Abstract
Children exposed prenatally to cocaine show deficits in emotion regulation and inhibitory control. While controlling for the measures of medical complication in the perinatal period, environmental risk, and prenatal polydrug exposure (alcohol, tobacco, and marijuana), we examined the effects of prenatal cocaine exposure and gender on attention and inhibitory control in 203 children at ages 6, 9, and 11. Cocaine exposure affected the performance of males, but not females. Heavily exposed males showed deficits in the attention and the inhibition tasks. In addition, a significantly greater proportion of heavily exposed males (21%) than unexposed males (7%) or heavily exposed females (7%) failed to complete the task (p < .01). Even without those poorest performing subjects, the overall accuracy for heavily exposed males (81%) was significantly reduced (p < .05) compared to lightly exposed males (87%) and unexposed males (89%). The findings highlight the importance of considering gender specificity in cocaine exposure effects. Processes by which cocaine effects may be specific to males are discussed.
Keywords: attention, inhibitory control, prenatal cocaine exposure
1. Introduction
Prenatal cocaine exposure affects brain development as measured by attention and inhibitory control [1,2,6,10–12,25,26,36,50–52,61,76]. A growing literature indicates that gender, however, moderates the negative effects of cocaine exposure as exposed males, but not exposed females, have been found to exhibit worse functioning in inhibitory control, intelligence, emotion regulation, aggression, and high risk behavior. Males prenatally exposed to cocaine have greater difficulty with frustration reactivity and emotion regulation at 4 years [25] and inhibitory control at 5 years [10], and exhibit more aggression and behavior problems at age 5 to 6 years [9,24]. Cognitively, exposed males have shown lower arithmetic scores on a preschool intelligence test [68] and lower IQs at ages 4 through 9 years [13,14]. Extending the adverse effects into preadolescence, exposure effects were found for males but not females in predicting risky behavior [15].
Animal studies further indicate that gender may moderate the negative effects of cocaine exposure as exposed male rats committed more omission errors than control males on trials that followed an error, which suggests impaired sustained attention and increased reactivity to committing an error [34]. Cocaine exposed males also were more likely than exposed females to commit a premature response after committing an error on a task of selective visual attention [35] and to exhibit impaired performance relative to unexposed males on a three-choice serial reversal task; these effects were not found for exposed females relative to unexposed females [32]. Collectively, the child and animal studies indicate that children, and males in particular, who were prenatally exposed to cocaine are at risk for attention and inhibitory control problems in childhood.
Inhibitory control refers to the ability to alter behavior in order to adapt to changing environmental demands [48]. It is a broad concept and there are different forms of inhibition in the perceptual/attention, cognitive, and motor domains [46]. The go/nogo paradigm has been frequently used to investigate motor inhibitory control [44]. Functional magnetic resonance imaging (fMRI) studies of the go/nogo task report a predominantly right hemisphere network that includes prefrontal, parietal and subcortical regions [18,31,38]. There are developmental differences in the brain regions used in response inhibition with children showing greater activation than adults in a fronto-striatal network [16] and greater activation of posterior regions [19]. With development, children show a shift in brain region activation; specifically children shift from attenuated activation in dorsolateral prefrontal cortical areas to increased focal activation in ventral prefrontal regions [28]. A review of developmental neuroimaging studies [49] showed that the inferior frontal gyrus (IFG) (BA45/46) and premotor regions (BA46) increase in activation with age in the go/nogo task [63,73]. FMRI studies of adults performing go/nogo tasks have also found gender differences in areas activated and in the extent of activation. For example, females show greater activation in many cortical regions including bilateral inferior parietal regions, right lentiform, precuneus and left middle frontal gyrus [31].
Several studies have examined inhibitory control in early and middle childhood in groups prenatally exposed to cocaine and have generally reported exposure effects, especially for heavy exposure to cocaine [10,12,57,62] although the findings may be subtle [64]. In addition, studies of inhibitory control rely on the attention abilities of subjects to develop a response to the stimulus that is then required to be inhibited under certain conditions. It is important, therefore, to establish the effects of prenatal cocaine exposure on attention abilities as well.
This study examines attention and inhibitory control abilities using a go/nogo reversal task at ages six, nine, and 11 years. We predicted that cocaine exposure would affect both attention and inhibitory control performance. In addition, we predicted that cocaine exposure would have a greater impact on males than females. Given the associations between poverty and prenatal substance abuse and between poverty and neurocognitive development [29,68], it was necessary to control for environmental risk factors. We controlled for other prenatal exposures (alcohol, tobacco, and marijuana) as well as for neonatal medical problems and environmental risk that also can negatively affect attention or inhibitory control [57,72].
2. Methods
2.1. General method
2.1.1. Participants
There were 203 participants in the study who were followed in an ongoing longitudinal study [8]. In the longitudinal study, participants were initially recruited at or prior to birth through hospital-based prenatal clinics or newly delivered women at three hospitals in Trenton, NJ or at the Medical College of Pennsylvania Hospital in Philadelphia. Children were excluded from the longitudinal study if they were born prior to 32 weeks of gestation, required special care or oxygen therapy for more than 24 hours, exhibited congenital anomalies, were exposed to opiates or phencyclidine in utero, or if their mothers were infected with HIV. Participation was voluntary, and incentives were provided in the form of $30 in vouchers per visit for use at local stores. All mothers were urban, clinic patients, predominantly African American (87%), with 10% Caucasian and 3% Hispanic.
The 203 participants were drawn from the longitudinal study that was recruited over a two year interval. A total of 384 pregnant women agreed to participate and 321 met the criteria for inclusion. A total of 258 completed the first laboratory visit at age 4 months and the active data set by age 11 included 210 participants, which was 81% of the sample at age 4 months. The sample in this study did not differ significantly in the percentage of children exposed to cocaine, gender or ethnicity compared to those lost to attrition from the cohort at age 4 months. Of these, there were 203 children who completed the task at one or more age points. Some children missed a laboratory visit at one age point. Overall, 90 participants (of 203, 44%) competed all three age points, 61 (30%) completed two visits, and 52 (26%) completed one visit. Therefore there were 444 scorable visits for analyses. Caretakers signed informed consent forms approved by the Institutional Review Boards of the University of Medicine and Dentistry of New Jersey and the Drexel University College of Medicine. The children gave verbal assent at age 6 and signed assent forms at ages 9 and 11.
2.1.2. Measures of cocaine exposure and covariates
Children and their mothers were assessed using several instruments to obtain measures of prenatal drug exposure, neonatal medical complications, and environmental risk [7].
2.1.2.1. Prenatal levels of drug exposure
Prenatal drug use by the mother was obtained through a semi-structured interview that was conducted either prenatally, in the mother’s room on the maternity ward if she had just delivered, in our laboratories near the hospitals, or in the mother’s home within two weeks of the child’s birth. Interviews were administered by trained interviewers and substance abuse counselors. The drug use interview contained questions about the frequency and amount of cocaine, alcohol, cigarettes, marijuana, opiates, and phencyclidine used during pregnancy. Prenatal cocaine exposure was categorized as light or heavy depending on the amount of cocaine used by the mother. Lightly exposed children were those whose mothers used cocaine less than twice weekly throughout pregnancy while heavily exposed children were those whose mother used cocaine twice or more weekly throughout pregnancy [11,43]. Cocaine use was confirmed by results of analysis of newborns’ meconium, which was screened with radioimmunoassay followed by confirmatory gas chromatography - mass spectrometry for the presence of benzoyl ecgonine (cocaine metabolite), cannabinoids, opiates, and PCP. Mothers showed no signs of PCP, heroin, or methadone use as determined by the assay and by self-report in repeated interviews. Prenatal cigarette exposure was assessed by maternal report at the time of birth, which has been shown to be a valid measure of maternal smoking during pregnancy [20]. There were seven mothers of cocaine exposed children in this study who did not complete the self report. The meconium assay was positive for cocaine for these seven children; the exposure level was set to lightly exposed for these seven participants.
