Abstract
Bone grafts are used for the reconstruction of congenital and acquired deformities of the facial skeleton and, as such, comprise a vital component of the craniofacial surgeon's armamentarium. A thorough understanding of bone graft physiology and the factors that affect graft behavior is therefore essential in developing a more intelligent use of bone grafts in clinical practice. This article presents a review of the basic physiology of bone grafting along with a survey of pertinent concepts and current research. The factors responsible for bone graft survival are emphasized.
Keywords: Wolff's law, bone graft, osteoinduction, osteoconduction, mechanotransduction
Bone grafting plays a central role in craniofacial surgery, in both reconstructive and aesthetic realms. Bone grafts are used to fill bony defects, impart structural support, and augment deficient projection in the craniofacial skeleton. Although commonly thought of as a static entity, bone is in a state of dynamic equilibrium and has the ability to regenerate, remodel, and replace itself. Unlike most tissues, bone has the potential to heal with virtually no scars. These unique properties make bone well-suited for grafting applications.
Scientific research has endeavored to explain the underlying processes of bone graft biology and to characterize the conditions that optimize survival. A better understanding of this basic biology will allow a more intelligent and predictable utilization of bone grafts in clinical practice. Although nonvascularized, autogenous bone grafts (i.e., free grafts) are the gold standard for craniofacial reconstruction, recent attention has been directed at vascularized grafts, allografts, bone substitutes, and osteoinductive factors in grafting applications. This article aims to provide a basic review of bone graft physiology as well as a survey of pertinent research and concepts in these fields.
HISTORY
The earliest attempt to repair bony defects was reported in the Edgar Smith papyrus, ca. 2000 BC.1 At that time, metals were used for osseous reconstruction. Van Meekren is credited with performing the first bone graft in 1668.2 He used a canine calvarial xenograft to repair a cranial defect in a Russian soldier. It was not until nearly 200 years later, however, that bone grafting was given further scientific consideration. In 1867, Ollier published his work, which emphasized the importance of periosteal preservation in bone grafting.3 Based on these principles, Macewen reconstructed an extensive humeral defect in a young girl using iliac crest autograft.4 The utility of the periosteum was challenged by Barth, who demonstrated that most donor osteocytes become necrotic after grafting.5 He suggested that healing in bone grafts occurs by resorption and replacement, in a process defined as “creeping substitution.” The importance of the periosteum was renewed with Axhausen's work, which demonstrated that periosteal cells are capable of inducing osteogenesis after transplantation.6 Further work demonstrated that endosteal cells may also contribute to this osteogenic effect, if the recipient site provided adequate nourishment for their survival.7 Superior survival was later demonstrated in cancellous grafts when compared with cortical grafts.8 These differences were attributed to the increased porosity of cancellous bone and its coincident augmented surface area, which facilitated diffusion and plasmatic imbibition in the graft microenvironment.
Mowlem expanded the clinical utility of bone grafting to a variety of indications.9 He emphasized the organic nature of bone grafting as a means to deliver viable osteoblasts to the recipient site. The modern concept of bone grafting incorporates these structural and organic principles defined by historical science, while adding the significance of biochemical phenomena such as growth and inductive factors (e.g., bone morphogenetic protein [BMP]) and the principles of mechanobiology.
BONE GRAFT PHYSIOLOGY
The healing of autogenous bone grafts parallels that of fracture repair. An important similarity in bone graft healing is that a substantial portion of the biological activity originates from the host. This occurs because most viable osteocytes within the graft itself necrose shortly after transplantation, rendering the graft relatively inert. Nonetheless, substantial biological interactions still remain between graft and host. This important biological interplay contributes to the final outcome of graft take.
Osteogenesis
Osteogenesis refers to the process that occurs when surviving osteogenic cells from within the graft produce new bone. This mechanism of graft healing relies on the transplanted osteogenic cells to retain viability and produce osteoid. These cells are believed to be derived from the periosteum, endosteum, marrow, and intracortical elements of the graft.10 The role of osteogenesis as a mechanism of new bone formation during nonvascularized bone graft healing, however, is thought to be of lesser significance than that of osteoconduction.
Osteoconduction
Nonvascularized bone grafts heal through a predictable sequence of events. Bone grafts initially undergo partial necrosis, followed by an inflammatory stage. During this phase, much of the grafted bone is replaced by new bone as a consequence of interactions between osteoclasts and osteoblasts, which are delivered through invading blood vessels. The term creeping substitution is used to describe this slow vessel invasion and bony replacement, a process formally known as osteoconduction. This mechanism may be conceptualized by envisioning the graft as a scaffold on which new bone is deposited.
Because of the surgical disruption of host soft tissues and the recipient bony bed, hematoma formation around the graft occurs shortly after bone graft transplantation. During this early stage, a small minority of cells on the graft's surface are able to survive, primarily as a result of plasmatic imbibition.11,12 An inflammatory reaction focused around the graft ensues after hematoma formation and lasts for 5 to 7 days. The inflammatory tissue is then reorganized into a dense fibrovascular stroma around the graft, and the onset of vascular invasion occurs at 10 to 14 days.13 Vascular invasion brings additional cells with osteogenic potential into the graft,14 as the interstices of the old bone act as a directive matrix. As osteoblasts deposit new bone, osteoclasts resorb necrotic bone and pave the way for the graft to be penetrated by vascular tissue.
Osteoinduction
Osteoinduction refers to the process by which active factors released from the grafted bone (e.g., BMP) stimulate osteoprogenitor cells from the host to differentiate and form new bone. Three phases of osteoconduction have been described: chemotaxis, mitosis, and differentiation. During chemotaxis, bone inductive factors direct the migration and activity of osteogenic cells via chemical gradients. The inductive factors then stimulate these osteoprogenitor cells to undergo intense mitogenic activity, followed by their differentiation into mature, osteoid-producing cellular elements (i.e., osteoblasts). Ultimately, the cells become revascularized by invading blood vessels and are incorporated as new bone. The ultrastructural character of the bone graft (i.e., cancellous versus cortical) determines the ability of revascularization to take place and, therefore, significantly impacts the process of incorporation.
Cancellous Bone Graft Incorporation
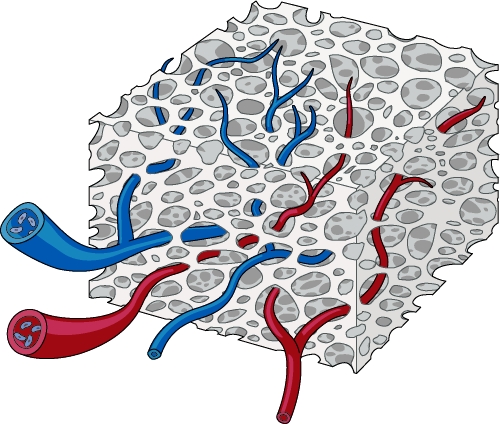
Cancellous bone grafts are more rapidly and completely revascularized than cortical bone grafts.15,16 The large spaces between trabeculae in cancellous grafts permit the unobstructed invasion of vascular tissue and the facile diffusion of nutrients from the host bed (Fig. 1). This is thought to promote osteogenic cell survival, imparting increased osteogenesis when compared with cortical grafts. Osteoprogenitor cells, brought in by the invading vessels, differentiate into osteoblasts and deposit a layer of new bone around the necrotic trabeculae. An osteoclastic phase ensues, wherein the entrapped cores of dead bone are resorbed. Cancellous bone grafts are completely revascularized and ultimately replaced with new bone over several weeks to months.
