Abstract
While endocannabinoid modulation of both GABAergic and glutamatergic synaptic transmission and plasticity has been extensively investigated, our understanding of the role of endocannabinoids in protecting neurons from harmful insults remains limited. 2-Arachidonoylglycerol (2-AG), the most abundant endogenous ligand and a full agonist for cannabinoid receptors, exhibits anti-inflammatory and neuroprotective effects via a CB1R-mediated mechanism. However, it is still not clear whether 2-AG is also able to protect neurons from beta-amyloid-induced neurodegeneration. Here, we demonstrate that exogenous application of 2-AG significantly protected hippocampal neurons in culture against beta-amyloid-induced neurodegeneration and apoptosis. This neuroprotective effect was blocked by SR141716 (SR-1), a selective CB1R antagonist, but not by SR144528 (SR-2), a selective CB2R antagonist, or capsazepine (CAP), a selective TRPV1 receptor antagonist. To determine whether endogenous 2-AG is capable of protecting neurons from beta-amyloid insults, hippocampal neurons in culture were treated with URB602 or JZL184, selective inhibitors of monoacylglycerol lipase (MAGL), the enzyme hydrolyzing 2-AG. MAGL inhibition that elevates endogenous levels of 2-AG also significantly reduced beta-amyloid-induced neurodegeneration and apoptosis. The 2-AG-produced neuroprotective effects appear to be mediated via CB1R-dependent suppression of ERK1/2 and NF-κB phosphorylation and cyclooxygenase-2 (COX-2) expression. Our results suggest that elevation of endogenous 2-AG by inhibiting its hydrolysis has potential as a novel efficacious therapeutic approach for preventing, ameliorating or treating Alzheimer’s disease.
Keywords: Neurodegeneration, neuroinflammation, endocannabinoids, phosphorylation, beta-amyloid, monoacylglycerol lipase, Alzheimer’s disease
Introduction
Alzheimer’s disease (AD) is a neurodegenerative disease characterized by progressive deterioration of cognitive function and loss of memory in association with widespread neuronal death. There is ample evidence indicating that production and accumulation of β-amyloid peptide (Aβ) are a primary event in the pathogenesis and neuropathology of AD. However, the few agents currently approved by the Food and Drug Administration for treating AD have demonstrated only modest effects in modifying the clinical symptoms for relatively short periods, and none have shown a clear effect on disease progression. Therefore, there is a great public health need to discover and develop new and efficacious therapeutic interventions for prevention and treatment of AD.
Endocannabinoids (eCBs) are endogenous lipid mediators involved in a variety of physiological, pharmacological and pathological processes (Kano et al., 2009). While eCB modulation of both GABAergic and glutamatergic synaptic transmission and plasticity via a CB1 receptor (CB1R)-dependent mechanism has been extensively investigated (Alger, 2002; Piomelli, 2003; Chevaleyre et al., 2006; Kano et al., 2009); our understanding of the role of eCB neuroprotective effects is still limited (Sarne and Mechoulam, 2005; van der Stelt and Di Marzo, 2005). Accumulated information suggests that eCBs possess anti-inflammatory and neuroprotective properties against harmful insults (Eljaschewitsch et al., 2006; Mackie, 2006; Panikashvili et al., 2001; 2005; 2006; Walter and Stella, 2004; Zhang & Chen, 2008). Because of their capability of anti-inflammation and neuroprotection, it has been proposed that eCBs have potential as a therapeutic approach for prevention and treatment of AD (Bisogno & Di Marzo, 2008; Centonze et al., 2007; Marchalant et al., 2008; Ramírez et al., 2005; Van der Stelt et al., 2006; Koppel & Davies, 2008).
2-Arachidonoylglycerol (2-AG), the most abundant endogenous cannabinoid and a full agonist for cannabinoid receptors, is mainly synthesized via diacylglycerol lipase-α (DGLα), and hydrolyzed via monoacylglycerol lipase (MAGL) and α-β-hydrolase domain 6 (ABHD6), and also oxidatively metabolized by cyclooxygenase-2 (COX-2) during inflammation (Blankman et al., 2007; Dinh et al., 2002; Gao et al., 2009; Kano et al., 2009; Kozak et al., 2004; Long et al., 2009a & b; Marrs et al., 2010; Sang & Chen, 2006). The neuroprotective effects of 2-AG are likely through a CB1R receptor-dependent inhibition of proinflmmatory cytokines, COX-2 and NF-κB (Panikashvili et al., 2001; 2005; 2006; Zhang & Chen, 2008). It has been shown that expression of CB1R is markedly reduced in AD brain (Ramírez et al., 2005) and the level of endogenous 2-AG in the hippocampus is elevated in response to administration Aβ42 (Van der Stelt et al., 2006). This means that endogenous 2-AG may play an important role in counteracting pathogenesis and neuropathology in AD, but direct evidence is still lacking. We demonstrated here that 2-AG is capable of preventing and suppressing Aβ-induced neurodegeneration and apoptosis. In particular, we provided evidence that elevation of endogenous 2-AG by inhibiting MAGL is able to protect neurons against Aβ insults. Our results suggest that elevation of endogenous 2-AG by inhibiting its hydrolysis may provide a novel therapeutic intervention for treating, ameliorating or preventing AD.
Experimental procedures
Cell culture
Primary hippocampal neurons from rat (Sprague Dawley) embryos (E18) or P0 were cultured as described previously (Sang et al., 2005; 2007; Zhang & Chen, 2008, Sang et al., 2010), according to the guidelines approved by the Institutional Animal Care and Use Committee of of Louisiana State University Health Sciences Center in New Orleans. Briefly, after suffocation with carbon dioxide, unconscious animals were decapitated. Embryos were taken out after 75% ethanol was sprayed on the abdominal region of the dam. Hippocampi were dissected out under microscope and triturated in serum-free culture medium after meninges were removed. Tissue was incubated in oxygenated trypsin for 10 minutes at 37°C and then mechanically triturated. Cells were spun down and resuspended in Neurobasal/B27 medium (Invitrogen) supplemented with 0.5 mM L-glutamine, penicillin/ streptomycin and 25 µM glutamate. Cells (1 × 106) were loaded into poly-D-lysine-coated 6-well plates for Western blot analysis. Cells (5 × 105) were plated on poly-D-lysine-coated glass coverslips for TUNEL staining. Medium was changed every three days with the same medium without glutamate until use. The percentage of astroglial cells in the culture was ~2 to 5% at 10 days in vitro (DIV), as estimated by staining with a neuronal marker NeuN, astrocytic marker GFAP, and microglial marker OX-42 in conjunction with the DAPI staining as described previously (Sang et al., 2005). Cultures were used between 10–21 DIV.
Immunocytochemistry
Immunostaining was performed in primary cultures of hippocampal neurons and astroglial cells as described previously (Sang et al., 2005; Zhang & Chen, 2008). The cells were rinsed with PBS after removal of the culture medium, and fixed with pre-warmed (37°C) 4% paraformaldehyde, 4% sucrose in 0.1 M phosphate buffer (pH 7.2) at room temperature. Then the cells were washed with ice-cold PBS four times, and incubated with blocking buffer (1% BSA and 10% normal goat serum) in PBS at room temperature for 1h. Primary antibodies at different dilutions (in PBS containing 1% BSA) were applied for overnight at 4°C. NeuN, GFAP and OX-42 were purchased from CHEMICON (Temecula, CA). After four 10-min washes with PBS containing 1% BSA, species-specific and highly cross-absorbed secondary antibodies coupled to Alexa 488 and CY3 (Molecular Probes), diluted 1/1000 in PBS containing 1% BSA, were applied for 1 h at room temperature. Cells were washed four times, 10 min each, with PBS containing 1% BSA, and rinsed with PBS. Images were taken by a deconvolution microscope using Slidebook 5.0 software.