2.1.2.2. Neonatal medical problems
Prenatal and neonatal medical data were abstracted by nurses from hospital records and used to complete a neonatal medical complications scale (MCS) consisting of 35 possible complications [40]. Variables included general factors (e.g., low birth weight, fetal anomalies, and feeding problems), respiratory complications (e.g., congenital pneumonia, apnea, and meconium aspiration syndrome), metabolic disorders (e.g., failure to gain weight and hypoglycemia), cardiac problems (e.g., murmur and cardiac anomalies), and CNS problems (e.g., CNS depression and seizures). Variables were weighted and summed to obtain the risk score and transformed to a log scale.
2.1.2.3. Environmental risk index
Several environmental risk variables were assessed by maternal interview at the 54, 84, 102, and 120 month visits of the longitudinal study. Risk variables were standardized, reverse coded if necessary so that higher scores reflected greater risk, and then converted into a composite environmental risk score (ERS) t-score [11]. This ERS was a composite of maternal life stress based on the Social Environment Inventory [59], maternal social support network size (Norbeck Social Support Questionnaire) [58], number of regular caregivers (greater number = higher risk), regularity of child’s schedule, stability of child’s surroundings (Family Chaos Scale) (A. Sameroff personal communication), single parent household (living alone with children = higher risk), maternal education, maternal race (non-European American = higher risk), and public assistance status (public assistance as main source of income = higher risk). Environmental risk was obtained by averaging the risk T-scores at the 54, 84, 102, and 120 month visits. In this study, only nine children had a history of foster care; all were males with six unexposed to prenatal cocaine, one lightly exposed to prenatal cocaine, and two heavily exposed. We did not have sufficient numbers of subjects in foster care to separate them out in the analyses.
2.1.3. Task
The Yale Child Study Center Attention Task [51] is a computerized go/nogo task that was used to assess attention and inhibitory control. We adjusted the timing and the number of go and nogo stimuli to produce go/nogo tasks that varied in difficulty. Images were displayed on a PC computer screen. Participants responded to go trials by pressing a button that was located on the desk next to their preferred hand. At all ages, the target image was presented for 20 trials to establish a prepotent response. Then 160 trials were presented with a random mixture of target and distractor images.
At six years, the target image was a house that was presented for1500 msec with a randomly varying inter-stimulus interval (ISI) of 500 to 5000 msec. The distractor images (book, fire truck, flag, jeep, moon, phone, scissors, sun, television, and tree) were shown for 1500 msec each with a randomly varying ISI of 500 to 5000 msec. In this condition, participants were to respond to the presentation of the image of a house by pressing a button (go trial). A failure to press the button within 2000 msec after the start of image presentation was coded as an attention error. They were to inhibit the response to the presentation of the distractor images (no-go trial). Pressing the button to a distractor image was coded as an inhibition error.
At nine and 11 years, the target image was a specific numeral (a “5” at age nine; a “6” at age 11) that was presented for 500 msec with a randomly varying ISI of 500 to 5000 msec. The timing of the presentation was reduced to 500 msec at nine and 11 years from the 1500 msec at age 6 in order to increase the demands of the task. The distractor images were other numbers also displayed for 500 msec with a varying ISI of 500 to 5000 msec. A failure to press the button within 1000 msec after the start of the image presentation was an attention error. Pressing the button to a distractor image was an inhibition error.
The experimenter instructed the participants and demonstrated the correct responses. Following successful practice trials, the stimuli were presented in three conditions. The first condition was a simple attention task in which all 20 trials were go trials, thereby setting up a prepotent response. If the child did not press the button within the permitted time (200 msec age 6; 1000 msec ages 9 and 11), repeated attempts were made to determine if the child learned this simple condition. The second condition was a distractor condition consisting of 80 trials (20 go-trials and 60 nogo-trials 1). In the distractor condition, participants were to respond to the presentation of the go image and not respond to the nogo images. The third condition was the inhibitory-response phase consisting of 80 trials. Participants were required to reverse their response pattern by inhibiting their response to the original go image (n = 20 trials) and instead respond to the presentation of any image except the previous go image (n = 60 trials).
2.1.4. Statistical analyses
The data collected in the first condition, the simple attention task, were used to screen participants for inclusion. We excluded those participant visits in which insufficient data were generated (e.g., instructions not followed after repeated attempts, subjects appeared asleep, or subjects were persistently delayed in responding within allotted time).
The two types of errors were attention errors (omissions), which were failures to respond to a go trial, and inhibitory errors (commissions), which were responses to a no-go trial. Error scores were converted to percentages to adjust for the variations in the number of trials. Both attention errors and inhibition errors were obtained across the combined trials of the distractor and reversal phases of the task. To begin the analysis an overall accuracy score across ages was obtained that combined both the attention and inhibitory trials. The 80 trials of the second condition and the 80 trials of the third condition were combined. The accuracy score was the percentage correct of the 160 trials in the task.
Polydrug exposure was measured by the amount of maternal use of alcohol, cigarettes, and marijuana during pregnancy. In order to reduce skewness of the substance exposure variables, maternal reports were transformed using natural logarithms. The covariates were natural log scale transformations of alcohol use, cigarette use, marijuana use and medical complications. The environmental risk was a T-scale transformation.
The effects of prenatal cocaine exposure and gender on accuracy were examined by a 2 X 3 mixed models analysis of covariance while controlling for the effects of polydrug exposure, medical complications and environmental risk [67]. This analysis (using SPSS, version 15.0 for Windows) was conducted on the 444 measures of overall accuracy. Missing data from missed sessions or from exclusion of visits due to poor performance was managed by the mixed model [77]. An individual does not need to be discarded if that individual missed some of the planned interviews. The mixed effects analysis uses all available data and finds model regression coefficients that maximize the likelihood of each individual’s observed data [77]. Essentially, via the mixed effects statistical model, gaps in the measurement of an individual at a given point are filled in from both data available for that individual at other time points, and from data available for other individuals who are not missing but are similar to individuals who are missing. Standard errors and confidence interval widths are sensitive to the total amount of data available, increasing with more missing data and decreasing with less missing data.
Another way of approaching a gender by exposure effect is by using hierarchical regression modeling [39]. Separate regression analyses were conducted for attention errors and for inhibition errors. Hierarchical linear regression analyses explored the relations of gender and exposure levels as they affect attention errors and inhibition errors while accounting for polydrug exposure, environmental risk, and medical complications. In the regression analyses, the change in R2 was examined at each stage. For both attention errors and inhibition errors, exposure level was entered in the first stage, followed by gender in the second stage. In the third stage, the polydrug exposure substances were entered, while environmental risk and medical complications were entered in the fourth stage.
3. Results
Table 1 presents the perinatal and demographic characteristics by cocaine exposure levels. Children who were prenatally exposed to cocaine were exposed to greater amounts of alcohol, cigarettes, and marijuana and had a greater number of neonatal medical complications. While there were no significant differences in environmental risk scores between unexposed and the entire group of cocaine-exposed children, the subgroup of heavily exposed children did have greater environmental risk scores.
Table 1.
