Abstract
Coronary artery ectasia (CAE) is generally diagnosed in patients undergoing arteriography for presumptive atherosclerotic coronary artery disease. CAE is commonly considered as a variant of atherosclerotic disease; however, recent studies suggest that CAE is the result of a systemic vascular disorder. There is increasing evidence that aneurysmal vascular disease is a systemic disorder characterized by enhanced expression of pro-inflammatory cytokines and increased synthesis of enzymes capable of degrading elastin and other components of the vascular wall. Matrix metalloproteinase-2 degrades a number of extracellular substrates, including elastin and has been shown to play a critical role in the development of abdominal aortic aneurysms. This study characterizes the development of CAE in a unique murine transgenic model with cardiac-specific expression of active MMP-2. Transgenic mice were engineered to express an active form of MMP-2 under control of the α-myosin heavy chain promoter. Coronary artery diameters were quantified, along with studies of arterial structure, elastin integrity and vascular expression of the MMP-2 transgene. Latex casts quantified total coronary artery volumes and arterial branching. Mid-ventricular coronary luminal areas were increased in the MMP-2 transgenics, coupled with foci of aneurysmal dilation, ectasia and perivascular fibrosis. There was no evidence for atherogenesis. Coronary vascular elastin integrity was compromised and coupled with inflammatory cell infiltration. Latex casts of the coronary arteries displayed ectasia with fusiform dilatation. The MMP-2 transgenic closely replicates human CAE and supports a critical and initiating role for this enzyme in the pathogenesis of this disorder.
Keywords: aneurysm, coronary artery ectasia, elastin, matrix metalloproteinase-2, transgenic
Coronary artery ectasia (CAE) refers to a localized or diffuse dilatation of the epicardial coronary arteries exceeding 1.5-fold of the luminal area of adjacent normal coronaries. The condition is usually unsuspected until performance of coronary arteriography in patients presenting with signs or symptoms of atherosclerotic coronary artery disease (Manginas & Cokkino 2006). There is a rather wide reported range of prevalence of CAE (1.2–4.9%), with the highest value of 4.9% reported in the Coronary Artery Surgery Study registry (Swaye et al. 1983; Rath et al. 1985). CAE has been generally regarded as a severe variant of coronary atherosclerotic disease (Swaye et al. 1983; Rath et al. 1985; Manginas & Cokkino 2006); however, a number of conditions may contribute to ectasia formation, including Kawasaki disease, connective tissue disorders and as a complication of coronary angioplasty (Manginas & Cokkino 2006).
Two recent reviews of CAE have challenged the commonly held assumption that this disorder simply represents a severe variant of coronary atherosclerotic disease. Ramappa et al. (2007) have proposed that the aetiology of CAE is more closely related to that of aortic aneurysmal disease and have suggested that similar genetic abnormalities may be involved. Yetkin and Waltenberger (2007) observed that CAE has different risk factors from atherosclerotic coronary artery disease and noted that CAE is associated with alterations in extracellular matrix turnover and inflammation. Further advancement in our understanding of the pathogenesis of CAE has been hampered by the absence of a reproducible animal model of this disease.
A considerable literature has documented the critical role of matrix metalloproteinases (MMPs) in the development of experimental and human aortic aneurysmal disease (Freestone et al. 1995; Davis et al. 1998; Longo et al. 2002; Thompson & Cockerill 2006; Raffetto & Khalil 2008). In particular, the activities of two specific metalloproteinases, MMP-2 and MMP-9, have been shown to act in concert for the development of experimental aortic aneurysms in transgenic mice with genetic ablation of MMP-2 and MMP-9 (Longo et al. 2002). In this model and in human aortic aneurysmal tissue (Freestone et al. 1995), MMP-2 initiates destruction of medial elastin, with subsequent release of chemoattractant peptides and perivascular infiltration by MMP-9-secreting inflammatory cells.