Immunoblot
Hippocampal neurons in cultures were extracted and immediately homogenized in a one-to-one volume of modified RIPA lysis buffer consisting of a number of protease inhibitors. Supernatants were fractionated on 10% SDS-PAGE gels (Bio-Rad), and transferred onto PVDF membranes (Bio-Rad). The membrane was incubated with anti-COX-2 polyclonal antibodies (dilution of 1:1,000, Cayman, Ann Arbor, MI), anti-rabbit caspase-3 and cleaved caspase-3, p44/42 and phospho-p44/42 MAPK, NF-κB65 and phospho-NF-κB antibodies (1:1,000, Cell Signaling, Danvers, MA) at 4°C overnight. The blot was washed and incubated with a secondary antibody (goat anti-rabbit 1:10000, Vector Laboratories, Burlingame, CA) at room temperature for 1 hr. Proteins were visualized by enhanced chemiluminescence (ECL, Amersham Biosciences, UK). The densities of specific bands were quantified by densitometry using FUJIFILM Multi Gauge software (version 3.0). Band densities were normalized to the total amount of protein loaded in each well as determined by mouse anti β-actin (1:4000, Sigma).
Cytotoxicity assays
Aβ25–35 and Aβ1–42 (American Peptide, Sunnyvale, CA) were diluted to 10 and 1 µM in culture medium from the stock solution (200 µM) that had been incubated in 37°C for 36 hrs. Cultures were incubated with Aβ or Locke’s solution alone for the specified time. We used DeadEnd™ fluorometric TUNEL system kit (Promega, Madison, WI) to determine degenerated neurons. The TUNEL-stained cultures also stained with DAPI were imaged using a Zeiss inverted deconvolution microscope with Slidebook 5.0 software. The changes in cell staining were analyzed and quantified using Image J software (NIH). The activation of caspase-3 was assayed using the Western immunoblot technique to detect neuronal apoptosis.
Data analysis
Data are presented as mean ± S.E.M. Unless stated otherwise, Student’s t-test and analysis of variance (ANOVA) with Fisher’s PLSD test or Student-Newman-Keuls test were used for statistical comparison when appropriate. Differences were considered significant when P< 0.05.
Results
Exogenous application of 2-AG prevents Aβ-induced neurodegeneration and apoptosis
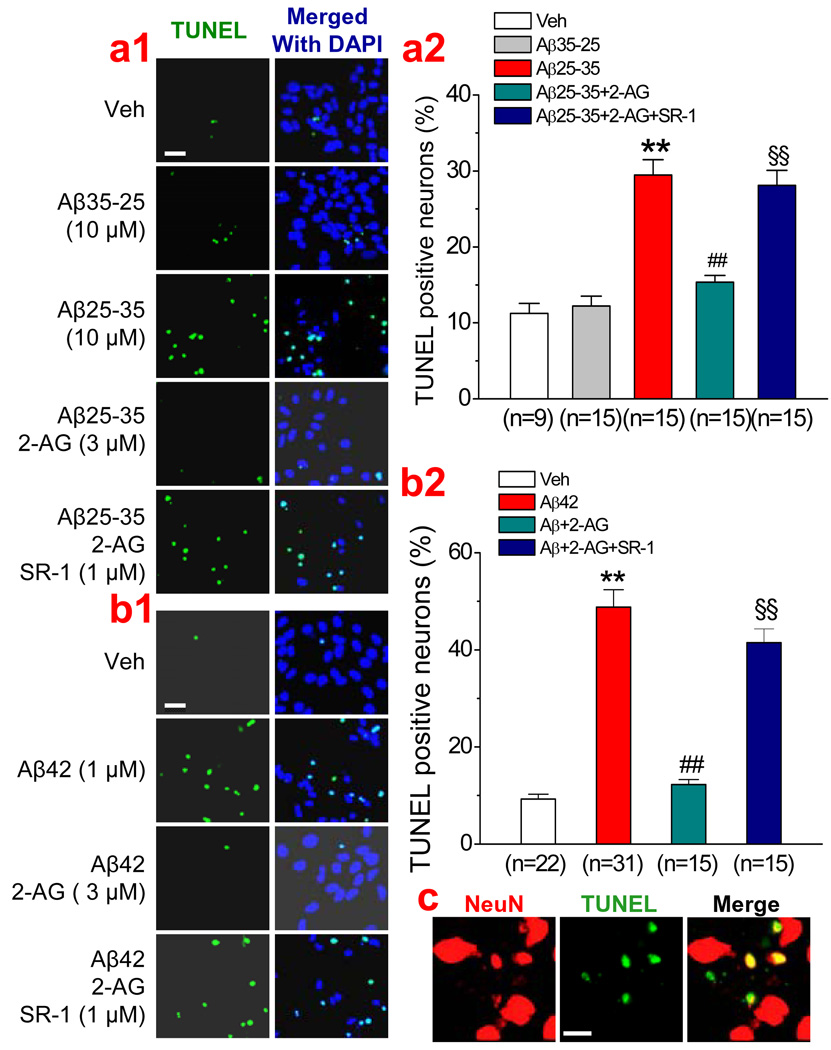
An important feature of AD is neuronal degeneration and death, which are largely due to the toxic effects of Aβ on neurons. To determine whether 2-AG is capable of protecting neurons from Aβ-induced degeneration, we determined TUNEL (neurodegenerative marker) positive neurons in cultured hippocampal neurons. As shown in Figure 1a, the number of TUNEL positive neurons were significantly increased in culture treated with Aβ25–35 (10 µM) for 24 hrs, but not with Aβ35–25. We demonstrated previously that 2-AG at 3 µM concentration is sufficient for protecting neurons from proinflammatory and excitotoxic insults (Zhang & Chen, 2008). Thus we used 2-AG at 3 µM concentration in the present study. It appeared that the Aβ25–35-induced neurodegeneration was prevented or reduced by application of 2-AG (3 µM). Similarly, treatment of the culture with Aβ1–42 (1 µM) for 72 hrs also robustly increased the number of TUNEL positive neurons and this increase was also prevented in the presence of 2-AG (3 µM). The protective effect of 2-AG against Aβ25–35 and Aβ1–42 appeared to be mediated by a CB1R since its effect was blocked by SR141716 (SR-1, 1 µM), a selective CB1R antagonist.
Figure 1.
Exogenous application of 2-arachidonoylglycerol (2-AG) protects hippocampal neurons against Aβ-induced degeneration. (a1). TUNEL images of hippocampal neurons in vehicle control, Aβ35–25 (10 µM), Aβ25–35 (10 µM), Aβ25–35+2-AG (3 µM), and Aβ25–35+2-AG+SR141716 (SR-1, 1 µM). Hippocampal neurons in culture were treated with Aβ25–35 in the absence and presence of 2-AG or SR-1 for 24 hrs. (a2). Percentages of TUNEL positive neurons under different treatments. (b1). TUNEL images of hippocampal neurons in vehicle control, Aβ1–42 (1 µM), Aβ1–42+2-AG (3 µM), and Aβ1–42+2-AG+SR-1 (1 µM). Hippocampal neurons in culture were treated with Aβ1–42 in the absence and presence of 2-AG or SR-1 for 72 hrs. (b2). Percentages of TUNEL positive neurons under different treatments. (c). Overlay of NeuN and TUNEL stained neurons from the culture treated with Aβ1–42 (1 µM) for 72 hrs). *P<0.01, compared with the control; ##P<0.01 compared with Aβ25–35 or Aβ1–42; §§P<0.01 compared with Aβ+2-AG. Images were taken using a Zeiss deconvolution microscope with Slidebook 5.0. Scar bars in a1 & b1: 20 µm, and in c: 10 µm.
