesting. Carol Stream, IL: Quintessence Publishing Co.; 2010. pp. 1–6.
- Marx R E. Bone and bone graft healing. Oral Maxillofac Surg Clin North Am. 2007;19:455–466, v. doi: 10.1016/j.coms.2007.07.008. [DOI] [PubMed] [Google Scholar]
- Winder J, Bibb R. Medical rapid prototyping technologies: state of the art and current limitations for application in oral and maxillofacial surgery. J Oral Maxillofac Surg. 2005;63:1006–1015. doi: 10.1016/j.joms.2005.03.016. [DOI] [PubMed] [Google Scholar]
- Mankovich N J, Cheeseman A M, Stoker N G. The display of three-dimensional anatomy with stereolithographic models. J Digit Imaging. 1990;3:200–203. doi: 10.1007/BF03167610. [DOI] [PubMed] [Google Scholar]
- Erben C, Vitt K D, Wulf J. First statistical analysis of data collected in the Phidias validation study of stereolithographic models. Phidias Newsletter. 2000;5:6–12. [Google Scholar]
- Erickson D M, Chance D, Schmitt S, Mathis J. An opinion survey of reported benefits from the use of stereolithographic models. J Oral Maxillofac Surg. 1999;57:1040–1043. doi: 10.1016/s0278-2391(99)90322-1. [DOI] [PubMed] [Google Scholar]
- Coward T J, Watson R M, Wilkinson I C. Fabrication of a wax ear by rapid-process modeling using stereolithography. Int J Prosthodont. 1999;12:20–27. [PubMed] [Google Scholar]
- Hannen E JM. Recreating the original contour in tumor deformed mandibles for plate adapting. Int J Oral Maxillofac Surg. 2006;35:183–185. doi: 10.1016/j.ijom.2005.07.012. [DOI] [PubMed] [Google Scholar]
- James W J, Slabbekoorn M A, Edgin W A, Hardin C K. Correction of congenital malar hypoplasia using stereolithography for presurgical planning. J Oral Maxillofac Surg. 1998;56:512–517. doi: 10.1016/s0278-2391(98)90726-1. [DOI] [PubMed] [Google Scholar]
- Toro C, Robiony M, Costa F, Zerman N, Politi M. Feasibility of preoperative planning using anatomical facsimile models for mandibular reconstruction. Head Face Med. 2007;3:5. doi: 10.1186/1746-160X-3-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kermer C, Rasse M, Lagogiannis G, Undt G, Wagner A, Millesi W. Colour stereolithography for planning complex maxillofacial tumour surgery. J Craniomaxillofac Surg. 1998;26:360–362. doi: 10.1016/s1010-5182(98)80068-1. [DOI] [PubMed] [Google Scholar]
- Kermer C, Lindner A, Friede I, Wagner A, Millesi W. Preoperative stereolithographic model planning for primary reconstruction in craniomaxillofacial trauma surgery. J Craniomaxillofac Surg. 1998;26:136–139. doi: 10.1016/s1010-5182(98)80002-4. [DOI] [PubMed] [Google Scholar]