Goodall et al. (2001) postulated that aortic aneurysmal disease is a component of a systemic disorder characterized by ubiquitous elevation of MMP-2 expression in the vasculature. Further support for a critical role of MMP-2 was provided by the observations of Papadakis et al. (2004), in which the incidence of CAE was five times more likely to be present together with ascending aortic aneurysms. Stajduhar et al. (1993) noted that 20.8% of patients operated on for abdominal aortic aneurysms had concurrent CAE compared with only 2.9% of patients operated on for occlusive peripheral vascular disease.
We have recently characterized the cardiac function of transgenic mice engineered to express active MMP-2 under control of the α-myosin heavy chain promoter (Wang et al. 2006; Bergman et al. 2007; Zhou et al. 2007). Transgenic mice expressing active MMP-2 develop severe ventricular remodelling with systolic dysfunction ultimately resulting in heart failure. During a systematic histological analysis of the MMP-2 transgenic mice we noted that the coronary arteries showed extensive areas of ectasia with many features characteristic of human CAE. The details of these observations are outlined in this report, which supports the hypothesis that CAE is pathophysiologically more closely related to aortic aneurysmal disease than atherosclerosis and that MMP-2 is the key mediator of this process. The transgenic mice described in this report could conceivably offer the first viable animal model of CAE suitable for the analysis of specific therapeutic interventions.
Materials and methods
Cardiac-specific MMP-2 transgenic mice
Cardiac-specific MMP-2 transgenic mice were generated as described in detail (Bergman et al. 2007). The transgene consists of the full-length MMP-2 cDNA encoding a constitutively active MMP-2 protein due to an introduced V107→G107 mutation in the prodomain sequence. To facilitate transgene visualization, a c-myc epitope tag was added to the C-terminus of the MMP-2 expression cassette. Cardiac-specific expression was driven by the α-myosin heavy chain promoter. Transgenic animals were maintained as heterozygotes within the outbred CD-1 background. All experiments and animal husbandry were carried out following the NIH Guide for the Care and Use of Laboratory Animals after local IACUC approval.
Tissue preparation and histology
Four- and 8-months-old transgenics and age-matched littermate controls were anaesthetized with ketamine (80 mg/kg i.p.) and xylazine (16 mg/kg i.p.). A laparotomy was performed to expose the suprarenal inferior vena cava. KCl (20 mM) was injected into the vena cava to induce diastolic arrest. A thoracotomy was performed and the left ventricle was cannulated with a 27-gauge needle and perfused with normal saline followed by 4 °C buffered paraformaldehyde at 20 mm Hg. Whole hearts were excised and immersion-fixed in paraformaldehyde overnight at 4 °C, followed by storage in 70% ethanol. Paraffin-embedded sections were prepared using standard methodology. The Masson trichrome and Verhoeff’s elastin stains were performed using standard methodology.
Measurement of arterial luminal areas
To determine coronary artery luminal areas mid-ventricular sections were examined at 100 × using OpenLab Software (Improvision, Coventry, UK) on six transgenics and six wild-type litter mate controls at 4 and 8 months of age. Femoral artery luminal areas were determined on sections from paraformaldehyde perfusion-fixed hind limbs on 8-month-old wild-type and transgenic mice. Luminal areas are expressed as μm2± SEM.
Coronary artery latex casting
Eight-month-old transgenics and littermate controls (n = 6 for each group) were anaesthetized and a thoracotomy performed. The left ventricle was cannulated with a 223/4 gauge needle and perfused with Batson’s # 17 liquid plastic (Polysciences, Inc., Warrington, PA, USA). The composition of the polymer was 5 ml of Batson’s # 17 base monomer with 5% red pigment supplemented with 1.2 ml of catalyst mixed with 5 ml of base monomer supplemented with 75 μl of promoter. The infrarenal vena cava was incised to allow venous drainage. After 2 h of polymerization, the heart, thoracic and abdominal aorta were excised en bloc and placed in saturated KOH for 2 weeks with regular solution changes. After tissue maceration was complete, the resulting latex cast of the heart and attached vasculature was digitally acquired. The number of primary and secondary branches from the major coronary arteries was determined by direct inspection. Relative coronary vascular volumes were determined by quantifying the red latex pixels using Image J software (National Institutes of Health, Bethesda, MD, USA).