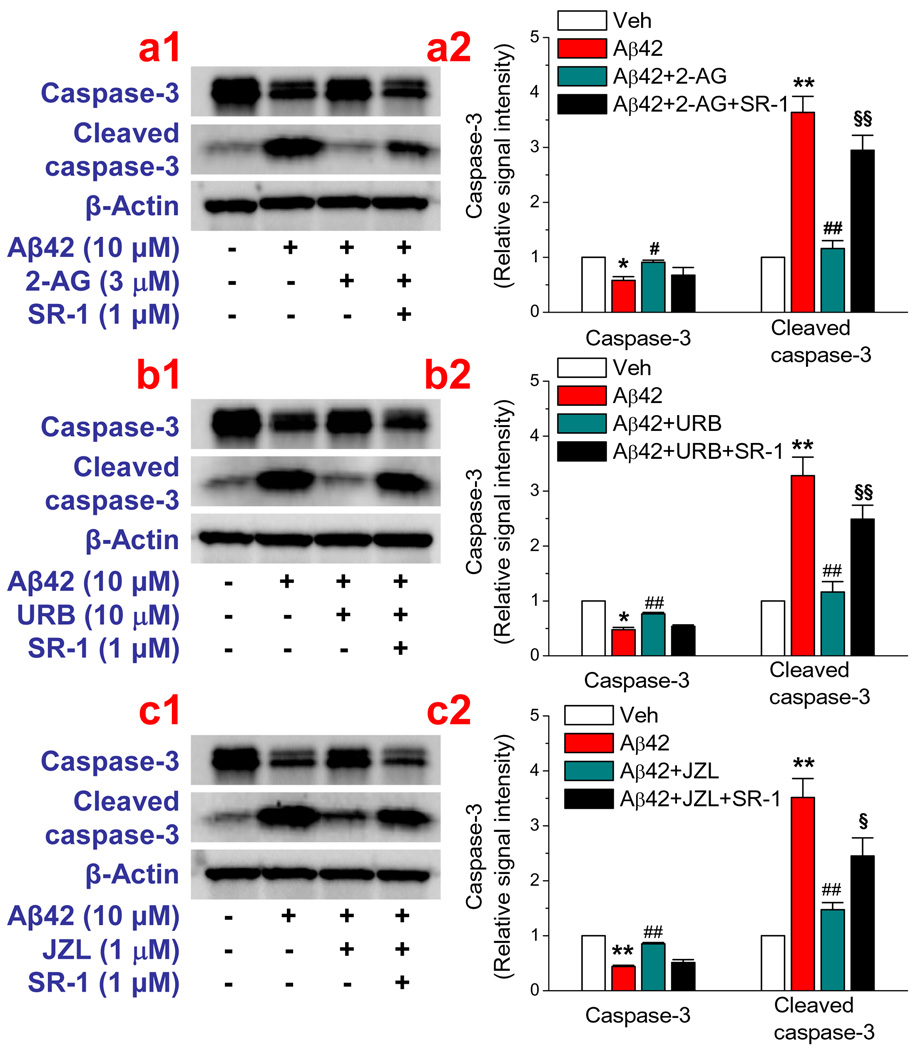
To determine whether 2-AG is also capable of preventing neuronal apoptosis from Aβ insults, we detected activation of caspase-3, an apoptotic marker. We first determined time courses for the Aβ-induced cleavage of caspase-3 in cultured hippocampal neurons and found that it took 24 hrs for Aβ25–35 at 10 µM concentration and 72 hrs for Aβ1–42 at 10 µM to maximally activate caspase-3 (Data not shown). Then we treated hippocampal neurons in culture with Aβ25–35 for 24 hrs or Aβ1–42 for 72 hrs in the absence and presence of 2-AG. As shown in Figure 2a, 2-AG significantly inhibited Aβ25–35- and Aβ1–42-induced cleavage of casapase-3, and this inhibition was eliminated by SR-1. These results indicate that exogenous administration of 2-AG is able to prevent or attenuate Aβ-induced neurodegeneration and apoptosis via a CB1R-dependent mechanism.
Figure 2.
2-AG prevents neuronal apoptosis from Aβ insults. Western blot analysis of cleaved caspase-3 in hippocampal neurons in culture treated with Aβ. (a) Exogenous application of 2-AG produces a CB1R-dependent inhibition of Aβ1–42-induced neuronal apoptosis (n=3–4). Neurons in culture treated with Aβ1–42 (10 µM) for 72 hrs. (b & c). Elevation of endogenous 2-AG by MAGL inhibitor URB602 or JZL184 attenuates Aβ-induced apoptosis (n=3). ##P<0.01 compared with Aβ1–42; §P<0.05; §§P<0.01 compared with Aβ1–42+2-AG, or URB602, or JZL184.