Figure 1.
Cancellous bone graft revascularization occurs rapidly and completely, owing to its open architecture.
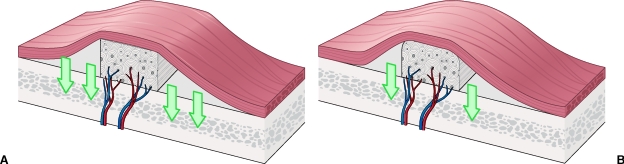
Cortical Bone Graft Incorporation
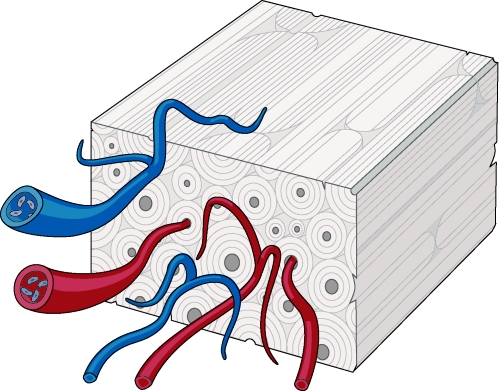
In contrast to cancellous bone grafts, cortical bone graft revascularization proceeds slowly and incompletely. Furthermore, although cancellous bone graft healing initiates with osteoblastic activity, revascularization of cortical bone grafts proceeds with initial osteoclastic activity. Osteoclastic enlargement of the haversian and Volkmann's canals must occur before vessels are able to penetrate the graft. Vascular invasion is limited by the dense lamellar structure of cortical bone, and the newly forming vasculature is constrained to invade the graft along these preexisting pathways (Fig. 2). This process begins at the graft periphery and progresses to the interior of the graft.17
Figure 2.
Cortical bone graft revascularization occurs slowly and incompletely, owing to its dense lamellar structure. Vessels must penetrate along haversian and Volkmann's canals.
Revascularization in cortical bone grafts may also be restricted by the limited number of endosteal cells that remain viable after transplantation. These cells are thought to contribute to end-to-end vessel anastomoses during bone graft revascularization.18
Ultimately, osteoblasts are brought in to deposit new bone, refilling the enlarged canal spaces. Although vessel invasion may begin within a few hours post-transplantation in cancellous grafts, in cortical grafts, the earliest vessels do not enter the graft until after ∼1 week. The entire revascularization process may then take months and is often incomplete. Incompletely revascularized regions of necrotic graft may persist indefinitely, sealed off from the viable regions of the graft. Accordingly, the final appearance of a cortical bone graft is frequently a patchwork of necrotic bone interspersed with areas of new bone. This admixture usually remains unaltered, even after a graft has become fully incorporated. Studies by Chen et al19 and Ozaki and Buchman20 confirm that cortical grafts in the onlay position show only superficial revascularization occurring in the first 10 to 21 days, and central revascularization by 8 to 16 weeks.
Biochemical Phenomena
GROWTH FACTORS
A myriad of growth factors and regulatory proteins direct the bone healing cascade.21 Among those studied, transforming growth factor-β (TGF-β) has been strongly implicated in the regulation of osteogenesis. Both immature and differentiated osteogenic cells have been shown to produce TGF-β during bone repair.22,23 At physiological doses, TGF-β has been shown to impart osteoblast migration and proliferation. It also has been shown to inhibit the differentiation of osteoprogenitor cells.
Experimentally, exogenous infusion of TGF-β has been shown to augment the repair of skull defects in the rabbit model. Hong et al24 and Arnaud et al25 both demonstrated that large doses of TGF-β augmented the healing potential of critical-size calvarial defects (i.e., those that would otherwise fail to heal) in rabbits when delivered with an organic carrier. These results appear to require high dosages with persistent treatment.26
Platelet-derived growth factor (PDGF), insulinlike growth factor (IGF), and fibroblast growth factor27 (FGF) have also been shown to play a role in the healing of bone and bone grafts, albeit to a lesser degree.
OSTEOINDUCTIVE FACTORS
A member of the TGF-β polypeptide superfamily, BMPs have demonstrated significant osteoinductive properties. They were discovered when Urist28 induced ectopic bone formation de novo with subcutaneous and submuscular placement of demineralized bone matrix. Bone morphogenetic proteins play a critical role in regulating the growth and differentiation of osteogenic precursors, and they can directly induce mesenchymal connective tissue to become bone-forming, osteoprogenitor cells.29
Bone morphogenetic proteins are initially released in low levels from the osteoblasts and osteoclasts along the edges of a fresh osteotomy defect.30 Osteoprogenitor cells in the cambium layer of the periosteum then respond by differentiating into osteoblasts. As these primitive cells mature, BMP expression decreases rapidly, becoming virtually absent in mature osteocytes.
Like TGF-β, BMP has also demonstrated the ability to heal critical-size defects in animal models. In the rhesus monkey, BMP facilitated the closure of critical-sized cranial defects, with nearly complete healing at 16 weeks.31 Similar findings in sheep and dogs have also been reported.32,33
Human studies using partially purified BMP have been used in combination with autologous and allogeneic grafts to successfully repair large cranial vault defects. Sailer and Kolb demonstrated vigorous bone development after placing BMP-laden implants into congenital and acquired skull defects.34 A multitude of additional clinical studies are underway to evaluate different BMP delivery systems that modulate the concentration, localization, and duration of administration.
FACTORS AFFECTING BONE GRAFT SURVIVAL
Graft Orientation
A bone graft's orientation in the recipient bed has a demonstrated impact on survival.35 Intuitively, survival is maximized when the graft's cancellous surface abuts the freshened edges of the host bone and the periosteal surface is in contact with the soft tissues. Mowlem described this orientation as orthotopic bone graft transplantation.36 This relationship is thought to improve revascularization of the transplanted graft.
Embryonic Origin
Bone grafts harvested from the craniofacial skeleton demonstrate superior volumetric maintenance and survival when compared with those taken from the rib, tibia, or iliac crest.37 The search for an explanation to this phenomenon has been a succession of research, speculation, and controversy. In 1951, Peer found that grafts harvested from vomer, nasal, or ethmoid bones, transplanted in contact with nonbony tissues (e.g., intramuscular and subcutaneous) retained their normal bony structure for up to 5 years.38 Conversely, rib, tibia, and iliac bone grafts were replaced by fibrous tissue in 6 to 8 months. He postulated that facial bones lacked regenerative potential, but were endowed with a tenacious ability to retain their structure. He did not elaborate on a mechanism for this hypothesis.
Using a rabbit model, Smith and Abramson proposed possible mechanisms for volumetric superiority of calvarial bone over iliac bone in grafting applications.39 After 1 year, the calvarial grafts had maintained their size and structure, whereas iliac grafts had lost at least 75% of their original volume. They postulated that mechanical stress was a key factor in graft survival, believing that iliac bone required mechanical stress to maintain its morphology. After transplantation, loss of this stress led to resorption when compared with grafts from low stress-bearing donor sites, such as the calvarium.