Statistics
All results are expressed as mean ± SE; a two-tailed unpaired t-test was used to examine differences between normal and transgenic vessels, with a P-value of < 0.05 defined as significant.
Results and discussion
Cardiac-specific transgenic expression of MMP-2
As recently reported in detail (Wang et al. 2006; Bergman et al. 2007; Zhou et al. 2007), we generated transgenic mice with cardiac-specific expression of MMP-2 using the α-myosin heavy chain promoter to drive expression of a murine MMP-2 cDNA. The expression cassette includes a C-terminus c-myc epitope tag to distinguish transgenic MMP-2 from intrinsic MMP-2 protein. The prodomain of the cDNA was mutated such that the expressed MMP-2 protein is enzymatically active. Ventricular lysates from the transgenic mice had a 3.9 ± 0.2-fold increase in MMP-2 expression levels as compared with controls and this increased further by 8 months of age (Bergman et al. 2007). The active MMP-2 transgene was diffusely expressed in cardiomyocytes, but enzyme activity was also detectable in the coronary vasculature (Wang et al. 2006). The primary phenotypic change observed in the MMP-2 transgenics was progressive ventricular remodelling and systolic dysfunction (Bergman et al. 2007).
Transgenic expression of active MMP-2 induces coronary ectasia
As shown in Figure 1, mid-ventricular cardiac sections using Masson trichrome show extensive coronary artery dilatation and increased branching in the 8-month-old MMP-2 transgenics as compared with the age-matched litter mate controls. In addition to the increases in coronary artery luminal area and branching, there was extensive perivascular fibrosis in the MMP-2 transgenic hearts. The coronary arteries in the MMP-2 transgenics did not demonstrate medial thickening and there was no evidence for atherosclerotic changes.
Figure 1.
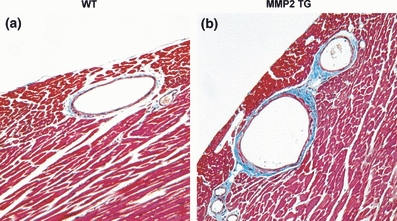
Masson trichrome stain of mid-ventricular coronary artery cross sections from wild-type (WT) and MMP-2 transgenic (MMP2 TG) mice at 8 months. (a) Cross section of WT coronary artery showing normal diameter, minimal branching and normal amounts of perivascular fibrosis. (b) Cross section of MMP-2 transgenic showing coronary artery dilatation, multiple primary and secondary branching arteries and large amounts of perivascular fibrosis (×200).
We performed a quantitative assessment of mid-ventricular coronary artery luminal areas and the results of these analyses are summarized in Figure 2. There were no significant differences in the coronary artery luminal areas between the wild-type (WT) and MMP-2 transgenic (TG) mice at 4 months of age (WT: 4319 ± 937 μm2vs. TG: 5659 ± 813 μm2, P = 0.29; n = 6 for each group). In contrast, at 8 months of age there were quantitatively significant differences in the coronary artery luminal areas between the wild-type and MMP-2 transgenic mice (WT: 6209 ± 894 μm2, TG: 10,184 ± 936 μm2, P < 0.05, n = 6 for each group). Thus, coronary artery dilation in the transgenic mice is an age-dependent acquired phenotypic change not related to developmental abnormalities of the coronary arteries.
Figure 2.