Endogenous 2-AG is capable of preventing and reducing Aβ -induced neurodegeneration and apoptosis
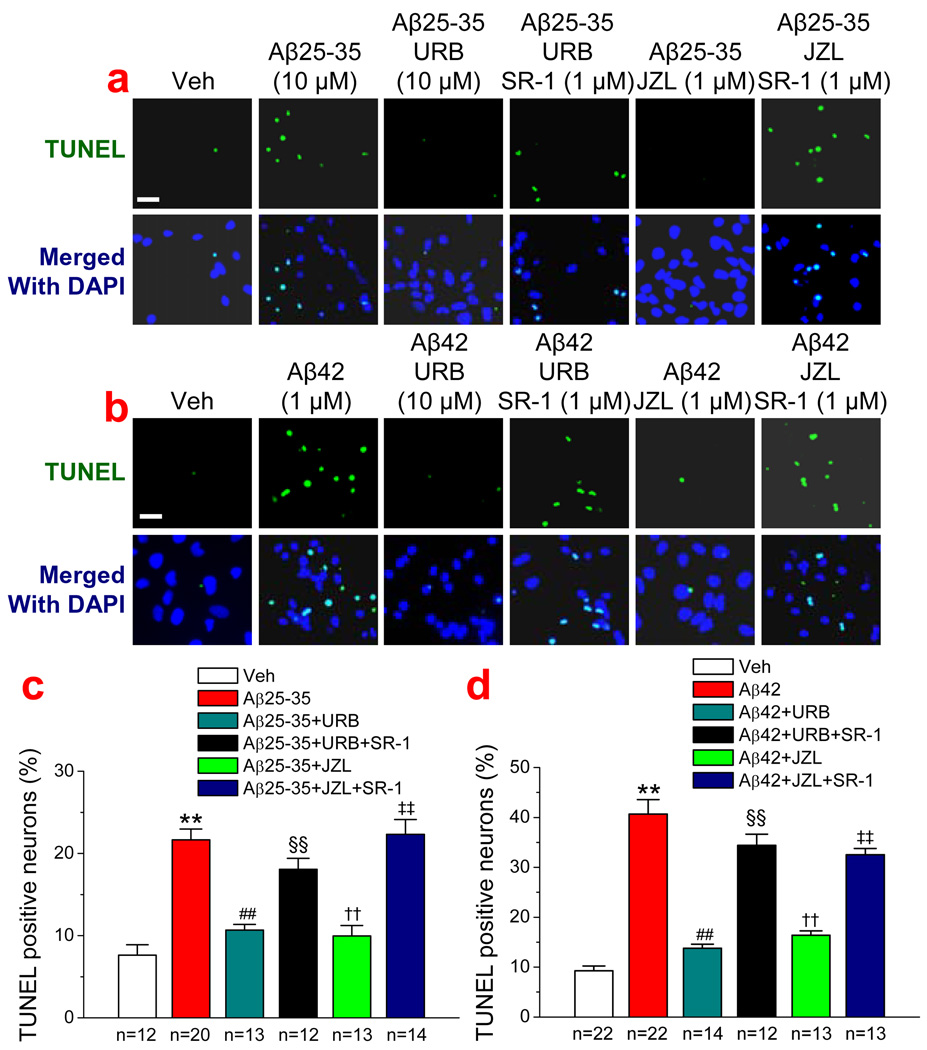
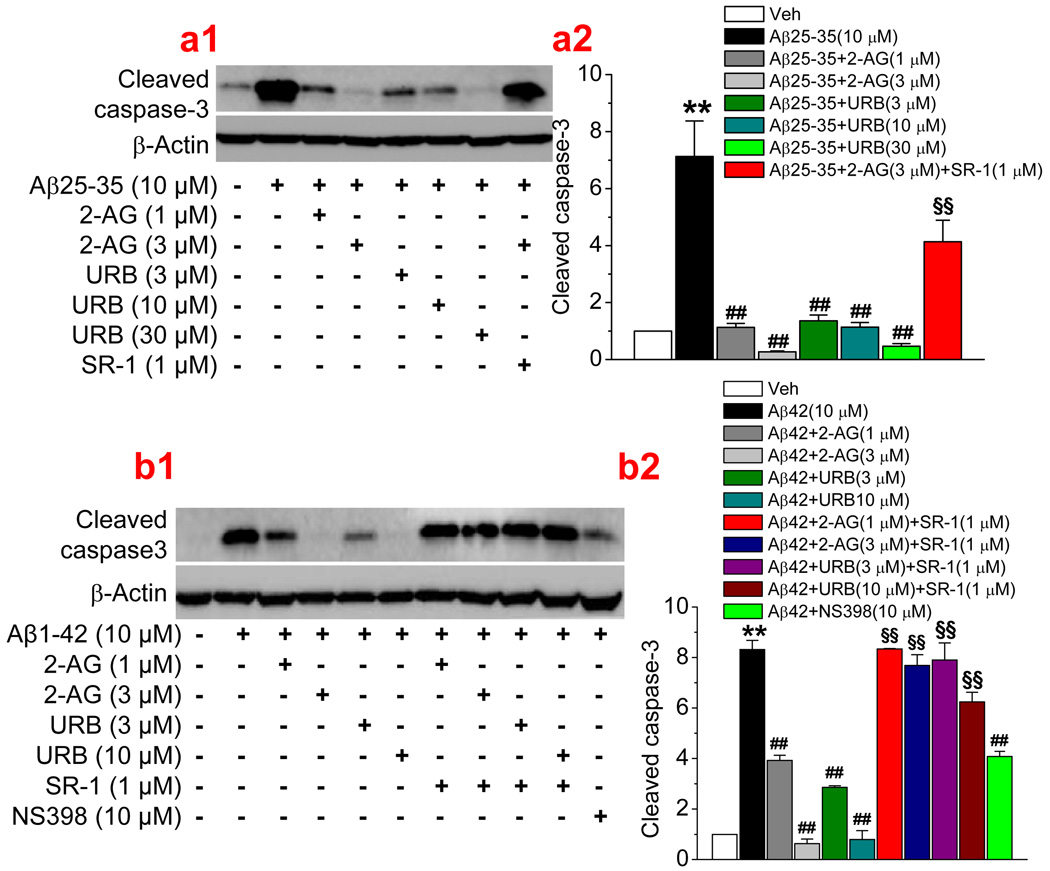
Since 2-AG was exogenously administrated in the experiments described above, we then sought to determine whether endogenously-released 2-AG also is able to suppress the Aβ-induced neurodegeneration and apoptosis. Monoacylglycerol lipase (MAGL) is the enzyme that hydrolyzes 85% of 2-AG in the brain (Blankman et al., 2007; Long et al., 2009a&b). Thus, an inhibition of MAGL with selective MAGL inhibitors will raise the levels of endogenous 2-AG. To this end, we used two selective MAGL inhibitor, URB 602, and JZL184, which have been shown to inhibit 2-AG hydrolysis and increase endogenous 2-AG levels (King et al., 2007; Comelli et al., 2007; Long et al., 2009a&b, Pan et al., 2009). As seen in Figure 3a & 3b, administration of URB 602 (10 µM) or JZL184 (1 µM) significantly reduced numbers of TUNEL positive neurons in cultures treated with Aβ25–35 or Aβ1–42, similar to that shown in Figure 1 where 2-AG was directly added to the culture. URB602- and JZL184-induced neuroprotective effects were blocked by SR-1, suggesting that CB1R mediates URB602- and JZL184-induced protective effects. Likewise, we observed that URB602 and JZL184 produced a CB1R-dependent inhibition of Aβ25–35- or Aβ1–42-induced cleavage of caspase-3 (Figure 2 b & c). Both exogenous and endogenous 2-AG-produced anti-apoptotic effects of Aβ25–35 or Aβ1–42 are concentration-dependent and mediated via CB1R (Figure 4). This means that an increase in 2-AG level either by inhibiting hydrolysis of endogenously released 2-AG or by exogenously supplying 2-AG is able to prevent or reduce Aβ-induced neurodegeneration and apoptosis. In addition, we observed that Aβ-induced apoptosis was also attenuated by NS398, a selective COX-2 inhibitor, suggesting that over-expression of COX-2 contributes to the Aβ-induced neuronal injury or death.
Figure 3.
Endogenous 2-AG protects hippocampal neurons against Aβ-induced neurodegeneration. (a). TUNEL images of hippocampal neurons in vehicle control, Aβ25–35 (10 µM), Aβ25–35+URB602 (10 µM), Aβ25–35+URB602+SR-1 (1 µM), Aβ25–35+JZL184 (1 µM), and Aβ25–35+JZL184+SR-1 (1 µM). (b). TUNEL images of hippocampal neurons in vehicle control, Aβ1–42 (1 µM), Aβ1–42+URB602 (10 µM), Aβ1–42+URB602+SR-1 (1 µM), Aβ1–42+JZL184 (1 µM), and Aβ1–42+ JZL184+SR-1 (1 µM). (c). Percentages of TUNEL positive neurons treated with Aβ25–35 in the absence and presence of URB or JZL. (d). Percentages of TUNEL positive neurons treated with Aβ1–42 in the absence and presence of URB or JZL. **P<0.01, compared with the vehicle control; ##P<0.01 compared with Aβ25–35 or Aβ1–42; §§P<0.01 compared with Aβ+URB602 or JZL; †† P<0.01 compared with Aβ25–35 or Aβ1–42; ‡‡ P<0.01 compared with Aβ+URB602 or JZL184. Scar bars in a1 & b1: 20 µm.