Mean Levels of Drug Exposure and Risk by Cocaine Exposure Levels
| Measure | Unexposed (n = 120) | Exposed (n = 83) | Lightly Exposed (n = 38) | Heavily Exposed (n = 45) | F-test (2, 200) | significance |
|---|---|---|---|---|---|---|
| Prenatal Exposure to Alcohol (drinks per day) | 0.024 | 1.49 | 0.944** | 1.953*** | 19.832 | <.001 |
| Prenatal Exposure to Cigarettes (cigarettes per day) | 1.358 | 9.450 | 8.900*** | 9.918*** | 36.671 | <.001 |
| Prenatal Exposure to Marijuana (joints per day) | 0.022 | 0.315 | 0.101 | 0.497 *** | 5.306 | .006 |
| Medical Complications Score – ln scale | 0.115 | 0.248 | 0.263 ** | 0.234 ** | 6.991 | .001 |
| Environmental Risk Score – T scale | 49.732 | 51.453 | 49.508 | 53.107 * | 21.267 | .106 |
Note:
p < .05;
p < .01;
p < .001 relative to unexposed
Table 2 presents the number of lab visits by gender and exposure as well as the percentage of lab visits which generated usable data. As shown in Table 2, the heavily exposed males had an inclusion rate of 79% compared to inclusion rates for all other groups that range from 93 to 97%. This group difference was significant, χ2 (5) = 14.85, p < .01. Given that the expected values in some cells were less than 10, a conservative measure using Yates correction also yielded a significant effect, χ2 (5) = 11.76, p < .05. The results that follow reflect only those who completed the task. In the analysis to follow we first look at overall accuracy followed by scores for attention and inhibitory errors.
Table 2.
Number of Lab Visits by Gender and Exposure Levels
| Gender | Exposure | Number of Lab Visits | Visits Excluded | Percentage Included Visits |
|---|---|---|---|---|
| Female | Unexposed | 136 | 9 | 93.4% |
| Lightly Exposed | 48 | 2 | 96.0% | |
| Heavily Exposed | 57 | 4 | 92.9% | |
| Male | Unexposed | 151 | 10 | 93.4% |
| Lightly Exposed | 40 | 1 | 97.5% | |
| Heavily Exposed | 48 | 10 | 79.2% | |
| Total | 480 | 36 | 92.5% |
3.1. Predictors of accuracy
Given (1) that gender effects have been found throughout our study of this sample and we predicted them, and (2) perhaps more importantly, the covariates have differential effects when examined separately by gender, exposure effects were assessed separately by gender. For example, while prenatal exposure to cigarettes was related to inhibition errors for females (p < .05) it was not for males. Likewise with medical complications, the relation with attention errors was significant for females (p < .01) but not for males. Of importance to this paper was that levels of cocaine exposure were more strongly related to attention errors in males (p = .008) than in females (p = .53). Given these differential effects of the covariates by gender, the gender by exposure effects on attention and inhibition errors may have been diluted. For these reasons the analyses of errors were treated separately by gender.
Table 3 presents the overall accuracy score by exposure level for females and males after adjusting the scores for the covariates of polydrug exposure, medical complications and environmental risk. A mixed model two-way ANCOVA (analysis of covariance) examined the effects of gender and exposure level (3 levels; unexposed, lightly exposed, heavily exposed) across age on accuracy. Gender, F (1, 160.9) = 2.91, p = .090, exposure level, F (2, 167.6) = 2.38, p = .096, and their interaction, F (2, 167.6) = 2.69, p = .071, approached a significance level of less than .05. As seen in Table 3, the heavily exposed males had the lowest performance relative to all other gender by exposure level groups. Posthoc examination using the confidence intervals of the means for gender and exposure levels showed that exposure level affected females and males differently. Exposure levels had no effect on accuracy for females. In contrast, exposure levels affected overall accuracy for males. Overall accuracy was lower for the heavily exposed males (M = 80.95, SE = 2.12, 95% CI = 76.78 to 85.13) than for the unexposed males (M = 89.22, SE = 1.25, 95% CI = 86.75 to 91.69, p < .05), and for the lightly exposed males (M = 87.49, SE = 2.01, 95% CI = 83.51 to 91.47, p < .05). There were no differences in overall accuracy between the unexposed and the lightly exposed males.
Table 3.
Overall Accuracy (Attention and Inhibition) by Gender and Cocaine Exposure Levels
| Gender | Exposure Level | Mean | Std Error | df | 95% CI |
|---|---|---|---|---|---|
| Male | Unexposed | 89.22 | 1.25 | 161.10 | 86.75, 91.69 |
| Lightly Exp | 87.49 | 2.01 | 145.17 | 83.51, 91.47 | |
| Heavily Exposed | 80.95 | 2.12 | 196.00 | 76.78, 85.13 | |
| Female | Unexposed | 87.70 | 1.17 | 162.98 | 85.39, 90.02 |
| Lightly Exp | 87.44 | 1.80 | 169.43 | 83.90, 90.99 | |
| Heavily Exposed | 87.84 | 2.11 | 173.08 | 83.68, 91.00 |
Note: Means are adjusted after accounting for effects of covariates of medical complications, alcohol exposure, cigarette exposure, marijuana exposure, and environmental risk
3.2. Predictors of attention errors
Figure 1 presents the percentage of attention errors. The mixed model ANCOVA analysis, conducted separately for females and for males, showed the effects of cocaine exposure on attention errors while controlling for the contributions of polydrug exposure, medical complications, and environmental risk. Levels of cocaine exposure were not significantly associated with attention errors for females, F (2, 94.76) < 1.00, p = .776 in that there was little variation in errors between the unexposed, the lightly exposed and the heavily exposed females.
Figure 1.
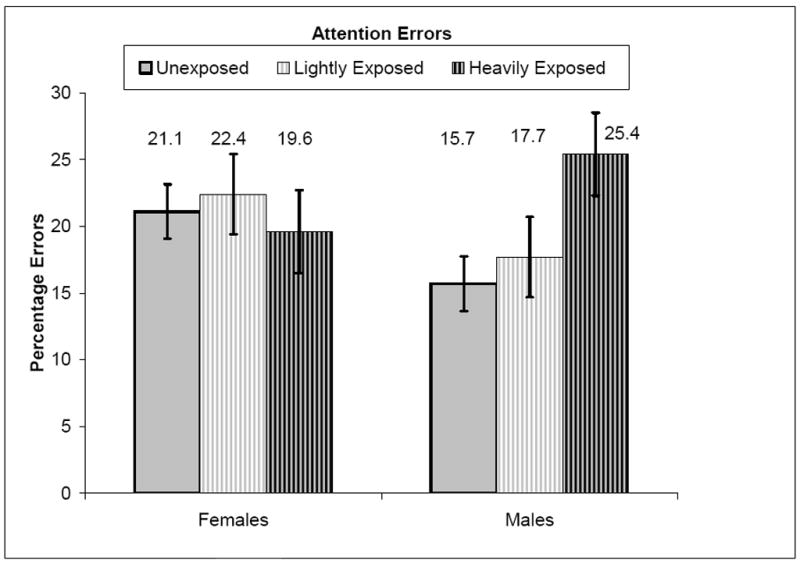
Percentage attention errors by gender and exposure level while controlling for polydrug exposure, medical complications, and environmental risk
In contrast to females, there were significant effects for males on attention errors due to cocaine exposure, F (2, 84.39) = 3.48, p < .05. Heavily exposed males had more attention errors (M = 25.44, SE = 3.11, 95% CI = 19.27 to 31.62) than did unexposed males (M = 15.73, SE= 1.64, 95% CI = 12.47 to 18.98), p < .02, and lightly exposed males (M = 17.71, SE = 3.01, 95% CI = 11.71 to 23.70), p < .05. There were no differences in attention errors between unexposed males and lightly exposed males.