In an effort to explain the differences in graft survival between cranial and noncranial sources, researchers focused on embryologic origin: bones of membranous origin (i.e., the cranial vault and most of the facial bones) arise directly from mesenchymal tissue, whereas bones of endochondral origin (i.e., the majority of long bones and the axial skeleton) undergo initial formation of cartilage and then become ossified.
In 1983, Zins and Whitaker published a study based on harvests from both endochondral and membranous donor sites.40 They found that membranous bone grafts retained their volume better than endochondral bone grafts. A specific mechanism to explain this “embryology hypothesis” was not given, and the concept of innate embryological bone graft behavior continued to be a matter of controversy.
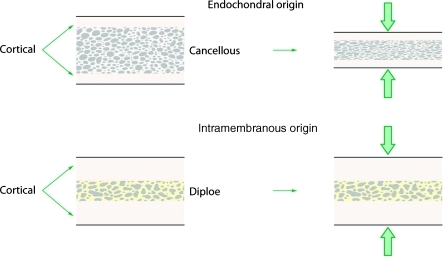
Hardesty and Marsh extended this concept into the architectural differences between bones of endochondral and intramembranous origin (Fig. 3). They hypothesized that the amount of cortical bone present in a graft is a key determinant in survival.41 Similarly, Chen et al showed that cancellous components of bone grafts had increased degrees of resorption.19 They also proposed that membranous bone grafts maintain better volume because of their larger cortical and smaller cancellous components.
Figure 3.
The embryologic origin of a graft is significant only in that it determines the relative proportion of cortical and cancellous bone within the graft. (Adapted from Hardesty RA, Marsh JL. Craniofacial onlay bone grafting: a prospective evaluation of graft morphology, orientation, and embryonic origin. Plast Reconstr Surg 1990;85(1):5–14.)
This “ultrastructural hypothesis” had only empiric evidence until Buchman and Ozaki demonstrated that the microarchitecture of a graft was indeed the basis for volume maintenance.42 These experiments isolated the cancellous and cortical components from both membranous and endochondral graft sources, and demonstrated no significant difference in resorption rates among cortical bone grafts based on embryonic origin. Cortical bone was thusly determined to be a superior onlay grafting material, regardless of its embryological origin. It is now generally acknowledged that the microarchitecture of a bone graft is perhaps the most important determinant of graft volume maintenance. The embryologic origin of a graft is significant only in that it determines the relative proportions of cortical and cancellous bone within the graft.
Periosteum
Periosteal preservation during bone graft transplantation has been shown to improve graft survival in the craniofacial region.43 Thompson and Casson studied these effects in a canine model and found that onlay bone grafts with preserved periosteum showed improved volumetric survival over those devoid of periosteum by a factor of 10%. They stressed the importance of early revascularization in bone graft survival and believed that the periosteum facilitated this process. Knize reported similar results with onlay bone grafts in a rabbit model.44 He also emphasized the importance of the periosteum for its role in revascularization and believed that periosteal cells provided a significant source of osteoprogenitor cells after transplantation. Using rib grafts in a canine model, Burstein et al described three layers of the periosteum: an outer vascular network, with communications to the internal portions of the bone; a middle layer of osteogenic reserve cells; and an inner cambial layer. They implicated the outer layer in enhanced graft revascularization through inosculation with the host's vasculature.45
Rigid Fixation
As in fracture healing, the use of rigid fixation in bone grafting has been shown to improve graft survival. Phillips and Rahn provided evidence for this in their comparison of cortical onlay bone grafting on the mandibles of sheep, demonstrating superior survival in grafts that underwent lag screw fixation.46 Further evidence for the benefits of rigid fixation was provided by Lin et al,47 who noted that rigid fixation improved graft volume retention in areas subject to high motion (femur), but provided no benefit in areas of low motion (cranium). Their rationale for these benefits of rigid fixation included increased primary bone healing and more rapid revascularization. Additionally, in a clinical study of 363 patients undergoing nasal reconstruction over a 14-year period, Jackson et al attributed their exceptional bone graft survival to the use of rigid interosseous stabilization.48
Revascularization
The revascularization of bone grafts has been theorized to be another important determinant of survival. Recipient bed conditions that adversely affect revascularization include the presence of necrotic bone, scarring, infection and prior irradiation. In a rabbit model, Lukash et al demonstrated that rib grafts placed in an irradiated tissue bed revascularized poorly, underwent resorption, and healed with a fibrous union.49 Conversely, rib grafts placed in nonirradiated tissues maintained their volumes and healed with bony union.
The type of soft tissue overlying the graft is also important, insofar as the vascularity of the tissue envelope affects the rate and extent of graft revascularization. Serving as an example, a study by Ermis and Poole noted that muscle coverage results in increased bone graft revascularization.50
As stated previously, the microarchitecture of a bone graft also has implications in the rate and completeness of revascularization. Cancellous bone grafts have been shown to revascularize quickly and completely, whereas cortical bone grafts revascularize slowly and incompletely.
The position of a bone graft (i.e., inlay or onlay) has additional effects on revascularization. Inlay grafts have extensive contact with the recipient bed; onlay grafts, however, are in contact through the base only. Increased contact results in more rapid and complete revascularization, which, in turn, imparts greater biological activity.
Recipient Site
The recipient site effects bone graft healing and incorporation. Studies have demonstrated that grafts placed in an avascular bed display poor survival.38 Other detrimental factors at the recipient site include prior irradiation, infection, and scarring. Although bone grafts are usually placed in contact with bone, some studies have investigated the effects of placing bone grafts in different tissue environments. Ermis and Poole placed endochondral bone grafts in subcutaneous and muscular pockets and found that bone graft resorption was greater when grafts were covered by muscle.50 They postulated that excessive graft movement and increased revascularization resulted in increased graft resorption.
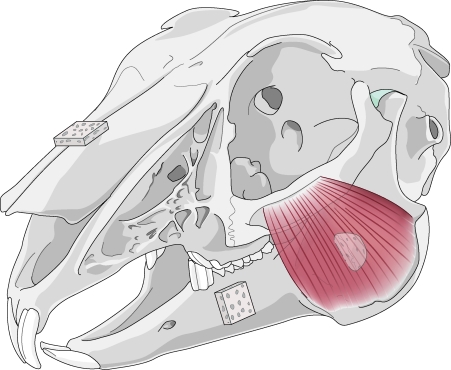
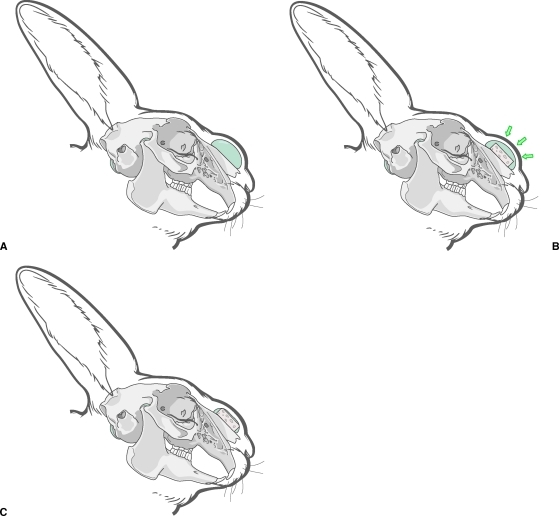
In 1982, Enlow imparted his theory of resorptive and depository fields.51 Through his hypotheses on transformative and translational growth in the craniofacial skeleton, he asserted that certain areas of the face remodel by bony deposition, whereas others remodel by bony resorption (Fig. 4). In an effort to extend Enlow's theories of craniofacial bone growth to grafting applications, Zins et al placed cortical-cancellous onlay grafts in resorptive and depository fields of rabbits.52 Their results showed significant differences in volume maintenance in accordance to the resorptive or depository nature of the recipient bed. Subsequent experiments elucidated the etiology for Enlow's fields through the underlying principles of mechanical stress.