Figure 4.
2-AG induces a dose-dependent inhibition of (a1&a2) Aβ25–35- and (b1&b2) Aβ1–42-induced neuronal apoptosis. Neurons in culture treated with Aβ25–35 (10 µM) for 24 hrs and Aβ1–42 (1 µM) for 72 hrs. 2-AG, URB602 (URB), SR141716 (SR-1) or NS398 were added into the cultures 30 min before application of Aβ (n=3). **P<0.01, compared with the vehicle control; ##P<0.01 compared with Aβ25–35 or Aβ1–42; §§P<0.01 compared with Aβ25–35 or Aβ1–42+2-AG, or URB602.
ERK and NF-κB signaling pathways are involved in 2-AG-produced neuroprotection against Aβ insults
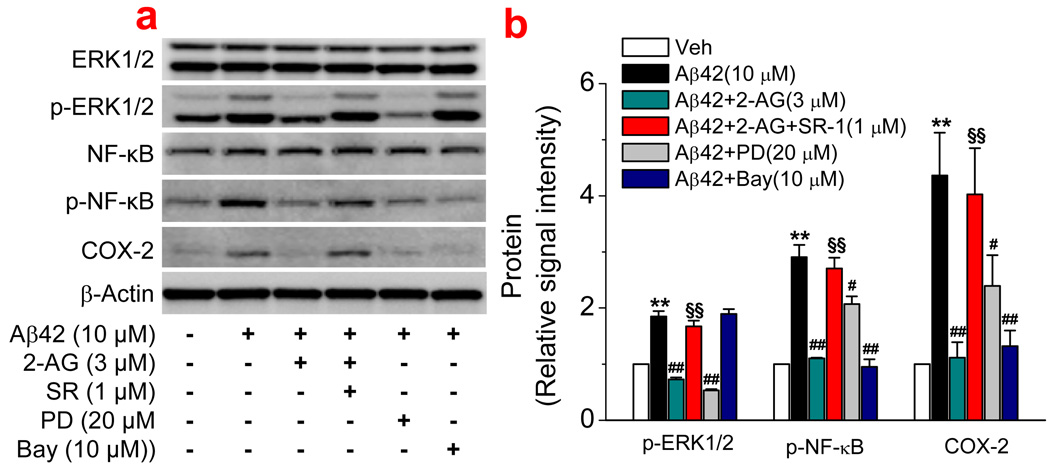
We previously reported that 2-AG-produced anti-inflammatory and neuroprotective effects are mediated via suppressions of ERK/p38MAPK and NF-κB phosphorylation and COX-2 expression (Zhang & Chen, 2008). To examine possible signal transduction mechanisms underlying the 2-AG-produced neuroprotection against Aβ toxicity, we probed the phosphorylation statuses of p38MAPK, ERK and NF-κB, and COX-2 expression in hippocampal neurons in culture. As indicated in Figure 5, treatment neurons with Aβ1–42 for 6 hrs significantly increased phosphorylation of ERK and NF-κB, but not p38MAPK (data not shown). The Aβ1–42-increased phosphorylation was inhibited by 2-AG, which was mediated via a CB1R. Application of PD98059 (PD), a p42/44 MAPK inhibitor, also blocked Aβ1–42-increased phosphorylation of ERK, but not NF-κB, indicating that NF-κB is downstream of ERK. This was confirmed by the fact that Bay11-7085 (Bay), an IκBα inhibitor, blocked Aβ1–42-increased phosphorylation of NF-κB, but not ERK. COX-2 has been long recognized as an important factor in initiating inflammation and inducing neurotoxicity and neurodegeneration. To determine whether Aβ induces an increase in COX-2 expression and whether 2-AG is able to suppress Aβ-induced COX-2 elevation, we detected expression of COX-2 in cultured hippocampal neurons. As shown in Figure 5, Aβ1–42 increased expression of COX-2. This increase was prevented or attenuated by 2-AG and inhibitors of ERK and NF- NF-κB. This information indicates that CB1R-depdendent suppressions of ERK/NF-κB phosphorylation and COX-2 expression may be involved in 2-AG-induced neuroprotection against Aβ insults.
Figure 5.
CB1R-dependent suppression of ERK and NF-κB phosphorylation and COX-2 expression is involved in 2-AG-induced neuroprotection. (a). Western blot analysis of ERK and NF-κB phosphorylation and COX-2 expression in hippocampal neurons treated with Aβ1–42 (10 µM) in the absence and presence of 2-AG (3 µM), SR (1 µM), PD98059 (PD, 20 µM), and Bay11-7085 (Bay, 10 µM). Phosphorylation of signaling proteins and COX-2 expression were detected 6 and 12hrs after the cultures were treated with Aβ1–42. (b). Quantifications of protein expressions under different treatments (n=3). ). **P<0.01, compared with the vehicle control; #P<0.05 and ##P<0.01 compared with Aβ1–42; §§P<0.01 compared with Aβ1–42+2-AG.
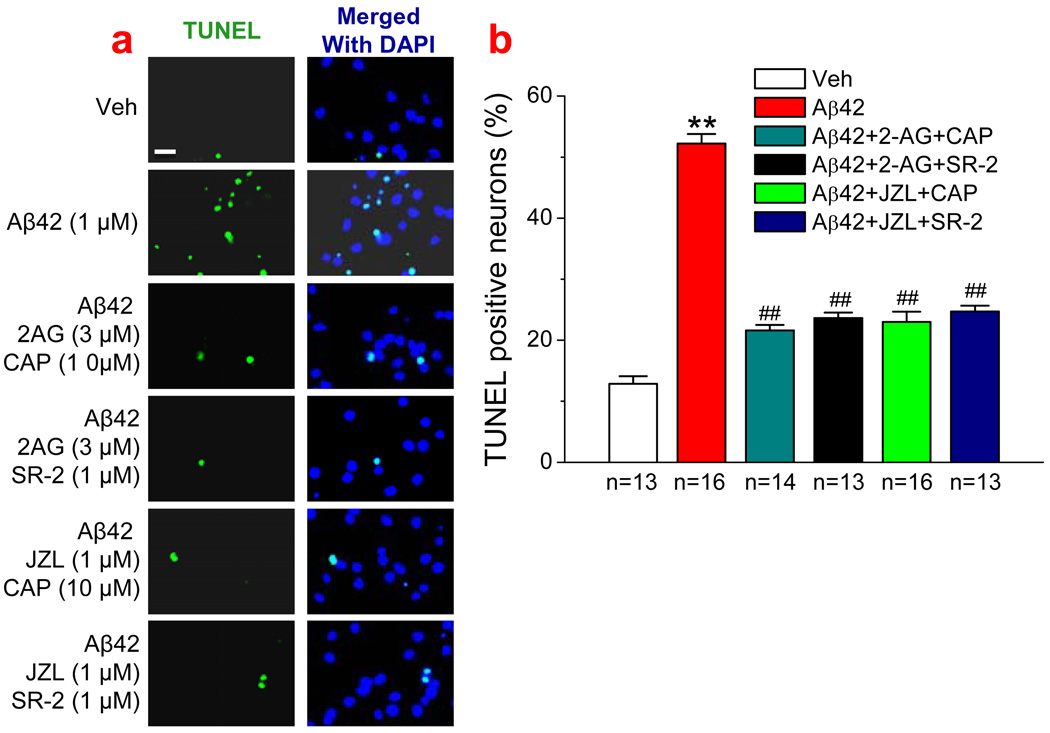
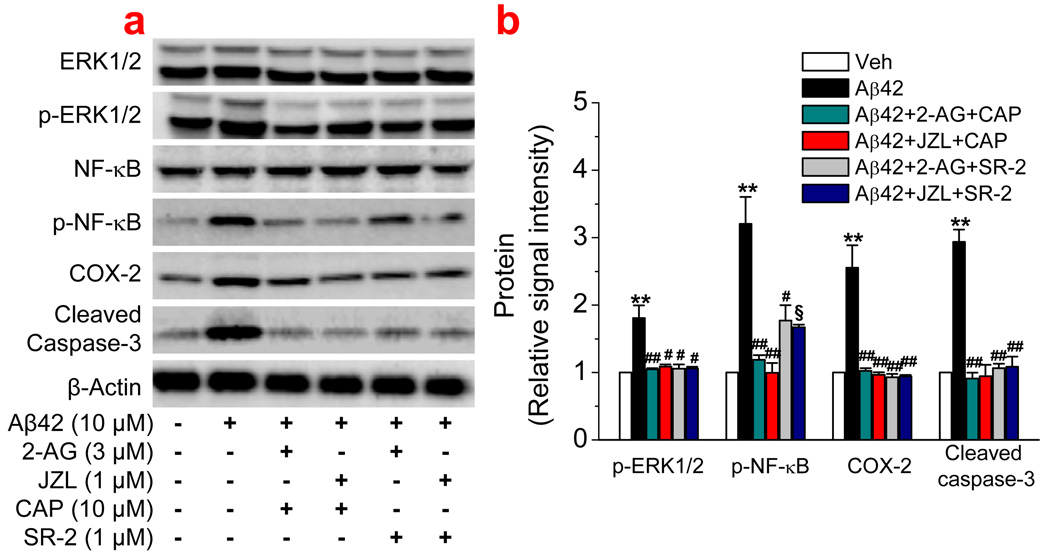
CB2R and TRPV1 may not contribute to the 2-AG-produced neuroprotection against Aβ toxicity
It was thought that the CB2 receptor is mainly present in the immune system. Recent evidence shows that CB2R is also present in the brain (astroglial cells and brainstem neurons, Cabral & Marciano-Cabral, 2005; Carrier et al., 2004; Eljaschewitsch et al., 2006; Gong et al., 2006; Pertwee, 2001; Van Sickle et al., 2005). To determine whether CB2R is involved in the 2-AG-produced suppression of COX-2, we used SR 144528 (SR-2), a selective CB2R antagonist. As shown in Figures 6 and 7, SR-2 failed to block 2-AG- and JZL184-induced neuroprotective effects against the Aβ-induced neurodegeneration and apoptosis. Since eCBs are partial agonists for transient receptor potential cation channels, subfamily V, member 1 (TRPV1), therefore, we also used capsazepine (CAP), a selective TRPV1 receptor antagonist, to determine whether TRPV1 receptors are involved in 2-AG-produced neuroprotection. It appeared that inhibition of TRPV1 did not block 2-AG- and JZL184-induced neuroprotection. In addition, antagonism of CB2R and TRPV1 receptors did not significantly affect 2-AG- and JZL184-induced suppressions of ERK1/2 and NF-kB phosphorylation and COX-2 expression. This information indicates that 2-AG-produced neuroprotection against the beta-amyloid toxicity is primarily mediated via the CB1 receptor.
Figure 6.