3.3. Predictors of inhibition errors
Figure 2 presents the inhibition errors. The mixed model ANOVA analysis, conducted separately for females and for males, showed the effects of cocaine exposure on inhibition errors while controlling for the contributions of polydrug exposure, medical complications, and environmental risk. For females, there were no significant effects on inhibition errors for levels of cocaine exposure, F (2, 102.91) < 1.00, p = .703, with little variation in errors between unexposed, lightly and heavily exposed females. In contrast to females, males showed a different pattern for the effects of cocaine exposure on inhibition errors. For males, cocaine exposure was not significantly related to inhibition errors, F (2, 57.41) = 1.43, p = .248, although there was a trend in that heavily exposed males made more inhibition errors (M = 13.75, SE= 3.59, 95% CI = 6.59 to 20.92) than either lightly exposed males (M= 8.00, SE= 3.39, 95% CI= 1.18 to 14.82) or unexposed males (M = 6.61, SE = 1.86, 95% CI= 2.89 to 10.34).
Figure 2.
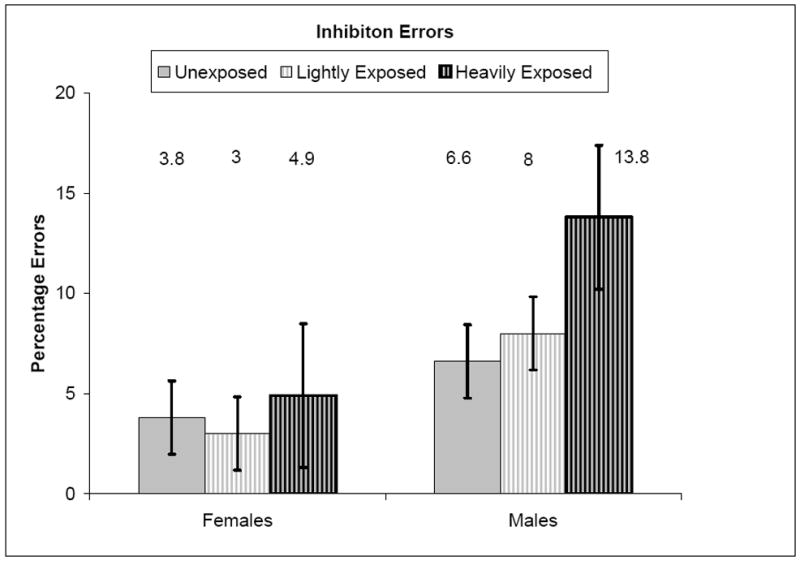
Percentage inhibitory errors by gender and exposure level while controlling for polydrug exposure, medical complications, and environmental risk.
3.4. Regression analyses of attention errors and inhibition errors
While the effects of the covariates were partialled out in the ANCOVA analyses, there was the possibility that the covariates had differential effects on trial types by gender. Therefore, the associations between the covariates and the types of errors, both attention and inhibition errors, were examined by gender. Table 4 presents the zero order interclass correlations between the dependent measures of attention errors and inhibition errors and the factor of exposure level and the covariates by gender. A notable gender specific finding was an association between cocaine levels and attention errors for males, not for females (p < .01). Medical complications were associated with both attention and inhibition errors for females, not for males (p < .05). Finally, prenatal exposure to cigarettes was related to inhibition errors for females, not for males, (p < .05).
Table 4.
The Zero-Order Interclass Correlations Between the Dependent Measures of Percentage Attention Errors and Percentage Inhibition Errors, Exposure Level and Covariates by Gender
| Attention Errors | Inhibition Errors | |||
|---|---|---|---|---|
| Variable | Females | Males | Females | Males |
| Cocaine Exposure Level: None, lightly, heavily | −.042 | .179** | .100 | −.007 |
| Prenatal Alcohol | .019 | .104 | .010 | −.085 |
| Prenatal Cigarette | .072 | .099 | .141 * | −.065 |
| Prenatal Marijuana | −.026 | .009 | .073 | −.097 |
| Medical Complications | .156 * | −.006 | .162 * | −.015 |
| Environmental Risk | .062 | −.103 | −.021 | −.017 |
Note:
p < .05,
p < .01
Table 5 presents the hierarchical standardized regression coefficients (β), the change in R2 for each stage, and the total model R2 for the prediction of attention errors and inhibition errors. For the analysis of attention errors, the final model significantly predicted attention errors, explaining 2% of the variance (p < .05). The model that was developed after Stage 1 showed that exposure level was significant, F (1, 442) = 5.49, p = .020, accounting for 1% of the variance. The addition of gender in Stage 2 led to a significant change, F (1, 441) = 4.97, p = .026, and accounted for an additional 1% of the variance. The models developed by adding polydrug exposure in Stage 3 (p = .683) and medical complications and environmental risk in Stage 4 (p = .288) did not improve the fit of the model. The most parsimonious model of attention errors included the predictors of cocaine exposure and gender. The greater the cocaine exposure, the greater the attention errors; male gender was associated with greater attention errors.
Table 5.
Hierarchical Regressions Predicting Attention Errors and Inhibition Errors
| Attention Errors | Inhibition Errors | |||||
|---|---|---|---|---|---|---|
| Predictor | Entry β | Stage β | Δ R2 | Entry β | Stage β | Δ R2 |
| Stage 1: Cocaine level | 0.111* | 0.012* | 0.018 | 0.033 | 0.000 | |
| Stage 2: Gender (1: male; 2:female) | 0.105* | 0.011* | −0.168*** | 0.028 | ||
| Cocaine level | 0.101* | 0.033 | ||||
| Stage 3: Polydrug exposure | ||||||
| Cigarette | 0.036 | 0.003 | 0.003 | 0.005 | ||
| Alcohol | −0.026 | −0.085 | ||||
| Marijuana | −0.052 | −0.017 | ||||
| Cocaine level | 0.109 | 0.086 | ||||
| Gender | 0.110* | −0.161*** | ||||
| Stage 4: Risks | ||||||
| Medical Complications | 0.073 | 0.006 | 0.062 | 0.004 | ||
| Environmental risk | −0.035 | −0.028 | ||||
| Cigarette | 0.021 | −0.010 | ||||
| Alcohol | −0.037 | −0.094 | ||||
| Marijuana | −0.054 | −0.019 | ||||
| Cocaine level | 0.113 | 0.089 | ||||
| Gender | 0.107* | −0.164*** | ||||
| Total model R2 | 0.032* | 0.037* | ||||
Note:
p < .05,
p <.001
For the analysis of inhibition errors, the final model included only gender, F (1, 442) = 12.32, p <.001. The model that was developed after Stage 1 showed that exposure level was not significant, F (1, 442) < 1.0, p = .703. The addition of gender in Stage 2 led to a significant change in F (1, 441) = 12.65, p < .001 and accounted for 2% of the variance. The models developed by adding polydrug exposure in Stage 3 (p =.548) and medical complications and environmental risk in Stage 4 (p = .410) did not improve the fit of the model. The most parsimonious model of inhibition errors included only gender as a predictor. Male gender was associated with greater inhibition errors.
5. Discussion
This study examined the effects of prenatal cocaine exposure on children’s attention and inhibitory control abilities at ages six, nine and 11. The task required children to remember instructions and to inhibit a prepotent response. Since there are many factors that contribute to the cognitive processes of attention and inhibition, polydrug prenatal exposure, neonatal medical complications, and environmental risk were controlled statistically. After controlling for these factors, cocaine exposure predicted both attention and inhibition, depending on gender. Specifically, cocaine-exposed males relative to unexposed males were less capable of completing the task, made more attention errors and made more inhibitory errors. These effects on performance were not evident for females, as prenatal cocaine exposure was not associated with attention or inhibition errors for females.