Figure 4.
Enlow's theory of resorptive and depository fields asserts that certain areas remodel by bony resorption (mandibular ramus), whereas others remodel by bony deposition (mandibular body and snout), as shown here in the rabbit skull.
Mechanical Stress
In 1989, Whitaker introduced the concept of the biological boundary,53 emphasizing that the soft tissue envelope has a genetically predetermined shape, which is inclined to remain constant. At its core, the biological boundary concept is an extension of Moss's functional matrix theory.54 Moss's theory states that as the craniofacial skeleton grows, the soft tissue environment shapes its morphology. The deforming effect of torticollis on a child's craniofacial development exemplifies this phenomenon, whereby an extrinsic muscle imbalance has a profound epigenetic effect on facial skeletal morphology. Whitaker applied Moss's functional matrix theory to bone grafting. He hypothesized that onlay grafts violate the body's natural soft tissue boundaries, which, in turn, elicits a homeostatic response to maintain the boundary and resorb the graft. Whitaker noted that inlay bone grafts did not disturb these biologic boundaries, resulting in less resorption.
Ultimately, all of these changes are applications of Wolff's Law, which states: “Remodeling of bone … occurs in response to physical stresses—or to the lack of them—in that bone is deposited in sites subjected to stress and is resorbed from sites where there is little stress.”55 In essence, a bone's form follows its function.
LaTrenta et al corroborated these theories, reporting that inlay bone grafts in a dog model maintained greater volume and weight than onlay grafts, and citing favorable remodeling forces of the inlay position.56 Rosenthal and Buchman performed a series of experiments on the survival patterns of inlayed cortical and cancellous bone grafts in a rabbit model.57 These experiments provided significant findings regarding the behavioral differences of inlay versus onlay grafts. Contrary to previous work, this study demonstrated that bone volume increases for inlay grafts at all time points. The data also demonstrated that cancellous inlay bone grafts increased their bone content to a greater extent than cortical inlay bone grafts, regardless of embryologic origin (Fig. 5).
Figure 5.
Cancellous bone grafts demonstrate favorable remodeling characteristics when placed in the inlay position.
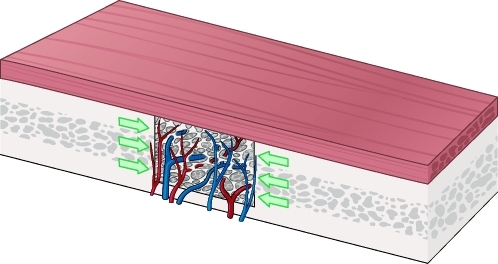
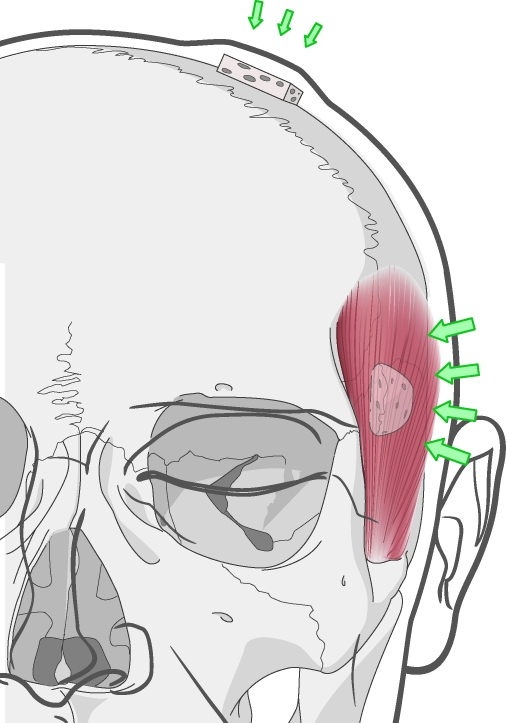
These scientific works revealed an essential variable in bone grafting, asserting that the mechanical environment is of paramount importance to graft survival. The recipient site's soft tissue envelope characteristics and the position in which a graft is placed (i.e., inlay versus onlay) dictate the mechanical forces on grafted bone. Onlay grafts within a tight tissue envelope will experience forces that are different from grafts within a loose soft tissue envelope. Onlay grafts that underlie muscle, a relatively tight tissue envelope, withstand a significant compressive force. For example, onlay grafts placed underneath the temporalis muscle invariably show significant resorption (Fig. 6).
Figure 6.
Onlay bone grafting in different regions of the face. Grafts placed under the temporalis muscle experience a greater magnitude of compressive force than those placed under the scalp. Clinically, onlay grafts placed under the temporalis undergo near-complete resorption.
Inlay bone grafts, in addition to being shielded from soft tissue recoil forces, benefit from receiving the identical physical stresses as the surrounding bone. The absence of recoil forces and the presence of these functional forces act to augment inlay graft survival.
A shielding effect (from the soft tissue recoil forces) for onlay grafts was perhaps inadvertently actualized when Goldstein et al used supraperiosteally placed tissue expanders to alter the recipient bed.58 Rigidly fixed, membranous onlay bone grafts were placed under pre-expanded soft tissue envelopes in rabbits, and were then compared with a control group of identical grafts placed in nonexpanded sites. The grafts placed within the pre-expanded pockets displayed superior survival, which was attributed to increases in local vascularity. Although this may be true, their results also support our shielding hypothesis59: that diminution of overlying soft tissue tensile forces lead to maintained bone graft volume (Fig. 7).
Figure 7.
(A–C) A “shielding” effect is demonstrated when tissue expansion proceeds bone grafting. This is hypothesized to reduce the compression forces on the graft, which in turn lead to resorption.
Presently, it is evident that two identical bone grafts placed in different locations in the craniofacial region will not behave in like fashion, owing to potential differences in the mechanical environment. The question arises as to how bone grafts “sense” these environmental forces. What signals are imparted, on a cellular level, that prompt bone to resorb when under certain mechanical stresses?