CB2 and TRPV1 receptors are not involved in 2-AG-produced neuroprotection against Aβ-induced neurodegeneration. (a). TUNEL images of hippocampal neurons in vehicle control, Aβ1–42 (1 µM), Aβ1–42+2-AG (3 µM)+ capsazepine (CAP, 10 µM), Aβ1–42+2-AG+ SR144528 (SR-2, 1 µM), Aβ1–42+JZL184 (1 µM)+CAP (10 µM), and Aβ1–42+JZL184+SR-2 (1 µM). the treatments of cultures with reagents were the same as described in Fig. 1. (b). Percentages of TUNEL positive neurons treated with Aβ1–42 in the absence and presence of 2-AG, JZL, CAP or SR-2 (n=3). **P<0.01, compared with the vehicle control; ##P<0.01 compared with Aβ1–42. Scar bar: 20 µm.
Figure 7.
CB2 and TRPV1 receptors do not contribute to the 2-AG-induced suppression of ERK and NF-κB phosphorylation, COX-2 expression, and apoptosis. (a). Western blot analysis of ERK and NF-κB phosphorylation, COX-2 expression, and cleaved caspase-3 in hippocampal neurons treated with Aβ1–42 (10 µM) in the absence and presence of 2-AG (3 µM), JZL184 (1 µM), SR-2 (1 µM), or CAP (10 µM). (b). Quantifications of protein expressions under different treatments (n=3). **P<0.01, compared with the vehicle control; #P<0.05 and ##P<0.01 compared with Aβ1–42; §P<0.01 compared with Aβ1–42+2-AG.
Discussion
In the present study, we provide the first evidence that 2-AG protects hippocampal neurons in culture against Aβ-induced neurodegeneration and apoptosis. In particular, we demonstrate that elevation of endogenous 2-AG by MAGL inhibition that prevents 2-AG breakdown is capable of protecting neurons from Aβ insults. We also show that CB1R-dependent suppression of ERK1/2 and NF-κB phosphorylation and COX-2 expression are likely involved in the 2-AG-produced neuroprotective effects against Aβ toxicity.
Arachidonoyl ethanolamide (AEA or anandamide) and 2-AG, two most studied eCBs, have been demonstrated to be involved in a variety of physiological and pathological processes (Chevaleyre et al., 2006; Freund et al., 2003; Piomelli, 2003; Sugiura et al., 2006; Kano et al., 2009). It has been proposed that AEA and 2-AG are likely important components of the endogenous system protecting neurons from harmful insults (Van der Stelt et al., 2005; Milton, 2002; Marsicano et al., 2002; 2003; Eljaschewitsch et al., 2006; Gopez et al., 2005; Melis et al., 2006; Panikashvili et al., 2001; 2005; Sarne and echoulam, 2005; van der Stelt and Di Marzo, 2005; Zhang & Chen, 2008). However, the endogenous protective system releases different eCBs in response to different assaults. For instance, stimulation of the Schaffer collateral synapses in rat hippocampal slices triggers endogenous release of 2-AG, but not AEA (Stella et al., 1997) and traumatic brain injury also significantly raises the brain level of 2-AG (Panikashvili et al., 2001). On the other hand, administration of kainic acid elevates the levels of AEA in the brain, but not 2-AG (Marsicano et al., 2003). This implies that the eCB system is an intrinsic protective system able to release eCBs on demand in response to different stimuli (Marsicano et al., 2003). Acute in vivo administration of Aβ increases the release of 2-AG in the brain (van der Stelt et al., 2006), suggesting that endogenous 2-AG plays an important role in protecting neurons from Aβ toxicity. It is likely that deficits or insufficiencies in eCB signaling may contribute to neuropathology in AD. In fact, it has been shown that the expression of the CB1 receptor is markedly reduced in human AD brain (Ramirez et al., 2005). Therefore, our results suggest that strengthening endogenous 2-AG signaling may exert neuroprotective effects against Aβ neurotoxicity. 2-AG-produced neuroprotection against Aβ insults observed in the present study appears to be mediated via CB1R since the protective effects of exogenous 2-AG application and elevation of endogenous 2-AG by inhibiting MAGL are blocked or attenuated by SR141716, a selective CB1R antagonist, but not by SR144528, a selective CB2R antagonist, or capsazepine (CAP), a selective TRPV1 receptor antagonist. We observed that hippocampal neurons in culture treated with Aβ significantly elevated phosphorylation of p38 MAPK and NF-κB and expression of COX-2. These elevations were inhibited or eliminated by 2-AG, suggesting that 2-AG-produced neuroprotective effects are mediated via CB1R-dependent suppressions of ERK1/2 and NF-κB phosphorylation and COX-2 expression. This is consistent with our previous observations where we demonstrated that 2-AG protects neurons from inflammatory and excitotoxic insults through CB1R-depedent suppression of ERK/MAPK/NF-κB phosphorylation and COX-2 expression (Zhang & Chen, 2008).