The effects of prenatal cocaine exposure on attention and inhibition are dose related. Heavily exposed males show the poorest performance relative to unexposed males and to lightly exposed males. Looking at task completion rates, the heavily exposed males had a completion rate of 79% compared to 93% for lightly exposed males and 97% for unexposed males. This is in contrast to completion rates of 93% for heavily exposed females. Clearly heavy exposure to prenatal cocaine removed a disproportionate number of male subjects from the subsequent analyses. Even without these poorest performing subjects, however, the overall accuracy for heavily exposed males was significantly reduced compared to lightly exposed males and unexposed males. If the subjects who were excluded were to be retained in the subsequent analyses, the effects of prenatal cocaine exposure by gender may have been even stronger as cocaine exposed boys were disproportionately among those whose data we excluded. The effects of cocaine exposure on attention and inhibitory control are consistent with prior reports [1,6,10,12,47,57,62]. In addition, males are more impacted than females, which is consistent with the gender specific impact of prenatal cocaine exposure shown in previous research with this cohort of children [9,13–15,25] as well as the work of others [5,68].
There are several possible explanations for the gender effects given the effects of prenatal exposure on brain development observed in animals and child neuroimaging studies. The brain structures that are activated in the tasks of attention and inhibition are among the structures compromised by prenatal exposure in animals. In addition prenatal cocaine exposure exerts deleterious effects on cerebral cortical development possibly by decreasing GABAergic neuronal migration from the ganglionic eminence to the cerebral wall, which then contributes to persistent structural and functional deficits observed in the exposed offspring [23]. Complementing these findings, in-vitro studies of the effects of prenatal cocaine exposure have shown that cocaine had direct dose-related inhibitory effects on brain cell differentiation [78].
Behavioral studies of animals also have shown the effects of prenatal cocaine exposure on attention, particularly in males. Rats were examined for the effects of exposure on learning in 2-choice and 3-choice paradigms [32]. Males were more affected by exposure than females on the 3-choice task implicating selective attention as the basis of impairment. Dose related attention errors in selective attention were found for males, not females, with greater errors associated with heavier exposure [34]. When omission errors are examined in rat studies, females are not affected by the dose of cocaine exposure; however, male rats are affected in that the more heavily exposed males made more omission errors than controls [35]. Cocaine-exposed male rats perform worse on selective attention tasks [32], which may be related to greater alterations in dopaminergic and adrenergic receptor binding in hippocampus, striatum and anterior cingulate regions [30,66] as well as reduced metabolic activity in the limbic system [26,27].
Looking at in-vivo animal models, prenatal cocaine exposure affects the dopamine receptors in the striatum of mice [74]. Specifically, there was an enhanced D1 dopamine receptor agonist-induced cAMP response in the striatum of adult male, but not female mice prenatally exposed to cocaine compared to controls. Effects on hippocampal pyramidal cells and granule cells have been shown in male rats with exposure to cocaine during the late gestation and early postnatal periods [41]. Also in rats, gender specific effects of exposure have been reported indicating that males are more affected than females. In one study, a gender-specific expression of cocaine-mediated alterations was displayed in the norepinephrine systems in adolescent rats that may be restricted to particular brain regions, and thus not reflect global brain alterations [17]. In another study, prenatal cocaine exposure reduced basal dopamine release from striata of juvenile male rats; this was not shown by juvenile female rats, with the trend continuing into adulthood [36]. Moreover, there are gender differences in the development of D1 receptors in exposed rats [30]. Specifically, there are sex-mediated alterations in D1 receptor binding in rats exposed to cocaine in utero suggesting that exposure differentially alters the DA systems that underlie sustained attention. These effects of exposure have also been shown in rabbits, where exposure to cocaine during time of peak corticogenesis produced long-term effects on the organization of neurons and interneurons in the anterior cingulate cortex [70].
Finally, neuroimaging studies of children exposed prenatally to cocaine have revealed some of the effects of exposure on brain structure and function. A diffusion tensor imaging (DTI) study of frontal brain regions has shown the effect of cocaine exposure on the development of white matter pathways [75]. The findings included subtle microstructural changes that suggested less mature development of frontal white matter pathways. The findings of under development in frontal regions were associated with poorer performance on tasks of executive functioning. The effects of exposure on brain functioning were demonstrated in an fMRI study of children [65]. Cocaine-exposed children showed greater activation in the right inferior frontal cortex and caudate during response inhibition, whereas non-exposed children showed greater activations in temporal and occipital regions. In a study of emotion regulation of gender and exposure effects using fMRI, children saw strong aversive stimuli and rated their experiences [79]. Exposure had the greatest impact on males. Specifically, relative to the unexposed males, the exposed males had the lower ratings of emotional reactions to the aversive stimuli and greater brain activation in ventro-medial prefrontal cortex. Exposed females relative to unexposed females did not show these effects. Both exposed females and exposed males showed less activation than unexposed males and unexposed females in hippocampal regions. Thus, there are effects of prenatal cocaine exposure, some of which are gender specific.
The presence of attention and inhibitory control deficits among cocaine exposed children suggests that they may be at risk for poor academic achievement and psychosocial adjustment in childhood and into adolescence [54]. Children and adolescents with poor performance on sustained attention and inhibitory control tasks such as those administered in the current study have poorer academic achievement and more social problems, substance use problems, and antisocial behavior [4,22,37,56,60]. For example, reading ability was found to be associated with inattention on a continuous performance task in pre-adolescent children [37]. Executive function abilities, including attention, have been shown to be associated with the adaptive behavior, communication, and socialization domains on the Vineland Adaptive Behavior Scale [22]. Executive control of attention is associated with self regulation, in that greater self regulation leads to greater resistance to the influence of a deviant peer and the development of antisocial behavior in adolescents [33]. Furthermore, poor response inhibition in childhood predicted the onset of alcohol use-related problems as well as illicit drug use in adolescents independent of family risk factors, with higher risk among children from alcohol-abusing families than nonalcoholic control families [56]. In addition, impaired inhibitory control in childhood is present in the clinical picture of adolescent substance abuse disorders [42]. Finally, neurocognitive impairments have been shown to be associated with antisocial behavior in adolescents [60]. If the attention and inhibitory control deficits are found prior to such outcomes in adolescence, then the early identification and implementation of interventions for these deficits may be helpful in enhancing future adjustment.
The present study has several strengths given that it controlled for potential confounding effects of environmental risk, medical complications, gender and prenatal exposure to other drugs. Nonetheless, several limitations deserve mention. Our findings are specific to the Yale Child Study Center Attention Task and need to be extended using other assessments of attention and inhibitory control. Second, while this study shows that attention and inhibitory deficits due to prenatal cocaine exposure continue into middle childhood, it remains to be seen whether these effects continue into adolescence, a time of increased risk taking behavior [71]. Third, this study was conducted with a low income, urban, predominantly African American sample and the findings may not necessarily generalize to other samples. Finally, while the present study controlled for a variety of environmental and neonatal medical risk factors, it is certainly likely that other unmeasured factors affect children’s development. For example, lead exposure has been shown to negatively affect drug exposed children’s cognitive function [53], although the effects of cocaine exposure on attention and other cognitive domains remain after controlling for lead exposure [6,55,69].
While different brain functions due to prenatal cocaine exposure are likely to have some effect on attention and inhibition, it is known that there are significant effects due to environmental risk, and perinatal medical complications [3,11,13,14,45]. In fact, environmental risk and perinatal medical complications exert differential effects on attention in children born premature, without exposure to cocaine [21]. Unless the other highly correlated effects of exposure to other substances, environmental risk, and perinatal medical complications are partialled out, the brain differences in structure and function cannot be assigned solely to the effects of prenatal cocaine exposure. Nevertheless, given that cocaine exposure appears to affect both attention and inhibition, with the effect stronger for males than for females, there exists the possibility there are differences in the impact of brain development due to exposure by gender. What would be required would be to look at gender differences at many levels, including in-vitro, behavioral, and neuroimaging levels; however, caution must be taken to remove the highly correlated effects of environmental risk and medical complications.