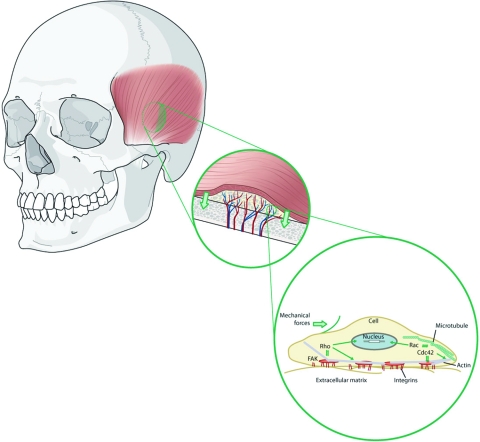
The answer lies in the principle of mechanotransduction. This concept, that mechanical forces can induce intracellular signals, was pioneered by Ingber et al through their work with integrin-β1.60 It has since been demonstrated in the osteoblast.61 Bierbaum and Notbohm documented an increase in osteoblastic tyrosine kinase activity as a reaction to mechanical stress on the β1 subunits of the integrin family.
The principle of mechanotransduction has been exploited for several decades in the process of distraction osteogenesis. Recently, the underlying molecular mechanisms for this modality have become elucidated.62 Rhee et al studied the cSrc mechanotransduction pathway in a rat model and measured resultant BMP expression. They found that cSrc expression closely approximated BMP expression during a cyclic distraction process, providing in vivo support that mechanical forces can result in the increased expression of BMP.63 Under this view, the seminal premise of Wolff's law may be envisioned in a new light, extending into the realm of mechanotransduction, thereby providing a molecular basis for the functional adaptation of bone (Fig. 8).
Figure 8.
Wolff's law, revisited: mechanotransduction. Mechanical forces induce intracellular signals, which dictate bone graft behavior.
CLINICAL APPLICATIONS
Autogenous bone grafts are considered the gold standard for reconstructing craniofacial bone defects, the nature of which dictates the type of graft used. Inlay bone grafts are used for the treatment of bone gaps, whereas onlay bone grafts are used to restore bone projection. Cancellous bone grafts are well-suited for inlay bone grafts because they revascularize quickly and stimulate significant new bone formation through osteoinduction. Conversely, cortical bone grafts are often used as onlay bone grafts in cases of volume deficiency (e.g., malar augmentation). These grafts survive without complete resorption and retain some mechanical strength after transplantation (Fig. 9).
Figure 9.
(A,B) Cortical bone grafts are preferred in the onlay position. Their dense structure resists compressive forces and revascularization, both of which would otherwise resorb the graft.
Bone grafting in craniofacial trauma was first proposed by Bonanno and Converse,64 and pioneered by Gruss65 and Manson.66 The use of interpositional bone grafts along the course of the native buttresses of the craniofacial skeleton provides the requisite support for restoring structure and function to this region. Plate and screw fixation or wiring may be used for graft stabilization, but rigid fixation is preferable in most settings.65
Donor Sites
In cases of traumatic craniofacial injury, fracture fragments should be reused as the primary bone graft source until they are exhausted, thereby limiting donor site morbidity. In severely contaminated wounds, or when tissue covering is inadequate or nonviable, bone grafting is generally contraindicated and may be deferred.
The calvarium, iliac crest, and rib are among the more commonly used donor sites for bone grafting in craniomaxillofacial surgery. The surgeon considers three variables when harvesting bone grafts: (1) bony characteristics, (2) ease of access, and (3) donor site morbidity.
Vascularized Bone Transfer
Vascularized bone transfers are most commonly used for postablative reconstruction in irradiated recipient beds, where standard bone grafts have been shown to be less viable. In general, bone flaps are used when bony defects larger than 6 cm are encountered or when composite tissues are required. Blair demonstrated the utility of bone flaps to the craniofacial skeleton after tumor ablation when a large mandibular defect was reconstructed with a vascularized osteocutaneous composite flap.67 Vascularized bone transfers are technically challenging and are not routinely required for craniofacial reconstruction.
Allogenic Bone Grafts
Allogenic bone grafts refer to the transplantation of bone from genetically nonidentical individuals. To date, bone allografts have been plagued by their unpredictable rates of resorption and bone formation. Allografts also carry the coincident risk of disease transmission. In 1997, the U.S. Food and Drug Administration (FDA) implemented an extensive donor screening protocol with the hope of reducing the transmission of HIV and Hepatitis B and C viruses.68 The rate of HIV transmission in allografts is exceedingly rare and is cited to be 1 in 1.6 million grafts.69 These low rates are the result of the extensive processing and preservation required to remove the cellular—and highly antigenic—elements. The same factors that reduce immunogenicity, however, also deactivate the osteoinductive factors that are so critical to survival. In addition, deep freezing ( − 70°C) and freeze-drying—the most common methods of preservation—may significantly alter the mechanical properties and strength of the graft.70 For these reasons, the use of allografts in craniofacial bone grafting has been limited.
Bone Substitutes
The development of alloplastic bone substitutes evolved out of the operative morbidities of autografts and the limitations of allografts. The most common bone substitutes are comprised of calcium sulfates, calcium phosphates, and methyl methacrylate.
CALCIUM SULFATES
The use of calcium sulfate hemihydrate (also known as plaster of Paris) can be traced back to Dreesman, who in 1892 treated bone gaps with a mixture of gypsum and 5% phenol.71 Its utility as a bone substitute in the craniofacial skeleton has remained limited, with a brief employment during the Vietnam War.72
CALCIUM PHOSPHATES
When compared with calcium sulfates, calcium phosphates more closely resemble the inorganic matrix of bone and, therefore, have greater utility as a bone substitute. Calcium phosphates are derived from naturally occurring corals but may also be synthesized in the laboratory. There are two major varieties of hydrated calcium phosphate (hydroxyapatite) preparations for use in bone applications: ceramics and cements.
Hydroxyapatite ceramic implants have been extensively used in dental applications within the fields of oral implantology73,74 and in oral and maxillofacial surgery.75 Owing to its brittle nature, the use of ceramic in craniofacial surgery has been limited to low-stress regions of the craniofacial skeleton such as the calvarium.76 Waite and Matukas successfully used a mixture of hydroxyapatite granules and autogenous collagen to augment deficient zygomatic bones after LeFort I osteotomy.77
Unlike ceramic forms, hydroxyapatite cements are moldable, allowing for intraoperative contouring. These forms have been used in large clinical trials for the reconstruction of cranial defects,78,79 where, again, their use was limited to low-stress regions of the craniofacial skeleton. A study by Friedman et al78 successfully used hydroxyapatite cement in this regard for 103 patients, reporting a surgical infection rate of 5.8% and an implant removal rate of 2.9%.
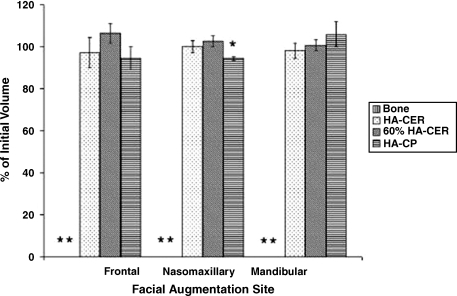
An animal study by Gosain et al80 demonstrated that onlay hydroxyapatite composites were more osseoconductive than autogenous bone grafts: the composites displayed less resorption and more bone replacement than the conventional grafts (Fig. 10). The authors concluded that this biomaterial has great promise for future research.
Figure 10.