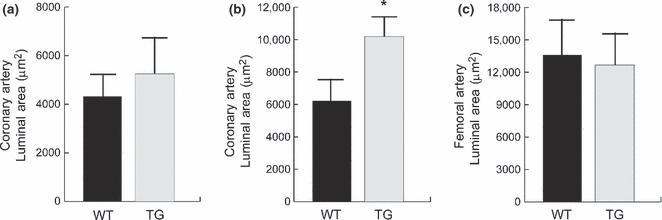
Luminal areas of coronary and femoral arteries of WT and MMP-2 TG mice (a) Coronary artery luminal areas of WT and MMP-2 TG mice at 4 months of age. There is a trend for increased coronary artery luminal diameters in the MMP-2 TG mice, but does not reach statistical significance. (b) Coronary artery luminal areas of WT and MMP-2 TG mice at 8 months of age: there is a significant increase in coronary artery luminal diameters (approximately 60%) in the MMP-2 TG mice. (c) Femoral artery luminal diameters of WT and MMP-2 TG mice at 8 months of age. There is no significant difference in the luminal areas between the two study groups.
We also evaluated femoral artery luminal areas at 8 months to determine whether arterial dilation in the MMP-2 transgenics was restricted to the coronary arteries and not representative of a systemic disorder. As shown in Figure 2c, there were no significant differences in the femoral artery luminal areas between wild-type and transgenic mice at 8 months (WT: 14,587 ± 2580 μm2vs. TG: 12,665 ± 3156 μm2, P = 0.67, n = 6).
MMP-2 transgene localization-recruitment to the coronary vasculature
The c-myc epitope tag on the C-terminus of the cDNA permits histochemical differentiation between transgenic and intrinsic MMP-2. Immunoperoxidase staining of MMP-2 transgenic mid-ventricular sections at 4 months revealed the expected cardiomyocyte distribution of the c-myc epitope, consistent with expression driven by the α-myosin heavy chain promoter, while no c-myc staining was evident in the controls (Figure 3a,b). There was no detectable c-myc staining within the coronary vasculature or adventitia at this time. At 8 months transgene expression was considerably more patchy in the cardiomyocytes, consistent with age-dependent transgene silencing, but c-myc epitope staining was readily detected in the coronary adventitial areas (Figure 3c) and within the vascular media as well (d). Transgene expression was most prominent in areas of vascular dilatation. Thus, at a time when coronary artery dilatation is present, there is evidence for active transgenic MMP-2 expression within both the vascular media and adventitia.
Figure 3.
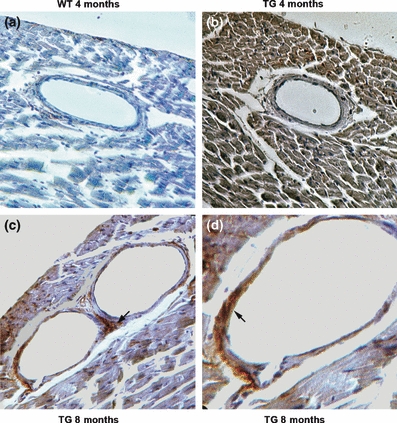
Immunohistochemical detection of c-myc-tagged MMP-2 transgene expression in coronary arteries of WT and MMP-2 TG mice at 4 and 8 months. (a) There is no detectable expression of the c-myc epitope in the WT heart and coronary arteries. (b) Dark brown staining for the c-myc epitope is widely present within the cardiomyocytes in 4-month-old hearts, whereas the cellular components of the coronary artery are not stained. (c) Prominent staining for the transgenic c-myc epitope tag is evident within the perivascular adventitia (arrow) of 8-month-old mice, whereas transgene expression is patchy in the cardiomyocytes due to age-dependent silencing. (d) Medial staining for the transgenic c-myc epitope is evident in this dilated coronary artery from an 8-month-old mouse. (a–c × 200; d × 400).
Coronary artery dilatation is associated with elastin and media degeneration
We performed Verhoeff’s elastin stain on ventricular cross sections from 8-month-old MMP-2 transgenics and age-matched litter mate controls to assess integrity of coronary artery elastin. Representative results of these studies are shown in Figure 4. In comparison with the intact elastin seen in the control coronary artery cross sections (a), there is degradation and fragmentation of the elastin in the coronary artery cross sections from the MMP-2 transgenics.
Figure 4.