Acknowledgments
Portions of this research were supported by grant USPHS R01-DA07109 from the National Institute on Drug Abuse to Michael Lewis, David S. Bennett, and Dennis P. Carmody. The sponsor agency had no involvement in the study design, collection, analysis, and interpretation of data; writing of this report; or in the decision to submit this report for publication.
Footnotes
Financial disclosure: The authors reported no financial interests or conflicts of interest.
The Yale Attention Task has a variable number of no-go trials in the distractor condition that ranges from 60 to 66. The variation in the number of trial types is inherent in the program that allowed quasi-randomization of the trial types. Given that the total number of trials, by adding together go and nogo trials, varied over participants from 80 to 86, the dependent measure of performance was obtained as the percentage of trials with correct responses.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Accornero VH, Amado AJ, Morrow CE, Xue L, Anthony JC, Bandstra ES. Impact of prenatal cocaine exposure on attention and response inhibition as assessed by continuous performance tests. J Dev Behav Pediatr. 2007;28:195–205. doi: 10.1097/01.DBP.0000268560.72580.f9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ackerman JP, Riggins T, Black MM. A review of the effects of prenatal cocaine exposure among school-aged children. Pediatrics. 2010;125:554–65. doi: 10.1542/peds.2009-0637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alessandri SM, Bendersky M, Lewis M. Cognitive functioning in 8- to 18-month-old drug-exposed infants. Dev Psychol. 1998;34:565–73. doi: 10.1037//0012-1649.34.3.565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Andrade BF, Brodeur DA, Waschbusch DA, Stewart SH, McGee R. Selective and sustained attention as predictors of social problems in children with typical and disordered attention abilities. J Atten Disord. 2009;12:341–52. doi: 10.1177/1087054708320440. [DOI] [PubMed] [Google Scholar]
- 5.Bailey BN, Sood BG, Sokol RJ, Ager J, Janisse J, Hannigan JH, Covington C, Delaney-Black V. Gender and alcohol moderate prenatal cocaine effects on teacher-report of child behavior. Neurotoxicol Teratol. 2005;27:181–189. doi: 10.1016/j.ntt.2004.10.004. [DOI] [PubMed] [Google Scholar]
- 6.Bandstra ES, Morrow CE, Anthony JC, Accornero VH, Fried PA. Longitudinal investigation of task persistence and sustained attention in children with prenatal cocaine exposure. Neurotoxicol Teratol. 2001;23:545–59. doi: 10.1016/s0892-0362(01)00181-7. [DOI] [PubMed] [Google Scholar]
- 7.Bendersky M, Alessandri S, Gilbert P, Lewis M. Characteristics of pregnant substance abusers in two cities in the northeast. Am J Drug Alcohol Abuse. 1996;22:349–62. doi: 10.3109/00952999609001664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bendersky M, Alessandri SM, Sullivan MW, Lewis M. Measuring the effects of prenatal cocaine exposure. In: Lewis M, Bendersky M, editors. Mothers, babies, and cocaine: The role of toxins in development. Erlbaum; Hillsdale, NJ: 1995. pp. 163–178. [Google Scholar]
- 9.Bendersky M, Bennett DS, Lewis M. Aggression at age 5 as a function of prenatal exposure to cocaine, gender, and environmental risk. J Pediatr Psychol. 2006;31:71–84. doi: 10.1093/jpepsy/jsj025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bendersky M, Gambini G, Lastella A, Bennett DS, Lewis M. Inhibitory motor control at five years as a function of prenatal cocaine exposure. J Dev Behav Pediatr. 2003;24:345–51. doi: 10.1097/00004703-200310000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bendersky M, Lewis M. Arousal modulation in cocaine-exposed infants. Dev Psychol. 1998;34:555–64. [PMC free article] [PubMed] [Google Scholar]
- 12.Bendersky M, Lewis M. Prenatal cocaine exposure and impulse control at two years. Ann N Y Acad Sci. 1998;846:365–367. [PubMed] [Google Scholar]
- 13.Bennett DS, Bendersky M, Lewis M. Children's cognitive ability from 4 to 9 years old as a function of prenatal cocaine exposure, environmental risk, and maternal verbal intelligence. Dev Psychol. 2008;44:919–28. doi: 10.1037/0012-1649.44.4.919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bennett DS, Bendersky M, Lewis M. Children's intellectual and emotional-behavioral adjustment at 4 years as a function of cocaine exposure, maternal characteristics, and environmental risk. Dev Psychol. 2002;38:648–58. doi: 10.1037//0012-1649.38.5.648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bennett DS, Bendersky M, Lewis M. Preadolescent health risk behavior as a function of prenatal cocaine exposure and gender. J Dev Behav Pediatr. 2007;28:467–72. doi: 10.1097/DBP.0b013e31811320d8. [DOI] [PubMed] [Google Scholar]
- 16.Booth JR, Burman DD, Meyer JR, Lei Z, Trommer BL, Davenport ND, Li W, Parrish TB, Gitelman DR, Mesulam MM. Neural development of selective attention and response inhibition. Neuroimage. 2003;20:737–51. doi: 10.1016/S1053-8119(03)00404-X. [DOI] [PubMed] [Google Scholar]
- 17.Booze RM, Wallace DR, Silvers JM, Strupp BJ, Snow DM, Mactutus CF. Prenatal cocaine exposure alters alpha2 receptor expression in adolescent rats. BMC Neurosci. 2006;7:33. doi: 10.1186/1471-2202-7-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Buchsbaum BR, Greer S, Chang WL, Berman KF. Meta-analysis of neuroimaging studies of the Wisconsin card-sorting task and component processes. Hum Brain Mapp. 2005;25:35–45. doi: 10.1002/hbm.20128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bunge SA, Dudukovic NM, Thomason ME, Vaidya CJ, Gabrieli JD. Immature frontal lobe contributions to cognitive control in children: evidence from fMRI. Neuron. 2002;33:301–11. doi: 10.1016/s0896-6273(01)00583-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Campbell E, Sanson-Fisher R, Walsh R. Smoking status in pregnant women assessment of self-report against carbon monoxide (CO) Addict Behav. 2001;26:1–9. doi: 10.1016/s0306-4603(00)00070-8. [DOI] [PubMed] [Google Scholar]
- 21.Carmody DP, Bendersky M, Dunn SM, DeMarco JK, Hegyi T, Hiatt M, Lewis M. Early risk, attention, and brain activation in adolescents born preterm. Child Dev. 2006;77:384–94. doi: 10.1111/j.1467-8624.2006.00877.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Clark C, Prior M, Kinsella G. The relationship between executive function abilities, adaptive behaviour, and academic achievement in children with externalising behaviour problems. J Child Psychol Psychiatry. 2002;43:785–96. doi: 10.1111/1469-7610.00084. [DOI] [PubMed] [Google Scholar]
- 23.Crandall JE, Hackett HE, Tobet SA, Kosofsky BE, Bhide PG. Cocaine exposure decreases GABA neuron migration from the ganglionic eminence to the cerebral cortex in embryonic mice. Cereb Cortex. 2004;14:665–75. doi: 10.1093/cercor/bhh027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Delaney-Black V, Covington C, Nordstrom B, Ager J, Janisse J, Hannigan JH, Chiodo L, Sokol RJ. Prenatal cocaine: Quantity of exposure and gender moderation. J Dev Behav Pediatr. 2004;25:254–263. doi: 10.1097/00004703-200408000-00005. [DOI] [PubMed] [Google Scholar]