Comparison of autologous bone grafts to hydroxyapatite composite grafts in various regions of the craniofacial skeleton. Results are displayed as a percentage of the initial graft volume after 1 year. The asterisks represent complete resorption of the autologous bone grafts. HA-CER, hydroxyapatite ceramic; 60% HA-CER, 60/40 hydroxyapatite/β-tricalcium phosphate; HA-CP, hydroxyapatite cement paste.
METHYL METHACRYLATE
A hydrophilic acrylic resin, methyl methacrylate has been used for craniofacial contouring and in the repair of traumatic defects of the craniofacial skeleton. Initially supplied as a powder, methyl methacrylate may be hydrated intraoperatively in a highly exothermic process. Accordingly, copious irrigation is required when the substitute is placed in situ to avoid thermal injury to the tissues. Its advantages include low cost, the ability to contour after placement, and biocompatibility. Thinning of the overlying skin with implant extrusion has been reported in pediatric craniofacial surgery.81 A custom fabricated methyl methacrylate composite implant is also available, which has demonstrated use in calvarial and orbital reconstruction.
Despite the diversity of alloplastic materials, the ideal bone substitute—one that is inert, inexpensive, structurally fortified, and fully osteoconductive—remains elusive.
CONCLUSION
Bone grafts are the building blocks of the craniofacial surgeon. To optimize their use, a clear understanding of graft physiology is required. Clinical success with bone grafting may be maximized with reverence to the established variables affecting free graft survival. Modern science has also provided bone graft alternatives and adjuvants in the form of bone substitutes and recombinant human growth factors. In addition, the phenomenon of mechanotransduction may take on new applications in de novo osteogenesis.
Although these modalities represent exciting adjuncts in the field, they have not—as of yet—uniformly supplanted the autogenous, nonvascularized bone graft as the gold standard in craniofacial skeletal reconstruction. A critical appraisal of these novel techniques will be required in the ongoing quest to restore the craniofacial skeleton.
References
- Frommelt H. Polymers for medical applications. Macromol Chem Makromol Symp. 1987;12:281. [Google Scholar]
- Meekren J Van. Observationes medicochirurgicae. Amsterdam: Henrici and Bloom; 1682.
- Ollier L. Traite Experimental et Linique de la Regeneration des Os et de la Production Artificielle du Tissue Osseux. Paris: Masson et Fils; 1867.
- Macewen W. The Growth of Bone. Glasgow: J Maclehose and Sons; 1912.
- Barth A. Uber histologische Befunde nach Knochenimplantation. Arch Klin Chir. 1893;46:409. [Google Scholar]
- Axhausen G. Histologische Untersuchungen uber Knochentransplantation am Menschen. Dtsch Z Chir. 1907;91:388. [Google Scholar]
- Phemister D. The fate of transplanted bone and regenerative power of its various constituents. Surg Gynecol Obstet. 1914;19:303. [Google Scholar]
- Gallie W, Robertson D. Transplantation of bone. JAMA. 1918;70:1134. [Google Scholar]
- Mowlem R. Cancellous chip bone grafts: report on 75 cases. Lancet. 1944;2:746. [Google Scholar]
- Burchardt H. Biology of bone transplantation. Orthop Clin North Am. 1987;18(2):187–196. [PubMed] [Google Scholar]
- Mulliken J B, Kaban L B, Glowacki J. Induced osteogenesis: the biological principle and clinical applications. J Surg Res. 1984;37:487–496. doi: 10.1016/0022-4804(84)90218-x. [DOI] [PubMed] [Google Scholar]
- Heslop B F, Zeiss I M, Nisbet N W. Studies on transference of bone: I. A comparison of autologous and homologous bone implants with reference to osteocyte survival, osteogenesis, and host reaction. Br J Exp Pathol. 1960;41:269–287. [PMC free article] [PubMed] [Google Scholar]
- Gross T P, Jinnah R H, Clarke H J, Cox Q G. The biology of bone grafting. Orthopedics. 1991;14(5):563–568. doi: 10.3928/0147-7447-19910501-11. [DOI] [PubMed] [Google Scholar]
- Schmitz J P, Hollinger J O. The critical size defect as an experimental model for craniomandibulofacial nonunions. Clin Orthop Relat Res. 1986;205:299–308. [PubMed] [Google Scholar]
- Stevenson S, Emery S E, Goldberg V M. Factors affecting bone graft incorporation. Clin Orthop Relat Res. 1996;(324):66–74. doi: 10.1097/00003086-199603000-00009. [DOI] [PubMed] [Google Scholar]
- Pinholt E M, Solheim E, et al. Revascularization of calvarial, mandibular, tibial, and iliac bone grafts in rats. Ann Plast Surg. 1994;33(2):193–197. doi: 10.1097/00000637-199408000-00012. [DOI] [PubMed] [Google Scholar]
- Burchardt H. The biology of bone graft repair. Clin Orthop Relat Res. 1983;(178):28–42. [PubMed] [Google Scholar]
- Heiple K G, Goldberg V M, Powell A E, Bos G D, Zika J M. Biology of cancellous bone grafts. Orthop Clin North Am. 1987;18(2):179–185. [PubMed] [Google Scholar]
- Chen N T, Glowacki J, Bucky L P, Hong H Z, Kim W K, Yaremchuk M J. The roles of revascularization and resorption on endurance of craniofacial onlay bone grafts in the rabbit. Plast Reconstr Surg. 1994;93(4):714–722. [PubMed] [Google Scholar]
- Ozaki W, Buchman S R. Volume maintenance of onlay bone grafts in the craniofacial skeleton: micro-architecture versus embryologic origin. Plast Reconstr Surg. 1998;102(2):291–299. doi: 10.1097/00006534-199808000-00001. [DOI] [PubMed] [Google Scholar]
- Reddi A H, Ma S S, Cunningham N S. Induction and maintenance of new bone formation by growth and differentiation factors. Ann Chir Gynaecol. 1988;77(5–6):189–192. [PubMed] [Google Scholar]
- Linkhart T A, Mohan S, Baylink D J. Growth factors for bone growth and repair: IGF, TGF beta, and BMP. Bone. 1996;19:1S–12S. doi: 10.1016/s8756-3282(96)00138-x. [DOI] [PubMed] [Google Scholar]
- Joyce M E, Jingushi S, Bolander M E. Transforming growth factor-beta in the regulation of fracture repair. Orthop Clin North Am. 1990;21:199–209. [PubMed] [Google Scholar]
- Hong L, Tabata Y, Miyamoto S, et al. Bone regeneration at rabbit skull defects treated with transforming growth factor-beta1 incorporated into hydrogels with different levels of biodegradability. J Neurosurg. 2000;92:315–325. doi: 10.3171/jns.2000.92.2.0315. [DOI] [PubMed] [Google Scholar]