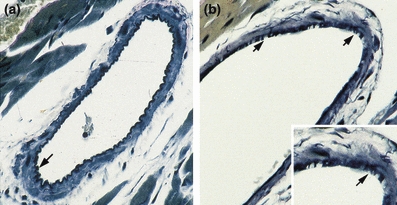
Verhoeff’s elastin stain of mid-ventricular coronary artery cross sections at 8 months of age. (a) WT control with intact internal elastic lamina (arrow). (b) MMP-2 TG: The internal elastic lamina is fragmented and disrupted (arrows). (a, b × 300, inset ×500).
While there was a generalized increase in coronary artery luminal diameters in the MMP-2 transgenics, examination of serial sections revealed regions of the coronary arteries with ectasia and aneurysmal dilatation. Representative examples are detailed in Figure 5. Figure 5a shows aneurysmal dilatation of the proximal left coronary artery with prominent perivascular fibrosis. In some areas, the degree of arterial ectasia was very marked (b), and higher power examination revealed foci of proliferating vascular smooth muscle cells with destruction of the medial layer (c). In addition, there were frequent foci of intense perivascular infiltration with inflammatory cells with the primary morphologic features of monocyte/macrophages (Figure 5d).
Figure 5.
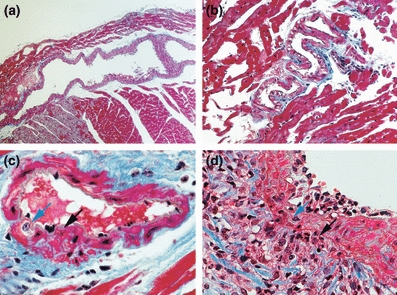
Characterization of coronary artery ectasia in MMP-2 TG mice. (a) Longitudinal section of a mid-ventricular coronary artery from an 8-month-old transgenic mice, demonstrating massive, localized arterial dilatation with ectasia. (b) Severely ectatic coronary artery with perivascular cellular infiltrates. (c) Focus of medial disruption (black arrow) immediately adjacent to proliferating vascular smooth muscle cell (blue arrow). (d) Focus of profound perivascular inflammatory cell infiltrate (black arrow) extending through the vascular media layer (blue arrow). (Masson trichrome stain; a × 50; b × 100; c, d × 400).
These findings are concordant with the two-step model of aneurysm formation proposed by Freestone et al. (1995), in which initial elastin degradation by MMP-2 is followed by a second phase of monocytes/macrophages which contribute further to vascular injury.
Three-dimensional assessment of coronary artery ectasia-latex casting
To gain a more complete perspective on the extent of coronary artery dilatation, ectasia and aneurysmal dilatation we performed latex casting of the coronary arteries from 8-month-old mice (wild-type and MMP-2 transgenic, n = 6 for each group) following retrograde infusion of Batson’s liquefied plastic and tissue maceration with saturated KOH. Representative examples are shown in Figure 6. As compared with the coronary artery tree in the age-matched litter mate controls (a), both proximal and distal branches of the coronary arteries revealed extensive ectasia (b). In addition, fusiform dilatation was frequently noted in the proximal coronary arteries (c).
Figure 6.
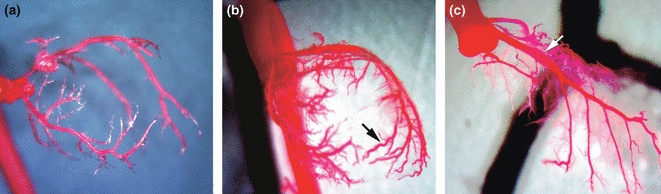
Latex casts of WT and MMP-2 TG coronary arterial trees from 8-month-old mice. (a) WT coronary arterial tree. (b) TG coronary arterial tree with a diffuse increase in diameter along with increased arterial branching and ectasia (black arrow). (c) Extensive fusiform dilatation of the left anterior descending coronary artery (white arrow). (a–c: ×10).