- 25.Dennis T, Bendersky M, Ramsay D, Lewis M. Reactivity and regulation in children prenatally exposed to cocaine. Dev Psychol. 2006;42:688–97. doi: 10.1037/0012-1649.42.4.688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dow-Edwards DL, Freed-Malen LA, Gerkin LM. Sexual dimorphism in the brain metabolic response to prenatal cocaine exposure. Brain Res Dev Brain Res. 2001;129:73–9. doi: 10.1016/s0165-3806(01)00184-5. [DOI] [PubMed] [Google Scholar]
- 27.Dow-Edwards DL, Freed LA, Fico TA. Structural and functional effects of prenatal cocaine exposure in adult rat brain. Brain Res Dev Brain Res. 1990;57:263–8. doi: 10.1016/0165-3806(90)90052-z. [DOI] [PubMed] [Google Scholar]
- 28.Durston S, Thomas KM, Yang Y, Ulug AM, ZRD, CBJ A neural basis for the development of inhibitory control. Dev Sci. 2002;5:F9–F16. [Google Scholar]
- 29.Farah MJ, Shera DM, Savage JH, Betancourt L, Giannetta JM, Brodsky NL, Malmud EK, Hurt H. Childhood poverty: specific associations with neurocognitive development. Brain Res. 2006;1110:166–74. doi: 10.1016/j.brainres.2006.06.072. [DOI] [PubMed] [Google Scholar]
- 30.Ferris MJ, Mactutus CF, Silvers JM, Hasselrot U, Beaudin SA, Strupp BJ, Booze RM. Sex mediates dopamine and adrenergic receptor expression in adult rats exposed prenatally to cocaine. Int J Dev Neurosci. 2007;25:445–54. doi: 10.1016/j.ijdevneu.2007.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Garavan H, Hester R, Murphy K, Fassbender C, Kelly C. Individual differences in the functional neuroanatomy of inhibitory control. Brain Res. 2006;1105:130–42. doi: 10.1016/j.brainres.2006.03.029. [DOI] [PubMed] [Google Scholar]
- 32.Garavan H, Morgan RE, Mactutus CF, Levitsky DA, Booze RM, Strupp BJ. Prenatal cocaine exposure impairs selective attention: evidence from serial reversal and extradimensional shift tasks. Behav Neurosci. 2000;114:725–38. [PubMed] [Google Scholar]
- 33.Gardner TW, Dishion TJ, Connell AM. Adolescent self-regulation as resilience: resistance to antisocial behavior within the deviant peer context. J Abnorm Child Psychol. 2008;36:273–84. doi: 10.1007/s10802-007-9176-6. [DOI] [PubMed] [Google Scholar]
- 34.Gendle MH, Strawderman MS, Mactutus CF, Booze RM, Levitsky DA, Strupp BJ. Impaired sustained attention and altered reactivity to errors in an animal model of prenatal cocaine exposure. Brain Res Dev Brain Res. 2003;147:85–96. doi: 10.1016/j.devbrainres.2003.10.002. [DOI] [PubMed] [Google Scholar]
- 35.Gendle MH, White TL, Strawderman M, Mactutus CF, Booze RM, Levitsky DA, Strupp BJ. Enduring effects of prenatal cocaine exposure on selective attention and reactivity to errors: evidence from an animal model. Behav Neurosci. 2004;118:290–7. doi: 10.1037/0735-7044.118.2.290. [DOI] [PubMed] [Google Scholar]
- 36.Glatt SJ, Trksak GH, Cohen OS, Simeone BP, Jackson D. Prenatal cocaine exposure decreases nigrostriatal dopamine release in vitro: effects of age and sex. Synapse. 2004;53:74–89. doi: 10.1002/syn.20036. [DOI] [PubMed] [Google Scholar]
- 37.Halperin JM, Sharma V, Greenblatt E, Schwartz ST. Assessment of the continuous performance test: Reliability and validity in a nonreferred sample. Psychol Assessment. 1991;3:603–608. [Google Scholar]
- 38.Hampshire A, Chamberlain SR, Monti MM, Duncan J, Owen AM. The role of the right inferior frontal gyrus: inhibition and attentional control. Neuroimage. 2010;50:1313–9. doi: 10.1016/j.neuroimage.2009.12.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hays WL. Statistics. 5. Holt, Rinehart, & Winston; Fort Worth, TX: 1994. [Google Scholar]
- 40.Hobel CJ, Hyvarinen MA, Okada DM, Oh W. Prenatal and intrapartum high-risk screening. I. Prediction of the high-risk neonate. Am J Obstet Gynecol. 1973;117:1–9. doi: 10.1016/0002-9378(73)90720-5. [DOI] [PubMed] [Google Scholar]
- 41.Ismail ZI, Bedi KS. Rats exposed to cocaine during late gestation and early postnatal life show deficits in hippocampal pyramidal and granule cells in later life. J Anat. 2007;210:749–60. doi: 10.1111/j.1469-7580.2007.00735.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ivanov I, Schulz KP, London ED, Newcorn JH. Inhibitory control deficits in childhood and risk for substance use disorders: a review. Am J Drug Alcohol Abuse. 2008;34:239–58. doi: 10.1080/00952990802013334. [DOI] [PubMed] [Google Scholar]
- 43.Jacobson JL, Jacobson SW. Methodological considerations in behavioral toxicology in infants and children. Dev Psychol. 1996;32:390–403. [Google Scholar]
- 44.Kelly AM, Hester R, Murphy K, Javitt DC, Foxe JJ, Garavan H. Prefrontal-subcortical dissociations underlying inhibitory control revealed by event-related fMRI. Eur J Neurosci. 2004;19:3105–12. doi: 10.1111/j.0953-816X.2004.03429.x. [DOI] [PubMed] [Google Scholar]
- 45.Kestler LP, Bennett DS, Carmody DP, Lewis M. Gender dependent effects of prenatal cocaine exposure. In: Lewis M, Kestler LP, editors. Gender differences in effects of prenatal substance exposure. American Psychological Association; Washington, DC: 2011. [Google Scholar]
- 46.Kok A, Ridderinkhof KR, Ullsperger M. The control of attention and actions: current research and future developments. Brain Res. 2006;1105:1–6. doi: 10.1016/j.brainres.2006.03.027. [DOI] [PubMed] [Google Scholar]
- 47.Leech SL, Richardson GA, Goldschmidt L, Day NL. Prenatal substance exposure: effects on attention and impulsivity of 6-year-olds. Neurotoxicol Teratol. 1999;21:109–18. doi: 10.1016/s0892-0362(98)00042-7. [DOI] [PubMed] [Google Scholar]
- 48.Logan GD, Cowan WB, Davis KA. On the ability to inhibit simple and choice reaction time responses: a model and a method. J Exp Psychol Hum Percept Perform. 1984;10:276–91. doi: 10.1037//0096-1523.10.2.276. [DOI] [PubMed] [Google Scholar]
- 49.Luna B, Padmanabhan A, O'Hearn K. What has fMRI told us about the development of cognitive control through adolescence? Brain Cogn. 2010;72:101–13. doi: 10.1016/j.bandc.2009.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Markowski VP, Cox C, Weiss B. Prenatal cocaine exposure produces gender-specific motor effects in aged rats. Neurotoxicol Teratol. 1998;20:43–53. doi: 10.1016/s0892-0362(97)00076-7. [DOI] [PubMed] [Google Scholar]
- 51.Mayes LC, Grillon C, Granger R, Schottenfeld R. Regulation of arousal and attention in preschool children exposed to cocaine prenatally. Ann N Y Acad Sci. 1998;846:126–43. doi: 10.1111/j.1749-6632.1998.tb09731.x. [DOI] [PubMed] [Google Scholar]
- 52.Mayes LC, Molfese DL, Key AP, Hunter NC. Event-related potentials in cocaine-exposed children during a Stroop task. Neurotoxicol Teratol. 2005;27:797–813. doi: 10.1016/j.ntt.2005.05.011. [DOI] [PubMed] [Google Scholar]