- Arnaud E, Morieux C, Wybier M, de Vernejoul M C. Potentiation of transforming growth factor (TGF-beta 1) by natural coral and fibrin in a rabbit cranioplasty model. Calcif Tissue Int. 1994;54:493–498. doi: 10.1007/BF00334331. [DOI] [PubMed] [Google Scholar]
- Barnes G L, Kostenuik P J, Gerstenfeld L C, Einhorn T A. Growth factor regulation of fracture repair. J Bone Miner Res. 1999;14(11):1805–1815. doi: 10.1359/jbmr.1999.14.11.1805. [DOI] [PubMed] [Google Scholar]
- Eppley B L, Doucet M, Connolly D T, Feder J. Enhancement of angiogenesis by bFGF in mandibular bone graft healing in the rabbit. J Oral Maxillofac Surg. 1988;46:391–398. doi: 10.1016/0278-2391(88)90223-6. [DOI] [PubMed] [Google Scholar]
- Urist M R. Bone: formation by autoinduction. Science. 1965;150:893–899. doi: 10.1126/science.150.3698.893. [DOI] [PubMed] [Google Scholar]
- Urist M R, DeLange R J, Finerman G A. Bone cell differentiation and growth factors. Science. 1983;220(4598):680–686. doi: 10.1126/science.6403986. [DOI] [PubMed] [Google Scholar]
- Spector J A, Luchs J S, Mehrara B J, et al. Expression of bone morphogenetic proteins during membranous bone healing. Plast Reconstr Surg. 2001;107:124–134. doi: 10.1097/00006534-200101000-00018. [DOI] [PubMed] [Google Scholar]
- Ferguson D, Davis W L, Urist M R, Hurt W C, Allen E P. Bovine bone morphogenetic protein (bBMP) fraction-induced repair of craniotomy defects in the rhesus monkey (Macaca speciosa) Clin Orthop Relat Res. 1987;(219):251–258. [PubMed] [Google Scholar]
- Lindholm T C, Lindholm T S, Alitalo I, Urist M R. Bovine bone morphogenetic protein (bBMP) induced repair of skull trephine defects in sheep. Clin Orthop Relat Res. 1988;227:265–268. [PubMed] [Google Scholar]
- Sato K, Urist M R. Induced regeneration of calvaria by bone morphogenetic protein (BMP) in dogs. Clin Orthop Relat Res. 1985;197:301–311. [PubMed] [Google Scholar]
- Sailer H F, Kolb E. Application of purified bone morphogenetic protein (BMP) preparations in cranio-maxillo-facial surgery: reconstruction in craniofacial malformations and post-traumatic or operative defects of the skull with lyophilized cartilage and BMP. J Craniomaxillofac Surg. 1994;22:191–199. doi: 10.1016/s1010-5182(05)80557-8. [DOI] [PubMed] [Google Scholar]
- Hardesty R A, Marsh J L. Craniofacial onlay bone grafting: a prospective evaluation of graft morphology, orientation, and embryonic origin. Plast Reconstr Surg. 1990;85:5–14. [PubMed] [Google Scholar]
- Mowlem R. Bone grafting. Br J Plast Surg. 1963;16:293–304. doi: 10.1016/s0007-1226(63)80133-2. [DOI] [PubMed] [Google Scholar]
- Sullivan W G, Szwajkun P R. Revascularization of cranial versus iliac crest bone grafts in the rat. Plast Reconstr Surg. 1991;87(6):1105–1109. doi: 10.1097/00006534-199106000-00013. [DOI] [PubMed] [Google Scholar]
- Peer L A. The fate of autogenous human bone grafts. Br J Plast Surg. 1951;3:233–243. doi: 10.1016/s0007-1226(50)80038-3. [DOI] [PubMed] [Google Scholar]
- Smith J D, Abramson M. Membranous vs endochondral bone autografts. Arch Otolaryngol. 1974;99(3):203–205. doi: 10.1001/archotol.1974.00780030211011. [DOI] [PubMed] [Google Scholar]
- Zins J E, Whitaker L A. Membranous versus endochondral bone: implications for craniofacial reconstruction. Plast Reconstr Surg. 1983;72(6):778–785. doi: 10.1097/00006534-198312000-00005. [DOI] [PubMed] [Google Scholar]
- Hardesty R A, Marsh J L. Craniofacial onlay bone grafting: a prospective evaluation of graft morphology, orientation, and embryonic origin. Plast Reconstr Surg. 1990;85(1):5–14. [PubMed] [Google Scholar]
- Buchman S R, Ozaki W. The ultrastructural and resorptive pattern of cancellous onlay bone grafts in the craniofacial skeleton. Ann Plast Surg. 1999;43:49–56. doi: 10.1097/00000637-199907000-00008. [DOI] [PubMed] [Google Scholar]
- Thompson N, Casson J A. Experimental onlay bone grafts to the jaws: a preliminary study in dogs. Plast Reconstr Surg. 1970;46(4):341–349. doi: 10.1097/00006534-197010000-00003. [DOI] [PubMed] [Google Scholar]
- Knize D M. The influence of periosteum and calcitonin on onlay bone graft survival: a roentgenographic study. Plast Reconstr Surg. 1974;53:190–199. doi: 10.1097/00006534-197402000-00011. [DOI] [PubMed] [Google Scholar]
- Burstein F D, Ariyan S, Chicarilli Z, Canalis R F. The effect of periosteal preservation on osteogenesis in a canine rib autograft model: tetracycline fluorescence incident photometry. J Craniofac Surg. 1994;5(3):161–171. doi: 10.1097/00001665-199407000-00005. [DOI] [PubMed] [Google Scholar]
- Phillips J H, Rahn B A. Fixation effects on membranous and endochondral onlay bone-graft resorption. Plast Reconstr Surg. 1988;82:872–877. doi: 10.1097/00006534-198811000-00023. [DOI] [PubMed] [Google Scholar]
- Lin K Y, Bartlett S P, Yaremchuk M J, Fallon M, Grossman R F, Whitaker L A. The effect of rigid fixation on the survival of onlay bone grafts: an experimental study. Plast Reconstr Surg. 1990;86(3):449–456. doi: 10.1097/00006534-199009000-00010. [DOI] [PubMed] [Google Scholar]
- Jackson I T, Choi H Y, Clay R, et al. Long-term follow-up of cranial bone graft in dorsal nasal augmentation. Plast Reconstr Surg. 1998;102:1869–1873. doi: 10.1097/00006534-199811000-00010. [DOI] [PubMed] [Google Scholar]
- Lukash F N, Zingaro E A, Salig J. The survival of free nonvascularized bone grafts in irradiated areas by wrapping in muscle flaps. Plast Reconstr Surg. 1984;74:783–788. doi: 10.1097/00006534-198412000-00008. [DOI] [PubMed] [Google Scholar]
- Ermis I, Poole M. The effects of soft tissue on bone graft resorption in the craniofacial region. Br J Plast Surg. 1992;45(1):26–29. doi: 10.1016/0007-1226(92)90110-j. [DOI] [PubMed] [Google Scholar]
- Enlow D H. The Handbook of Facial Growth. Philadelphia: WB Saunders; 1982.
- Zins J E, Kusiak J F, Whitaker L A, Enlow D H. The influence of the recipient site on bone grafts to the face. Plast Reconstr Surg. 1984;73(3):371–381. doi: 10.1097/00006534-198403000-00005. [DOI] [PubMed] [Google Scholar]
- Whitaker L A. Biological boundaries: a concept in facial skeletal restructuring. Clin Plast Surg. 1989;16:1–10. [PubMed] [Google Scholar]
- Moss M L. Facial growth: the functional matrix concept. In: In: Grabb WC, Rosenstein SW, Bzoch KR, editor. Cleft Lip and Palate. Boston: Little, Brown & Co; 1971. pp. 97–107. [Google Scholar]
- Salter R B. Textbook of Disorders and Injuries of the Musculoskeletal System. 1st ed. Baltimore: Williams & Wilkins; 1970.