We quantified the extent of primary and secondary arterial branching from the left main coronary arteries of wild-type and MMP-2 transgenic mice and note that there was an approximate 50% increase in branching of this vessel in the transgenic mice (Figure 7a). In addition, total coronary artery volumes were increased by nearly 100% (b). These quantitative assessments are consistent with enhanced arteriogenesis in the MMP-2 transgenics. Along with a generalized increase in the volumes of individual coronary vessels, this finding correlates with the enlarged coronary artery diameters quantified in Figure 2. Thus, in addition to MMP-2-mediated coronary ectasia, there is also adaptive coronary arteriogenesis, presumably because of the development of systolic dysfunction and ventricular remodelling in this transgenic model. In this regard, Cai et al. (2004) have emphasized the critical role of MMP-2 for the remodelling of the vascular tunica media essential for arteriogenesis in the canine heart.
Figure 7.
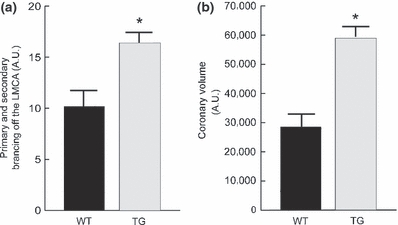
Quantification of coronary arterial vasculature branching and volumes of latex casts from 8-month-old mice. (a) Primary and secondary branches extending from the left main coronary artery (LMCA). There is a significant increase in the extent of primary and secondary arterial branching in the MMP-2 TG mice. (b) Total coronary arterial volumes as determined by quantitative densitometry of digitized images of the coronary artery latex casts. There is a significant, greater than two-fold increase in total coronary artery volumes in the MMP-2 TG mice.
These experiments provide proof of principle for a direct role of MMP-2 for the initiation and progression of CAE in the absence of atherosclerotic disease. The findings are consistent with the concept that arterial aneurysmal disease is a systemic disorder in which elevated MMP-2 expression is the common and mediating feature.
Optimally, one would wish to determine the effects of MMP-2 inhibition on the formation or resolution of coronary ectasia in our transgenic model. Unfortunately, we have been unable to obtain a highly selective MMP-2 inhibitor that does not exhibit inhibitory activities against other members of the large MMP gene family, of which there are 28. Doxycycline is frequently used in rodent studies for MMP inhibition, but this compound is non-selective and has other activities not related to inhibition of proteolytic activity (Sapadin & Fleischmajer 2006).
An important question not directly addressed in this study concerns the pathophysiological mechanisms driving enhanced vascular synthesis of MMP-2. Finkelstein et al. (2005) noted an association between proteolytic activities and elevation of inflammatory and neurohumoral markers in patients with CAE. Li et al. (2007) have postulated a linkage between systemic inflammation and CAE.
While frequently mischaracterized as constitutive in nature, MMP-2 synthesis is subject to multiple levels of tissue-specific and cytokine-driven transcriptional and translational regulation. For example, cardiac ischemia/reperfusion injury induces MMP-2 transcription and synthesis in all cardiac cell types, including the coronary endothelium, via induction of the AP-1 transcription factors FosB and JunB (Alfonso-Jaume et al. 2006).
Genetic variation in the MMP-2 gene could also be involved in enhanced MMP-2 synthesis. Lamblin et al. (2002) examined single nucleotide polymorphisms (SNPs) in the promoter regions of several MMP genes, including MMP-2, in a case–control study of CAE. While no significant associations between a –1306 C/T MMP-2 promoter SNP variant was observed, the analytic power of this study was limited by the use of a single SNP as opposed to a statistically much more robust promoter haplotype analysis. Functional MMP-2 promoter haplotypes have been described in terms of lung cancer susceptibility and MMP-2 transcriptional responses to oestrogen (Harendza et al. 2003; Zhou et al. 2005), suggesting that future analyses of MMP-2 promoter haplotypes in the setting of CAE may be more informative.