2-AG has been shown to protect neurons from brain ischemia, traumatic brain injury and proinflammatory stimuli (Gopez et al., 2005; Melis et al., 2006; Panikashvili et al., 2001; 2005; 2006). We also showed previously that exogenous and endogenous 2-AG is able to suppress COX-2 elevation and protect neurons from proinflammatory and excitotoxic stimuli (Zhang & Chen, 2008). In this study, we demonstrated that endogenous 2-AG is also able to protect neurons in vitro against Aβ toxicity. If the neuroprotective effects against Aβ insults prove to be valid in an in vivo animal of AD, then this means that strengthening endogenous 2-AG signaling by inhibiting its hydrolysis or facilitating its synthesis or directly administering 2-AG may lead to potential interventions for preventing, alleviating and treating AD.
Research highlights.
Exogenous and endogenous endocannabinoid 2-AG protects neurons against Aβ insults.
2-AG-induced neuroprotection is mediated via a CB1 receptor.
ERK1/2, NF-κB and COX-2 are involved in 2-AG-produced neuroprotection.
Acknowledgement
The authors thank NIH Metal Health Institute Chemical Synthesis and Drug Supply Program for providing SR141716. This work was supported by National Institutes of Health grant R01NS054886 and the Alzheimer’s Association grant IIRG-05-13580.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alger BE. Retrograde signaling in the regulation of synaptic transmission: focus on endocannabinoids. Prog Neurobiol. 2002;68:247–286. doi: 10.1016/s0301-0082(02)00080-1. [DOI] [PubMed] [Google Scholar]
- Bisogno T, Di Marzo V. The role of the endocannabinoid system in Alzheimer's disease: facts and hypotheses. Curr Pharm Des. 2008;14:2299–3305. doi: 10.2174/138161208785740027. [DOI] [PubMed] [Google Scholar]
- Blankman JL, Simon GM, Cravavtt BF. A comprehensive profile of brain enzymes that hydrolyze the endocannabinoid 2-arachidonylglycerol. Chem Biol. 2007;14:1347–1356. doi: 10.1016/j.chembiol.2007.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabral GA, Marciano-Cabral F. Cannabinoid receptors in microglia of the central nervous system: immune functional relevance. J Leukoc Biol. 2005;78:1192–1197. doi: 10.1189/jlb.0405216. [DOI] [PubMed] [Google Scholar]
- Carrier EJ, Kearn CS, Barkmeier AJ, Breese NM, Yang W, Nithipatikom K, Pfister SL, Campbell WB, Hillard CJ. Cultured rat microglial cells synthesize the endocannabinoids 1-arachidonylglycerol, which increases proleferation via a CB2 receptor-dependent mechanism. Mol Pharmacol. 2004;65:999–1007. doi: 10.1124/mol.65.4.999. [DOI] [PubMed] [Google Scholar]
- Centonze D, Finazzi-Agro A, Bernardi G, Maccarrone M. The endocannabinoid system in targeting inflammatory neurodegenerative diseases. Trends in Pharmacol Sci. 2007;28:180–187. doi: 10.1016/j.tips.2007.02.004. [DOI] [PubMed] [Google Scholar]
- Chevaleyre V, Takahashi KA, Castillo PE. Endocannabinoid-Mediated Synaptic Plasticity in the CNS. Ann Rev Neurosci. 2006;29:37–76. doi: 10.1146/annurev.neuro.29.051605.112834. [DOI] [PubMed] [Google Scholar]
- Comelli F, Giagnoni G, Bettoni I, Colleoni M, Costa B. The inhibition of monoacylglycerol lipase by URB602 showed an anti-inflammatory and anti-nociceptive effect in a murine model of acute inflammation. Br J Pharmacol. 2007;152:787–794. doi: 10.1038/sj.bjp.0707425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinh TP, Carpenter D, Leslie FM, Freund TF, Katona I, Sensi SI, Kathuria S, Piomelli D. Brain monoglyceride lipase participating in endocannabinoid inactivation. Proc Natl Acad Sci USA. 2002;99:10819–10824. doi: 10.1073/pnas.152334899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eljaschewitsch E, et al. The endocannabinoid anandamide protects neurons during CNS inflammation by induction of MKP-1 in microglial cells. Neuron. 2006;49:67–79. doi: 10.1016/j.neuron.2005.11.027. [DOI] [PubMed] [Google Scholar]
- Freund TF, Katona I, Piomelli D. Role of endogenous cannabinoids in synaptic signaling. Physiol Rev. 2003;83:1017–1066. doi: 10.1152/physrev.00004.2003. [DOI] [PubMed] [Google Scholar]
- Gao Y, et al. Loss of retrograde endocannabinoid signaling and reduced adult neurogenesis in diacylglycerol lipase knock-out mice. J Neurosci. 2010;30(6):2017–2024. doi: 10.1523/JNEUROSCI.5693-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gopez JJ, et al. Cyclooxygenase-2-specific inhibitor improves functional outcomes, provides neuroprotection, and reduces inflammation in a rat model of traumatic brain injury. Neurosurgery. 2005;56:590–604. doi: 10.1227/01.NEU.0000154060.14900.8F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong JP, Onaivi ES, Ishiguro H, Liu QR, Tagliaferro PA, Brusco A, Uhl GR. Cannabinoid CB2 receptors: Immunohistochemical localization in rat brain. Brain Res. 2006;1071:10–23. doi: 10.1016/j.brainres.2005.11.035. [DOI] [PubMed] [Google Scholar]
- Kano M, Ohno-Shosaku T, Hashimotodani Y, Uchigashima M, Watanabe M. Endocannabinoid-mediated control of synaptic transmission. Physiol Rev. 2009;89:309–380. doi: 10.1152/physrev.00019.2008. [DOI] [PubMed] [Google Scholar]
- King AR, et al. URB602 inhibits monoacylglycerol lipase and selectively blocks 2-arachidonoylglycerol degradation in intact brain slices. Chem Biol. 2007;14:1357–1365. doi: 10.1016/j.chembiol.2007.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koppel J, Davies P. Targeting the endocannabinoid system in Alzheimer's disease. J Alzheimers Dis. 2008;15:495–504. doi: 10.3233/jad-2008-15315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozak KR, Prusakiewicz JL, Marnett LJ. Oxidative metabolism of endocannabinoids by COX-2. Curr Pharmaceut Design. 2004;10:659–667. doi: 10.2174/1381612043453081. [DOI] [PubMed] [Google Scholar]
- Long JZ, Normura DK, Cravatt BF. Characterization of monoacylglycerol lipase inhibition reveals differences in central and peripheral endocannabinoid metabolism. Chem Biol. 2009a;16:744–753. doi: 10.1016/j.chembiol.2009.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long JZ, et al. Selective blockade of 2-arachidonoylglycerol hydrolysis producs cannabinoid behavioral effects. Nat Chem Biol. 2009b;5:37–44. doi: 10.1038/nchembio.129. 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackie K. Cannabinoid receptors as therapeutic targets. Annu Rev Pharmacol Toxicol. 2006;46:101–122. doi: 10.1146/annurev.pharmtox.46.120604.141254. [DOI] [PubMed] [Google Scholar]