- LaTrenta G S, McCarthy J G, Breitbart A S, May M, Sissons H A. The role of rigid skeletal fixation in a bone-graft augmentation of the craniofacial skeleton. Plast Reconstr Surg. 1989;84(4):578–588. [PubMed] [Google Scholar]
- Rosenthal A H, Buchman S R. Volume maintenance of inlay bone grafts in the craniofacial skeleton. Plast Reconstr Surg. 2003;112(3):802–811. doi: 10.1097/01.PRS.0000069713.62687.F5. [DOI] [PubMed] [Google Scholar]
- Goldstein J, Mase C, Newman M H. Fixed membranous bone graft survival after recipient bed alteration. Plast Reconstr Surg. 1993;91(4):589–596. doi: 10.1097/00006534-199304000-00003. [DOI] [PubMed] [Google Scholar]
- Buchman S R, Tong L. Facial bone grafts: contemporary science and thought. J Craniomaxillofac Trauma. 2000;6(1):31–41. [PubMed] [Google Scholar]
- Wang N, Butler J P, Ingber D E. Mechanotransduction across the cell surface and through the cytoskeleton. Science. 1993;260(5111):1124–1127. doi: 10.1126/science.7684161. [DOI] [PubMed] [Google Scholar]
- Bierbaum S, Notbohm H. Tyrosine phosphorylation of 40 kDa proteins in osteoblastic cells after mechanical stimulation of beta1-integrins. Eur J Cell Biol. 1998;77(1):60–67. doi: 10.1016/s0171-9335(98)80102-7. [DOI] [PubMed] [Google Scholar]
- Rhee S T, El-Bassiony L, Buchman S R. Extracellular signal-related kinase and bone morphogenetic protein expression during distraction osteogenesis of the mandible: in vivo evidence of a mechanotransduction mechanism for differentiation and osteogenesis by mesenchymal precursor cells. Plast Reconstr Surg. 2006;117(7):2243–2249. doi: 10.1097/01.prs.0000224298.93486.1b. [DOI] [PubMed] [Google Scholar]
- Rhee S T, Buchman S R. Colocalization of c-Src (pp60src) and bone morphogenetic protein 2/4 expression during mandibular distraction osteogenesis: in vivo evidence of their role within an integrin-mediated mechanotransduction pathway. Ann Plast Surg. 2005;55(2):207–215. doi: 10.1097/01.sap.0000164576.10754.aa. [DOI] [PubMed] [Google Scholar]
- Bonanno P C, Converse J M. Primary bone grafting in management for facial fractures. N Y State J Med. 1975;75:710–712. [PubMed] [Google Scholar]
- Gruss J S, Mackinnon S E, Kassel E E, Cooper P W. The role of primary bone grafting in complex craniomaxillofacial trauma. Plast Reconstr Surg. 1985;75:17–24. doi: 10.1097/00006534-198501000-00005. [DOI] [PubMed] [Google Scholar]
- Manson P N, Crawley W A, et al. Midface fractures: advantages of immediate extended open reduction and bone grafting. Plast Reconstr Surg. 1985;76(1):1–12. [PubMed] [Google Scholar]
- Blair V. Surgery and Disease of the Mouth and Jaws. St. Louis: CV Mosby; 1918.
- Screening and Testing of Donors of Human Tissue Intended for Transplantation. Washington, DC: U.S. Department of Health and Human Services, Food and Drug Administration, Center for Biologics Evaluation and Research; 1997.
- Buck B E, Malinin T I, Brown M D. Bone transplantation and human immunodeficiency virus: an estimate of risk of acquired immunodeficiency syndrome (AIDS) Clin Orthop Relat Res. 1989;(240):129–136. [PubMed] [Google Scholar]
- Voggenreiter G, Ascherl R, Blümel G, Schmit-Neuerburg K P. Effects of preservation and sterilization on cortical bone grafts: a scanning electron microscopic study. Arch Orthop Trauma Surg. 1994;113:294–296. doi: 10.1007/BF00443821. [DOI] [PubMed] [Google Scholar]
- Dreesman J. Uber Knochenplombierung. Beiter Klin Chir. 1892;9:804–810. [Google Scholar]
- Mehrara B J, McCarthy J C. Repair and Grafting of Bone in Mathes' Plastic Surgery. 2nd ed. Philadelphia: Elsevier; 2006. p. 696.
- Kwon H J, el Deeb M, Morstad T, Waite D. Alveolar ridge maintenance with hydroxylapatite ceramic cones in humans. J Oral Maxillofac Surg. 1986;44:503–508. doi: 10.1016/s0278-2391(86)80089-1. [DOI] [PubMed] [Google Scholar]
- El Deeb M, Tompach P C, Morstad A T, Kwon P. Long-term follow-up of the use of nonporous hydroxyapatite for augmentation of the alveolar ridge. J Oral Maxillofac Surg. 1991;49:257–261. doi: 10.1016/0278-2391(91)90215-8. [DOI] [PubMed] [Google Scholar]
- Wolford L M, Wardrop R W, Hartog J M. Coralline porous hydroxyapatite as a bone graft substitute in orthognathic surgery. J Oral Maxillofac Surg. 1987;45:1034–1042. doi: 10.1016/0278-2391(87)90160-1. [DOI] [PubMed] [Google Scholar]
- Ono I, Tateshita T, Satou M, et al. Treatment of large complex cranial bone defects by using hydroxyapatite ceramic implants. Plast Reconstr Surg. 1999;104:339–349. doi: 10.1097/00006534-199908000-00003. [DOI] [PubMed] [Google Scholar]
- Waite P D, Matukas V J. Zygomatic augmentation with hydroxyapatite: a preliminary report. J Oral Maxillofac Surg. 1986;44:349–352. doi: 10.1016/s0278-2391(86)80029-5. [DOI] [PubMed] [Google Scholar]
- Friedman C D, Costantino P D, Takagi S, Chow L C. BoneSource hydroxyapatite cement: a novel biomaterial for craniofacial skeletal tissue engineering and reconstruction. J Biomed Mater Res. 1998;43:428–432. doi: 10.1002/(sici)1097-4636(199824)43:4<428::aid-jbm10>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- Burstein F D, Cohen S R, Hudgins R, Boydston W, Simms C. The use of hydroxyapatite cement in secondary craniofacial reconstruction. Plast Reconstr Surg. 1999;104:1270–1275. doi: 10.1097/00006534-199910000-00005. [DOI] [PubMed] [Google Scholar]
- Gosain A K, Riordan P A, Song L, et al. A 1-year study of hydroxyapatite-derived biomaterials in an adult sheep model: III. Comparison with autogenous bone graft for facial augmentation. Plast Reconstr Surg. 2005;116:1044–1052. doi: 10.1097/01.prs.0000178402.77445.44. [DOI] [PubMed] [Google Scholar]
- Eppley B L. Alloplastic implantation. Plast Reconstr Surg. 1999;104:1761–1785. doi: 10.1097/00006534-199911000-00025. [DOI] [PubMed] [Google Scholar]