In summary, we have described a unique experimental model of CAE that delineates a critical mediating role for MMP-2. The study provides further support for the hypothesis that arterial aneurysmal disease is a systemic disorder characterized by enhanced vascular synthesis of MMP-2. Finally, the availability of a viable animal model of CAE will provide an opportunity to evaluate potential non-surgical therapies.
Acknowledgments
This work was supported by National Heart, Lung and Blood Institute Grant PO-1-HL-68738 to J. S. Karliner and D. H. Lovett.
Disclosure statement
None of the authors has a conflict of interest to declare.
References
- Alfonso-Jaume MA, Bergman MR, Mahimkar R, et al. Cardiac ischemia-reperfusion injury induces matrix metalloproteinase-2 expression through the AP-1 components FosB and JunB. Am. J. Physiol. Heart Circ. Physiol. 2006;291:H1838–H1846. doi: 10.1152/ajpheart.00026.2006. [DOI] [PubMed] [Google Scholar]
- Bergman MR, Teerlink JR, Mahimkar R, et al. Cardiac matrix metalloproteinase-2 expression independently induces marked ventricular remodeling and systolic dysfunction. Am. J. Physiol. Heart Circ. Physiol. 2007;292:H1847–H1860. doi: 10.1152/ajpheart.00434.2006. [DOI] [PubMed] [Google Scholar]
- Cai WJ, Kocsis E, Wu X, et al. Remodeling of the vascular tunica media is essential for development of collateral vessels in the canine heart. Mol. Cell. Biochem. 2004;264:201–210. doi: 10.1023/b:mcbi.0000044389.65590.57. [DOI] [PubMed] [Google Scholar]
- Davis V, Persidskaia R, Baca-Regen L, et al. Matrix metalloproteinase-2 production and its binding to the matrix are increased in abdominal aortic aneurysms. Arterioscler. Thromb. Vasc. Biol. 1998;18:1625–1633. doi: 10.1161/01.atv.18.10.1625. [DOI] [PubMed] [Google Scholar]
- Finkelstein A, Michowitz Y, Abashidze A, Miller H, Keren G, George J. Temporal association between circulating proteolytic, inflammatory and neurohormonal markers in patients with coronary ectasia. Atherosclerosis. 2005;179:353–359. doi: 10.1016/j.atherosclerosis.2004.10.020. [DOI] [PubMed] [Google Scholar]
- Freestone T, Turner RJ, Coady A, Higman DJ, Greenhalgh RM, Powell JT. Inflammation and matrix metalloproteinases in the enlarging abdominal aortic aneurysm. Arterioscler. Thromb. Vasc. Biol. 1995;15:1145–1151. doi: 10.1161/01.atv.15.8.1145. [DOI] [PubMed] [Google Scholar]
- Goodall S, Crowther M, Hemingway DM, Bell PR, Thompson MM. Ubiquitous elevation of matrix metalloproteinase-2 expression in the vasculature of patients with abdominal aneurysms. Circulation. 2001;104:304–309. doi: 10.1161/01.cir.104.3.304. [DOI] [PubMed] [Google Scholar]
- Harendza S, Lovett DH, Panzer U, Lukacs Z, Kuhno P, Stahl RA. Linked common polymorphisms in the gelatinase A promoter are associated with diminished transcriptional response to estrogen and genetic fitness. J. Biol. Chem. 2003;278:20490–20499. doi: 10.1074/jbc.M211536200. [DOI] [PubMed] [Google Scholar]
- Lamblin N, Bauters C, Hermant X, Lablanche J-M, Helbecque N, Amouyel P. Polymorphisms in the promoter regions of MMP-2, MMP-3, MMP-9 and MMP-12 genes as determinants of aneurysmal coronary artery disease. J. Am. Coll. Cardiol. 2002;40:43–48. doi: 10.1016/s0735-1097(02)01909-5. [DOI] [PubMed] [Google Scholar]