- Marchalanta Y, Brothersa HM, Wenka GL. Inflammation and aging: can endocannabinoids help? Biomed Pharmacother. 2008;62:212–217. doi: 10.1016/j.biopha.2008.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marrs WR, et al. The serine hydrolase ABHD6 controls the accumulation and efficacy of 2-AG at cannabinoid receptors. Nat Neurosci. 2010;13:951–957. doi: 10.1038/nn.2601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsicano G, Moosmann B, Hermann H, Lutz B, Behl C. Neuroprotetive properties of cannabinoids against oxidative stress: role of the cannabinoid receptor CB1. J Neurochem. 2002;80 doi: 10.1046/j.0022-3042.2001.00716.x. 448-28. [DOI] [PubMed] [Google Scholar]
- Marsicano G, et al. CB1 cannabinoid receptors and on-demand defense against excitotoxicity. Science. 2003;302:84–88. doi: 10.1126/science.1088208. [DOI] [PubMed] [Google Scholar]
- Melis M, et al. Protective activation of the endocannabinoid system during ischemia in dopamine neurons. Neurobiol Dis. 2006;24:15–27. doi: 10.1016/j.nbd.2006.04.010. [DOI] [PubMed] [Google Scholar]
- Milton NGN. Anandamide and noladin ether prevent neurotoxicity of the human amyloid-β peptide. Neurosci lett. 2002;332:127–130. doi: 10.1016/s0304-3940(02)00936-9. [DOI] [PubMed] [Google Scholar]
- Pan B, Wang W, Long JZ, Sun D, Hillard CJ, Cravatt BF, Liu QS. Blockade of 2-arachidonoylglycerol hydrolysis by selective monoacylglycerol lipase inhibitor 4-nitrophenyl 4- (dibenzo[d][1,3]dioxol-5-yl(hydroxy)methyl)piperidine-1-carboxylate JZL184) Enhances retrograde endocannabinoid signaling. J Pharmacol Exp Ther. 2009;331(2):591–597. doi: 10.1124/jpet.109.158162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panikashvili D, Simeonidou C, Ben-Shabat S, Hanus L, Breuer A, Mechoulam R, Shohami E. An endogenous cannabinoid (2-AG) is neuroprotective after brain injury. Nature. 2001;413:427–531. doi: 10.1038/35097089. [DOI] [PubMed] [Google Scholar]
- Panikashvili D, Mechoulam R, Beni SM, Alexandrovich A, Shohami E. CB1 cannabinoid receptors are involved in neuroprotection via NF-kappa B inhibition. J Cereb Blood Flow Metab. 2005;25:477–484. doi: 10.1038/sj.jcbfm.9600047. [DOI] [PubMed] [Google Scholar]
- Panikashvili D, Shein NA, Mechoulam R, Trembovler V, Kohen R, Alexandrovich A, Shohami E. The endocannabinoid 2-AG protects the blood-brain barrier after closed head injury and inhibits mRNA expression of proinflammatory cytokines. Neurobiol Dis. 2006;22:257–264. doi: 10.1016/j.nbd.2005.11.004. [DOI] [PubMed] [Google Scholar]
- Piomelli D. The molecular logic of endocannabinoid signaling. Nat Rev Neurosci. 2003;4:873–884. doi: 10.1038/nrn1247. [DOI] [PubMed] [Google Scholar]
- Ramírez BG, Blazquez C, del Pulgar TG, Guzman M, de Ceballos ML. Prevention of Alzheimer’s disease pathology by cannabinoids: Neuroprotection medicated by blockade of microglial activation. J Neurosci. 2005;25:1904–1913. doi: 10.1523/JNEUROSCI.4540-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pertwee RG. Cannabinoid receptors and pain. Prog Neurobiol. 2001;63:569–611. doi: 10.1016/s0301-0082(00)00031-9. [DOI] [PubMed] [Google Scholar]
- Sang N, Zhang J, Marcheselli V, Bazan NG, Chen C. Postsynaptically synthesized prostaglandin E2 modulates hippocampal synaptic transmission via a presynaptic PGE2 EP2 receptor. J Neurosci. 2005;25:9858–9870. doi: 10.1523/JNEUROSCI.2392-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sang N, Chen C. Lipid signaling and synaptic plasticity. Neuroscientist. 2006;12:425–434. doi: 10.1177/1073858406290794. [DOI] [PubMed] [Google Scholar]
- Sang N, Zhang J, Chen C. COX-2 oxidative metabolite of endocannabinoid 2-AG enhances excitatory glutamatergic synaptic transmission and induces neurotoxicity. J Neurochem. 2007;103:1966–1977. doi: 10.1111/j.1471-4159.2007.04668.x. [DOI] [PubMed] [Google Scholar]
- Sang N, Zhang J, Chen C. Anandamide potentiation of miniature spontaneous excitatory synaptic transmission is mediated via IP3 pathway. Neurochem Intl. 2010;56:590–596. doi: 10.1016/j.neuint.2010.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarne Y, Mechoulam R. Cannabinoids: between neuroprotection and neurotoxicity. Curr. Drug Target- CNS Neurol Dis. 2005;4:677–684. doi: 10.2174/156800705774933005. [DOI] [PubMed] [Google Scholar]
- Stella N, Schweitzer P, Piomelli D. A second endogenous cannabinoid that modulates long-term potentiation. Nature. 1997;388:773–778. doi: 10.1038/42015. [DOI] [PubMed] [Google Scholar]
- Sugiura T, kishimoto S, Oka S, Gokoh M. Biochemistry, pharmacology and physiology of 2-arachidonoylglycerol, an endogenous cannabinoid receptor ligand. Prog Lipid Res. 2006;45:405–446. doi: 10.1016/j.plipres.2006.03.003. [DOI] [PubMed] [Google Scholar]
- Van der Stelt M, Di Marzo V. Cannabinoid receptors and their role in neuroprotection. NeuroMolecular Med. 2005;7:37–50. doi: 10.1385/NMM:7:1-2:037. [DOI] [PubMed] [Google Scholar]
- van der Stelt M, et al. Endocannabinoids and beta-amyloid-induced neurotoxicity in vivo: effect of pharmacological elevation of endocannabinoid levels. Cell Mol Life Sci. 2006;63:1410–1424. doi: 10.1007/s00018-006-6037-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Sickle MD, et al. Identification and functional characterization of brainstem cannabinoid CB2 receptors. Science. 2005;310:329–332. doi: 10.1126/science.1115740. [DOI] [PubMed] [Google Scholar]
- Walter L, Stella N. Cannabinoids and neuroinflammation. Br J Pharmacol. 2004;141:775–785. doi: 10.1038/sj.bjp.0705667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Chen C. Endocannabinoid 2-arachidonoylglycerol protects neurons by limiting COX-2 elevation. J Biol Chem. 2008;283:22601–22611. doi: 10.1074/jbc.M800524200. [DOI] [PMC free article] [PubMed] [Google Scholar]