- 53.Min MO, Singer LT, Kirchner HL, Minnes S, Short E, Hussain Z, Nelson S. Cognitive development and low-level lead exposure in poly-drug exposed children. Neurotoxicol Teratol. 2009;31:225–31. doi: 10.1016/j.ntt.2009.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Minnes S, Singer LT, Kirchner HL, Short E, Lewis B, Satayathum S, Queh D. The effects of prenatal cocaine-exposure on problem behavior in children 4–10 years. Neurotoxicol Teratol. 2010 doi: 10.1016/j.ntt.2010.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Morrow CE, Vogel AL, Anthony JC, Ofir AY, Dausa AT, Bandstra ES. Expressive and receptive language functioning in preschool children with prenatal cocaine exposure. J Pediatr Psychol. 2004;29:543–54. doi: 10.1093/jpepsy/jsh056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Nigg JT, Wong MM, Martel MM, Jester JM, Puttler LI, Glass JM, Adams KM, Fitzgerald HE, Zucker RA. Poor response inhibition as a predictor of problem drinking and illicit drug use in adolescents at risk for alcoholism and other substance use disorders. J Am Acad Child Adolesc Psychiatry. 2006;45:468–475. doi: 10.1097/01.chi.0000199028.76452.a9. [DOI] [PubMed] [Google Scholar]
- 57.Noland JS, Singer LT, Short EJ, Minnes S, Arendt RE, Kirchner HL, Bearer C. Prenatal drug exposure and selective attention in preschoolers. Neurotoxicol Teratol. 2005;27:429–38. doi: 10.1016/j.ntt.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 58.Norbeck JS, Lindsey AM, Carrieri VL. The development of an instrument to measure social support. Nurs Res. 1981;30:264–9. [PubMed] [Google Scholar]
- 59.Orr ST, James SA, Casper R. Psychosocial stressors and low birth weight: development of a questionnaire. J Dev Behav Pediatr. 1992;13:343–7. [PubMed] [Google Scholar]
- 60.Raine A, Moffitt TE, Caspi A, Loeber R, Stouthamer-Loeber M, Lynam D. Neurocognitive impairments in boys on the life-course persistent antisocial path. J Abnorm Psychol. 2005;114:38–49. doi: 10.1037/0021-843X.114.1.38. [DOI] [PubMed] [Google Scholar]
- 61.Richardson GA, Conroy ML, Day NL. Prenatal cocaine exposure: effects on the development of school-age children. Neurotoxicol Teratol. 1996;18:627–34. doi: 10.1016/s0892-0362(96)00121-3. [DOI] [PubMed] [Google Scholar]
- 62.Rose-Jacobs R, Waber D, Beeghly M, Cabral H, Appugleise D, Heeren T, Marani J, Frank DA. Intrauterine cocaine exposure and executive functioning in middle childhood. Neurotoxicol Teratol. 2009;31:159–68. doi: 10.1016/j.ntt.2008.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rubia K, Smith AB, Woolley J, Nosarti C, Heyman I, Taylor E, Brammer M. Progressive increase of frontostriatal brain activation from childhood to adulthood during event-related tasks of cognitive control. Hum Brain Mapp. 2006;27:973–93. doi: 10.1002/hbm.20237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Savage J, Brodsky NL, Malmud E, Giannetta JM, Hurt H. Attentional functioning and impulse control in cocaine-exposed and control children at age ten years. J Dev Behav Pediatr. 2005;26:42–7. [PubMed] [Google Scholar]
- 65.Sheinkopf SJ, Lester BM, Sanes JN, Eliassen JC, Hutchison ER, Seifer R, Lagasse LL, Durston S, Casey BJ. Functional MRI and response inhibition in children exposed to cocaine in utero. Preliminary findings. Dev Neurosci. 2009;31:159–66. doi: 10.1159/000207503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Silvers JM, Wallace DR, Harrod SB, Mactutus CF, Booze RM. Prenatal cocaine alters dopamine and sigma receptor binding in nucleus accumbens and striatum in dams and adolescent offspring. Neurotoxicol Teratol. 2006;28:173–80. doi: 10.1016/j.ntt.2006.01.009. [DOI] [PubMed] [Google Scholar]
- 67.Singer JD, Willett JB. Applied longitudinal data analysis: Modeling change and event occurrence. Oxford University Press; New York: 2003. [Google Scholar]
- 68.Singer LT, Minnes S, Short E, Arendt R, Farkas K, Lewis B, Klein N, Russ S, Min MO, Kirchner HL. Cognitive outcomes of preschool children with prenatal cocaine exposure. JAMA. 2004;291:2448–2456. doi: 10.1001/jama.291.20.2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Singer LT, Nelson S, Short E, Min MO, Lewis B, Russ S, Minnes S. Prenatal cocaine exposure: drug and environmental effects at 9 years. J Pediatr. 2008;153:105–11. doi: 10.1016/j.jpeds.2008.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Stanwood GD, Washington RA, Levitt P. Identification of a sensitive period of prenatal cocaine exposure that alters the development of the anterior cingulate cortex. Cereb Cortex. 2001;11:430–40. doi: 10.1093/cercor/11.5.430. [DOI] [PubMed] [Google Scholar]
- 71.Steinberg L. Risk taking in adolescence: what changes, and why? Ann N Y Acad Sci. 2004;1021:51–8. doi: 10.1196/annals.1308.005. [DOI] [PubMed] [Google Scholar]
- 72.Streissguth AP, Sampson PD, Olson HC, Bookstein FL, Barr HM, Scott M, Feldman J, Mirsky AF. Maternal drinking during pregnancy: attention and short-term memory in 14-year-old offspring--a longitudinal prospective study. Alcohol Clin Exp Res. 1994;18:202–18. doi: 10.1111/j.1530-0277.1994.tb00904.x. [DOI] [PubMed] [Google Scholar]
- 73.Tamm L, Menon V, Reiss AL. Maturation of brain function associated with response inhibition. J Am Acad Child Adolesc Psychiatry. 2002;41:1231–1238. doi: 10.1097/00004583-200210000-00013. [DOI] [PubMed] [Google Scholar]
- 74.Tropea TF, Guerriero RM, Willuhn I, Unterwald EM, Ehrlich ME, Steiner H, Kosofsky BE. Augmented D1 dopamine receptor signaling and immediate-early gene induction in adult striatum after prenatal cocaine. Biol Psychiatry. 2008;63:1066–74. doi: 10.1016/j.biopsych.2007.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Warner TD, Behnke M, Eyler FD, Padgett K, Leonard C, Hou W, Garvan CW, Schmalfuss IM, Blackband SJ. Diffusion tensor imaging of frontal white matter and executive functioning in cocaine-exposed children. Pediatrics. 2006;118:2014–24. doi: 10.1542/peds.2006-0003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wood RD, Spear LP. Prenatal cocaine alters social competition of infant, adolescent, and adult rats. Behav Neurosci. 1998;112:419–31. doi: 10.1037//0735-7044.112.2.419. [DOI] [PubMed] [Google Scholar]
- 77.Wothke W. Longitudinal and multigroup modeling with missing data. In: Little TD, Schnabel KU, Baumert J, editors. Modeling longitudinal and multilevel data: Practical issues, applied approaches and specific examples. Lawrence Erlbaum; Mawah, NJ: 2000. pp. 219–240. [Google Scholar]
- 78.Zachor D, Cherkes JK, Fay CT, Ocrant I. Cocaine differentially inhibits neuronal differentiation and proliferation in vitro. J Clin Invest. 1994;93:1179–85. doi: 10.1172/JCI117071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Zhang Y, Carmody DP, Dettwiler A, Lewis M. Decreased neural activation in prenatal cocaine-exposed children during emotional processing: An fMRI study. Neuroimage. 2009;47(Supplement 1):181. [Google Scholar]