- Li J-J, Li Z, Li J. Is any link between inflammation and coronary artery ectasia? Med. Hypotheses. 2007;69:678–683. doi: 10.1016/j.mehy.2006.09.071. [DOI] [PubMed] [Google Scholar]
- Longo GM, Xiong W, Greiner TC, Zhao Y, Fiotti N, Baxter BT. Matrix metalloproteinases 2 and 9 work in concert to produce aortic aneurysms. J. Clin. Invest. 2002;11:625–632. doi: 10.1172/JCI15334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manginas A, Cokkino DV. Coronary artery ectasias: imaging, functional assessment and clinical implications. Eur. Heart J. 2006;27:1026–1031. doi: 10.1093/eurheartj/ehi725. [DOI] [PubMed] [Google Scholar]
- Papadakis M, Leontiadis E, Manginas A, et al. Frequency of coronary artery ectasias in patients operated on for ascending aortic aneurysms. Am. J. Cardiol. 2004;94:1433–1435. doi: 10.1016/j.amjcard.2004.08.019. [DOI] [PubMed] [Google Scholar]
- Raffetto JD, Khalil RA. Matrix metalloproteinases and their inhibitors in vascular remodeling and vascular disease. Biochem. Pharmacol. 2008;75:346–359. doi: 10.1016/j.bcp.2007.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramappa P, Koitam A, Kuivanemi H, Thatai D. Coronary artery ectasia-is it time for a reappraisal? Clin. Cardiol. 2007;30:214–217. doi: 10.1002/clc.20002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rath S, Har-Zahav Y, Battler A, et al. Fate of nonobstructive aneurysmatic coronary artery disease: angiographic and clinical follow-up report. Am. Heart J. 1985;109:785–791. doi: 10.1016/0002-8703(85)90639-8. [DOI] [PubMed] [Google Scholar]
- Sapadin AN, Fleischmajer R. Tetracyclines: nonantibiotic properties and their clinical implications. J. Am. Acad. Derm. 2006;54:258–265. doi: 10.1016/j.jaad.2005.10.004. [DOI] [PubMed] [Google Scholar]
- Stajduhar KC, Laird JR, Rogan KM, Wortham DC. Coronary arterial ectasia: increased prevalence in patients with abdominal aortic aneurysm as compared to occlusive atherosclerotic peripheral vascular disease. Am. Heart J. 1993;125:86–92. doi: 10.1016/0002-8703(93)90060-m. [DOI] [PubMed] [Google Scholar]
- Swaye PS, Fisher LD, Litwin P, et al. Aneurysmal coronary artery disease. Circulation. 1983;67:134–138. doi: 10.1161/01.cir.67.1.134. [DOI] [PubMed] [Google Scholar]
- Thompson M, Cockerill G. Matrix metalloproteinase-2: the forgotten enzyme in aneurysm pathogenesis. Ann. N.Y. Acad. Sci. 2006;1085:170–174. doi: 10.1196/annals.1383.034. [DOI] [PubMed] [Google Scholar]
- Wang GY, Bergman MR, Nguyen AP, et al. Cardiac transgenic matrix metalloproteinase-expression directly induces impaired contractility. Cardiovasc. Res. 2006;69:688–696. doi: 10.1016/j.cardiores.2005.08.023. [DOI] [PubMed] [Google Scholar]
- Yetkin E, Waltenberger J. Novel insights into an old controversy. Is coronary artery ectasia a variant of coronary atherosclerosis? Clin. Res. Cardiol. 2007;96:331–339. doi: 10.1007/s00392-007-0521-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, Yu C, Miao X, et al. Functional haplotypes in the promoter of matrix metalloproteinase-2 and lung cancer susceptibility. Carcinogenesis. 2005;26:1117–1121. doi: 10.1093/carcin/bgi057. [DOI] [PubMed] [Google Scholar]
- Zhou HZ, Ma X, Gray MO, et al. Transgenic MMP-2 expression induces latent cardiac mitochondrial dysfunction. Biochem. Biophys. Res. Commun. 2007;358:189–195. doi: 10.1016/j.bbrc.2007.04.094. [DOI] [PMC free article] [PubMed] [Google Scholar]


